Abstract
The field of nanoscience is extending the applications of physics, chemistry and biology into previously unapproached infinitesimal length scales. Understanding the behavior and manipulating the positions and properties of single atoms and molecules hold great potential to improve areas of science as disparate as medicine and computation, and communication and orbiting satellites. Yet, in the race to develop novel, previously unavailable nanoparticles, there is an opportunity for scientists in this field to digress and to apply their growing understanding of nanoscience and the tools of nanotechnology to one of the most pressing problems in all of human biology—diseases related to lipoproteins. Although not appreciated outside the field of lipoprotein biology, variations in the compositions, structures and properties of these nanoscale-sized, blood-borne particles are responsible for most of the variations in health, morbidity and mortality in the Western world. If the lipoproteins could be understood at the nanometer length scale with precise details of their structures and functions, scientists could understand a wide range of perplexing physiological processes and also address the dysfunctions in normal lipoprotein biology that lead to such diseases as hypercholesterolemia, heart disease, stroke and neurodegenerative diseases. Furthermore, if the capabilities of nanoscience to assemble and manipulate nanometer-sized particles could be recruited to studies of lipoproteins, these biological particles would provide a new dimension to therapeutic agents, and these natural particles could be designed to carry out many specialized beneficial tasks.
Keywords: Nanotechnology, Nanoscience, Nanoparticles, Lipoproteins
1. Introduction
Nanoscience is the new field embracing the sciences and engineering needed for designing, synthesizing and describing materials and devices at the level of atoms, molecules and supramolecular structures while leveraging on the unique properties and phenomena of matter at the nanometer length scale. Nanoscience has great promise to revolutionize fields as disparate as computer electronics, communication, energy production and medicine. Medical research is beginning to explore nanoscience with applications to the development of nanodevices as diagnostics, sensors and therapeutic delivery systems. Nanoscaled devices are in many situations envisioned to be manufactured to resemble nature's nanodevices—proteins, DNA, membranes (Table 1) and even to incorporate natural biomolecules in their assembly. However, the potential to produce particles or constituents that are not found in nature raises the important consideration of their side effects and the environmental and/or personal hazards associated with disseminating these particles into routine practice [7]. The potential long-term toxicity of new classes of nanosubstances to biological organisms has yet to be assessed. Therefore, building a regulatory infrastructure for this technology will be just one of the hurdles that will delay bringing nanoscience applications to practice. However, nanoscience, if used to understand existing biological structures at the nanometer length scale, would potentially revolutionize several fields of biological science that are struggling with the challenges of working at such small length scales. In particular, the field of lipoprotein biology is critical to human health, but the field is substantially disabled by the lack of scientific tools to study the basic structures that constitute the core of lipoprotein biology—the lipoproteins themselves. The tools of nanoscience and the key questions of lipoprotein biology are well matched. Nanoscience is striving to understand materials at the length scale of atoms and molecules with sufficient clarity to assemble devices on this nanometer scale. Lipoprotein biologists would like to understand lipoproteins on the same length scale. To achieve controlled assembly of nanometer-sized devices and particles, nanoscientists will need to develop means of measuring, isolating and manipulating molecules at this dimension. To understand the structures and functions of lipoproteins, biologists need to be able to measure, isolate and manipulate in quantitative and precise terms lipoproteins as individual nanometer-sized particles. Certainly, it can be seen that the two fields of science potentially overlap; however, what are the arguments that investing in understanding lipoproteins will bring greater value to the human condition than racing ahead to the first proofs-of-principle of nanodevices using carbon nanotubes—self-assembling synthetic particles and atomic-sized memory devices? This review highlights the challenges and opportunities that could emerge from directing nanoscience towards biology's most vexing nanoparticles—lipoproteins.
Table 1.
Nanoscale lengths in biology
| Biological structures | Diameter or length (nm) | References |
|---|---|---|
| Hydrogen atom | 0.1 | [1] |
| Sugars, amino acids, nucleotides | 0.5–1 | [2] |
| Phospholipid bilayer thickness | 4.5–5.5 | [3] |
| Globular proteins | 2–10 | [2] |
| HDL | 7–13 | [4] |
| LDL | 21–27 | [3] |
| Ribosome | 30 | [2] |
| VLDL | 30–90 | [5] |
| Lysosomes | 200–500 | [2] |
| Chylomicron | 200–600 | [3] |
| Mitochondrion | 1000–2000 | [1] |
| Nucleus | 3000–10,000 | [2] |
| Capillary diameter | 8000 | [6] |
| Animal cell | 10,000–30,000 | [2] |
2. Nanoscience
The intent of this article is not to review or discuss nanoscience nor its applications to the technologies of nanoparticles. Nonetheless, the vision provided by Richard Feynman [8••] that is now being realized by scientists around the world provides an unprecedented path to improve the human condition. Science is beginning to retrace the same steps, principles and complexes that led to the emergence of life itself. Molecular evolution first mastered the nanoworld, and from this mastery, emerged life. The optimism for this new science is justifiably high. But as with all new scientific fields, the practical applications of the science are not usually as clear as are the first questions of the basic research. Many of the most obvious benefits that can be imagined by nanoscience, and particularly its applications into nanotechnology, have also been recognized to be potentially hazardous and contribute to overall environmental toxicity [7].
Interestingly, nanoscientists have accurately grasped the principle that biology mastered the nanometer length scale as the means to produce life and all its manifestations (espoused by Richard Feynman in his seminal paper [8••]). As a result, scientists have appreciated that many of the molecules of life are appropriate building blocks for nanodevices. However, although the tools of biology are being used to build nanodevices, the tools of nanoscience are not yet being used to understand biology. If the principles and technologies emerging from nanoscience could be leveraged to address the subject of lipoprotein biology, these would form the necessary enabling means to revolutionize our understanding of lipoprotein particles and their roles in health. In fact, if nanoscience in this generation could provide solely an understanding of lipoproteins sufficient for precise and accurate diagnostics, direct effective therapeutics, and design prevention strategies to individual humans, the investment in nanoscience would be more than returned.
3. Lipoproteins
Lipoproteins, the colloidal particles that transport insoluble lipids within blood, lymph and cerebral spinal fluid, are among the most studied structures in biology. There are excellent reasons for this intense interest. Dysregulations of lipoprotein metabolism, even when expressed as crudely as inordinately high or low concentrations of individual components of these complex particles in human blood (e.g., cholesterol), are responsible for considerable morbidity and mortality within human populations in the Western world. Literally thousands of studies have followed the raising and lowering of the lipid constituents of lipoprotein particles in humans and surrogate animal models as a function of experimental variables, including genetics, diet, drugs, lifestyle, toxins and exercise. Despite this massive effort, very little is understood of the precise structures and functions of lipoprotein particles in the nanometer length scale at which they function.
Two generations of scientists have attacked the complexity, diversity and physiology of lipoproteins with brilliant insights and ingenuity in spite of the technological limitations. One over-riding problem has been the inability to manipulate the critical lipoprotein variables–size, composition and structure–in a truly scientific and independent manner. Scientists have had to use ingenious methods to manipulate the biology underlying the synthesis, release and clearance of the lipoproteins in order to generate information about them. Unfortunately, as a result of the complexity of the biology itself, it has been impossible to isolate these particle variables as orthogonal experimental variables. As a result, our knowledge is fragmented and confounded. As one example, it has been suggested for over 10 years that low-density lipoproteins (LDL), which differ in their size and density, are more or less atherogenic (associated with heart disease) [9•]. However, it is still not known precisely why smaller size is more atherogenic, nor what changes to the particles would reverse their increased risk. A more detailed understanding will require fundamentally new means to isolate, describe, manipulate and ultimately craft lipoprotein particles as independent scientific variables.
3.1. Isolation of lipoproteins
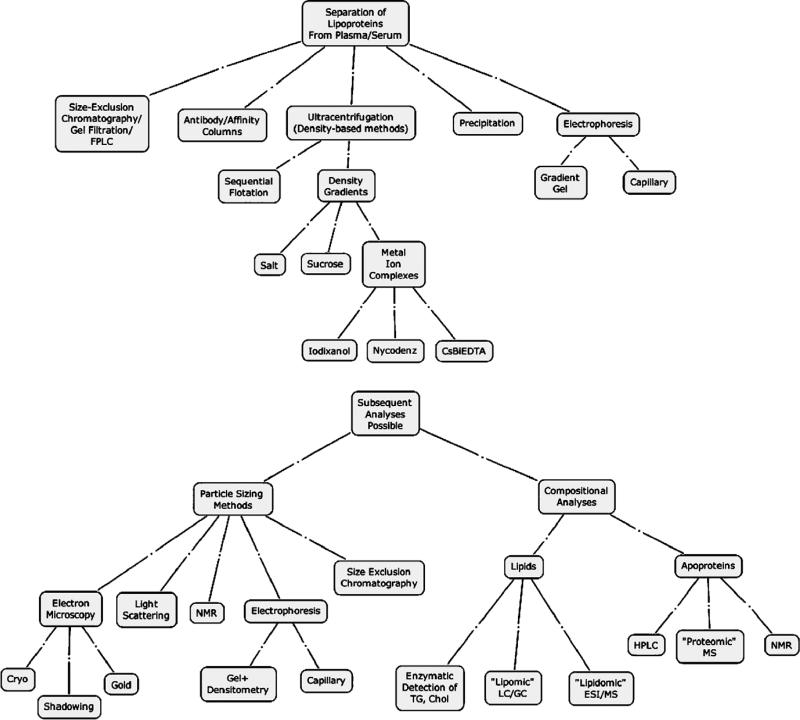
The methods to prepare lipoproteins (Fig. 1) have been pursued for decades, and the major advances have been made in isolating the broad classes of particles based primarily on bulk properties (e.g., diameter, density and charge). Notably, the methods are bulk and do not recognize–much less take advantage of–the diversity of lipoprotein size and structure, and are thus incapable at present of developing the level of structure/function understanding that is necessary to predict function and metabolic dysfunction within individuals.
