Abstract
Selective drugs targeting dysregulated oncogenic pathways are promising cancer therapies. Because the mammalian target of rapamycin complex 1 (mTORC1) pathway is hyperactivated in human follicular thyroid cancer (FTC), we hypothesized that its inhibition could block cancer development and progression. We, therefore, analyzed the effect of a treatment with a specific mTORC1 inhibitor (RAD001) in a faithful mouse model of FTC with constitutive mTORC1 activation (TRβPV/PVPten+/− mice). The treatment did not prevent capsular and vascular invasion of the thyroid and the occurrence of lung metastasis. However, it substantially decelerated thyroid tumor growth, thereby prolonging TRβPV/PVPten+/− mouse life span. RAD001 efficiently inhibited mTORC1 activity, as shown by the reduced phosphorylation of its downstream targets involved in the activity of the translation machinery, such as ribosomal S6 kinase (p70S6K), eukaryotic translation initiation factor 4E binding protein (4E-BP1) and the eukaryotic translation initiation factors eIF-4B and eIF-4G. Whereas mTORC1 signaling inhibition did not alter cell apoptosis, it induced a significant decrease in cell proliferation that was associated with the reduced abundance and altered activity of key regulators of cell cycle progression. Altogether, our data indicate that mTORC1 signaling plays a major role in the integration of the mitogenic signal in FTC. Therefore, our preclinical study with a relevant mouse model of FTC demonstrates for the first time that RAD001 efficaciously stabilizes cancer growth although it does not prevent its fatal outcome. In conclusion, our work underscores that in the treatment of FTC patients, RAD001 can only be used in combination with drugs and therapies inducing tumor shrinkage and blocking metastasis.
Introduction
Thyroid cancer accounts for a large proportion of endocrine-related cancer deaths each year. The origin of the vast majority of these cancers is the follicular epithelium, for which there are three morphological subtypes: papillary thyroid carcinoma, follicular thyroid carcinoma (FTC) and anaplastic carcinoma (1). The differentiated thyroid cancers, papillary thyroid carcinoma and FTC constitute ∼90% of thyroid cancers (2–4). The standard management of thyroid cancer includes surgical resection, radiotherapy, radioactive iodine and chemotherapy (5). Although the patients with differentiated thyroid cancer have a good prognosis, recurrence can develop in 20–40% of patients, and with occurrence of extensive local invasion and distant metastases, the prognosis is much poorer despite treatment (3). Cytotoxic chemotherapy has limited effectiveness and is generally limited to patients with rapidly progressing thyroid cancer unresponsive or unsuitable for surgery, radioiodine and external beam radiotherapy (6). Elucidating the molecular etiology of thyroid cancer is, thus, critical to develop novel therapeutic strategies to treat thyroid cancer patients.
The development of a mouse model of FTC (TRβPV/PV mouse) has provided us with the opportunity to dissect the complex alteration of molecular pathways driving thyroid carcinogenesis (7). The TRβPV/PV mouse was created by a targeted mutation of thyroid hormone receptor β gene (TRβPV) via homologous recombination and the Cre-LoxP system (7). The TRβPV mutation was discovered in a heterozygous patient with syndrome of resistance to thyroid hormone, a disease where dysfunction of the pituitary–thyroid axis leads to high circulating levels of thyroid-stimulating hormone (TSH) in the face of high circulating levels of thyroid hormones (8). Because of the critical role of TR in metabolism, growth and development, only one homozygous resistance to thyroid hormone patient, who died at an early age, has been reported (9). However, somatic mutations of TRs have been observed in a wide array of human cancers, suggesting that mutant TRs could activate oncogenic pathways (10). Indeed, as TRβPV/PV mice age, in addition to severe resistance to thyroid hormone, they all develop FTC similar to human thyroid cancer, with pathological progression from hyperplasia to vascular invasion, capsular invasion, anaplasia and eventually metastasis to the lung (11). Our extensive molecular analyses have shown that thyroid cancers in TRβPV/PV mice exhibit similar molecular defects as in patients, including constitutive activation of phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt) (12–15), repression of peroxisome proliferator-activated receptor γ signaling (16,17) and aberrant accumulation of both pituitary tumor transforming gene (PTTG) (18) and β-catenin (19). Thus, our mouse model faithfully recapitulates the molecular aberrations found in human thyroid cancer and is suitable for preclinical studies.
In the past years, the extensive use of PI3K inhibitors in preclinical and clinical studies has clearly demonstrated the crucial role of this pathway in tumorigenesis (20). Using our mouse model of FTC, we have shown that the potent PI3K inhibitor LY294002 significantly prolongs mouse survival and diminishes thyroid cancer progression (21). In contrast, further activation of the PI3K/Akt pathway in TRβPV/PV mice heterozygous for Pten (phosphatase and tensin homolog deleted from chromosome 10) (TRβPV/PVPten+/− mice), a tumor suppressor gene that opposes the PI3K/Akt signaling, considerably exacerbates the aggressiveness of the thyroid cancer (22). However, because the PI3K/Akt pathway regulates a number of signaling pathways, including that of the mammalian target of rapamycin complex 1 (mTORC1) (Figure 4A), the exact role of the PI3K/Akt downstream pathways in the development and progression of thyroid cancer remains elusive. In particular, mTORC1 is constitutively activated in human cancers, but whether mTORC1 is a critical driver of thyroid carcinogenesis remains to be addressed (23,24).
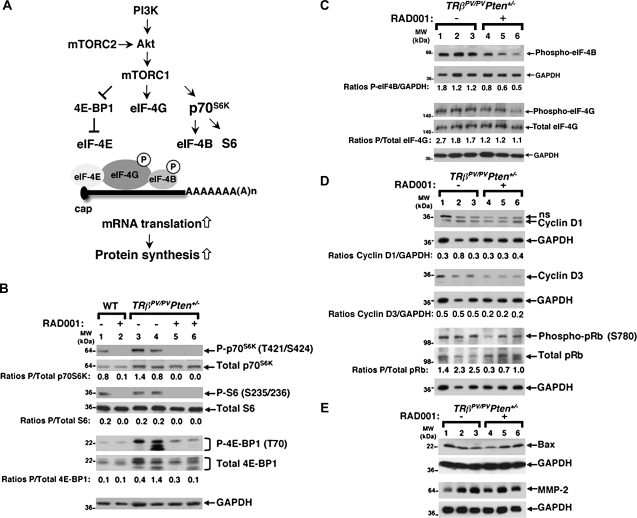
Fig. 4.
