Abstract
Intrinsically photosensitive retinal ganglion cells (ipRGCs) project to the suprachiasmatic nucleus (SCN), and are essential for normal photic entrainment of global circadian rhythms in physiology and behavior. The effect of light on the central clock is dependent on circadian phase, and the retina itself contains intrinsic circadian oscillators that can alter its sensitivity to light. This raises the possibility that the ipRGCs, and hence the photoentraining signals in the retinohypothalamic tract, are subject to circadian modulation. Although the ipRGC photopigment, melanopsin, reportedly exhibits circadian variations in expression, there has been no direct test of the hypothesis that ipRGC sensitivity is under circadian control. Here, we provide such a test by measuring the sensitivity of intrinsic photoresponses of rat ipRGCs at four circadian times (CTs) using multielectrode array (MEA) recording. We observe little if any circadian modulation in the threshold of intrinsic ipRGC photoresponses. We do observe, however, that very bright light evoked significantly more spiking early in the subjective night (CT12-13) than at other circadian phases. Thus, the gain of the melanopsin-driven response is slightly increased in the early night, at roughly the circadian phase when melanopsin synthesis is thought to be elevated. However, this gain change is probably too modest to contribute much to shape the phase response curve for behavioral photoentrainment.
Keywords: ipRGC, melanopsin, entrainment, sensitivity, gain, retina
Introduction
Circadian clocks enable organisms to predict daily environmental changes and to optimize their activity accordingly. In mammals, the master biological pacemaker resides in the suprachiasmatic nucleus of the hypothalamus (SCN) (Antle and Silver, 2005; Morin and Allen, 2006). Peripheral circadian oscillators can also be found in many organs and tissues, including the retina (Anderson and Green, 2000; Green and Besharse, 2004; Reppert and Weaver, 2002; Tosini and Fukuhara, 2002). The retina exhibits robust daily oscillations in diverse structural features and functional processes, including disk shedding by rod outer segments (Goldman et al., 1980; LaVail, 1976; Terman et al., 1993), cell proliferation (Chiu et al., 1995), melatonin and dopamine synthesis (Doyle et al., 2002; Nguyen-Legros et al., 1996; Tosini and Menaker, 1996; Wirz-Justice et al., 1984), amplitude of electroretinogram (ERG) components (Brandenburg et al., 1983), and retinal sensitivity (Bassi and Powers, 1986, 1987; Li and Dowling, 1998; Walker and Olton, 1979). These are true circadian processes, not merely effects of light exposure, because they are observable even when lighting conditions are held constant. Moreover, they are intrinsic to the retina because the rhythmicity is maintained for multiple circadian cycles in culture (Cahill and Besharse, 1993; Ruan et al., 2008; Tosini and Menaker, 1996).
The retina plays a key role in the regulation of the central circadian pacemaker through direct axonal projections to the SCN. This pathway can advance or delay the central clock to keep it synchronized to the environmental light cycle. These signals can be traced to a specialized population of retinal ganglion cells that express the photopigment melanopsin and are capable of responding directly to light (Berson et al., 2002; Fu et al., 2005; Gooley et al., 2001; Hannibal et al., 2002; Hattar et al., 2002; Melyan et al., 2005; Newman et al., 2003; Panda et al., 2005; Provencio et al., 2002; Qiu et al., 2005; Warren et al., 2003). These intrinsically photosensitive retinal ganglion cells (ipRGCs) are essential for normal photoentrainment of the central pacemaker, and for other non-image-forming visual functions, such as the pupillary light reflex and suppression of the nocturnal synthesis of melatonin (Goz et al., 2008; Guler et al., 2008; Hatori et al., 2008; Hattar et al., 2003; Lucas et al., 2003; Panda et al., 2003; Panda et al., 2002; Ruby et al., 2002; Semo et al., 2003; Zhu et al., 2007).
The impact of intraretinal circadian modulation on ipRGCs and, through them, on the central pacemaker is poorly understood. The effect of light on SCN clockwork depends on circadian phase, and it is possible that at least some of this dependence derives from circadian modulation of ipRGC photoresponses. Indeed, ipRGCs exhibit a circadian variation in levels of melanopsin mRNA and protein (Hannibal et al., 2005; Sakamoto et al., 2005; Sakamoto et al., 2004). It remains unknown whether the rhythm in melanopsin expression, or alternative mechanisms, translate into circadian variations in the light sensitivity of ipRGCs. Nor is it known to what extent ipRGCs may participate in regulating intraretinal circadian rhythms. Several lines of evidence suggest that ipRGCs are involved in circadian modulation of retinal signaling as reflected in the electroretinogram (Barnard et al., 2006; Hankins and Lucas, 2002) and, indeed, that they may be required for such modulation (Barnard et al., 2006). This raises the possibility that ipRGCs may function as retinal circadian pacemakers, but other evidence casts doubt on this. For example, the rhythm in melanopsin expression is abolished after degeneration of rods and cones (Sakamoto et al., 2004), suggesting it is imposed by synaptic networks and circadian clockworks extrinsic to the ipRGCs. Moreover, ipRGCs lack detectable expression of the clock gene mPer1 (Witkovsky et al., 2003), though it should be noted that this gene is not required for a functioning clock (Bae et al., 2001; Zheng et al., 2001).
In this study, we used multielectrode array recordings of rat ipRGCs pharmacologically isolated from synaptic influences to determine whether their intrinsic, melanopsin-based photoresponses are subject to circadian variations in sensitivity. We observe a modest increase in the gain of ipRGC phototransduction early in the subjective night, but no appreciable circadian modulation of sensitivity. This is in keeping with the view that photic effects on circadian phase are gated primarily in the suprachiasmatic nucleus rather than within the retina.
Materials and Methods
Animals
Adult male Sprague-Dawley rats (Charles River, Wilmington, MA, USA) 2–3 months of age and weighing 325–375 g were used in this study. Use and handling of animals were strictly in accordance to National Institutes of Health guidelines for work with laboratory animals and were approved by the Institutional Animal Care and Use Committee at Brown University. Animals were housed for at least 7 days in a 12 hr light: 12 hr dark (LD) cycle. White fluorescent light (40 lux) was presented from Zeitgeber time 0 (ZT0) to ZT12. Animals were then transferred into constant darkness (DD) at least 12 hours and typically less than 48 hours before the retinal recordings were made. This design allowed us to uncouple circadian phase from recent lighting history, which are linked under LD conditions, and to test sensitivity in fully dark adapted retinas at all circadian phases. The time in darkness did not differ significantly among the four circadian phases tested (one way ANOVA; p = 0.32), namely early day (CT0-1), midday (CT6-7), early night (CT12-13), and midnight (CT18-19). The ages of animals were also comparable among the four groups. Because the time in DD was short (<48 hours), the circadian time (CT) at the time of recording could be inferred to a good approximation by extrapolating from the prior LD cycle to which the animals had been fully entrained (CT=ZT), using the free-running circadian period (tau) of 24.09 hours in DD conditions in this strain of rats (Summer et al., 1984). For example, a recording made 25 hours after the previous light onset (ZT0) would be assumed to be taken at CT1.
Retinal preparation
Retinas were prepared for electrophysiological recording under very dim red light. Only one retina was tested in each animal. Retinas were harvested about 1 hr before the CT phase to be tested (e.g., at about CT23-0 for recordings at CT0-1). The rats were sacrificed by carbon dioxide asphyxiation and the eyes immediately enucleated. The cornea, lens, and vitreous body were removed and the eye cup submerged in bicarbonate-buffered Ames’ medium (Sigma, St. Louis, MO, USA) equilibrated with 95% O2 and 5% CO2. The retina was dissected from the eye cup and placed photoreceptor side down on a piece of nylon mesh. Three to four small cuts were made to flatten the retina.