Fig. 1.
Analytical techniques for isolation and characterization of lipoprotein nanoparticles. FPLC, fast protein liquid chromatography; CsBiEDTA, cesium bisethylenediaminetetraacetic acid; TG, triglycerides; Chol, cholesterol; LC/GC, liquid chromatography/gas chromatography; ESI/MS, electrospray ionization/mass spectrometry; HPLC, high pressure liquid chromatography; MS, mass spectrometry; NMR, nuclear magnetic resonance.
3.1.1. Density
Density gradient ultracentrifugation continues to be the “gold standard” for isolation of lipoprotein fractions 50 years after publication of the method [10]. This method relies on the observation that, depending on the ratios of the denser constituents (i.e., proteins) compared with the less dense constituents (i.e., lipids, especially triglycerides (TG)), different fractions of lipoproteins can be separated either with sequential centrifugation steps or with a continuous density gradient. The TG-rich very low-density lipoproteins (VLDL) “float” first, followed by intermediate-density lipoproteins (IDL), then by LDL and finally by high-density lipoprotein (HDL) particles. It has long been recognized that there are always several subgroups within each lipoprotein density class that must be resolved with further centrifugation or other separation techniques, each of which creates potential problems as well.
3.1.2. Size
Separating lipoproteins by bulk properties limits the precise size differences that can be ascribed to them—most obviously there is an overlap between equally dense particles that belong to functionally different lipoproteins. Chylomicrons (lipoproteins that carry dietary fats from the intestine) are relatively large and buoyant immediately following a meal, and are easily separated from smaller, more dense VLDL. However, as the TG from these chylomicrons are hydrolyzed and taken up into tissues, chylomicron remnants are formed that closely resemble VLDL and their remnants in density and diameter. Likewise, there can be overlap of lipoproteins in various physiological states where impaired clearance from the blood and other metabolic defects result in abnormal lipoprotein metabolism [10]. One of the challenges that a new generation of nanotools must address is how to separate the lipoproteins by the multiple criteria that distinguish them biologically. As early studies have shown, mixing different species in any observational or interventional experiment confounds the biological information that can be derived from measurements. Once isolated, subsequent steps can separate lipoproteins to analyze their composition, properties, etc. (Fig. 1), but none is completely satisfactory. Further ultracentrifugation can be performed to achieve separation of subgroups, as has been done with LDL [11] and VLDL [12]. Non-denaturing polyacrylamide gel-gradient electrophoresis [13], high-performance gel-filtration chromatography [14] and capillary isotachophoresis [15] can be used to separate lipoproteins by diameter within a lipoprotein fraction. There are several specific diameter subgroups within the LDL fraction, ranging from 18 to 28 nm [13]. However, agreement between methods remains difficult. For instance, when the gel electrophoretic method was compared with particle sizing via nuclear magnetic resonance, a poor correlation (r2 = 0.39) reflected differences in peak particle diameter estimates of up to 5.38 nm [16].
3.1.3. Apoprotein-based separation
A more biologically based approach to separate lipoproteins is to bind and remove them via affinity matrices built to recognize the proteins (apolipoproteins) that are present on their surfaces. The apolipoproteins are the “brains” of the lipoprotein system, regulating most of the myriad events in the dynamic processes of assembly remodeling and uptake [17]. Apolipoproteins are proteins bound onto the surface of the lipoprotein complex that in turn bind to specific enzymes or transport proteins on the cell membranes, directing the lipoprotein to the proper site of metabolism. Apolipoproteins are grouped by function in four classes—A, B, C and E (the former apoD is now apoAIII). Apolipoprotein A is a class of apolipoproteins–apoA-I, -II, -III and -IV–that occurs primarily in HDL and in lesser amounts in chylomicrons; apoA-I is the activator of lecithin–cholesterol acyltransferase (LCAT), which forms cholesteryl esters in HDL. Apolipoprotein B is a class of apolipoproteins recognized by specific cell-surface receptors that mediate endocytosis of lipoprotein particles; apoB-100 on VLDL, IDL and LDL is recognized by LDL receptors on liver and extrahepatic cells; apoB-48 on chylomicrons is recognized by chylomicron remnant receptors on liver cells. The apolipoprotein C class of apolipoproteins–apoC-I, -II and -III–occur in VLDL, HDL and chylomicrons; apoC-II activates lipoprotein lipase, which hydrolyzes TG for transfer from VLDL and chylomicrons to tissues. Apolipoprotein E–apoE2, E3 and E4–occurs in all classes of lipoproteins, and it may be involved in the conversion of VLDL to IDL and its clearance from the circulation.
Because most apolipoproteins can, and are likely intended to, exchange between lipoprotein particles while circulating in the blood, the strategy of using apolipoproteins as a means to separate lipoproteins is not as discriminating as could be hoped. Nonetheless, the apoB proteins are intrinsic, non-exchangeable structural elements of the lipoprotein particles on which they are found (chylomicrons, VLDL, IDL and LDL) and are intimate to their structures. In humans, chylomicrons are formed in the intestine with a truncated form of the protein—apoB-48. Thus, chylomicron particles and their remnants can be differentiated from the VLDL lipoproteins and their derivatives, which are synthesized with the full size protein, apoB-100. Immunoaffinity chromatography [18–22], immunoaffinity gel electrophoresis [23] and precipitation techniques [24,25] have been used to effect such separations. A tantalizing glimpse of the information that could be acquired by examining lipoproteins, based not simply on the content of apoB proteins but on the actual structure of the protein on a particular lipoprotein, was reviewed by Gustafsson and Boren [26]. In a series of articles, it was revealed that alterations in the content/dimension of the lipoprotein contorted the apolipoprotein sufficiently to modify its tendency to bind to endothelial cell surface glycoproteins. Because such binding is thought to influence disease processes, knowing what the actual structure of lipoproteins and the proteins on them–again in the dimensional detail anticipated for nanoscience–is necessary to understanding the inter-relations between lipoprotein structure and disease function.
3.1.4. Stability
The tools of nanoscience must address the problems of stability that bedevil attempts to measure lipoproteins ex vivo. The basic nature of lipoproteins is to exchange constituents during their circulation in plasma. Therefore, to accurately assess the compositional status of lipoproteins at any point in time, it is necessary to measure their composition as it occurs in blood. Unfortunately, ultracentrifugation can introduce artifacts by modifying particles and their constituents due to the prolonged times and high g forces involved during separation [27–29]. The sequential flotation method (density gradient ultracentrifugation) requires extremely long ultracentrifugation times [30], with the isolation of all major lipoprotein fractions requiring days (72 h) to complete.
3.1.5. Composition
Lipoprotein particles contain a variety of biomolecules depending on a number of factors: (1) their synthesis within the cell that produces them, (2) active remodeling within the blood or lymph compartment during their circulation and (3) artifactual modifications occurring during isolation. Nonetheless, isolated populations of lipoprotein particles have been analyzed for their molecular compositions (Table 2). All of the methods currently in routine practice for isolating and measuring lipoproteins are designed to prepare broad classes of particles. It speaks to the importance of the lipoproteins to overall metabolic health that these broadly based and crude separation methods are still capable of distinguishing dramatically different health outcomes associated with these lipoprotein measurements. Discouragingly for the potential of lipoproteins as health assessment indicators, their separation by the methods in both clinical practice and scientific investigation–the most selective of which is still density gradient ultracentrifugation–is poorly quantitative. During the process of sample preparation and handling, a substantial amount of lipid may be lost. Recovery is not uniform across lipid classes, and an acceptable fractionation often recovers ~90% of cholesterol present in whole plasma and ~70% of the triacylglycerol (Paul A. Davis, University of California, Davis, personal communication).
Table 2.