RAD001 efficiently suppresses mTORC1 signaling and reduces the abundance of Cyclin D3 and the phosphorylation of pRb. (A) The mTOR kinase is the catalytic component of two distinct multiprotein complexes called mTORC1 and mTORC2. By interacting with mTORC1- or mTORC2-specific proteins, mTOR acquires different substrate specificities and cell function. mTORC2 directly phosphorylates and activates Akt. In contrast, mTORC1 is phosphorylated and activated by the PI3K/Akt pathway, and in turn, it phosphorylates a number of target proteins involved in messenger RNA translation, such as the 4E-BP1, the eukaryotic translation initiation factor (eIF)-4G (eIF-4G) and p70S6K. Upon mTORC1 activation, hyperphosphorylated 4E-BP1 dissociates from eIF-4E, resulting in the recruitment of the scaffold protein eIF-4G to the 5′ end of the messenger RNA and thereby allowing translation initiation to proceed. mTORC1 also phosphorylates eIF-4G, and this may increase eIF-4G activity. The activation of p70S6K leads to increased phosphorylation of the ribosomal protein S6 (S6) and to the hyperphosphorylation of eIF4-B, which then promotes the ‘unwinding’ of the RNA secondary structures. Thus, mTORC1 is a master regulator of components of the translation machinery, thereby controlling protein synthesis. (B) Total protein extracts were prepared from thyroids of wild-type mice (lanes 1 and 2) and thyroid tumors of TRβPV/PVPten+/− mice (lanes 3–6) treated or not with RAD001, as described in Materials and Methods. Western blot analysis of phosphorylated p70S6K, total p70S6K, phosphorylated S6, total S6, phosphorylated 4E-BP1, total 4E-BP1 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as a loading control. The ratios of phosphorylated protein to total protein levels, after quantification of the band intensities for each sample, are indicated. (C) Total protein extracts were prepared from thyroid tumors of TRβPV/PVPten+/− mice receiving the placebo (lanes 1–3) or RAD001 (lanes 3–6), as described in Materials and Methods. Western blot analysis of phosphorylated eIF-4B, phosphorylated eIF-4G, total eIF-4G and GAPDH as a loading control. The ratios of phosphorylated eIF-4B to GAPDH and those of phosphorylated eIF-4G to total eIF-4G protein levels, after quantification of the band intensities for each sample, are indicated. (D) Total protein extracts were prepared from thyroid tumors of TRβPV/PVPten+/− mice receiving the placebo (lanes 1–3) or RAD001 (lanes 3–6), as described in Materials and Methods. Western blot analysis of Cyclin D1, Cyclin D3, phosphorylated pRb, total pRb and GAPDH as a loading control; ns, non-specific band. (E) Western blot analysis of Bax, MMP-2 and GAPDH as a loading control.
The recent development of rapamycin analogs, which are selective mTORC1 inhibitors with antitumor activity and relatively minor toxicities, offers novel promising opportunities to better define the role of mTORC1 in oncogenesis and to treat thyroid cancer patients. mTORC1 is composed of Raptor (regulatory associated protein of mTOR) and mLST8 (mammalian Lethal with SEC13 protein 8). Rapamycin and its derivatives bind to an abundant intracellular protein, FKBP-12, forming a complex that inhibits mTORC1 signaling. Although the precise mechanisms of this inhibition are still unclear, it has been proposed that these drugs would perturb the interaction of the active mTOR kinase with Raptor, thereby displacing the substrates from the mTOR catalytic domain (24).
We, therefore, took advantage of the TRβPV/PVPten+/− mouse to determine the involvement of mTORC1 signaling in the development and progression of thyroid cancer. The mTORC1 signaling was inhibited by treating TRβPV/PVPten+/− mice with the mTORC1 inhibitor RAD001 (Everolimus), which is currently being tested in vivo on various animal cancer models and in clinical trials (20) but not on FTC. Here, we report that RAD001 treatment did not prevent capsular and vascular invasion of the thyroid and the occurrence of distant metastasis to the lung of TRβPV/PVPten+/− mice. However, the treatment prolonged the survival of TRβPV/PVPten+/− mice by significantly reducing thyroid cancer growth. In conclusion, our results suggest that mTORC1 signaling is essentially involved in cell proliferation. This limited efficacy of RAD001 in preventing thyroid cancer demonstrated in the faithful and unique in vivo model of FTC indicates that mTORC1 inhibitors would be most beneficial in combination with other therapies inducing tumor shrinkage and preventing metastasis.
Materials and methods
Animals and treatments
The care and handling of the animals used in this study were approved by the National Cancer Institute Animal Care and Use Committee. Mice harboring the TRβPV gene (TRβPV/PV mice) were prepared via homologous recombination, and genotyping was carried out using the polymerase chain reaction method, as described previously (7). Pten+/− mice were kindly provided by Dr Ramon Parsons (Columbia University, New York, NY) (25). TRβPV/PVPten+/− mice were obtained by crossing Pten+/− mice with TRβPV/+ mice and then TRβPV/+Pten+/− with TRβPV/+Pten+/+ mice (22). RAD001 (Sigma–Aldrich Corp., St Louis, MO) was dissolved in 1.25% ethanol in water and given by oral gavage twice a week at a dose of 10 mg/kg body wt starting at the age of 6 weeks. This protocol of intermittent drug administration was chosen based on previous works showing the long-term effect of biweekly administration of RAD001 (5 mg/kg) on mTORC1 signaling inhibition, with minimal immunosuppressive properties (26,27). Mice were monitored until they became moribund with rapid weight loss, hunched posture and labored breathing. The thyroids were collected for weighing, histological analysis and biochemical studies.
Immunohistochemistry and in situ apoptosis detection
Formalin-fixed paraffin thyroid sections were deparaffinized, rehydrated, heated to 98°C in 0.05% citraconic anhydride, pH 7.4 (Sigma-Aldrich Corp.), for 45 min and then blocked for 1 h in 10% goat serum at room temperature. After washing in phosphate-buffered saline, slides were incubated overnight with Ki-67 primary antibody (Thermo Scientific, Fremont, CA; #RB-9043-P0). After washing, slides were incubated with goat anti-rabbit secondary antibody for 90 min at room temperature and rinsed in phosphate-buffered saline. Slides were then incubated in 3,3′-diaminobenzidene (DAB substrate kit for peroxidase; Vector Laboratories, Burlingame, CA; SK-4100), and after staining development, they were counterstained in Gill's Hematoxylin, rinsed and mounted in Permount (Fisher Scientific, Pittsburgh, PA). Ki-67 immunostaining was used to compare the proliferative index in placebo- and RAD001-treated TRβPV/PVPten+/− mice. Cells were considered as positive when they displayed a yellow/brown-stained nucleus. Three representative fields of a thyroid section were photographed for each animal (n = 4–5 animals per group). The proliferative index was calculated as the percentage of Ki-67-positive nuclei to the total number of nuclei on the thyroid section (1500–2500 total nuclei were counted per microphotograph). Counting was performed using National Institutes of Health (NIH) IMAGE software (ImageJ 1.34s; Wayne Rasband, NIH, Bethesda, MD) (http://rsb.info.nih.gov/ij).