MEA recording
The retina was then transferred into the recording chamber of an MEA-1060 multielectrode array (Multi Channel System MCS GmbH, Reutlingen, Germany), with the ganglion cell layer facing the recording electrodes (100 μm in diameter, at a center-to-center spacing of 700 μm). A stainless steel ring was used to anchor the retina. The retina was continuously superfused at 3–4 ml min−1 with oxygenated bicarbonate-buffered Ames’ medium and maintained at 32°C with a temperature controller (TC-324B, Warner Instruments, Hamden, CT, USA). Amplified voltage data were digitized at 25 kHz using a PC-based A/D interface card and MC_Rack software (MCS), and stored on a personal computer. The signals were bandpass filtered between 200 Hz and 3 kHz.
Chemicals
Once spike amplitudes had stabilized (about 30 min after the retina was placed in the chamber), a pharmacological cocktail (see below) was continuously applied through the perfusion system for the remainder of the experiment to block signaling from rod and cone photoreceptors to the inner retina. Under these conditions, the light responses of ipRGCs were generated entirely by their own melanopsin-based phototransduction, permitting us to assess whether their intrinsic photosensitivity undergoes circadian modulation. It should be noted, however, that this pharmacological regime may also have masked any such modulation dependent upon retinal networks, as discussed later.
Data acquisition started 15 to 20 min after drug application. The cocktail for synaptic isolation included: 50 μM L (+) -2-amino-4-phosphonobutyrate (L-AP4, group III metabotropic glutamate receptor agonist), 40 μM 6,7-dinitroquinoxaline-2,3-dione (DNQX, AMPA/kainate receptor antagonist), 30 μM D-2-amino-5-phosphonovalerate (D-AP5, NMDA receptor antagonist). In some cases, we augmented the cocktail with one of two agents known to block gap junctions, namely 100 μM 3β-Hydroxy-11-oxoolean-12-en-30-oic acid 3-hemisuccinate (carbenoxolone), or 200 μM meclofenamic acid (MFA). All pharmacological reagents were freshly prepared from aqueous stock solutions by dilution with Ames’ medium. L-AP4, DNQX, and D-AP5 were obtained from Tocris (Ellisville, MO, USA). Carbenoxolone and MFA were obtained from Sigma-Aldrich (St. Louis, MO, USA).
Light stimulation
Once in the chamber, retinas were kept in complete darkness except when probed with test stimuli described here. Full-field white light stimuli were generated with a tungsten-halogen microscopy illuminator (model EW-09741-50, Cole-Parmer Instruments, Vernon Hills, IL, USA) equipped with a custom made electromechanical shutter, and delivered onto the retina by a fiber optic cable. The unattenuated (‘0 log’) intensity at the surface of the preparation was 4.3 mW cm−2 or 1.04 × 1016 photons cm−2 s−1 sampled at 480 nm. As expected for a tungsten source, the spectral power distribution of this white light stimulus rose as a nearly linear function of wavelength, with unattenuated intensity of 0.36 mW cm−2 at 400 nm and 23.9 mW cm−2 at 600 nm. Stimulus intensities were adjusted by introducing neutral density filters (Newport Oriel Instruments, Stratford, CT, USA) into the light path. Stimuli were presented in a series that was monotonically ascending in intensity and identical for all experiments. Interstimulus intervals varied within the series, being shortest between the dimmest stimuli (3 min) and longest for the highest intensities (30 min), and the entire irradiance-response process lasted for about 70 min. The intent of this design was to minimize the effects of light adaptation on the intrinsic light response (Wong et al., 2005) while completing the series as quickly as practical so as to avoid response rundown.
Data analysis
Offline data analysis was performed using Offline Sorter software (Plexon Inc., Dallas, TX, USA), which uses cluster analysis to discriminate the action potentials from individual neurons. We derived two measures of response amplitude from the spike data: “peak frequency”, calculated from the maximum number of spikes in a one sec bin; and “total spikes”, comprising the sum of all action potentials occurring during the stimulus. We did not correct these measures for the background discharge rate because >98% of cells (135/137 cells) entirely lacked spontaneous firing; the two exceptional cells fired spontaneously at <5 Hz. We encountered a few cells with higher background discharge, but these had weak and inconsistent light responses and were excluded from this analysis. For the purposes of Table 1, the rare cells (2 of 137) that fired spontaneously in the dark were considered to be light responsive only when the sum of spikes during the 10 sec light stimulus was at least twice that during the 10 sec of darkness preceding the stimulus. All other cells were considered light responsive if they spiked even once during the 60 sec following stimulus onset. Data were further processed with OriginPro 7.0 (OriginLab Corp., Northampton, MA, USA) to generate intensity-response plots and to perform statistical analysis. Raw data were also converted into .ABF files with MC DataTool software (MCS), and then viewed with Clampfit 10.0 (Axon Instruments, UnionCity, CA, USA) or processed with OriginPro 7.0 to generate Fig. 1.
Table 1.
Summary of the proportion of ipRGCs responding as a function of light intensity and circadian phase
| Retinas | ipRGCs | % ipRGCs responding | ||||
|---|---|---|---|---|---|---|
| −2 log I | −3 log I | −3.5 log I | −4 log I | |||
| early day | 9 | 35 | 100 | 97 | 69 | 17 |
| midday | 11 | 33 | 100 | 97 | 58 | 30 |
| early night | 10 | 34 | 100 | 91 | 74 | 24 |
| midnight | 11 | 35 | 100 | 100 | 60 | 23 |
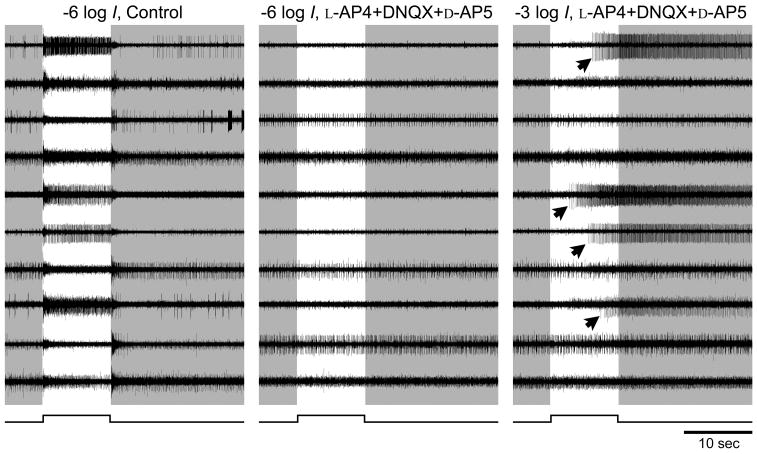
Figure 1.
Pharmacological isolation of ipRGCs. Raw voltage traces from a rat whole mount retina preparation recorded simultaneously from ten electrodes of a multielectrode array under three different conditions. Most electrodes recorded spikes originating from more than one cell. Left and middle columns: Spike responses to a dim flash (−6 log I) recorded either in control Ames’ medium (left) or after 15 min in a pharmacological cocktail that blocks rod and cone signaling to ganglion cells (middle). Timing of light stimulus is indicated by the step below the traces and by the light background behind the traces. The brisk light-evoked responses evident in control conditions (left) are blocked by the cocktail at this light intensity (middle), suggesting that they are mediated by extrinsic input from retinal networks driven by rods and cones. Some spontaneous spiking persists. Right column: in the same cocktail, a light stimulus of 1000-fold higher intensity (−3 log I) evoked spiking in several channels (arrows). These exhibited the long onset latency and persistent post-stimulus discharge typical of melanopsin-mediated intrinsic responses.