Average number of protein and lipid molecules of lipoproteinsa
| Molecules | HDL3 [31] | HDL2 [31] | LDL | VLDL1 [32] | VLDL2 [32] |
|---|---|---|---|---|---|
| ApoA | 4 | 5 | N/A | N/A | N/A |
| ApoB | N/A | N/A | 1 [33] | 1 | 1 |
| ApoC | ± | 1 | N/A | 90 | 36 |
| ApoE | 0.8 [34] | 1 [34] | 0.1 [35] | 1–2 | 1–3 |
| Phospholipids | 93 | 189 | 700 [36] | 4700 | 2600 |
| Free cholesterol | 11 | 52 | 600 [36] | 1200 | 800 |
| Cholesterol ester | 44 | 109 | 1600 [36] | 2400 | 1600 |
| Triglycerides | 9 | 18 | 170 [36] | 19,000 | 6600 |
References are shown in brackets.
To pursue a more detailed understanding of the lipoproteins as discrete particles and how their specific compositions, structures and functions dictate health and disease, it will be necessary to isolate, measure and ultimately engineer compositions and structures of lipoproteins. Methods to date have relied on biological models of lipoprotein preparation, and invariably, several compositional features end up confounded. If sufficient molecular dexterity could be recruited to this task, lipoproteins could be constructed with precise compositions of lipids, proteins and tracers, and their functions, fates and consequences studied in molecular detail. What is known now about the structural diversity of lipoproteins and the consequences of this diversity on health provides considerable confidence that greater understanding of lipoproteins at the length scales at which they function, i.e., nanoscale, will provide a major advantage to the study of the influence of lipoprotein structure on human health. The challenge and also the opportunities to be gained by understanding lipoprotein biology can be seen in the extensive scientific work to date on the broad classes of lipoprotein particles.
3.2. Very low-density and the triglyceride-rich lipoproteins
VLDL are the primary transport particles for delivering energy-rich but insoluble TG to tissues throughout the body. VLDL are synthesized in the liver by a complex multi-step process requiring multiple proteins to achieve the final structure and composition that is secreted into blood (for review see [37]). Although still being actively studied, the process is considered to involve two discrete pathways to obtain ostensibly two separate particles that are then brought together prior to secretion [38]. These various steps are thought to be responsible for ensuring the integrity, composition and successful release of these marvels of biological engineering. Nonetheless, depending on various genetic and environmental factors (diet, age, health, etc.), a range of sizes of VLDL particles is secreted from the liver. Secreted VLDL particles are typically between 30 and 80 nm in diameter, as estimated by electron microscopy, high-performance gel-filtration chromatography, polyacrylamide gradient gel-electrophoresis, light-scattering methods and nuclear magnetic resonance. Recognizing that VLDL are secreted with different sizes and densities and varying compositions as a result of different metabolic conditions has energized the field of TG-rich lipoproteins. Relatively crude separation by density yields two distinct classes of VLDL termed VLDL1 (larger and less dense) and VLDL2 (smaller and more dense). Recent research indicating that the condition of type 2 diabetes leads to greater production of VLDL1 [39•] will likely stimulate the development of VLDL speciation as a clinical diagnostic.
3.2.1. Composition
The composition of VLDL is somewhat dependent on the metabolic state of the liver synthesizing them, however, the general composition is relatively constant (Table 2). They are ostensibly emulsion particles with a monolayer phospholipid surface, around which is interwoven the major apolipoprotein on VLDL–apoB-100–and a core of neutral lipids of TG, cholesterol esters and a variety of minor fat-soluble molecules depending on the nutritional status of the individual. The relative proportions of TG and cholesterol esters are emerging as a particularly important consequence of the metabolic status of individuals and their diets. Interestingly, those with high carbohydrate diets tend to be associated with larger, TG-rich VLDL1, and those with diets low in carbohydrates and higher in fat tend to be smaller, but higher in cholesterol ester—VLDL2 [39•]. This variation in VLDL composition is believed to be the causal mechanism behind the production of LDL of varying sizes as well [4]. VLDL are assembled in a two-step process: in the first step, an apoB-100-containing particle is assembled on the endoplasmic reticulum; subsequently, in the second step, the small particle is joined with a large TG-rich, apolipoprotein-free particle to form the mature VLDL destined for secretion. Considerable debate continues, however, as to how these assembly steps are performed. Most of the elements that are thought to be involved (and there are dozens of proteins at least) have been identified by ingenious experiments that genetically or pharmacologically eliminate one of the participating proteins together with its enzymatic or transfer activities. Nonetheless, however ingenious, such experiments are unable as yet to guide scientists to a molecular, mechanistic understanding. The entire biological scientific community would rejoice if the internal workings of the cell could be isolated and manipulated with the dexterity promised by even very early stages of nanoscience.
Once released into the circulation, VLDL become active participants in the highly dynamic process of blood flow in which apolipoproteins are removed or added, lipid constituents are transferred in and out and, within seconds, the VLDL encounters a capillary bed on which is sitting the most active agent in all of lipid delivery—lipoprotein lipase. Lipoprotein lipase is a surface-active 1,3-triacylglycerol esterase that binds along one of its surfaces to the glycoprotein-rich endothelial face, and by another of its faces to the surface of a passing VLDL. An active complex of lipoprotein lipase and an activating protein, apoCIII, on the VLDL surface then cooperate to hydrolyze the TG from the VLDL, converting these insoluble lipids to soluble products that apparently diffuse unaided through the blood and to the adjacent endothelial surface where fatty acid transport proteins prompt their uptake. What wonders could be learned by a molecule-by-molecule disassembly of these capillary wall events. When done successfully at the blood interface to adipose, muscle, mammary, skin and lungs, these tissues are fueled, rebuilt and remodeled. When any aspect appears to fail, the consequences to the overall health can be devastating.
3.2.2. Functions
The biological genius of VLDL particles is their ability to deliver both their energy-rich TG and other insoluble components (e.g., fat-soluble vitamins) to various tissues according to the structure of the lipoproteins, their composition and the acute demands of the tissues through which they circulate. Perhaps one of the most perplexing directions of multi-tissue animal evolution was to develop the pathway that hydrolyzes VLDL particles at the blood vessel wall surface rather than taking them up intact into cells. Remarkably, many of the components necessary to this regulated off-loading of constituents at the vessel wall are not part of the particles when produced, but are acquired during their circulation within the blood. This highly dynamic remodeling process remains poorly understood even though it is critical to such physiological processes as energy metabolism, nutrient delivery, immune functions and cholesterol trafficking. If methodologies with the spatial precision needed by nanoscience could be recruited to analyzing how lipoproteins are dynamically remodeled in blood, considerable insights could be gained into how the processes work and why they fail. Furthermore, learning how biology remodels lipoproteins would provide insights to innovative biological ways to restructure a variety of multi-molecular aggregates in highly dynamic states in various subsequent applications of nanoscience in medicine and pharmaceuticals.
3.2.3. Consequences of size
The TG-rich lipoproteins begin as large (in nanoterms) emulsion particles and are progressively reduced in size during clearance, hence, variations in size and composition were not originally thought to be critical to their functions and health risks. Recent research is proving that simplifying assumption is wrong. Subfractions of TG-rich lipoproteins isolated from healthy males were studied for their interaction with macrophages—the immune cells involved in the pathogenesis of atherosclerosis [40]. Particles corresponding to the larger VLDL1 fraction induced significantly more TG accumulation within macrophages, and exhibited greater binding affinity compared with the smaller VLDL2 fraction. Disease states also appear to influence VLDL properties. In patients with apoB mutations, hepatic VLDL secretion is impaired and often leads to fatty liver—the excessive accumulation of fat in the liver (steatosis). The extent of steatosis is related to the size and number of VLDL particles produced within this patient population [41]. Depending on the particular apoB mutation, either smaller particles carrying lower than normal amounts of TG or a smaller number of particles carrying higher than normal amounts of TG are secreted. These two types of particles display different kinetics in the blood compartment, and cause different types of dyslipidemia.
3.3. Low-density lipoproteins
LDL are not only among the most studied molecular aggregates in science, but their relationship to health has anointed them among the most recognized of scientific terms by the public. Early epidemiological studies documented that levels of blood cholesterol predicted heart disease [42]. Subsequent studies that made it possible to separate the insoluble metabolite cholesterol into the colloidal lipoprotein particles that carry it throughout the body further documented that LDL was the lipoprotein culpable in this quantitatively impressive prediction of future health and disease accounting for 50% of atherosclerotic heart disease [42]. LDL are not synthesized in any organ or tissue, but are the product of extracellular remodeling activities. The remnants of VLDL, termed IDL, when returning to the liver, are either taken up via receptor-mediated endocytosis specifically by the LDL receptor, or are substrates for the lipase expressed on the surface of hepatocytes—hepatic lipase. According to current models, if hepatic lipase acts upon IDL to remove the remaining TG from the core of the lipoprotein particle, the cholesterol ester-rich, apoB-100-containing particle emerges from the liver as intact LDL. The first clinical indication that LDL do not all provide the same risk was provided by Austin et al. [43]. These investigators first demonstrated that individuals with LDL particles that were smaller and denser were at 3-fold elevated risk of heart disease. Since that seminal paper was published, the concept of varying lipoprotein size and health risk has been pursued for all lipoproteins. The mechanisms to explain the basis of the different risks associated with small, dense particles are still being debated. Ostensibly, the questions are, “Are small, dense particles genuinely more atherogenic, or does a disproportionate number of small, dense LDL in an individual reflect a metabolic state that is itself the basis of higher risk? Can one individual have small, dense LDL that are atherogenic and other small dense LDL that are not?”