Apoptosis was detected using the ApopTag plus Peroxydase In Situ Apoptosis Detection kit from Chemicon International (Millipore, Bedford, MA; S7101) according to the manufacturer's protocol. Tissue section from a rodent mammary gland after weaning of pups was used as a positive control and showed ∼1% of apoptotic cells on the section, as expected.
Histological analysis
Thyroid glands, lungs, hearts and lymph nodes were dissected and embedded in paraffin. Five micrometer thick sections were prepared and stained with hematoxylin and eosin. For each animal, single random sections through the thyroid, through the lung and through the heart were examined. For thyroids, morphological evidence of hyperplasia, capsular invasion, vascular invasion and anaplasia was routinely counted in that single section. Hyperplasia was generally diffuse throughout the gland. Evidence of any of these changes in any section was counted as positive for that change. On average, in those cases with capsular invasion and/or vascular invasion, these morphological changes were seen in multiple locations (usually two or three) in any one single thyroid section. The presence of a single microscopic focus of metastatic follicular carcinoma in the lung was counted as a metastatic lesion in that animal. Assessment of the number of metastatic cells was performed by counting cells in each lung lesion per section, under a light microscope.
Western blot analysis
Preparation of whole-cell lysates from thyroid glands has been described previously (12). The protein sample (50 μg) was loaded and separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis. After electrophoresis, the protein was electrotransferred to a polyvinylidene difluoride membrane (Immobilon-P; Millipore Corp., Bedford, MA). Antibodies used according to the manufacturers’ manuals include phosphorylated Akt (Ser473, #9271), Akt (#9272), Cyclin D3 (#2936), phosphorylated eIF-4B (#3591), phosphorylated eIF-4G (#2441), total eIF-4G (#2469), phosphorylated glycogen synthase kinase (GSK) 3α/β (#9331), total GSK3β (#9315), phosphorylated mTOR (Ser2448, #2971), total mTOR (#2972), phosphorylated p70S6K (ribosomal S6 kinase) (Thr421/Ser424, #9204), total p70S6K (#9202), phosphorylated S6 (ribosomal protein S6) (Ser235/236, #4857), total S6 (#2217), phosphorylated eukaryotic translation initiation factor 4E binding protein (4E-BP1) (Thr70, #9455), total 4E-BP1 (#9644) and phosphorylated retinoblastoma protein (pRb) (Thr70, #9307), all from Cell Signaling Technologies (Beverly, MA) and used at a 1:1000 dilution. Cyclin D1 (sc-450), Bax (sc-7480), matrix metalloprotease-2 (MMP-2) (sc-10736) and pRb (sc-50) antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA) and used at a 1:200 dilution. Secondary antibodies used were horseradish peroxidase-conjugated goat anti-mouse or anti-rabbit IgG (Amersham Biosciences, Piscataway, NJ) and detected using the Western Lightning chemiluminescence reagent plus system (PerkinElmer Life Sciences, Boston, MA). The blots were stripped with Re-Blot Plus (Chemicon, Temecula, CA) and reprobed with rabbit polyclonal antibodies to glyceraldehyde-3-phosphate dehydrogenase (#2118). Band intensities were quantified by using NIH IMAGE software (ImageJ 1.34s; Wayne Rasband, NIH) (http://rsb.info.nih.gov/ij).
Statistical analysis
Data are expressed as mean ± standard error of the mean. Statistical analysis was performed with the use of analysis of variance, and P < 0.05 was considered significant unless otherwise specified. GraphPad Prism 5.0a (San Diego, CA) was used to perform Kaplan–Meier cumulative survival analysis and unpaired Student's t-test, with Welch's correction when variances were unequal.
Results
The inhibition of mTORC1 signaling by RAD001 significantly augments survival by inhibiting thyroid tumor growth
To test the hypothesis that mTORC1 could be a potential target for preventing the progression of thyroid cancer, cohorts of 6-week-old TRβPV/PVPten+/− mice received orally either the placebo or the RAD001 biweekly until they became moribund with palpable tumors, rapid weight loss, hunched posture or labored breathing (n = 12 in each group). At 6 weeks, TRβPV/PVPten+/− mice displayed early thyroid hyperplasia, which is a benign lesion (data not shown).
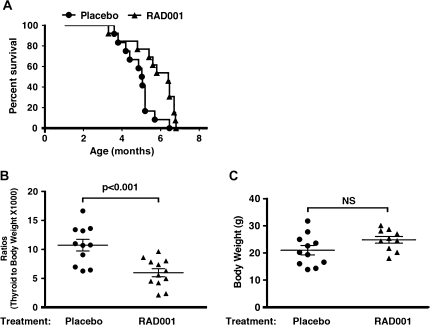
Analysis of the survival curve shows that RAD001-treated TRβPV/PVPten+/− mice died at a significantly older age (50% survival age: 6.4 months) than placebo-treated TRβPV/PVPten+/− mice (50% survival age: 5.0 months) (P < 0.01) (Figure 1A). These results indicate that RAD001 was effective in prolonging the survival of TRβPV/PVPten+/− mice. In all placebo- and RAD001-treated TRβPV/PVPten+/− moribund mice, the trachea was compressed due to the enlargement of the thyroid. The thyroid weight was determined and expressed as the ratios of thyroid to body weight (Figure 1B). The delayed morbidity of RAD001-treated TRβPV/PVPten+/− mice was associated with a substantial decrease in thyroid weight as compared with placebo-treated TRβPV/PVPten+/− mice (P < 0.001) to reduce the compression of the trachea. This decrease in thyroid weight was not accompanied with any significant changes in the body weight (Figure 1C, P = 0.096, data not significant).
