Abstract
Emerging evidence indicates that intrinsic differences and induced changes in aerobic capacity are probably to play a critical role in the development of chronic diseases like cancer. This study was initiated: (i) to determine how citrate synthase activity, which is routinely used as a marker of aerobic capacity and mitochondrial density in skeletal muscle, was affected by voluntary running on either a motorized activity wheel or a non-motorized free wheel and (ii) to investigate the association between aerobic capacity and the carcinogenic response induced in the mammary gland by intraperitoneal injection of 1-methyl-1-nitrosurea. Overall, wheel running reduced cancer incidence (96 versus72%, P = 0.0006) and the number of cancers per animal (2.84 versus 1.78, P < 0.0001) and induced citrate synthase activity (276 versus 353 U/mg, P < 0.0001, sedentary control versus wheel running,respectively). Both motorized and free wheel running increased citrate synthase activity (373 ± 24, 329 ± 11 and 276 ± 9 U/mg protein, P < 0.0001) and reduced the average number of cancers per rat (2.84, 1.96 and 1.63, P < 0.01), sedentary control, free wheel and motorized wheel, respectively. However, regression analyses failed to provide evidence of a significant association between citrate synthase activity and either cancer incidence or cancer multiplicity. Citrate synthase activity is a single measure in a complex pathway that determines aerobic capacity. The multifaceted nature of intrinsic and inducible aerobic capacity limits the usefulness of citrate synthase activity alone in elucidating the relationship between aerobic capacity and the carcinogenic response.
Introduction
Aerobic capacity is an integrated measure of the ability of the cardiovascular system to deliver oxygen and the capacity of mitochondria to utilize oxygen for energy production (1). As summarized in (2), low aerobic capacity is a strong predictor of early mortality and emerging evidence also indicates that aerobic capacity is inversely associated with the risk for becoming overweight and obese and for the occurrence of type-2 diabetes and heart disease (3). Low aerobic capacity has also been associated with death due to breast cancer in a cohort of >14 000 healthy women followed for a mean of 16.4 years with 68 breast cancer deaths (4). In physically inactive individuals, it is estimated that genetic inheritance accounts for up to 70% of the variation in intrinsic aerobic capacity (5). In most cases, adoption of a physically active life style improves aerobic capacity; however, activity-induced responses are grossly heterogeneous (6,7). Interestingly, recent evidence suggests that variation in intrinsic aerobic capacity classified by treadmill testing of either animals or humans is also reflected biochemically in tissues other than muscle and that the differences observed may impact energy metabolism, fat accumulation and host factors influenced by insulin sensitivity and cytokines produced by adipose tissue (2,8). Moreover, these factors could contribute to the risk for chronic diseases, including cancer.
The intensity of individuals’ patterns of physical activity affect functional measures such as aerobic capacity and it has been argued that measurement of aerobic capacity provides a more reliable index of overall levels of physical activity than self report, although such measures are expensive and not feasible to evaluate in large population studies. However, assessment of aerobic capacity may be possible in intervention studies in which compliance to a protocol is essential to measure, and such functional measures are clearly achievable in investigations that use animal models for physical activity and cancer. Maximal citrate synthase activity is routinely used as a marker of aerobic capacity and mitochondrial density in skeletal muscle in experiments with humans and animals (9). A classic marker of mitochondrial density, citrate synthase is an enzyme of the tricarboxylic acid cycle that catalyzes the condensation reaction of acetyl CoA and oxaloacetate to form citrate (1).
Epidemiological investigations provide strong evidence that individuals who are most physically active have a lower incidence of breast cancer and lower mortality rates than individuals who report the lowest levels of physical activity (10–14). Moreover, the intensity of physical activity is linked to protective activity against cancer (15). Animal models for physical activity and for cancer have been developed in an effort to determine mechanisms that account for cancer preventive activity, and it has been shown that forced exercise on a treadmill and voluntary wheel running inhibit experimentally induced breast cancer (16). While the intensity of exercise on a treadmill has been reported to be inversely related to the carcinogenic response and positively correlated with aerobic capacity measured as VO2max (17,18), little is known about intensity of physical activity in rats that run on an activity wheel. As reviewed in ref. 19, the running characteristics of animals that are exercised on a treadmill according to an investigator-defined training protocol differ markedly from those of animals given access to an activity wheel and in which animals decide on the intensity, duration of each bout of running, the time interval between bouts of running and the total distance run per day.