Results
Pharmacological isolation of intrinsic photoresponses of ipRGCs
When the retina was perfused with regular Ames’ medium, a relatively dim (−6 log I) flash elicited, in almost every channel brisk, robust responses at light onset, light offset or both (Fig. 1, left column). After synaptic blockade, the same dim flash failed to evoke any spikes (Fig. 1, middle column), indicating the loss of rod/cone-driven synaptic input to the ganglion cell layer. Under such blockade, a 1000-fold brighter flash (−3 log I) elicited spike discharges in several (usually 5 to 10) channels. These were typical of sluggish melanopsin-mediated intrinsic responses, exhibiting long onset latencies, sustained spiking during the stimulus, and very slow response termination, with post-stimulus discharges lasting >10 sec (Fig. 1, right-hand column, arrows). Typically, a few units in each retina had to be omitted from further analysis due to excessive spontaneous discharge, inconsistent intrinsic responses or low spike amplitude. The remaining cells, numbering 1 to 8 per retina, provided the data of this report.
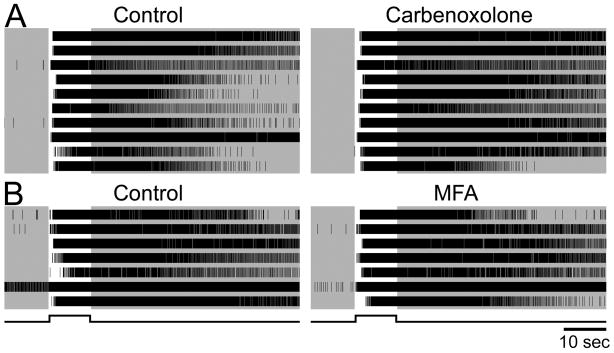
We used this pharmacological blockade in all further experiments to assess melanopsin-based responses in isolation. However, it would be premature to assume light-evoked spiking under these conditions invariably reflects phototransduction within the recorded cell. An earlier calcium-imaging study suggested that electrical coupling through gap junctions permits melanopsin-driven signals in ipRGCs to propagate to other neurons of the ganglion-cell layer (Sekaran et al., 2003). To determine whether some of the light-evoked spiking we observed under synaptic blockade reflected such electrical coupling, we infused the same gap-junction blocker used in the earlier report and at the same concentration (carbenoxolone; 100 μM). To our surprise, this did not abolish or significantly attenuate the light response in any of the 25 identified pharmacologically isolated cells recorded in 4 retinas (Fig. 2A). We also tested the effects of MFA, another potent non-selective gap junction inhibitor reported to completely eliminate coupling among other retinal neurons (Pan et al., 2007). Like carbenoxolone, MFA failed to appreciably affect the light response during rod/cone signaling blockade (Fig. 2B, 5 retinas, 23 cells). We thus conclude that under glutamatergic blockade sufficient to interrupt rod and cone signals to the inner retina, all light-evoked spiking derives directly from ipRGCs and reflects their intrinsic phototransduction.
Figure 2.
Gap junction blockers do not suppress light-evoked spiking driven by inner-retinal photoreception. The cocktail blocking rod and cone signaling was present in all cases, so all light-evoked spiking was presumably driven by melanopsin-based phototransduction. Single units were identified by offline spike sorting. A: Raster plots of intrinsic light responses in ten single ipRGCs recorded simultaneously on a multielectrode array before (left) and during (right) bath application of carbenoxolone, a gap junction blocker. The lack of effect of carbenoxolone suggests that spiking was driven by phototransduction within each light-responsive cell, and not indirectly through electrical synapses with ipRGCs. B: Same as A except that an alternative gap junction blocker was used (meclofenamic acid, 200 μM). Cell represented by the second trace from the bottom had an unusually high rate of spontaneous spiking in darkness (~10 Hz) but exhibited a robust light response nonetheless (~40 Hz). Data in A and B are from two different retinas. The stimulus was a −2 log I flash.
Weak circadian modulation of the intrinsic light response
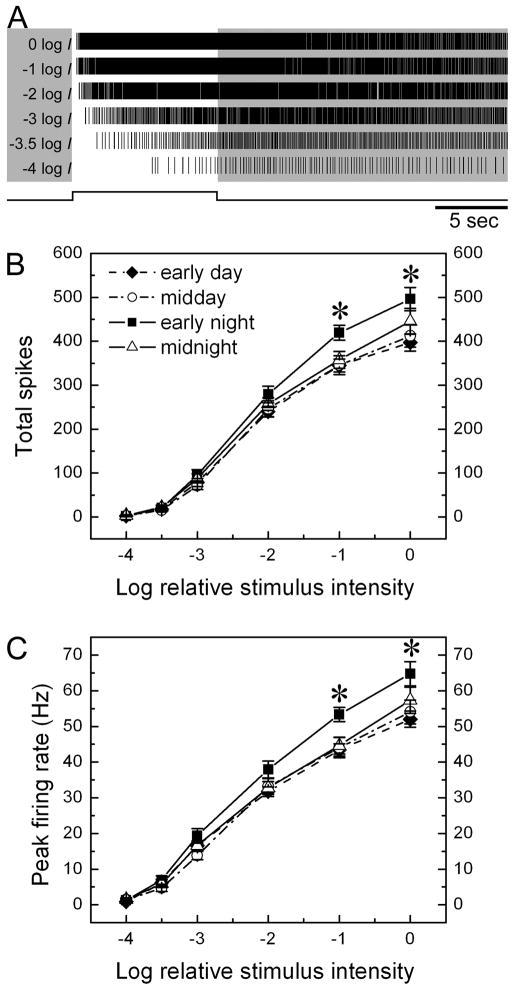
Intrinsic melanopsin-mediated phototransduction drives spiking in ipRGCs at rates that are a monotonic function of stimulus intensity. This is shown in the raster plots of Fig. 3A for a representative, pharmacologically isolated ipRGC. We pooled such data across cells to generate the irradiance-response functions shown in Figs. 3B and 3C. These plots differ only in the measure of response amplitude used, either the total number of spikes evoked during the stimulus (Fig. 3B) or the peak firing rate (Fig. 3C).
Figure 3.
Effect of circadian phase on intensity-response behavior of ipRGCs. A: raster plots of the spiking of a single representative ipRGC evoked by an ascending series of stimulus intensities, dimmest stimulus at bottom. Note that latency decreases and spike rate increases as a function of light intensity. B and C: Intensity-response relationships of ipRGCs at four circadian times. The two plots differ only in the measure of response used, either total spikes during the stimulus (B) or peak firing rate (C). Each point represents the mean value among 33–35 cells (see Table 1 for details); error bars represent standard error of the mean (SEM). Asterisks indicate significant difference detected by one way ANOVA (*p<0.05).
Four curves are displayed in both of these plots; each summarizes irradiance-response data obtained at one of the four circadian phases tested. The curves are virtually identical at midnight, early day, and midday. However, the fourth curve, for the early subjective night, displayed an elevated response for the two brightest stimuli tested (0 log I and −1 log I). Both peak firing rate and total evoked spikes were elevated roughly 20% above the values obtained at other circadian phases. One way ANOVA analysis reveals a significant difference among the four circadian groups for these two brightest stimuli (p = 0.036 (0 log I) and 0.011 (−1 log I) for spike number, and p = 0.031 (0 log I) and 0.006 (−1 log I) for peak firing rate).