Fig. 1.
mTORC1 signaling inhibition by RAD001 augments life span by decreasing thyroid tumor growth. (A) Kaplan–Meier survival curves for TRβPV/PVPten+/− mice treated or not with RAD001. TRβPV/PVPten+/− mice received the placebo or RAD001 (10 mg/kg body wt) twice a week by oral gavage from 6 weeks of age until they had to be euthanized because of sickness. (B) Thyroid glands of TRβPV/PVPten+/− mice, treated or not with RAD001, were dissected and weighed. The data are presented as ratios of thyroid weight to body weight. The difference in the thyroid weight between TRβPV/PVPten+/− mice treated or not with RAD001 is significant (P < 0.001), as determined by Student's t-test analysis. (C) Body weights of TRβPV/PVPten+/− mice receiving placebo or RAD001. Student's t-test analysis did not show any significant difference (P = 0.096; NS, not significant).
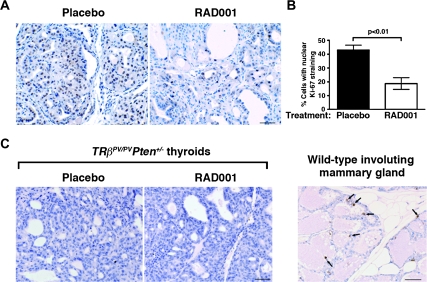
We then evaluated whether RAD001-induced reduction in thyroid growth resulted from either decreased cell proliferation or increased cell apoptosis or both. The antiproliferative effect of RAD001 was clearly illustrated by the reduced distribution of the cell proliferation marker Ki-67 on thyroid sections of RAD001-treated TRβPV/PVPten+/− mice as compared with the placebo group (Figure 2A). The quantification of Ki-67-stained nuclei showed a ∼2-fold decrease in their percentage in the thyroids after RAD001 treatment (Figure 2B, P < 0.01).
Fig. 2.
Inhibition of mTORC1 signaling reduces cell proliferation without affecting cell apoptosis in FTC. (A) Representative microphotographs of Ki-67 immunohistochemistry on sections of placebo- and RAD001-treated TRβPV/PVPten+/− thyroids counterstained by hematoxylin; bar, 50 μm. (B) Thyroid cell proliferative index, determined by Ki-67 immunohistochemistry in the two groups, shows that there is a significant reduction in the percentage of proliferating cells in the thyroids of RAD001-treated TRβPV/PVPten+/− mice (n = 4 mice) as compared with placebo-treated TRβPV/PVPten+/− mice (n = 5 mice). (C) Representative microphotographs showing no increase in apoptosis in RAD001-treated animals after prolonged treatment, as measured by terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling assay. Involuting wild-type female mammary gland, used as a positive control for terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling assay, shows apoptotic epithelial cells (depicted by arrows); bar, 50 μm.
Assessment of cell apoptosis by terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling assay confirmed that the basal level of cellular apoptosis is dramatically low in the thyroids of placebo-treated TRβPV/PVPten+/− mice (22) (Figure 2C). RAD001 treatment did not result in any changes in the apoptotic activity, as apoptotic cells were also barely observed in thyroid lesions (Figure 2C). As a positive control, an involuting mammary gland from a wild-type female mouse after pup weaning exhibited numerous apoptotic cells (arrows in Figure 2C). Altogether, our results indicate that RAD001 exerts an antiproliferative rather than a proapoptotic effect to decelerate tumor growth in the thyroid.
The inhibition of mTORC1 signaling by RAD001 does not prevent thyroid cancer development and aggressiveness
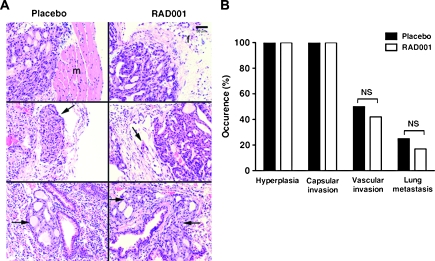
Whether the decreased thyroid tumor growth induced by RAD001 was associated with the prevention of thyroid cancer development and aggressiveness was analyzed by histology. Figure 3 shows representative pathological features of the thyroids and lungs of placebo- and RAD001-treated TRβPV/PVPten+/− mice. All placebo- and RAD001-treated mice displayed thyroid cancer as shown by the occurrence of advanced thyroid hyperplasia and capsular invasion (Figure 3A, top rows, and Figure 3B). Vascular invasion of the thyroid (Figure 3A, arrows in middle rows) and metastatic spread to the lung (Figure 3A, arrows in lower rows) occurred at the same percentage in placebo- and RAD001-treated TRβPV/PVPten+/− mice (Figure 3B). In both groups, lung metastasis consisted of micro-metastases. Further analyses indicated that there was no difference in the extent of lung metastasis between the two groups, in terms of number of lesions and metastatic cells (data not shown). Therefore, the inhibition of mTORC1 signaling neither prevented the development of thyroid cancer nor did it block the progression of thyroid cancer in TRβPV/PVPten+/− mice.
Fig. 3.
mTORC1 inhibition does not block capsular and vascular invasion of the thyroid and metastasis in TRβPV/PVPten+/− mice. (A) Hematoxylin and eosin staining of thyroids (top and middle rows) and lungs (lower rows) from TRβPV/PVPten+/− mice receiving the placebo or RAD001. Hyperplastic thyroids show capsular invasion in the surrounding tissue in placebo-treated TRβPV/PVPten+/− mice (top row, in the muscle, shown as m) and in RAD001-treated TRβPV/PVPten+/− mice (top row, in the fat tissue, shown as f). Both groups displayed vascular invasion as shown by the thyroid cells in vessels (pointed by the arrows in middle rows). Lung metastasis was observed in both placebo- and RAD001-treated TRβPV/PVPten+/− mice (depicted by arrows in lower rows). (B) Sections of thyroids and lungs from placebo- and RAD001-treated TRβPV/PVPten+/− mice were stained with hematoxylin and eosin and analyzed for pathological progression of thyroid cancer, i.e. advanced hyperplasia, capsular invasion, vascular invasion of the thyroid and metastasis spread to the lung. The data are expressed as the percentage of occurrence of total mutant mice examined; NS, not significant (P > 0.05).