3.3.1. Composition
LDL particles are considered to be the primary carriers of cholesterol in the plasma, and their composition reflects this dedication to purpose (Table 2). These are particles with remarkably little complexity in spite of their well-described deleterious properties. In contrast to the other lipoproteins in which a complex suite of apolipoproteins alternately “jump” onto and off of the lipoprotein surfaces at various times during synthesis, circulation and disassembly, LDL are seemingly just apoB-100 plus the surface lipids left from VLDL, plus a core of cholesterol esters. Their basic structure has been studied for decades with increasingly detailed models, but the distance yet to go was highlighted by Teerlink et al. [44], who recently provided evidence that LDL are discoidal rather than spherical.
3.3.2. Functions
The primary function of LDL appears to be the delivery of cholesterol to cholesterol-requiring tissues in a quantitatively impressive, receptor-mediated process. Indeed, cells that require cholesterol for such processes as growth, hormone synthesis or metabolism to bile acids express significant quantities of the LDL-specific receptors on the surfaces of their cells. Unlike TG, which are delivered by enzymatic hydrolysis to fatty acids, upon leaving the lipoprotein in the blood compartment, LDL-cholesterol is delivered as the intact lipoprotein particle with all of its free cholesterol and cholesterol ester in one impressive package.
3.3.3. Consequences of composition and size
The fact that LDL were associated epidemiologically with heart disease, and could be shown histologically to be present in atherosclerotic plaques, did not explain how these normally benign carriers of cholesterol, a valuable cellular building block and precursor for many biological functions and compounds, could become so devastating to the health of artery walls in humans. The major conceptual breakthrough was provided by Steinberg et al. [45] when it was shown that, indeed, normal LDL were not taken up by cells in the artery wall, but that compositionally modified LDL were. The compositional modification of LDL that Steinberg documented was initiated by the oxidation of the polyunsaturated fatty acids in the particles, but the principle has been generalized since that time. The resulting LDL modification theory states that the normal, i.e., compositionally normal, LDL circulate and are taken up in a highly regulated, receptor-mediated process consistent with the requirement (or lack thereof) of cholesterol by tissues. However, compositionally modified LDL are not substrates for uptake by the LDL receptor but are removed by scavenger receptors on phagocytes, most notably for the development of atherosclerosis on macrophages in the subendothelium of artery walls. The cellular dilemma of the macrophages in the subendothelial space is that whereas they do not express LDL receptors and thus do not typically acquire cholesterol-rich LDL particles, they constitutively express (i.e., cannot shut down) scavenger receptors on their surface. In essence, according to the LDL modification theory [45], once an LDL is compositionally modified, it is rapidly taken up by cells that are ill-suited to manage the bolus of cholesterol that it contains. Because this bolus of cholesterol, accompanied–according to the theory–by oxidized lipids, etc., is an activating and disrupting stimulus to the subendothelial macrophages, a progressive, inflammatory and ultimately destructive chain of events in the artery wall eventually leads to a debilitating plaque.
The LDL modification theory has become so well established that a large range of scientific pursuits has been designed solely to block various steps in the modification process. Most notably, antioxidants that are capable of slowing oxidation of LDL are among the most studied of dietary components in the past 10 years. What is particularly disturbing, given the investment in this research, is how little evidence exists to support the modification process in molecular and mechanistic detail. Literally dozens of clinical trials have attempted to “prove” the antioxidant theory of atherosclerosis prevention with conspicuously little success. Once again, the discouraging lack of detailed knowledge at the nanolength scale of the composition, structural and functional consequences of LDL modification, such as could occur in vivo, is blocking sufficient understanding to act therapeutically in this direction. Although oxidation of LDL certainly occurs in vitro, attempts to document the same process in vivo have been unsuccessful. If oxidation is so critical in vivo, why are antioxidant therapies/preventatives such failures [46]? Is simple oxidation genuinely the problem or are related events ultimately the cause? Or, are antioxidants, either through diets or at pharmacological doses, simply unable to work their way through the complex metabolic steps needed to enrich the actual protection of LDL in vivo? Interestingly, in the case of LDL, composition and size are both metabolically and physically confounded.
Differences in LDL particle size that can be detected with current methods capable of discriminating on the level of 1–4 nm were proposed to make a significant difference in disease risk [47]. Such differences were shown, for example, by the increased incidence of coronary artery disease in men and women with smaller, denser LDL (pattern B with peak diameter 24–25.5 nm) compared with larger and less dense LDL (pattern A with a peak diameter 26–28 nm) [47]. Furthermore, in the postprandial state–the period of time immediately after consumption of a meal–individuals with pattern B had larger increases in the levels of cholesterol and TG in the VLDL1 compared with pattern A individuals [48]. This difference in response to a dietary fat load may be what leads to the formation of the small, dense-LDL pattern B as opposed to the large, buoyant LDL-pattern A in this metabolic setting. However, even though metabolic factors of VLDL assembly can be shown to produce LDL with different sizes and densities, this is not the only way that LDL can “get small.” Another school of thought has proposed that because LDL are continuously acted upon by blood-borne and surface-attached catalytic and transfer activities, LDL that circulate for longer periods get smaller. Furthermore, LDL that circulate longer become more susceptible to oxidation [49•]. Hence, at the present time, even though good evidence has shown that LDL can be of different size due to metabolic differences in their formation and in their clearance, it is not yet established whether either of these mechanisms is truly the basis for increased risk. In fact, since smaller LDL are likely to circulate longer, the truth may be that the explanation for the causes of small LDL being more atherogenic is a combination of both altered metabolic production and delayed clearance. Methods to characterize LDL at the scale on which they are importantly different (nanolength) do not yet exist.
Studies have attempted to bring lines of evidence to explore the variation in LDL size with risk. A logical conclusion is that the ability of an individual particle to penetrate the endothelial barrier into the subendothelial space is dependent on its size. The tendency of particles to adhere to subendothelial surfaces, which in turn depends on the structure of the apoB protein on the LDL particle, is likely the critical aspect of particle properties [50,51]. Thus, it has been proposed that the basis of size-dependent changes in risk of disease associated with LDL can be linked to very specific structural events. However, due to a lack of precision in assessing, much less creating, these defined differences as experimental variables, it is still not understood why different sizes of LDL are associated with such variations in risk, and, therefore, what the most effective means to reverse it would be.
Recent studies using cryoelectron microscopy showed that variations in the ratio of TG to cholesterol in the core of LDL particles resulted in overt differences in the lamellar structure of the particle, its transition temperature and surface component composition [52]. This difference in cholesterol to TG ratio in the core of the particle may also alter protein conformation, which can in turn affect the affinity of the apolipoprotein for its receptor [53].
3.4. High-density lipoproteins
The lipoprotein class that epitomizes the treasure to be gained by mastering the nanoworld is HDL. The benefits of HDL to protection from heart disease are increasingly well understood [54]. However, HDL are also protective against other health problems, particularly infection. The apoA protein on HDL binds to bacterial lipopolysaccharide (endotoxin) and, once bound, the HDL-endotoxin complex is either removed by the liver and the lipopolysaccharide excreted as bile or, apparently benignly, taken up by macrophages. Interestingly, when HDL become limiting during severe infection, these endotoxin-clearing properties are apparently also carried out by the other lipoproteins [55]. Because of the unique physical and chemical properties of HDL, combined with their myriad biological roles, HDL research holds considerable potential for improving health. The challenges to that research are proportionately high as well. HDL are heterogeneous in every aspect of their composition, (apolipoproteins, phospholipids, cholesterol, cholesterol esters and TG), they are in every physical and biological sense dynamic, they are unstable and very, very small. HDL particles are so remarkably heterogeneous as a result of the disparate synthetic steps that produce them, and the continuous dynamic metabolic processes that remodel them within the circulation. Each particle undergoes remodeling resulting in multiple apolipoprotein and lipid compositions, size, density and charge. The precise means of assembling HDL are still being studied. However, it is clear that HDL are not formed de novo within cells but rather are assembled largely within the dynamic bloodstream itself.