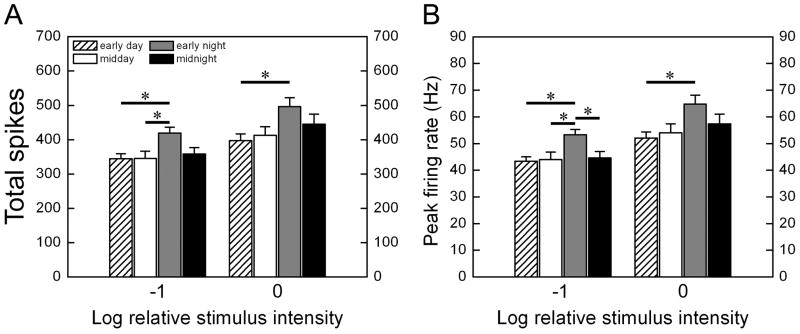
This difference is presumably due to the elevated response to strong stimuli in the early night and we confirmed this by post hoc Tukey tests (Fig. 4). The response in the early night was invariably significantly stronger than in the early day, regardless of both the response measure chosen and of which of the two strongest stimulus intensities was considered (0 or −1 log I). Responses were also stronger in the early night than around midday, but the difference reached significance only for the less intense of the two strong intensities considered (−1 log I). The difference between early night and midnight was less obvious, only significant at −1 log I when peak firing rate was compared.
Figure 4.
Summary of post hoc Tukey tests across circadian phases. In each panel, data are shown for the two highest intensities tested, −1 log I and 0 log I. The two panels differ only in the measure of response used, either total evoked spikes during the stimulus (A) or peak firing frequency (B). (mean ± SEM; *p<0.05).
The increased response rates, on average, in the early night reflect a generalized increase in responsiveness among cells recorded at this time, rather than the presence of a few cells with unusually high activity. One indication of this is that the variance was small and comparable for all circadian phases (Fig. 4). Furthermore, analysis of the distribution of response amplitudes showed a general shift toward stronger responses in the early subjective night, regardless of stimulus intensity or the response measure used (data not shown). For example, 91 % of cells in early night fired more than 300 spikes during 10 sec −1 log I stimulus, whereas at other times of day no more than 69% of cells fired this many spikes.
If this response enhancement were attributable to a change in sensitivity, one would expect to see a uniform leftward shift of the intensity-response functions of Fig. 3B and 3C, and thus an elevation of response for weaker as well as stronger stimuli. Instead, the responses at the lower end of the dynamic range were indistinguishable among the four circadian phases tested (one way ANOVA, p ≫ 0.05 in all cases). The same point is underscored in Table 1, which compares the proportion of ipRGCs responding to moderate to weak light stimuli (≤ −2 log I) at each of the circadian phases tested.
Taken together, the data indicate relatively modest circadian modulation of the intrinsic light response of ipRGCs. This consists of an increased slope of the irradiance-response function in the early subjective night, a modest augmentation of response gain without an increase in sensitivity or a reduction in threshold.
Lack of evidence for multiple functional subtypes
We mined our data for evidence that rat ipRGCs might comprise multiple functional subtypes, as has been reported for mice (Schmidt and Kofuji, 2009; Tu et al., 2005). We lumped our sample across circadian phase and examined three response measures that have previously distinguished subtypes among murine ipRGCs: response amplitude (total spikes or peak firing rate), response latency and poststimulus response persistence. All of these response measures proved to be unimodally distributed (data not shown). Our data thus provide no evidence for the existence of ipRGC subtypes in adult rat retina, though other measures may reveal them. The question should be reexamined in younger rats, since the functional distinctions among subtypes are apparently more apparent in early postnatal than in adult mice (Tu et al., 2005).
Discussion
The central finding of this study is that the intrinsic, melanopsin-based light responses of rat ganglion cell photoreceptors lack cell-autonomous circadian modulation in sensitivity, although there is a modest increase in response gain in the early subjective night.
Intraretinal clocks and the phase-response curve for photoentrainment
The main effect of this circadian modulation of ipRGC physiology is to moderately enhance the firing rate of these cells when exposed to bright light in the early night. This is the circadian phase when light has its maximal effect on the rat’s circadian system, and when the effect of activation of retinal input to the SCN is to phase-advance the clock (Bauer, 1992).
Although ipRGCs play an essential role in photoentrainment of the central pacemaker (Goz et al., 2008; Guler et al., 2008; Hatori et al., 2008), the circadian modulation of their intrinsic photoresponses plays little role in determining the phase-dependence of the pacemaker’s response to light stimulation. The form of the phase-response curve (PRC), which describes the phase resetting effect of light as a function of circadian phase, is not predictable from the magnitude of intrinsic light responses. For example, light evokes little if any phase shift during the subjective day (Bauer, 1992; Shibata and Moore, 1993; Shibata et al., 1994), despite the fact that ipRGCs exhibit robust light response at this time. Further, the polarity of the photically driven phase shift is inverted between early night (phase delay) and late night (phase advance), while the light response of ipRGCs is excitatory at both times.
The PRC is instead shaped primarily by diverse mechanisms intrinsic to the SCN that modulate the size and polarity of photic effects as a function of circadian phase (Lundkvist and Block, 2005). For example, there are phase-dependent alterations in the efficacy of retinohypothalamic synaptic transmission (Colwell, 2001; Pennartz et al., 2001), and in the intrinsic excitability of SCN cells (Cloues and Sather, 2003; Itri et al., 2005). These may partly account for the observation that the circadian modulation of light-evoked spiking is much more dramatic in SCN cells (Meijer et al., 1998) than we find in the intrinsic photoresponses of ipRGCs, and exhibits a somewhat different phase relationship. There are numerous additional downstream mechanisms that gate the effect of light input on circadian phase resetting, including phase-dependent coupling of retinohypothalamic synaptic activity to the expression of immediate early genes (Rusak et al., 1992) or to multiple intracellular signaling cascades (Gillette and Mitchell, 2002).
The modest size of the circadian modulation of ipRGC phototransduction may seem surprising, because circadian phase is a strong determinant of retinal sensitivity at many levels (Mangel, 2001). Viewed from the perspective of circadian biology, however, this makes intuitive sense, in that it buffers a primary zeitgeber from the influences of the clocks it is intended to calibrate. However, such speculation is not only teleological but also premature. In these studies we have blocked synaptic influences onto ipRGCs, and our conclusions thus apply mainly to cell-autonomous circadian modulation of intrinsic photosensitivity. Extrinsic, network-mediated circadian modulation of intrinsic phototransduction in these cells would probably have been masked by the synaptic blockade we used, unless they are exerted through mechanisms with slow time constants and thus large inertia, such as changes in transcription or translation of transduction-related genes. Also masked by our methods would be any circadian modulation in the behavior of rod/cone networks driving ipRGC firing through synaptic networks (Dacey et al., 2005; Wong et al., 2007). Rod and cone signals reach the SCN (Aggelopoulos and Meissl, 2000; Drouyer et al., 2007) and can shift its circadian phase when melanopsin phototransduction is silenced (Panda et al., 2002; Ruby et al., 2002). Indeed, ipRGCs are apparently an obligatory conduit of rod/cone input to the circadian system (Goz et al., 2008; Guler et al., 2008; Hatori et al., 2008). Further research will be needed to determine whether the excitatory rod/cone influences on ipRGCs are subject to circadian modulation. It will also be of interest to learn whether circadian phase affects the impact of light in the other functional outputs of ipRGCs (e.g., pupillary responses, melatonin regulation or sleep propensity) and whether such effects can be traced to the circadian modulation identified here.