The inhibition of mTORC1 signaling by RAD001 affects the translation initiation machinery and key regulators of cell cycle progression
Signaling through the PI3K/Akt/mTORC1 pathway leads to an increase in protein translation, particularly of proteins involved in cell cycle progression. To understand how RAD001 decreased thyroid tumor growth, we examined the activity of components of mTORC1 signaling (Figure 4A). RAD001 treatment strongly inhibited p70S6K activity, as shown by its reduced phosphorylation in the wild-type as in the TRβPV/PVPten+/− thyroids (Figure 4B). Consistently, the phosphorylation of a p70S6K downstream target, i.e. the ribosomal protein S6, was also strongly decreased (Figure 4B). The present western blot analysis of the 4E-BP1 showed several bands of the phosphorylated and total proteins, an observation that has been reported by others (28) (Figure 4B). The abundance of 4E-BP1 was very low in the wild-type thyroids, and RAD001 did not consistently affect its phosphorylation levels (Figure 4B, lanes 1 and 2). However, RAD001 treatment reduced the phosphorylation of 4E-BP1 in TRβPV/PVPten+/− thyroids (Figure 4B, compare lanes 3 and 4 with lanes 5 and 6). It also slightly affected the total levels of 4E-BP1, suggesting that in the TRβPV/PVPten+/− thyroids, RAD001 may regulate the translation of this protein. Altogether, our data clearly indicate that RAD001 treatment suppressed the activity of mTORC1 signaling, thereby affecting the activity of proteins involved in messenger RNA translation.
We then studied the phosphorylation of eukaryotic translation initiation factor (eIF) 4B (eIF-4B) and 4G (eIF-4G), two proteins that are directly involved in the activity of the translation machinery (Figure 4A). Consistent with the decreased mTORC1 activity in RAD001-treated animals, there was a modest but significant decrease in the phosphorylation of eIF-4B and eIF-4G (Figure 4C, compare lanes 3 and 4 with lanes 5 and 6).
The findings that mTORC1 inhibition affected cell proliferation (Figure 2A and B) in TRβPV/PVPten+/− thyroids prompted us to study the abundance and activity of proteins involved in G0/G1 to S phase transition. mTORC1 has been reported to regulate the translation of the cell cycle effectors Cyclin D1 (29,30) and Cyclin D3 (29–32). The present western blot analyses showed that RAD001 did not significantly affect Cyclin D1 abundance (Figure 4D, compare lanes 1–3 with lanes 4–6) but reduced Cyclin D3 protein levels by ∼2.5-fold in TRβPV/PVPten+/− thyroids (Figure 4D, compare lanes 1–3 lanes with 4–6). We also tested the hypothesis that the inhibition of mTORC1 pathway by RAD001 would decrease the phosphorylation of the pRb, which, in its unphosphorylated state, is a negative regulator of cell cycle progression by binding to and inhibiting critical regulatory proteins, including members of the E2F family of transcription factors. Cyclin D complexed with cyclin-dependent kinase 4 is essential for the phosphorylation of pRb, thereby releasing E2F family of transcription factors to propel cell cycle progression (33,34). In addition, the mTORC1 signaling can induce cyclin-dependent kinase 4 phosphorylation and thereby regulate pRb activity (32,33). We found a significant diminution in the phosphorylation levels of pRb in the thyroids of TRβPV/PVPten+/− mice (Figure 4D, compare lanes 1–3 with lanes 4–6). Altogether, our results indicate that the inhibition of mTORC1 signaling affects the translation and the activity of proteins involved in cell proliferation.
That mTORC1 inhibition by RAD001 did not alter cell apoptosis in TRβPV/PVPten+/− thyroids was reflected by the absence of changes in the protein levels of the proapoptotic factors Bax (Figure 4E, compare lanes 1–3 with lanes 4–6) and Bim (data not shown). Consistent with the absence of detection of apoptotic cells on thyroid sections (Figure 2C), we were unable to detect cleaved caspase-3 by western blot (data not shown). In addition, the lack of effect of RAD001 on capsular and vascular invasion (Figure 3A and B) is associated with virtually unaltered abundance of MMP-2, which plays an important role in cell invasion and metastasis (Figure 4E, compare lanes 1–3 with lanes 4–6).
mTORC1 inhibition has moderate effect on Akt-signaling pathway and does not alter mitogen-activated protein kinase (extracellular signal-regulated kinase 1/2) pathway
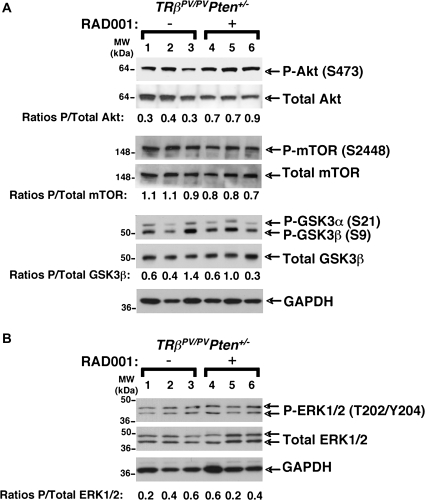
In several cancer cell types, the existence of a negative feedback loop wherein the mTORC1-activated p70S6K decreases PI3K/Akt activity has been uncovered (35,36). In these cells, treatment with RAD001 disrupts the feedback loop, thereby leading to PI3K/Akt signaling activation. This mechanism would explain the limited efficacy of mTORC1 inhibitors in some cancers. On the other hand, prolonged treatment with rapamycin may perturb the assembly of mTOR into mTORC2 complex, and in ∼20% of cancer cell lines, the drop in intact mTOR is sufficient to strongly inhibit Akt signaling (37,38). We, therefore, studied the effect of prolonged RAD001 treatment on the activity of Akt signaling in the thyroids. We observed a modest increase in the activity of Akt in the thyroids of RAD001-treated TRβPV/PVPten+/− mice (Figure 5A, compare lanes 1–3 with lanes 4–6). That Akt activity was increased by RAD001 treatment in the thyroids of TRβPV/PVPten+/− mice prompted us to assess whether the activity of its downstream targets, such as mTOR and GSK3β, was also affected. However, neither the phosphorylation levels of mTOR nor those of GSK3β were consistently altered by the treatment (Figure 5A, compare lanes 1–3 with lanes 4–6).
Fig. 5.
mTORC1 signaling inhibition by RAD001 does not alter PI3K/Akt and mitogen-activated protein kinase (ERK1/2) pathways in the thyroid tumors of TRβPV/PVPten+/− mice. Total protein extracts were prepared from thyroid tumors of TRβPV/PVPten+/− mice receiving the placebo (lanes 1–3) or RAD001 (lanes 3–6), as described in Materials and Methods. (A) Western blot analysis of phosphorylated Akt, total Akt, phosphorylated mTOR, total mTOR, phosphorylated GSK3β, total GSK3β and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as a loading control. The ratios of phosphorylated protein to total protein levels, after quantification of the band intensities for each sample, are indicated. (B) Western blot analysis of phosphorylated ERK1/2, total ERK1/2 and GAPDH as a loading control.