3.4.1. Composition
The basic composition of HDL (Table 2) does not reflect their diversity, their dynamic nature, nor the consequences of this diversity. Whereas size is implicated in directing the metabolic fate of HDL, the protein and phospholipid composition of the particle greatly influence its metabolic outcome. The most commonly studied HDL–reconstituted HDL (rHDL)–are discoidal particles containing apoAI and a single type of phosphatidylcholine (PC) [3]. However, PC composition of native HDL is complex. HPLC analysis demonstrated stark differences in the fatty acid composition of PC in plasma of humans that are deficient in the lecithin–cholesterol acyltransferase protein compared with normal controls containing this enzyme [56]. Preβ1-HDL circulate throughout interstitial fluid and acquire PC from association with apoAI and the cell membrane's transport system (ABCA1). Prior to complexing with the cell membrane's lipid transporter, sphingomyelin concentrations of discoidal HDL are high and PC concentrations are low. Subsequently, the converse is observed, sphingomyelin concentrations decrease and PC concentrations increase [57]. Plasma LCAT activity is inefficient on HDL with a high sphingomyelin content [58]. The phospholipid composition of HDL may act as a signal that reflects the metabolic state of HDL. Particles with a higher PC content that acquire cellular cholesterol may, in turn, be preferentially acted on by LCAT. This regulation would increase the efficiency of reverse cholesterol transport. If HDL particles with specific compositions could be produced in vitro, in vivo studies infusing rHDL with phospholipid concentrations would be able to resolve which compositions were most effective for particular functions.
Various combinations of apolipoproteins that are associated with HDL particles will exert unique metabolic effects. HDL2 are predominantly associated with apoAI and less with apoAII, whereas HDL3 contain appreciable quantities of both [59]. ApoAII exhibits metabolic roles not shared with apoAI. ApoAII is less efficient than apoAI at activating LCAT, inhibits lipid transfer between HDL and TG-rich lipoproteins and, paradoxically, increases hepatic lipase association with HDL while inhibiting lipid hydrolysis [60•]. The association of apoAI may implicate HDL2 as the driving particles in the reverse cholesterol transport process and partially explain why this subclass is associated with improved cardiovascular health [58]. Other exchangeable apolipoproteins found on HDL might influence the metabolic fate of HDL particles. Enzyme kinetic studies demonstrated increased rates of TG and phospholipid hydrolysis from hepatic lipase when apoE (isoform dependent) was present on spherical human rHDL [61]. Thus, apolipoprotein compositional differences associated with HDL subclasses may reflect metabolic differences between particles.
3.4.2. Functions
The process of reverse cholesterol transport involves the dynamic acceptance of cellular lipids by HDL in the interstitial space, exchange of lipids between lipoproteins and HDL within the plasma compartment and final delivery of HDL lipids to the liver for excretion. The size and composition of HDL influences the success of this process. Reverse cholesterol transport involves circulation of lipid-poor HDL particles containing apoAI. These particles acquire cellular free cholesterol and phospholipids by engaging with ATP-binding cassette transporter protein (ABCA1) present on the cell's exofacial membrane. The active plasma enzyme LCAT esterifies the cellular-derived free cholesterol associated with the small HDL particle, and facilitates the transformation of lipid-poor discs into mature, spherical, larger particles. The plasma proteins, cholesterol ester transfer protein and phospholipid transfer protein facilitate the transfer of lipids between lipoproteins and HDL particles, furthering the maturation of HDL. Mature HDL transfer cholesterol to the liver via docking to the SR-B1 receptor, and HDL TG and phospholipids are hydrolyzed by hepatic lipase. Subsequently, large, mature HDL particles are converted to smaller HDL and apoAI lipid-poor particles [62].
Although most research has focused on their role in reverse cholesterol transport, HDL appear to be multi-functional transporters that move lipids, proteins and insoluble vitamins around the body. Both native HDL and rHDL protect against atherosclerosis by preventing oxidation of LDL [63], promote endothelial production of nitric oxide [64], prevent inflammation [63], bind and neutralize lipopolysaccharide of Gram negative bacteria [65] and modulate the expression of endothelial cell adhesion molecules [66]. Understanding how the structure and composition of each HDL reflect the particle's disparate functions would be invaluable to diagnosing the consequences of varying HDL and for designing strategies to improve HDL functions for particular health problems.
Generating rHDL has shed light on the metabolic outcomes of HDL in reverse cholesterol transport and other metabolic functions. Methods have been developed for the preparation of small micellular complexes of apoAI and phospholipids to mimic discoidal and spherical HDL in diameter, shape, composition and functional properties. Reconstituted HDL have proven to be useful tools in building the understanding of HDL as it now exists. However, these are at best blunt tools to date. They are spontaneously synthesized by generating mixed micelles between detergent and phospholipids of choice, followed by adding an exchangeable apolipoprotein. The diameter of rHDL particles is manipulated by the ratio of PC/apoAI [3].
3.4.3. Consequences of size
Longitudinal, cross-sectional and mechanistic studies demonstrate the myriad functions of HDL subclasses in reverse cholesterol transport. Striking is the heterogeneity of its size (7–12 nm). The varying efficiencies of HDL sizes and transport have been suggested to reflect the necessity to reach various intercellular spaces [67]. Based on their β-migration on the gel, spherical, lipid-rich HDL particles are fractionated into five subclasses ranging from small, dense HDL3c, HDL3b and HDL3a to larger HDL2a and HDL2b (Table 3) [68], whereas lipid-poor HDL exhibit preβ-mobility and are distinguished as preβ1, preβ2 and preβ3 [69]. The smallest, preβ-HDL and preβ1-HDL, are avid acceptors of cellular free cholesterol. Subsequent to its interaction with LCAT and plasma proteins involved in exchanging lipids, nascent discoidal preβ1 HDL transform into mature spherical HDL2.
Table 3.
Principal HDL subclasses based on density and particle diameter [4]
| HDL | Density (g/ml) | Diameter (nm) |
|---|---|---|
| 2b | 1.063–1.100 | 9.8–12.9 |
| 2a | 1.100–1.125 | 8.8–9.8 |
| 3a | 1.125–1.147 | 8.2–8.8 |
| 3b | 1.147–1.167 | 7.7–8.2 |
| 3c | 1.167–1.200 | 7.2–7.7 |
HDL subclass distribution is associated with the progression of cardiovascular disease. In dyslipidemic patients with insulin resistance and visceral obesity, HDL subclass distribution is altered to contain an increased concentration of small, dense HDL (HDL3 subclass) and decreased large HDL particles (HDL2 subclass) [13]. Case control studies demonstrate an inverse relationship between coronary heart disease risk and HDL2b levels and a positive relationship to HDL3c and HDL3b levels [68]. Studies suggest that HDL subclass distribution may result from differential rates of clearance from circulation, in which case the rate of HDL2 catabolism may exceed the rate of synthesis [13].
Even though scientists do not yet have precise means to accurately assemble HDL particles, studies that have attempted to do so have given a glimpse of the knowledge that could be gained were it possible. Pulse chase studies of free cholesterol efflux from 3H free cholesterol-labeled cells identified that the major acceptor of cell-derived cholesterol was preβ1-HDL followed by larger preβ particles. Mature, spherical HDL rich in cholesterol esters were poor acceptors of cellular cholesterol compared with lipid-poor HDL [57]. Alternatively, Lamarche et al. [70] demonstrated that large human HDL radiolabeled and injected into rabbits was cleared more rapidly than small HDL.
3.4.4. Structure determines function
HDL phospholipid composition influences the metabolic fate of the particle, yet studies implicate that structure and composition of HDL's exchangeable apolipoproteins may also be involved. ApoAI plays a prominent role in the anti-atherogenic activity of HDL as it is involved in the myriad processes involved in reverse cholesterol transport. ApoAI is the preferential acceptor of cell cholesterol–a cofactor for LCAT–and is a ligand for SRB1, the HDL receptor [71]. ApoAI over-expression in animals increases circulating HDL particles and is protective against diet and gene-induced atherosclerosis. The amino acid sequence of ApoAI contains 11 repeat units of amino acids that form amphipathic helical structures conferring active binding with lipid structures and to the HDL receptor.
Apolipoproteins on HDL particles appear to be flexible molecules whose conformation changes in response to changing lipid content and composition of the particles. In rHDL particles, the alpha helix content of associated apoAI molecules is increased in response to increased phospholipid to apoAI ratio of the particle [67]. Increasing the protein's alpha helix content increases the protein's hydrophobic binding capacity. ApoAI configuration is also regulated by the degree of unsaturation of PC acyl chains and by concentration of cholesterol in the rHDL particle. Synthetic amphipathic helical segments whose primary sequence is unrelated to that of native apoAI can be effective mimics of native apoAI in binding phospholipid, promoting cholesterol efflux from cells and activating the formation of cholesterol esters by LCAT [57]. Thus, apolipoprotein structure mediates the metabolic outcome of the HDL particle.