Mechanisms of circadian modulation of ipRGCs
The modest circadian variation of melanopsin-based light-evoked spiking of ipRGCs that we detect must be due to modulation either of the phototransduction cascade or of the intrinsic excitability of the ipRGC membrane. There is some evidence that signal transduction in ipRGCs may be modulated through circadian regulation of melanopsin transcription and/or translation. The increase in phototransduction gain reported here occurs in the early subjective night, close to the time (CT10-14) that melanopsin mRNA levels peak in rats under DD conditions (Sakamoto et al., 2005; Sakamoto et al., 2004). Unfortunately, the corresponding protein levels were not assessed, and these could lag behind the peak of transcription by several hours. Sakamoto et al. (2005) did report a peak in melanopsin protein levels at ZT18, but this observation appears to have been made under an LD cycle, which would not permit direct light effects to be distinguished from circadian processes. Hannibal et al. (2005) attempted to assess circadian variations in protein levels, but were thwarted by a strong upregulation of melanopsin protein levels in constant darkness.
Even if substantial circadian modulation of melanopsin protein levels can be confirmed, however, it is not clear that this can account for our findings. Increasing the abundance of this photopigment in ipRGCs would be expected to elevate the probability of photon catch, and thus an increase in sensitivity and a reduction in threshold. By contrast, we observe an upward scaling of the response with no change in threshold, that is, an increase in gain but not in sensitivity. This is more easily explained by modulation of a process downstream of photopigment activation, such as enhanced amplification within the signaling cascade or changes in the abundance or properties of light-gated or voltage-gated ion channels.
The locus of the clock driving this modulation is unclear. Available evidence, though scanty, would seem to weight against a cell-autonomous process. Melanopsin-expressing ganglion cells do not express mPer1 (Witkovsky et al., 2003), a well established core clock gene, albeit not essential for a working clock (Bae et al., 2001; Zheng et al., 2001). An exogenous origin of the modulatory effect would help to explain why massive loss either of rods and cones (in retinally degenerate rats) or of cells in the inner nuclear layer (after intraocular injection of kainic acid) suppresses the circadian rhythm in melanopsin mRNA (Sakamoto et al., 2005).
Dopaminergic amacrine cells are one plausible exogenous source of the circadian modulation of ipRGCs. These neurons are the sole source of retinal dopamine, a neuromodulator that has been heavily implicated in circadian modulation of the retina at many levels (Witkovsky, 2004). Dopaminergic amacrine cells express core clock genes and may be autonomously rhythmic (Ruan et al., 2006; Witkovsky et al., 2003). There is an intrinsic retinal rhythm of dopamine release, with peak levels during the subjective day. Dopaminergic amacrine cells appear to make synaptic contacts with ipRGCs (Viney et al., 2007; Vugler et al., 2007) and these may be reciprocated (Zhang et al., 2008). Melanopsin-containing ganglion cells have been reported to express D2 dopamine receptors, and systemic application of a D2 receptor agonist increased melanopsin mRNA levels (Sakamoto et al., 2005). Though generally supportive of a dopaminergic role in circadian modulation of ipRGCs, the foregoing data do not lend themselves to a fully consistent functional model. Because high levels of daytime dopamine, acting at D2 receptors, would be expected to elevate melanopsin expression during the day, this scheme appears to be at odds with the observation that both melanopsin mRNA levels (Sakamoto et al., 2004) and the gain of intrinsic phototransduction (present results) are highest at night.
These amacrine cells could potentially modulate ipRGC excitability not only through dopaminergic mechanisms, but also by GABAergic inhibition. They coexpress GABA (Nguyen-Legros et al., 1997) and are presynaptic to GABA receptors on inner retinal neurons (Contini and Raviola, 2003); ipRGCs express functional ionotropic GABA receptors (Perez-Leon et al., 2006; Wong et al., 2007). If dopaminergic amacrine cells increase their GABA release during the circadian day, the resulting ionotropic inhibition of ipRGCs should reduce the gain of the ipRGC photoresponse by making these cells less excitable and attenuating their light-driven receptor potential and associated spiking.
Note, however that circadian modulation of the release of GABA or dopamine from these cells can explain our observations only if such release persists in the presence of the glutamatergic blockade we used to isolate melanopsin-driven photoresponses. Such blockade could disfacilitate the amacrine cells, and could also block any circadian regulatory network involving bipolar cells. On the other hand, the antagonists would not have interfered with any cell-autonomous circadian modulation of the membrane potential of these amacrine cells or of their release of GABA or dopamine.
A note on electrical coupling in ipRGCs
Electrical coupling through gap junction has been found between many types of retinal neurons (Sohl et al., 2005; Vaney, 1991), but relatively little is known about the gap junctions in ganglion-cell photoreceptors. It has been suggested that melanopsin-based photoresponses in ipRGCs may propagate through gap junctions to drive cells that do not express melanopsin (Sekaran et al., 2003; Sekaran et al., 2005). Our study, though not directly concerned with this issue, did provide some data apparently inconsistent with this view. In the original observations (Sekaran et al., 2003; Sekaran et al., 2005), a minority of cells in the ganglion cell layer exhibited light-evoked calcium signals that could not have been driven by rods and cones. Application of the gap junction blocker carbenoxolone reduced the number of cells exhibiting such Ca2+ signals by more than half. This suggested that certain ganglion cells exhibit rod-and-cone-independent light responses not because they express melanopsin themselves but because they are coupled by gap junctions to cells that do (i.e., to ipRGCs). In the present study, however, we found that carbenoxolone did not reduce the number of ganglion cells exhibiting light-evoked spiking during blockade of chemical synapses. We do not believe that the divergence between our findings and those of Sekaran and colleagues can be attributed to our failure to block gap junctions. We used the same dosage as employed in the Sekaran study and obtained the same result with MFA, an extremely effective non-specific gap junction blocker in rabbit retina (Pan et al., 2007). There is corroborating evidence that this drug affects transmission of rod pathways as predicted for a gap-junction blocker (Veruki and Hartveit, 2009).
The explanation for the apparent discrepancy between our result and the Ca2+ imaging findings is unclear. It may lie in the different output measures used in the two studies. For example, it is possible that calcium signals propagate from ipRGCs to other ganglion cells without affecting spiking in those cells, or to displaced amacrine cells that are not able to discharge spikes. A more likely possibility, though, is that carbenoxolone suppresses Ca2+ signals in a subset of melanopsin-expressing ipRGCs without affecting their light-evoked spiking. A possible mechanism for this is direct blockade of voltage-gated calcium channels by carbenoxolone (Vessey et al., 2004). In ipRGCs, these channels are the primary source of the elevated intracellular Ca2+ evoked by light (Hartwick et al., 2007). In any case, our findings suggest that when rod and cone function is lost or chemical synapses are blocked, light-evoked spiking is limited to ganglion cells that are truly intrinsically photosensitive.
Acknowledgments
This project was supported by grant R01 EY17137 to DMB. We thank Dianne Boghossian and Michael Jibitsky for technical assistance, and Michael Berry, Ning Tian, and Hui Chen for advice on multielectrode array technique.