The existence of another mTORC1-p70S6K negative feedback loop that represses mitogen-activated protein kinase [extracellular signal-regulated kinase 1/2 (ERK1/2)] pathway has been reported recently (39). Indeed, the use of RAD001 in a mouse model of prostate cancer and in some cancer patients leads to an increase in mitogen-activated protein kinase signaling activity, thereby limiting the efficacy of the treatment (39). We, thus, determined whether RAD001 stimulated the activity of ERK1/2 in the TRβPV/PVPten+/− thyroids (Figure 5B). We did not detect any consistent changes in the ratios of phosphorylated to total ERK1/2 (Figure 5B, compare lanes 1–3 with lanes 4–6). Altogether, these data indicate that in the thyroids of TRβPV/PVPten+/− mice, RAD001-repressed mTORC1 signaling has moderate effect on PI3K/Akt signaling and does not alter ERK1/2 signaling.
Discussion
The clinical development of mTORC1 inhibitors has offered the new opportunity to target mTORC1-signaling pathway in cancer therapy and to better understand its role in cancer. These immunosuppressive compounds possess antiproliferative and antitumor activities both in vitro and in vivo. Their efficacy, either alone or in combination with other agents, is currently being investigated in a large number of preclinical studies and of Phase I–III oncology clinical trials (20). Whereas preclinical studies and clinical trials have focused on their potential benefits for the treatment of a wide array of cancers, there is still, to our knowledge, no report on thyroid cancer.
The study of the effects of mTORC1 inhibition in a unique murine model of thyroid cancer, the TRβPV/PVPten+/− mouse, which displays a dramatic activation of mTORC1 signaling (22), has provided us with the opportunity to evaluate for the first time the role of this signaling pathway on the development and progression of thyroid cancer. RAD001 treatment was started at 6 weeks, an age when TRβPV/PVPten+/− mice display mild thyroid hyperplasia but no thyroid cancer. Here, we show that although mTORC1 inhibition did not prevent the occurrence of thyroid cancer, it markedly decreased thyroid cancer growth, thereby prolonging the life span of the mice. To ascertain that the effect of RAD001 in delaying thyroid cancer growth did not involve the pituitary hormone TSH, which is a major stimulator of thyrocyte growth, we compared serum TSH levels in mice with or without RAD001 treatment (supplementary Figure 1 is available at Carcinogenesis Online). However, we did not observe any significant differences in serum TSH levels between groups. These results indicate that the delayed cancer growth of TRβPV/PVPten+/− thyroids induced by mTORC1 inhibition did not involve the participation of TSH.
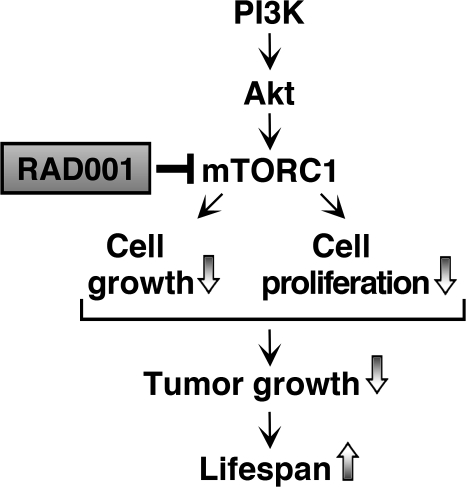
The present biochemical studies indicate that RAD001 strongly suppressed mTORC1 activity in the thyroid, as shown by the reduced phosphorylation of its downstream targets involved in the activity of the translation machinery, i.e. p70S6K, 4E-BP1 and the eukaryotic translation initiation factors eIF-4B and eIF-4G. The inhibition of mTORC1 by RAD001 specifically triggered tumor growth by decreasing cell growth and proliferation without affecting cell apoptosis, as shown in other studies (30,40–42) (Figure 6). In the thyroid cancer of TRβPV/PVPten+/− mice, we found that inhibition of mTORC1 signaling reduced the levels of Cyclin D3 and decreased the phosphorylation of pRb, thereby consistently diminishing cell cycle progression. Finally, that Cyclin D1 abundance, in contrast with that of Cyclin D3, was not regulated by mTORC1 in thyroid cancer of TRβPV/PVPten+/− mice has been observed in other cancer cells (32). These results suggest that these two related proteins can be regulated by distinct signaling pathways.
Fig. 6.
Proposed model for the effect of the inhibition of mTORC1 signaling by RAD001 in the thyroids of TRβPV/PVPten+/− mice. RAD001 significantly inhibits the activity of the mTORC1 downstream targets belonging to the translation initiation machinery. Whereas the treatment did not inhibit cell migration to prevent cancer progression or exert noticeable proapoptotic actions, it consistently reduced cell proliferation, thereby reducing thyroid cancer growth to augment mouse life span.
The present findings are consistent with the observations obtained in mice that have selectively lost the Pten gene in the thyroid [PtenL/L;TPO-Cre mice (43)]. The loss of Pten in thyroid cells in PtenL/L;TPO-Cre mice leads to increased activation of the Akt signaling and induces mild thyroid hyperplasia (43). RAD001 treatment of PtenL/L;TPO-Cre mice leads to reduced thyroid growth by affecting cell proliferation, but it does not reverse thyroid abnormalities (43). Importantly, our mouse model of thyroid cancer shows that despite a significant reduction in tumor growth, mTORC1 signaling inhibition did not fully prevent thyroid cancer progression as shown by similar occurrence of advanced hyperplasia, capsular extension and vascular invasion in the thyroids and lung metastasis. That mTORC1 inhibition had a limited effect on thyroid cancer progression prompted us to determine whether this could be due to the activation of the PI3K/Akt or mitogen-activated protein kinase-signaling pathway via a negative feedback loop arising from p70S6K, as described by others (23,24,35,36,39). However, the present biochemical studies indicate that RAD001 did not consistently affect the activity of either signaling pathways in the thyroid cancer of TRβPV/PVPten+/− mice. For instance, the activity of the downstream PI3K/Akt target, GSK3β, which is involved in the regulation of Cyclin D1 abundance, was not altered. In addition, the abundance of PI3K/Akt downstream effectors such as Bax, which is involved in cell apoptosis, and MMP-2, which is involved in cell migration, was not affected. Therefore, in the thyroid cancer of TRβPV/PVPten+/− mice, the activity of mTORC1-p70S6K negative feedback loop is negligible and does not account for the limited efficacy of RAD001 on thyroid cancer progression.