An improved understanding of the structural interactions between lipids and apolipoproteins on HDL would clearly provide targets for modulating metabolic effects. Interestingly, natural mutations of the apoAI gene associated with improved cardiovascular health have received significant attention in determining the role of apoAI in lipoprotein metabolism. The apoAIMilano mutation is a naturally occurring single amino acid mutation of the apoAI gene, whereby a cysteine amino acid is substituted for an arginine residue forming a disulfide dimer [72••]. The distinctive attributes shared by all carriers of this mutation include elevated plasma TG and very low levels of plasma HDL (10–30 mg/dl) [72••]. Paradoxically, these individuals have less or no increased risk of cardiovascular disease [5]. These findings indicate that its unique structural and functional properties may mediate anti-atherogenicity beyond that of apoAI. Recombinant HDL particles that contain apoAIMilano may be more efficient in promoting cholesterol efflux from cells, less reactive substrates for LCAT and cleared from circulation at a slower rate compared with apoAI particles [71]. Studies in mice and rabbits demonstrate reductions in atherosclerotic plaques in response to infusion of recombinant apoAIMilano complexed to phospholipids [72••]. The results of a prospective, randomized, double-blinded, placebo-controlled clinical trial in which recombinant apoAIMilano complexed to phospholipids was infused into patients with acute coronary syndromes were remarkable. Intravascular ultrasound and angiographic analysis demonstrated that five weekly infusions of recombinant apoAIMilano significantly reduced atheroma volume in just 2 weeks compared with placebo treatment [72••]. However the fact that the placebo was a saline injection rather than a control HDL particle infusion makes it difficult to interpret the specific active components in these potentially biotherapeutic particles.
3.4.5. Non-plasma high-density lipoprotein particle
HDL particles are highly dynamic and heterogeneous lipoproteins whose most studied role entails accepting cholesterol from peripheral tissues and delivering it via specific receptors primarily to the liver for excretion in bile. Interestingly, cerebrospinal fluid contains lipoprotein particles distinct from plasma particles and these comprise a unique subclass of HDL particles that are apoE and apoAI rich [73]. ApoE lipoproteins are speculated to exert important roles in central nervous system cholesterol metabolism, and this process is up-regulated in response to acute brain injury. In vivo data are lacking, but conjecture confers apoE-containing lipoproteins deliver cholesterol to injured neurons during the phase of recovery that facilitates synaptogenesis. Compared with cerebrospinal fluid lipoprotein particle concentrations of controls, subjects recovering from subarachnoid hemorrhage exhibited a dramatic increase in lipid and apoAI content in addition to increased levels of smaller apoAI-containing particles. The authors suggested that smaller diameter apoAI-containing lipoproteins identified in subarachnoid hemorrhage cerebrospinal fluid would be preferred substrates for the formation of cholesterol ester by LCAT and thus would facilitate efficient reverse cholesterol transport.
4. Potential for nanoscience to improve our understanding and applications of lipoproteins
The study of lipoproteins has been one of the most active aspects of metabolic health research, diagnostics and intervention over the past 50 years. During that time, the importance of varying lipoprotein concentration, size and composition has been well recognized. However, the complexity of understanding these colloidal particles has been a daunting research endeavor. The most difficult aspects of lipoprotein research have been in understanding the particles at the molecular scale. If the tools of nanoscience could be brought rapidly to bear on the study of lipoproteins, several aspects could be rapidly implemented to the entire field. First, nanoscience could revolutionize lipoprotein measurements as diagnostics. The variation in human lipoproteins is clearly in part responsible for differences in health outcomes from all chronic diseases, from heart disease to neurodegenerative diseases, from infections to cancer. Until it is possible to measure the nanometer length scale differences reliably in routine human samples, it will be impossible to assign these differences to cause and effect, or to identify specific individuals with inordinately effective or ineffective lipoprotein particles. Second, nanoscience could revolutionize our understanding of cause and effect of the myriad functions of different particles. Why are small dense LDL so atherogenic? Why are small, PC-rich HDL so effective in reverse cholesterol transport? Third, nanoscience could revolutionize intervention. Because it has not been possible to measure lipoproteins with sufficient accuracy to link assembly to composition to metabolism, it has been virtually impossible to design strategies, either pharmacologic or dietary, to manipulate lipoprotein composition effectively. With HDL this lack is particularly obvious in so much as there is not yet an effective means to significantly increase HDL levels in general, much less enhance specific HDL functions. Finally, nanoscience could revolutionize our ability to build lipoproteins as therapeutic agents. It is not a coincidence that most non-polar biological molecules are transported, delivered and removed from tissues by lipoproteins. If nanoscience could develop the means to assemble in high throughput, with highly accurate compositions and structures, specific HDL, these particles would represent a next generation bio-delivery system matching the utility of the natural particles in vivo.
5. Conclusions
The field of nanoscience will invariably produce novel molecular components bringing considerable benefits to the human condition. Nevertheless, before these benefits can be achieved, the field and the novel particles that can be produced will require a long period of testing, validation and regulatory scrutiny. Instead of developing proofs-of-principle and examples of breathtaking molecular dexterity with novel materials, the field of nanoscience could embrace a very common, everyday example of particles whose very existence already lies in the nanometer length scale—lipoproteins. Nanoscientists may not appreciate just how powerful their new approaches would be to unraveling the mysteries of lipoprotein biology, from their biosynthesis and assembly to their myriad functions in normal health. Furthermore, variations and dysfunctions in lipoprotein biology are responsible for much of the chronic and degenerative diseases that are plaguing the world today. Understanding the causes of these diseases, and building effective therapies and preventative strategies will require considerable dexterity in the nanoscience realm.
Acknowledgements
The authors gratefully acknowledge the writing support of C. J. Dillard and discussions with Rosemary L. Walzem.
Abbreviations
- apo
apolipoprotein
- ABCA1
ATP-binding cassette transporter protein
- HDL
high-density lipoproteins
- IDL
intermediate-density lipoproteins
- LCAT
lecithin–cholesterol acyltransferase
- LDL
low-density lipoproteins
- PC
phosphatidylcholine
- rHDL
reconstituted high-density lipoproteins
- TG
triglyceride
- VLDL
very low-density lipoprotein
References and recommended reading
• Of special interest.
•• Of outstanding interest.
- 1.Lodish H, Berk A, Zipursky LS, Matsudaira P, Baltimore D, Darnell J. Molecular Cell Biology. 3rd ed. W.H. Freeman; New York, NY: 1995. [Google Scholar]
- 2.Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular Biology of the Cell. 3rd ed. Garland Science; New York, NY: 1994. pp. 18–19.pp. 89–191. [Google Scholar]
- 3.Jonas A. Lipoprotein structure. In: Vance DE, Vance JE, editors. Biochemistry of lipids, lipoproteins and membranes. 4th ed. Elsevier; Amsterdam: 2002. pp. 483–504. [Google Scholar]
- 4.Krauss RM. Lipids and lipoproteins in patients with type 2 diabetes. Diabetes Care. 2004;27:1496–504. doi: 10.2337/diacare.27.6.1496. [DOI] [PubMed] [Google Scholar]
- 5.Rensen P. Recombinant lipoproteins: lipoprotein-like lipid particles for drug targeting. Adv Drug Deliv Rev. 2001;47:251–76. doi: 10.1016/s0169-409x(01)00109-0. [DOI] [PubMed] [Google Scholar]
- 6.Barron B. Cardiovascular System—Blood Vessels. [9 November 2005]; available on the Internet: www.biosbcc.net/barron/anatomy/pdf; 2005.
- 7.Seaton A, Donaldson K. Nanoscience, nanotoxicology, and the need to think small. Lancet. 2005;365(9463):923–4. doi: 10.1016/S0140-6736(05)71061-8. [DOI] [PubMed] [Google Scholar]
- 8 ••.Feynman RP. There's Plenty of Room at the Bottom. A transcript of the classic talk that Richard Feynman gave on December 29, 1959, at the annual meeting of the American Physical Society at the California Institute of Technology (Caltech) was first published in the February 1960 issue of Caltech's Engineering and Science, which owns the copyright. [22 October 2005]; now available on the Internet at http://www.zyvex.com/nanotech/feynman.html.