References
- Aggelopoulos NC, Meissl H. Responses of neurones of the rat suprachiasmatic nucleus to retinal illumination under photopic and scotopic conditions. J Physiol. 2000;523(Pt 1):211–222. doi: 10.1111/j.1469-7793.2000.t01-1-00211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson FE, Green CB. Symphony of rhythms in the Xenopus laevis retina. Microsc Res Tech. 2000;50:360–372. doi: 10.1002/1097-0029(20000901)50:5<360::AID-JEMT5>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Antle MC, Silver R. Orchestrating time: arrangements of the brain circadian clock. Trends Neurosci. 2005;28:145–151. doi: 10.1016/j.tins.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Bae K, Jin X, Maywood ES, Hastings MH, Reppert SM, Weaver DR. Differential functions of mPer1, mPer2, and mPer3 in the SCN circadian clock. Neuron. 2001;30:525–536. doi: 10.1016/s0896-6273(01)00302-6. [DOI] [PubMed] [Google Scholar]
- Barnard AR, Hattar S, Hankins MW, Lucas RJ. Melanopsin regulates visual processing in the mouse retina. Curr Biol. 2006;16:389–395. doi: 10.1016/j.cub.2005.12.045. [DOI] [PubMed] [Google Scholar]
- Bassi CJ, Powers MK. Daily fluctuations in the detectability of dim lights by humans. Physiol Behav. 1986;38:871–877. doi: 10.1016/0031-9384(86)90056-9. [DOI] [PubMed] [Google Scholar]
- Bassi CJ, Powers MK. Circadian rhythm in goldfish visual sensitivity. Invest Ophthalmol Vis Sci. 1987;28:1811–1815. [PubMed] [Google Scholar]
- Bauer MS. Irradiance responsivity and unequivocal type-1 phase responsivity of rat circadian activity rhythms. Am J Physiol. 1992;263:R1110–1114. doi: 10.1152/ajpregu.1992.263.5.R1110. [DOI] [PubMed] [Google Scholar]
- Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- Brandenburg J, Bobbert AC, Eggelmeyer F. Circadian changes in the response of the rabbits retina to flashes. Behav Brain Res. 1983;7:113–123. doi: 10.1016/0166-4328(83)90008-6. [DOI] [PubMed] [Google Scholar]
- Cahill GM, Besharse JC. Circadian clock functions localized in xenopus retinal photoreceptors. Neuron. 1993;10:573–577. doi: 10.1016/0896-6273(93)90160-s. [DOI] [PubMed] [Google Scholar]
- Chiu JF, Mack AF, Fernald RD. Daily rhythm of cell proliferation in the teleost retina. Brain Res. 1995;673:119–125. doi: 10.1016/0006-8993(94)01411-a. [DOI] [PubMed] [Google Scholar]
- Cloues RK, Sather WA. Afterhyperpolarization regulates firing rate in neurons of the suprachiasmatic nucleus. J Neurosci. 2003;23:1593–1604. doi: 10.1523/JNEUROSCI.23-05-01593.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colwell CS. NMDA-evoked calcium transients and currents in the suprachiasmatic nucleus: gating by the circadian system. Eur J Neurosci. 2001;13:1420–1428. doi: 10.1046/j.0953-816x.2001.01517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contini M, Raviola E. GABAergic synapses made by a retinal dopaminergic neuron. Proc Natl Acad Sci U S A. 2003;100:1358–1363. doi: 10.1073/pnas.0337681100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacey DM, Liao HW, Peterson BB, Robinson FR, Smith VC, Pokorny J, Yau KW, Gamlin PD. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature. 2005;433:749–754. doi: 10.1038/nature03387. [DOI] [PubMed] [Google Scholar]
- Doyle SE, Grace MS, McIvor W, Menaker M. Circadian rhythms of dopamine in mouse retina: the role of melatonin. Vis Neurosci. 2002;19:593–601. doi: 10.1017/s0952523802195058. [DOI] [PubMed] [Google Scholar]
- Drouyer E, Rieux C, Hut RA, Cooper HM. Responses of suprachiasmatic nucleus neurons to light and dark adaptation: relative contributions of melanopsin and rod-cone inputs. J Neurosci. 2007;27:9623–9631. doi: 10.1523/JNEUROSCI.1391-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Zhong H, Wang MH, Luo DG, Liao HW, Maeda H, Hattar S, Frishman LJ, Yau KW. Intrinsically photosensitive retinal ganglion cells detect light with a vitamin A-based photopigment, melanopsin. Proc Natl Acad Sci U S A. 2005;102:10339–10344. doi: 10.1073/pnas.0501866102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillette MU, Mitchell JW. Signaling in the suprachiasmatic nucleus: selectively responsive and integrative. Cell Tissue Res. 2002;309:99–107. doi: 10.1007/s00441-002-0576-1. [DOI] [PubMed] [Google Scholar]
- Goldman AI, Teirstein PS, O’Brien PJ. The role of ambient lighting in circadian disc shedding in the rod outer segment of the rat retina. Invest Ophthalmol Vis Sci. 1980;19:1257–1267. [PubMed] [Google Scholar]
- Gooley JJ, Lu J, Chou TC, Scammell TE, Saper CB. Melanopsin in cells of origin of the retinohypothalamic tract. Nat Neurosci. 2001;4:1165. doi: 10.1038/nn768. [DOI] [PubMed] [Google Scholar]
- Goz D, Studholme K, Lappi DA, Rollag MD, Provencio I, Morin LP. Targeted destruction of photosensitive retinal ganglion cells with a saporin conjugate alters the effects of light on mouse circadian rhythms. PLoS ONE. 2008;3:e3153. doi: 10.1371/journal.pone.0003153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green CB, Besharse JC. Retinal circadian clocks and control of retinal physiology. J Biol Rhythms. 2004;19:91–102. doi: 10.1177/0748730404263002. [DOI] [PubMed] [Google Scholar]
- Guler AD, Ecker JL, Lall GS, Haq S, Altimus CM, Liao HW, Barnard AR, Cahill H, Badea TC, Zhao H, Hankins MW, Berson DM, Lucas RJ, Yau KW, Hattar S. Melanopsin cells are the principal conduits for rod-cone input to non-image-forming vision. Nature. 2008;453:102–105. doi: 10.1038/nature06829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankins MW, Lucas RJ. The primary visual pathway in humans is regulated according to long-term light exposure through the action of a nonclassical photopigment. Curr Biol. 2002;12:191–198. doi: 10.1016/s0960-9822(02)00659-0. [DOI] [PubMed] [Google Scholar]
- Hannibal J, Georg B, Hindersson P, Fahrenkrug J. Light and darkness regulate melanopsin in the retinal ganglion cells of the albino Wistar rat. J Mol Neurosci. 2005;27:147–155. doi: 10.1385/JMN:27:2:147. [DOI] [PubMed] [Google Scholar]