We have recently shown that the treatment of TRβPV/PV mice with a potent and specific PI3K inhibitor, LY294002, significantly delays tumor progression and efficiently blocks tumor invasion and metastasis (21). The effect of the LY294002 treatment on TRβPV/PV thyroid cancer results from alterations in multiple pathways downstream of the PI3K signaling. Indeed, the activity of mTORC1-p70S6K pathway that regulates cell proliferation is attenuated in LY294002-treated TRβPV/PV mice. In addition, the activity of other PI3K downstream targets regulating cell migration and cell survival, such as MMP-2 and Bad, respectively, is altered to decrease cell migration and increase cell apoptosis (21). Altogether, the comparison of the present study using the mTORC1 inhibitor RAD001 with our previous work evaluating the effect of LY294002 further emphasizes the idea that mTORC1 pathway plays an important role in thyroid tumor growth by regulating cell growth and proliferation but that PI3K-dependent mTORC1-independent pathways are critical in mediating tumor progression and invasiveness.
In conclusion, this study indicates that the mTORC1 signaling, via its action on the translation initiation machinery, regulates thyroid cancer growth by stimulating cell growth and proliferation (Figure 6). However, mTORC1-signaling pathway does not regulate cell survival and cell migration, two processes that contribute to cancer growth, progression and metastasis. Therefore, our work strongly supports the idea that the signaling pathways involved in tumor growth and metastasis are different. This preclinical study, which may pave the way for clinical trials on thyroid cancer, suggests that optimal therapy in thyroid cancer with overactivated PI3K/Akt signaling could be obtained if mTOR inhibitors are used in combination with other drugs or therapies that target cell apoptosis and block cell migration.
Supplementary material
Supplementary Figure 1 can be found at http://carcin.oxfordjournals.org/
Funding
Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research
Supplementary Material
Acknowledgments
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- Akt
protein kinase B
- eIF
eukaryotic translation initiation factor
- ERK1/2
extracellular signal-regulated kinase 1/2
- 4E-BP
eukaryotic translation initiation factor 4E binding protein
- FTC
follicular thyroid cancer
- GSK
glycogen synthase kinase
- MMP-2
matrix metalloprotease-2
- mTORC1
mammalian target of rapamycin complex 1
- NIH
National Institutes of Health
- PI3K
phosphatidylinositol 3-kinase
- pRb
retinoblastoma protein
- TSH
thyroid-stimulating hormone
References
- 1.Gillenwater AM, et al. Thyroid carcinoma. Cancer Treat. Res. 1997;90:149–169. doi: 10.1007/978-1-4615-6165-1_8. [DOI] [PubMed] [Google Scholar]
- 2.Hundahl SA, et al. A National Cancer Data Base report on 53,856 cases of thyroid carcinoma treated in the U.S., 1985-1995 [see comments] Cancer. 1998;83:2638–2648. doi: 10.1002/(sici)1097-0142(19981215)83:12<2638::aid-cncr31>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 3.Sherman SI, et al. Prospective multicenter study of thyroidcarcinoma treatment: initial analysis of staging and outcome. National Thyroid Cancer Treatment Cooperative Study Registry Group. Cancer. 1998;83:1012–1021. doi: 10.1002/(sici)1097-0142(19980901)83:5<1012::aid-cncr28>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 4.Schlumberger M, et al. [Papillary and follicular cancers of the thyroid] Presse Med. 1998;27:1479–1481. [PubMed] [Google Scholar]
- 5.Fitzgibbons SC, et al. The treatment of thyroid cancer. Am. Surg. 2008;74:389–399. [PubMed] [Google Scholar]
- 6.Sherman SI. Advances in chemotherapy of differentiated epithelial and medullary thyroid cancers. J. Clin. Endocrinol. Metab. 2009;94:1493–1499. doi: 10.1210/jc.2008-0923. [DOI] [PubMed] [Google Scholar]
- 7.Kaneshige M, et al. Mice with a targeted mutation in the thyroid hormone beta receptor gene exhibit impaired growth and resistance to thyroid hormone. Proc. Natl Acad. Sci. USA. 2000;97:13209–13214. doi: 10.1073/pnas.230285997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weiss RE, et al. Resistance to thyroid hormone. Rev. Endocr. Metab. Disord. 2000;1:97–108. doi: 10.1023/a:1010072605757. [DOI] [PubMed] [Google Scholar]
- 9.Ono S, et al. Homozygosity for a dominant negative thyroid hormone receptor gene responsible for generalized resistance to thyroid hormone. J. Clin. Endocrinol. Metab. 1991;73:990–994. doi: 10.1210/jcem-73-5-990. [DOI] [PubMed] [Google Scholar]
- 10.Guigon CJ, et al. Novel oncogenic actions of TRbeta mutants in tumorigenesis. IUBMB Life. 2009;61:528–536. doi: 10.1002/iub.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suzuki H, et al. Mice with a mutation in the thyroid hormone receptor beta gene spontaneously develop thyroid carcinoma: a mouse model of thyroid carcinogenesis. Thyroid. 2002;12:963–969. doi: 10.1089/105072502320908295. [DOI] [PubMed] [Google Scholar]
- 12.Kim CS, et al. AKT activation promotes metastasis in a mouse model of follicular thyroid carcinoma. Endocrinology. 2005;146:4456–4463. doi: 10.1210/en.2005-0172. [DOI] [PubMed] [Google Scholar]
- 13.Furuya F, et al. Activation of phosphatidylinositol 3-kinase signaling by a mutant thyroid hormone beta receptor. Proc. Natl Acad. Sci. USA. 2006;103:1780–1785. doi: 10.1073/pnas.0510849103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ringel MD, et al. Overexpression and overactivation of Akt in thyroid carcinoma. Cancer Res. 2001;61:6105–6111. [PubMed] [Google Scholar]
- 15.Miyakawa M, et al. Increased expression of phosphorylated p70S6 kinase and Akt in papillary thyroid cancer tissues. Endocr. J. 2003;50:77–83. doi: 10.1507/endocrj.50.77. [DOI] [PubMed] [Google Scholar]
- 16.Ying H, et al. Mutant thyroid hormone receptor beta represses the expression and transcriptional activity of peroxisome proliferator-activated receptor gamma during thyroid carcinogenesis. Cancer Res. 2003;63:5274–5280. [PubMed] [Google Scholar]