- 9 •.Dreon DM, Fernstrom HA, Miller B, Krauss RM. Low-density lipoprotein subclass patterns and lipoprotein response to a reduced-fat diet in men. FASEB J. 1994;8:121–6. [This article rekindled optimism for HDL as an artery disease reversal agent and spawned a wealth of studies around the world to manipulate HDL by diet, drugs and biotherapeutics.] [PubMed] [Google Scholar]
- 10.Havel RJ, Eder HA, Bragdon JH. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955;34:1345–53. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chapman MJ, Laplaud PM, Luc G, Forgez P, Bruckert E, Goulinet S, et al. Further resolution of the low density lipoprotein spectrum in normal human plasma: physicochemical characteristics of discrete subspecies separated by density gradient ultracentrifugation. J Lipid Res. 1988;29:442–58. [PubMed] [Google Scholar]
- 12.Zhao SP, Bastiaanse EM, Ha MF, Smel AH, Gevers Leuven JA, Van der Laars A, et al. Separation of VLDL subfractions by density gradient ultracentrifugation. J Lab Clin Med. 1995;125:641–9. [PubMed] [Google Scholar]
- 13.Krauss RM, Burke DJ. Identification of multiple subclasses of plasma low density lipoproteins in normal humans. J Lipid Res. 1982;23:97–104. [PubMed] [Google Scholar]
- 14.Scheffer PG, Bakker SJ, Heine RJ, Teerlink T. Measurement of low-density lipoprotein particle size by high-performance gel-filtration chromatography. Clin Chem. 1997;43:1904–12. [PubMed] [Google Scholar]
- 15.Schlenck A, Herbeth B, Siest G, Visvikis S. Characterization and quantification of serum lipoprotein subfractions by capillary isotachophoresis: relationships with lipid, apolipoprotein, and lipoprotein levels. J Lipid Res. 1999;40:2125–33. [PubMed] [Google Scholar]
- 16.Witte DR, Taskine MR, Perttunen-Nio H, Van Tol A, Livingstone S, Colhoun HM. Study of agreement between LDL size as measured by nuclear magnetic resonance and gradient gel electrophoresis. J Lipid Res. 2004;45:1069–76. doi: 10.1194/jlr.M300395-JLR200. [DOI] [PubMed] [Google Scholar]
- 17.Gursky O. Apolipoprotein structure and dynamics. Curr Opin Lipidol. 2005;16:287–94. doi: 10.1097/01.mol.0000169348.61191.ac. [DOI] [PubMed] [Google Scholar]
- 18.McVicar JP, Kunitake ST, Hamilton RL, Kane JP. Characteristics of human lipoproteins isolated by selected-affinity immunosorption of apolipoprotein A-I. Proc Natl Acad Sci U S A. 1984;81:1356–60. doi: 10.1073/pnas.81.5.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hennessy LK, Kunitake ST, Kane JP. Apolipoprotein A-I-containing lipoproteins, with or without apolipoprotein A-II, as progenitors of pre-beta high-density lipoprotein particles. Biochemistry. 1993;32:5759–65. doi: 10.1021/bi00073a006. [DOI] [PubMed] [Google Scholar]
- 20.Hennessy LK, Kunitake ST, Jarvis M, Hamilton RL, Endeman G, Protter A, et al. Isolation of subpopulations of high density lipoproteins: three particle species containing apoE and two species devoid of apoE that have affinity for heparin. J Lipid Res. 1997;38:1859–68. [PubMed] [Google Scholar]
- 21.Rapp JH, Lespine A, Hamilton RL, Colyvas N, Chaumeton AH, Tweedie-Hardman J, et al. Triglyceride-rich lipoproteins isolated by selected-affinity anti-apolipoprotein B immunosorption from human atherosclerotic plaque. Arterioscler Thromb. 1994;14:1767–74. doi: 10.1161/01.atv.14.11.1767. [DOI] [PubMed] [Google Scholar]
- 22.Campos E, Kotite L, Blanche P, Mitsugi Y, Frost PH, Masharani U, et al. Properties of triglyceride-rich and cholesterol-rich lipoproteins in the remnant-like particle fraction of human blood plasma. J Lipid Res. 2002;43:365–74. [PubMed] [Google Scholar]
- 23.Nakajima K, Saito T, Tamura A, Suzuki M, Nakano T, Adachi M, et al. Cholesterol in remnant-like lipoproteins in human serum using monoclonal anti apo B-100 and anti apo A-I immunoaffinity mixed gels. Clin Chim Acta. 1993;223:53–71. doi: 10.1016/0009-8981(93)90062-9. [DOI] [PubMed] [Google Scholar]
- 24.Adler L, Hill JS, Frohlich J. Chemical precipitation of apolipoprotein B-containing lipoproteins facilitates determination of LDL particle size. Clin Biochem. 2000;33:187–90. doi: 10.1016/s0009-9120(00)00061-8. [DOI] [PubMed] [Google Scholar]
- 25.Gibson JC, Rubinstein A, Brown WV. Precipitation of apo E-containing lipoproteins by precipitation reagents for apolipoprotein B. Clin Chem. 1984;30:1784–8. [PubMed] [Google Scholar]
- 26.Gustafsson M, Boren J. Mechanism of lipoprotein retention by the extracellular matrix. Curr Opin Lipidol. 2004;15:505–14. doi: 10.1097/00041433-200410000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Schaefer EJ, Eisenberg S, Levy RI. Lipoprotein apoprotein metabolism. J Lipid Res. 1978;19:667–87. [PubMed] [Google Scholar]
- 28.Kunitake ST, Kane JP. Factors affecting the integrity of high density lipoproteins in the ultracentrifuge. J Lipid Res. 1982;23:936–40. [PubMed] [Google Scholar]
- 29.Puppione DL, Kunitake ST, Hamilton RL, Phillips ML, Schumaker VN, Davis LD. Characterization of unusual intermediate density lipoproteins. J Lipid Res. 1982;23:283–90. [PubMed] [Google Scholar]
- 30.Schumaker VN, Puppione DL. Sequential flotation ultracentrifugation. Methods Enzymol. 1986;128:155–70. doi: 10.1016/0076-6879(86)28066-0. [DOI] [PubMed] [Google Scholar]
- 31.Eisenberg S. High density lipoprotein metabolism. J Lipid Res. 1984;25:1017–58. [PubMed] [Google Scholar]
- 32.Bjorkegren J, Karpe F, Vitols S, Tornvall P, Hamsten A. Transient triglyceridemia in healthy normolipidemic men increases cellular processing of large very low density lipoproteins by fibroblasts in vitro. J Lipid Res. 1998;39:423–36. [PubMed] [Google Scholar]
- 33.Durrington PN. Can measurement of apolipoprotein B replace the lipid profile in the follow-up of patients with lipoprotein disorders? Clin Chem. 2000;48:401–2. [PubMed] [Google Scholar]
- 34.Wilson HM, Griffin BA, Watt C, Skinner ER. The isolation and characterization of high-density-lipoprotein subfractions containing apolipoprotein E from human plasma. Biochem J. 1992;284(Pt 2):477–81. doi: 10.1042/bj2840477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dergunov AD, Smirnova EA, Merched A, Visvikis S, Siest G, Yakushkin VV, et al. Structural peculiarities of the binding of very low density lipoproteins and low density lipoproteins to the LDL receptor in hypertriglyceridemia: role of apolipoprotein E. Biochim Biophys Acta. 2000;1484:29–40. doi: 10.1016/s1388-1981(99)00197-3. [DOI] [PubMed] [Google Scholar]
- 36.Hevonoja T, Pentikainen MO, Hyvonen MT, Kovanen PT, Ala-Korpela M. Structure of low density lipoprotein (LDL) particles: basis for understanding molecular changes in modified LDL. Biochim Biophys Acta. 2000;1488:189–210. doi: 10.1016/s1388-1981(00)00123-2. [DOI] [PubMed] [Google Scholar]
- 37.Shelness GS, Ledford AS. Evolution and mechanism of apolipoprotein B-containing lipoprotein assembly. Curr Opin Lipidol. 2005;16:325–32. doi: 10.1097/01.mol.0000169353.12772.eb. [DOI] [PubMed] [Google Scholar]
- 38.Shelness GS, Sellers JA. Very-low-density lipoprotein assembly and secretion. Curr Opin Lipidol. 2001;12:151–7. doi: 10.1097/00041433-200104000-00008. [DOI] [PubMed] [Google Scholar]
- 39 •.Adiels M, Boren J, Caslake MJ, Stewart P, Soro A, Westerbacka J, et al. Overproduction of VLDL1 driven by hyperglycemia is a dominant feature of diabetic dyslipidemia. Arterioscler Thromb Vasc Biol. 2005;25:1697–703. doi: 10.1161/01.ATV.0000172689.53992.25. [DOI] [PubMed] [Google Scholar]
- 40.Palmer AM, Nova E, Anil E, Jackson K, Bateman P, Wolstencroft E, et al. Differential uptake of subfractions of triglyceride-rich lipoproteins by THP-1 macrophages. Atherosclerosis. 2005;180:233–44. doi: 10.1016/j.atherosclerosis.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 41.Schonfeld G, Patterson BW, Yablonskiy DA, Tanoli TS, Averna M, Elias N, et al. Fatty liver in familial hypobetalipoproteinemia: triglyceride assembly into VLDL particles is affected by the extent of hepatic steatosis. J Lipid Res. 2003;44:470–8. doi: 10.1194/jlr.M200342-JLR200. [DOI] [PubMed] [Google Scholar]