- Hannibal J, Hindersson P, Knudsen SM, Georg B, Fahrenkrug J. The photopigment melanopsin is exclusively present in pituitary adenylate cyclase-activating polypeptide-containing retinal ganglion cells of the retinohypothalamic tract. J Neurosci. 2002;22:RC191. doi: 10.1523/JNEUROSCI.22-01-j0002.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwick AT, Bramley JR, Yu J, Stevens KT, Allen CN, Baldridge WH, Sollars PJ, Pickard GE. Light-evoked calcium responses of isolated melanopsin-expressing retinal ganglion cells. J Neurosci. 2007;27:13468–13480. doi: 10.1523/JNEUROSCI.3626-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatori M, Le H, Vollmers C, Keding SR, Tanaka N, Schmedt C, Jegla T, Panda S. Inducible ablation of melanopsin-expressing retinal ganglion cells reveals their central role in non-image forming visual responses. PLoS ONE. 2008;3:e2451. doi: 10.1371/journal.pone.0002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science. 2002;295:1065–1070. doi: 10.1126/science.1069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattar S, Lucas RJ, Mrosovsky N, Thompson S, Douglas RH, Hankins MW, Lem J, Biel M, Hofmann F, Foster RG, Yau KW. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 2003;424:76–81. doi: 10.1038/nature01761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itri JN, Michel S, Vansteensel MJ, Meijer JH, Colwell CS. Fast delayed rectifier potassium current is required for circadian neural activity. Nat Neurosci. 2005;8:650–656. doi: 10.1038/nn1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaVail MM. Rod outer segment disk shedding in rat retina: relationship to cyclic lighting. Science. 1976;194:1071–1074. doi: 10.1126/science.982063. [DOI] [PubMed] [Google Scholar]
- Li L, Dowling JE. Zebrafish visual sensitivity is regulated by a circadian clock. Vis Neurosci. 1998;15:851–857. doi: 10.1017/s0952523898155050. [DOI] [PubMed] [Google Scholar]
- Lucas RJ, Hattar S, Takao M, Berson DM, Foster RG, Yau KW. Diminished pupillary light reflex at high irradiances in melanopsin-knockout mice. Science. 2003;299:245–247. doi: 10.1126/science.1077293. [DOI] [PubMed] [Google Scholar]
- Lundkvist GB, Block GD. Role of neuronal membrane events in circadian rhythm generation. Methods Enzymol. 2005;393:623–642. doi: 10.1016/S0076-6879(05)93033-4. [DOI] [PubMed] [Google Scholar]
- Mangel SC. Circadian clock regulation of neuronal light responses in the vertebrate retina. Prog Brain Res. 2001;131:505–518. doi: 10.1016/s0079-6123(01)31040-3. [DOI] [PubMed] [Google Scholar]
- Meijer JH, Watanabe K, Schaap J, Albus H, Detari L. Light responsiveness of the suprachiasmatic nucleus: long-term multiunit and single-unit recordings in freely moving rats. J Neurosci. 1998;18:9078–9087. doi: 10.1523/JNEUROSCI.18-21-09078.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melyan Z, Tarttelin EE, Bellingham J, Lucas RJ, Hankins MW. Addition of human melanopsin renders mammalian cells photoresponsive. Nature. 2005;433:741–745. doi: 10.1038/nature03344. [DOI] [PubMed] [Google Scholar]
- Morin LP, Allen CN. The circadian visual system, 2005. Brain Res Rev. 2006;51:1–60. doi: 10.1016/j.brainresrev.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Newman LA, Walker MT, Brown RL, Cronin TW, Robinson PR. Melanopsin forms a functional short-wavelength photopigment. Biochemistry. 2003;42:12734–12738. doi: 10.1021/bi035418z. [DOI] [PubMed] [Google Scholar]
- Nguyen-Legros J, Chanut E, Versaux-Botteri C, Simon A, Trouvin JH. Dopamine inhibits melatonin synthesis in photoreceptor cells through a D2-like receptor subtype in the rat retina: biochemical and histochemical evidence. J Neurochem. 1996;67:2514–2520. doi: 10.1046/j.1471-4159.1996.67062514.x. [DOI] [PubMed] [Google Scholar]
- Nguyen-Legros J, Versaux-Botteri C, Savy C. Dopaminergic and GABAergic retinal cell populations in mammals. Microsc Res Tech. 1997;36:26–42. doi: 10.1002/(SICI)1097-0029(19970101)36:1<26::AID-JEMT3>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Pan F, Mills SL, Massey SC. Screening of gap junction antagonists on dye coupling in the rabbit retina. Vis Neurosci. 2007;24:609–618. doi: 10.1017/S0952523807070472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda S, Nayak SK, Campo B, Walker JR, Hogenesch JB, Jegla T. Illumination of the melanopsin signaling pathway. Science. 2005;307:600–604. doi: 10.1126/science.1105121. [DOI] [PubMed] [Google Scholar]
- Panda S, Provencio I, Tu DC, Pires SS, Rollag MD, Castrucci AM, Pletcher MT, Sato TK, Wiltshire T, Andahazy M, Kay SA, Van Gelder RN, Hogenesch JB. Melanopsin is required for non-image-forming photic responses in blind mice. Science. 2003;301:525–527. doi: 10.1126/science.1086179. [DOI] [PubMed] [Google Scholar]
- Panda S, Sato TK, Castrucci AM, Rollag MD, DeGrip WJ, Hogenesch JB, Provencio I, Kay SA. Melanopsin (Opn4) requirement for normal light-induced circadian phase shifting. Science. 2002;298:2213–2216. doi: 10.1126/science.1076848. [DOI] [PubMed] [Google Scholar]
- Pennartz CM, Hamstra R, Geurtsen AM. Enhanced NMDA receptor activity in retinal inputs to the rat suprachiasmatic nucleus during the subjective night. J Physiol. 2001;532:181–194. doi: 10.1111/j.1469-7793.2001.0181g.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Leon JA, Warren EJ, Allen CN, Robinson DW, Lane Brown R. Synaptic inputs to retinal ganglion cells that set the circadian clock. Eur J Neurosci. 2006;24:1117–1123. doi: 10.1111/j.1460-9568.2006.04999.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provencio I, Rollag MD, Castrucci AM. Photoreceptive net in the mammalian retina. This mesh of cells may explain how some blind mice can still tell day from night. Nature. 2002;415:493. doi: 10.1038/415493a. [DOI] [PubMed] [Google Scholar]
- Qiu X, Kumbalasiri T, Carlson SM, Wong KY, Krishna V, Provencio I, Berson DM. Induction of photosensitivity by heterologous expression of melanopsin. Nature. 2005;433:745–749. doi: 10.1038/nature03345. [DOI] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- Ruan GX, Allen GC, Yamazaki S, McMahon DG. An autonomous circadian clock in the inner mouse retina regulated by dopamine and GABA. PLoS Biol. 2008;6:e249. doi: 10.1371/journal.pbio.0060249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan GX, Zhang DQ, Zhou T, Yamazaki S, McMahon DG. Circadian organization of the mammalian retina. Proc Natl Acad Sci U S A. 2006;103:9703–9708. doi: 10.1073/pnas.0601940103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby NF, Brennan TJ, Xie X, Cao V, Franken P, Heller HC, O’Hara BF. Role of melanopsin in circadian responses to light. Science. 2002;298:2211–2213. doi: 10.1126/science.1076701. [DOI] [PubMed] [Google Scholar]