- 17.Kato Y, et al. PPARgamma insufficiency promotes follicular thyroid carcinogenesis via activation of the nuclear factor-kappaB signaling pathway. Oncogene. 2006;25:2736–2747. doi: 10.1038/sj.onc.1209299. [DOI] [PubMed] [Google Scholar]
- 18.Ying H, et al. Aberrant accumulation of PTTG1 induced by a mutated thyroid hormone beta receptor inhibits mitotic progression. J. Clin. Invest. 2006;116:2972–2984. doi: 10.1172/JCI28598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guigon CJ, et al. Regulation of beta-catenin by a novel nongenomic action of thyroid hormone beta receptor. Mol. Cell. Biol. 2008;28:4598–4608. doi: 10.1128/MCB.02192-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yap TA, et al. Targeting the PI3K-AKT-mTOR pathway: progress, pitfalls, and promises. Curr. Opin. Pharmacol. 2008;8:393–412. doi: 10.1016/j.coph.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 21.Furuya F, et al. Inhibition of phosphatidylinositol 3-kinase delays tumor progression and blocks metastatic spread in a mouse model of thyroid cancer. Carcinogenesis. 2007;28:2451–2458. doi: 10.1093/carcin/bgm174. [DOI] [PubMed] [Google Scholar]
- 22.Guigon CJ, et al. PTEN deficiency accelerates tumour progression in a mouse model of thyroid cancer. Oncogene. 2009;28:509–517. doi: 10.1038/onc.2008.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sabatini DM. mTOR and cancer: insights into a complex relationship. Nat. Rev. Cancer. 2006;6:729–734. doi: 10.1038/nrc1974. [DOI] [PubMed] [Google Scholar]
- 24.Bjornsti MA, et al. The TOR pathway: a target for cancer therapy. Nat. Rev. Cancer. 2004;4:335–348. doi: 10.1038/nrc1362. [DOI] [PubMed] [Google Scholar]
- 25.Podsypanina K, et al. Mutation of Pten/Mmac1 in mice causes neoplasia in multiple organ systems. Proc. Natl Acad. Sci. USA. 1999;96:1563–1568. doi: 10.1073/pnas.96.4.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boulay A, et al. Antitumor efficacy of intermittent treatment schedules with the rapamycin derivative RAD001 correlates with prolonged inactivation of ribosomal protein S6 kinase 1 in peripheral blood mononuclear cells. Cancer Res. 2004;64:252–261. doi: 10.1158/0008-5472.can-3554-2. [DOI] [PubMed] [Google Scholar]
- 27.Lane HA, et al. Future directions in the treatment of hormone-sensitive advanced breast cancer: the RAD001 (Everolimus)-letrozole clinical program. Semin. Oncol. 2006;33:S18–S25. doi: 10.1053/j.seminoncol.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 28.Gingras AC, et al. Regulation of 4E-BP1 phosphorylation: a novel two-step mechanism. Genes Dev. 1999;13:1422–1437. doi: 10.1101/gad.13.11.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pervin S, et al. Nitric oxide-induced cytostasis and cell cycle arrest of a human breast cancer cell line (MDA-MB-231): potential role of cyclin D1. Proc. Natl Acad. Sci. USA. 2001;98:3583–3588. doi: 10.1073/pnas.041603998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yeager N, et al. Mammalian target of rapamycin is the key effector of phosphatidylinositol-3-OH-initiated proliferative signals in the thyroid follicular epithelium. Cancer Res. 2008;68:444–449. doi: 10.1158/0008-5472.CAN-07-3030. [DOI] [PubMed] [Google Scholar]
- 31.Prabhu S, et al. A novel mechanism for Bcr-Abl action: Bcr-Abl-mediated induction of the eIF4F translation initiation complex and mRNA translation. Oncogene. 2007;26:1188–1200. doi: 10.1038/sj.onc.1209901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paternot S, et al. Combined inhibition of MEK and mammalian target of rapamycin abolishes phosphorylation of cyclin-dependent kinase 4 in glioblastoma cell lines and prevents their proliferation. Cancer Res. 2009;69:4577–4581. doi: 10.1158/0008-5472.CAN-08-3260. [DOI] [PubMed] [Google Scholar]
- 33.Lundberg AS, et al. Functional inactivation of the retinoblastoma protein requires sequential modification by at least two distinct cyclin-cdk complexes. Mol. Cell. Biol. 1998;18:753–761. doi: 10.1128/mcb.18.2.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paternot S, et al. Distinct specificities of pRb phosphorylation by CDK4 activated by cyclin D1 or cyclin D3: differential involvement in the distinct mitogenic modes of thyroid epithelial cells. Cell Cycle. 2006;5:61–70. doi: 10.4161/cc.5.1.2265. [DOI] [PubMed] [Google Scholar]
- 35.O'Reilly KE, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500–1508. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang H, et al. PDGFRs are critical for PI3K/Akt activation and negatively regulated by mTOR. J. Clin. Invest. 2007;117:730–738. doi: 10.1172/JCI28984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zeng Z, et al. Rapamycin derivatives reduce mTORC2 signaling and inhibit AKT activation in AML. Blood. 2007;109:3509–3512. doi: 10.1182/blood-2006-06-030833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Copp J, et al. TORC-specific phosphorylation of mammalian target of rapamycin (mTOR): phospho-Ser2481 is a marker for intact mTOR signaling complex 2. Cancer Res. 2009;69:1821–1827. doi: 10.1158/0008-5472.CAN-08-3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carracedo A, et al. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J. Clin. Invest. 2008;118:3065–3074. doi: 10.1172/JCI34739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hernando E, et al. The AKT-mTOR pathway plays a critical role in the development of leiomyosarcomas. Nat. Med. 2007;13:748–753. doi: 10.1038/nm1560. [DOI] [PubMed] [Google Scholar]
- 41.Yang L, et al. PTEN loss does not predict for response to RAD001 (Everolimus) in a glioblastoma orthotopic xenograft test panel. Clin. Cancer Res. 2008;14:3993–4001. doi: 10.1158/1078-0432.CCR-07-4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Papewalis C, et al. Role of the novel mTOR inhibitor RAD001 (everolimus) in anaplastic thyroid cancer. Horm. Metab. Res. 2009;41:752–756. doi: 10.1055/s-0029-1224116. [DOI] [PubMed] [Google Scholar]
- 43.Yeager N, et al. Pten loss in the mouse thyroid causes goiter and follicular adenomas: insights into thyroid function and Cowden disease pathogenesis. Cancer Res. 2007;67:959–966. doi: 10.1158/0008-5472.CAN-06-3524. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.