- 42.Anonymous, Department of Health and Human Services . The Surgeon General's report on nutrition and health. U.S. Government Printing Office; Washington, DC: 1988. DHHS publication [PHS] 88-50210. [Google Scholar]
- 43.Austin MA, Breslow JL, Hennekens CH, Buring JE, Willett WS, Krauss RM. Low-density lipoprotein subclass patterns and risk of myocardial infarction. JAMA. 1988;260:1917–21. [PubMed] [Google Scholar]
- 44.Teerlink T, Scheffer PG, Bakker SJ, Heine RJ. Combined data from LDL composition and diameter measurement are compatible with a discoid particle shape. J Lipid Res. 2004;45:954–66. doi: 10.1194/jlr.M300521-JLR200. [DOI] [PubMed] [Google Scholar]
- 45.Steinberg D, Parthasarathy S, Carew TE, Khoo JC, Witztum JL. Beyond cholesterol, Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989;320:915–24. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- 46.Lonn E, Bosch J, Yusuf S, Sheridan P, Pogue J, Arnold JM, et al. HOPE and HOPE-TOO Trial Investigators. Effects of long-term vitamin E supplementation on cardiovascular events and cancer: a randomized controlled trial. J Am Med Assoc. 2005;293:1338–47. doi: 10.1001/jama.293.11.1338. [DOI] [PubMed] [Google Scholar]
- 47.Gardner CD, Fortmann SP, Krauss RM. Association of small low-density lipoprotein particles with the incidence of coronary artery disease in men and women. JAMA. 1996;276:875–81. [PubMed] [Google Scholar]
- 48.Koba S, Hirano T, Murayama S, Kotani T, Tsunoda F, Iso Y, et al. Small dense LDL phenotype is associated with postprandial increases of large VLDL and remnant-like particles in patients with acute myocardial infarction. Atherosclerosis. 2003;170:131–40. doi: 10.1016/s0021-9150(03)00245-4. [DOI] [PubMed] [Google Scholar]
- 49 •.Walzem RL, Watkins S, Frankel EN, Hansen RJ, German JB. Older plasma lipoproteins are more susceptible to oxidation: a linking mechanism for the lipid and oxidation theories of atherosclerotic cardiovascular disease. Proc Natl Acad Sci U S A. 1995;92:7460–4. doi: 10.1073/pnas.92.16.7460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen GC, Liu W, Duchateau P, Allaart J, Hamilton RL, Mendel CM, et al. Conformational differences in human apolipoprotein B-100 among subspecies of low density lipoproteins (LDL). Association of altered proteolytic accessibility with decreased receptor binding of LDL subspecies from hypertriglyceridemic subjects. J Biol Chem. 1994;269:29121–8. [PubMed] [Google Scholar]
- 51.Skalen K, Gustafsson M, Rydberg EK, Hulten LM, Wiklund O, Innerarity TL, et al. Subendothelial retention of atherogenic lipoproteins in early atherosclerosis. Nature. 2002;417(6890):750–4. doi: 10.1038/nature00804. [DOI] [PubMed] [Google Scholar]
- 52.Sherman MB, Orlova EV, Decker GL, Chiu W, Pownall HJ. Structure of triglyceride-rich human low-density lipoproteins according to cryoelectron microscopy. Biochemistry. 2003;42:14988–93. doi: 10.1021/bi0354738. [DOI] [PubMed] [Google Scholar]
- 53.McKeone BJ, Patsch JR, Pownall HJ. Plasma triglycerides determine low density lipoprotein composition, physical properties, and cell-specific binding in cultured cells. J Clin Invest. 1993;91:1926–33. doi: 10.1172/JCI116411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Linsel-Nitschke P, Tall AR. HDL as a target in the treatment of atherosclerotic cardiovascular disease. Nat Rev Drug Discov. 2005;4:193–205. doi: 10.1038/nrd1658. [DOI] [PubMed] [Google Scholar]
- 55.Kitchens RL, Thompson PA, Munford RS, O'Keefe GE. Acute inflammation and infection maintain circulating phospholipid levels and enhance lipopolysaccharide binding to plasma lipoproteins. J Lipid Res. 2003;4:2339–48. doi: 10.1194/jlr.M300228-JLR200. [DOI] [PubMed] [Google Scholar]
- 56.Subbaiah PV, Pritchard PH. Molecular species of phosphatidylcholine in familial lecithin–cholesterol acyltransferase deficiency: effect of enzyme supplementation. Biochim Biophys Acta. 1989;1003:145–50. doi: 10.1016/0005-2760(89)90248-8. [DOI] [PubMed] [Google Scholar]
- 57.Fielding CJ, Fielding PE. Cellular cholesterol efflux. Biochim Biophys Acta. 2001;1533:175–89. doi: 10.1016/s1388-1981(01)00162-7. [DOI] [PubMed] [Google Scholar]
- 58.Miller M, Zhan M. Genetic determinants of low high-density lipoprotein cholesterol. Curr Opin Cardiol. 2004;19:380–4. doi: 10.1097/01.hco.0000126584.12520.b5. [DOI] [PubMed] [Google Scholar]
- 59.Patsch JR, Karlin JB, Scott LW, Smith LC, Gotto AM., Jr Inverse relationship between blood levels of high density lipoprotein subfraction 2 and magnitude of postprandial lipemia. Proc Natl Acad Sci U S A. 1983;80:1449–53. doi: 10.1073/pnas.80.5.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Boucher J, Ramsamy TA, Braschi S, Sahoo D, Neville TA, Sparks DL. Apolipoprotein A-II regulates HDL stability and affects hepatic lipase association and activity. J Lipid Res. 2004;45:849–58. doi: 10.1194/jlr.M300431-JLR200. [DOI] [PubMed] [Google Scholar]
- 61.Hime NJ, Drew KJ, Hahn C, Barter PJ, Rye KA. Apolipoprotein E enhances hepatic lipase-mediated hydrolysis of reconstituted high-density lipoprotein phospholipid and triacylglycerol in an isoform-dependent manner. Biochemistry. 2004;43:12306–14. doi: 10.1021/bi036305i. [DOI] [PubMed] [Google Scholar]
- 62.Rye KA, Clay MA, Barter PJ. Remodeling of high density lipoproteins by plasma factors. Atherosclerosis. 1999;145:227–38. doi: 10.1016/s0021-9150(99)00150-1. [DOI] [PubMed] [Google Scholar]
- 63.Barter PJ, Nicholls S, Rye KA, Anantharamaiah GM, Navab M, Fogelman AM. Antiinflammatory properties of HDL. Circ Res. 2004;95:764–72. doi: 10.1161/01.RES.0000146094.59640.13. [DOI] [PubMed] [Google Scholar]
- 64.Mineo C, Shaul PW. HDL stimulation of endothelial nitric oxide synthase. Trends Cardiovasc Med. 2003;6:226–31. doi: 10.1016/s1050-1738(03)00098-7. [DOI] [PubMed] [Google Scholar]
- 65.Levine DM, Parker TS, Donnelly TM, Walsh A, Rubin AL. In vivo protection against endotoxin by plasma high density lipoprotein. Proc Natl Acad Sci U S A. 1993;90:12040–4. doi: 10.1073/pnas.90.24.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Calabresi L, Vecchio G, Frigerio F, Vavassori L, Sirtori CR, Franceschini G. Reconstituted high-density lipoproteins with a disulfide-linked apolipoprotein A-I dimmer: evidence for restricted particle size heterogeneity. Biochemistry. 1997;36:12428–33. doi: 10.1021/bi970505a. [DOI] [PubMed] [Google Scholar]
- 67.Davidson WS, Rodrigueza WV, Lund-Katz S, Johnson WJ, Rothbla GH, Phillips MC. Effects of acceptor particle size on the efflux of cellular free cholesterol. J Biol Chem. 1995;270:17106–13. doi: 10.1074/jbc.270.29.17106. [DOI] [PubMed] [Google Scholar]
- 68.Williams PT, Superko HR, Haskell WL, Alderman EL, Blanche PJ, Holl LG, et al. Smallest LDL particles are most strongly related to coronary disease progression in men. Arterioscler Thromb Vasc Biol. 2003;23:314–21. doi: 10.1161/01.atv.0000053385.64132.2d. [DOI] [PubMed] [Google Scholar]
- 69.Xu Y, Fu M. Alterations of HDL subclasses in hyperlipidemia. Clin Chim Acta. 2003;332:95–102. doi: 10.1016/s0009-8981(03)00138-4. [DOI] [PubMed] [Google Scholar]
- 70.Lamarche B, Tchernof A, Mauriege P, Cantin B, Dagenai GR, Lupien PJ, et al. Fasting insulin and apolipoprotein B levels and low-density lipoprotein particle diameter as risk factors for ischemic heart disease. JAMA. 1998;279:1955–61. doi: 10.1001/jama.279.24.1955. [DOI] [PubMed] [Google Scholar]
- 71.Sirtori CR, Calabresi L, Franceschini G. Recombinant apolipoproteins for the treatment of vascular diseases. Atherosclerosis. 1999;142:29–40. doi: 10.1016/s0021-9150(98)00247-0. [DOI] [PubMed] [Google Scholar]
- 72 ••.Nissen SE, Tsunoda T, Tuzcu EM, Schoenhagen P, Cooper CJ, Yasin M, et al. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. JAMA. 2003;290:2292–300. doi: 10.1001/jama.290.17.2292. [DOI] [PubMed] [Google Scholar]
- 73.Kay AD, Day SP, Nicoll JA, Packar CJ, Caslak MJ. Remodelling of cerebrospinal fluid lipoproteins after subarachnoid hemorrhage. Atherosclerosis. 2003;170:141–6. doi: 10.1016/s0021-9150(03)00249-1. [DOI] [PubMed] [Google Scholar]