- Rusak B, McNaughton L, Robertson HA, Hunt SP. Circadian variation in photic regulation of immediate-early gene mRNAs in rat suprachiasmatic nucleus cells. Brain Res Mol Brain Res. 1992;14:124–130. doi: 10.1016/0169-328x(92)90019-8. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, Liu C, Kasamatsu M, Pozdeyev NV, Iuvone PM, Tosini G. Dopamine regulates melanopsin mRNA expression in intrinsically photosensitive retinal ganglion cells. Eur J Neurosci. 2005;22:3129–3136. doi: 10.1111/j.1460-9568.2005.04512.x. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, Liu C, Tosini G. Classical photoreceptors regulate melanopsin mRNA levels in the rat retina. J Neurosci. 2004;24:9693–9697. doi: 10.1523/JNEUROSCI.2556-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt TM, Kofuji P. Functional and morphological differences among intrinsically photosensitive retinal ganglion cells. J Neurosci. 2009;29:476–482. doi: 10.1523/JNEUROSCI.4117-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekaran S, Foster RG, Lucas RJ, Hankins MW. Calcium imaging reveals a network of intrinsically light-sensitive inner-retinal neurons. Curr Biol. 2003;13:1290–1298. doi: 10.1016/s0960-9822(03)00510-4. [DOI] [PubMed] [Google Scholar]
- Sekaran S, Lupi D, Jones SL, Sheely CJ, Hattar S, Yau KW, Lucas RJ, Foster RG, Hankins MW. Melanopsin-dependent photoreception provides earliest light detection in the mammalian retina. Curr Biol. 2005;15:1099–1107. doi: 10.1016/j.cub.2005.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semo M, Peirson S, Lupi D, Lucas RJ, Jeffery G, Foster RG. Melanopsin retinal ganglion cells and the maintenance of circadian and pupillary responses to light in aged rodless/coneless (rd/rd cl) mice. Eur J Neurosci. 2003;17:1793–1801. doi: 10.1046/j.1460-9568.2003.02616.x. [DOI] [PubMed] [Google Scholar]
- Shibata S, Moore RY. Neuropeptide Y and optic chiasm stimulation affect suprachiasmatic nucleus circadian function in vitro. Brain Res. 1993;615:95–100. doi: 10.1016/0006-8993(93)91118-c. [DOI] [PubMed] [Google Scholar]
- Shibata S, Watanabe A, Hamada T, Ono M, Watanabe S. N-methyl-D-aspartate induces phase shifts in circadian rhythm of neuronal activity of rat SCN in vitro. Am J Physiol. 1994;267:R360–364. doi: 10.1152/ajpregu.1994.267.2.R360. [DOI] [PubMed] [Google Scholar]
- Sohl G, Maxeiner S, Willecke K. Expression and functions of neuronal gap junctions. Nat Rev Neurosci. 2005;6:191–200. doi: 10.1038/nrn1627. [DOI] [PubMed] [Google Scholar]
- Summer TL, Ferraro JS, McCormack CE. Phase-response and Aschoff illuminance curves for locomotor activity rhythm of the rat. Am J Physiol. 1984;246:R299–304. doi: 10.1152/ajpregu.1984.246.3.R299. [DOI] [PubMed] [Google Scholar]
- Terman JS, Reme CE, Terman M. Rod outer segment disk shedding in rats with lesions of the suprachiasmatic nucleus. Brain Res. 1993;605:256–264. doi: 10.1016/0006-8993(93)91748-h. [DOI] [PubMed] [Google Scholar]
- Tosini G, Fukuhara C. The mammalian retina as a clock. Cell Tissue Res. 2002;309:119–126. doi: 10.1007/s00441-002-0578-z. [DOI] [PubMed] [Google Scholar]
- Tosini G, Menaker M. Circadian rhythms in cultured mammalian retina. Science. 1996;272:419–421. doi: 10.1126/science.272.5260.419. [DOI] [PubMed] [Google Scholar]
- Tu DC, Zhang D, Demas J, Slutsky EB, Provencio I, Holy TE, Van Gelder RN. Physiologic diversity and development of intrinsically photosensitive retinal ganglion cells. Neuron. 2005;48:987–999. doi: 10.1016/j.neuron.2005.09.031. [DOI] [PubMed] [Google Scholar]
- Vaney DI. Many diverse types of retinal neurons show tracer coupling when injected with biocytin or Neurobiotin. Neurosci Lett. 1991;125:187–190. doi: 10.1016/0304-3940(91)90024-n. [DOI] [PubMed] [Google Scholar]
- Veruki ML, Hartveit E. Meclofenamic acid blocks electrical synapses of retinal AII amacrine and ON-cone bipolar cells. J Neurophysiol. 2009 doi: 10.1152/jn.00112.2009. [DOI] [PubMed] [Google Scholar]
- Vessey JP, Lalonde MR, Mizan HA, Welch NC, Kelly ME, Barnes S. Carbenoxolone inhibition of voltage-gated Ca channels and synaptic transmission in the retina. J Neurophysiol. 2004;92:1252–1256. doi: 10.1152/jn.00148.2004. [DOI] [PubMed] [Google Scholar]
- Viney TJ, Balint K, Hillier D, Siegert S, Boldogkoi Z, Enquist LW, Meister M, Cepko CL, Roska B. Local retinal circuits of melanopsin-containing ganglion cells identified by transsynaptic viral tracing. Curr Biol. 2007;17:981–988. doi: 10.1016/j.cub.2007.04.058. [DOI] [PubMed] [Google Scholar]
- Vugler AA, Redgrave P, Semo M, Lawrence J, Greenwood J, Coffey PJ. Dopamine neurones form a discrete plexus with melanopsin cells in normal and degenerating retina. Exp Neurol. 2007;205:26–35. doi: 10.1016/j.expneurol.2007.01.032. [DOI] [PubMed] [Google Scholar]
- Walker JA, Olton DS. Circadian rhythm of luminance detectability in the rat. Physiol Behav. 1979;23:17–21. [PubMed] [Google Scholar]
- Warren EJ, Allen CN, Brown RL, Robinson DW. Intrinsic light responses of retinal ganglion cells projecting to the circadian system. Eur J Neurosci. 2003;17:1727–1735. doi: 10.1046/j.1460-9568.2003.02594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirz-Justice A, Da Prada M, Reme C. Circadian rhythm in rat retinal dopamine. Neurosci Lett. 1984;45:21–25. doi: 10.1016/0304-3940(84)90323-9. [DOI] [PubMed] [Google Scholar]
- Witkovsky P. Dopamine and retinal function. Doc Ophthalmol. 2004;108:17–40. doi: 10.1023/b:doop.0000019487.88486.0a. [DOI] [PubMed] [Google Scholar]
- Witkovsky P, Veisenberger E, LeSauter J, Yan L, Johnson M, Zhang DQ, McMahon D, Silver R. Cellular location and circadian rhythm of expression of the biological clock gene Period 1 in the mouse retina. J Neurosci. 2003;23:7670–7676. doi: 10.1523/JNEUROSCI.23-20-07670.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong KY, Dunn FA, Berson DM. Photoreceptor adaptation in intrinsically photosensitive retinal ganglion cells. Neuron. 2005;48:1001–1010. doi: 10.1016/j.neuron.2005.11.016. [DOI] [PubMed] [Google Scholar]
- Wong KY, Dunn FA, Graham DM, Berson DM. Synaptic influences on rat ganglion-cell photoreceptors. J Physiol. 2007;582:279–296. doi: 10.1113/jphysiol.2007.133751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DQ, Wong KY, Sollars PJ, Berson DM, Pickard GE, McMahon DG. Intraretinal signaling by ganglion cell photoreceptors to dopaminergic amacrine neurons. Proc Natl Acad Sci U S A. 2008 doi: 10.1073/pnas.0803893105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B, Albrecht U, Kaasik K, Sage M, Lu W, Vaishnav S, Li Q, Sun ZS, Eichele G, Bradley A, Lee CC. Nonredundant roles of the mPer1 and mPer2 genes in the mammalian circadian clock. Cell. 2001;105:683–694. doi: 10.1016/s0092-8674(01)00380-4. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Tu DC, Denner D, Shane T, Fitzgerald CM, Van Gelder RN. Melanopsin-dependent persistence and photopotentiation of murine pupillary light responses. Invest Ophthalmol Vis Sci. 2007;48:1268–1275. doi: 10.1167/iovs.06-0925. [DOI] [PubMed] [Google Scholar]