Our laboratory has recently completed a series of investigations in which the effects of wheel running on experimentally induced breast cancer were evaluated using either a non-motorized free wheel or a motorized activity wheel (20,21). For the study reported herein, all animals from those experiments for which skeletal muscle was available from a bank of tissue snap frozen in liquid nitrogen at necropsy were included to create a group of animals with a wide range of physical activity exposures across which to explore the linkage between aerobic capacity and the carcinogenic response. By doing this, a unique opportunity was created such that for each animal included in the analysis, data were available on distance run in the activity wheel, aerobic capacity measured as skeletal muscle citrate synthase activity and breast tumor occurrence. Using these animals, three questions were addressed: (i) how does running on a non-motorized free wheel versus motorized activity wheel affect aerobic capacity measured as skeletal muscle citrate synthase activity; (ii) does aerobic capacity mediate inhibition of the carcinogenic response associated with wheel running and (iii) can aerobic capacity measured as citrate synthase activity reliably discriminate between wheel runners and non-wheel runners?
Materials and methods
Experimental design
This preclinical model has been reported in detail in (21,22). Briefly, female Sprague–Dawley rats were obtained at 20 days of age and housed in solid-bottomed polycarbonate cages. At 21 days of age, rats were injected (intraperitoneally) with 50 mg 1-methyl-1-nitrosurea per kilogram body weight as described previously (21). At 28 days of age, 1 week post carcinogen injection, rats were randomized by weight to one of three groups: (i) a non-motorized free wheel; (ii) a motorized activity wheel or (iii) sedentary control. The motorized wheel rats ran at a constant speed (40 m/min), whereas the free wheel rats self-determined the speed at which they ran. The rats were not forced to run in either circumstance. Rather, when the rat entered the wheel, a proximity sensor detected the presence of the animal. In the case of the motorized wheel, the wheel began to turn at a constant speed but it stopped turning when the animal exited the wheel. In the free wheel, the rat turned the wheel by running. In both cases, the revolutions run were counted and food reward was delivered for the amount of distance run. Even though in both circumstances, running behavior was reinforced by giving a food reward delivered using a food pellet dispenser, the rats in both groups still determined the length of each running bout, the interval between running bouts and the total distance run per day. Since no fitness goals were set for the animals, we do not consider that this work modeled the effects of exercise training. Rather, our view is that rats given free access to activity wheels model a population of individuals with different physical activity levels, and that accordingly, these animals would have a range of aerobic capacities. Rats were fed a purified pelleted diet (Research Diet, New Brunswick, NJ). A computer device attached to the activity wheel monitored distance run, which was recorded daily.
At necropsy, rats were skinned and the skin to which mammary gland chains were attached was examined under translucent light for detectable mammary pathologies. All grossly detectable mammary gland pathologies were excised and prepared for histological classification, only confirmed mammary carcinomas are reported. The experimental protocols were reviewed and approved by the Institutional Animal Care and Use Committee and conducted according to the committee guidelines.
Muscle tissue preparation
Following an overnight fast, rats at rest were euthanized over a 3 h time interval via inhalation of gaseous carbon dioxide and cervical dislocation. The euthanization sequence was stratified across groups to minimize any treatment-associated effects due to order. Once rats were euthanized, the entire gastrocnemius muscle was collected and immediately frozen between stainless steel calipers precooled in liquid nitrogen. Excised tissue was stored at −80°C.
Gastrocnemius muscle was prepared (15% wt/vol; 0.15 mg/ml) in extraction buffer (175 mM KCl, 2 mM ethylenediaminetetraacetic acid and 10 mM Tris, pH 7.4) by homogenizing the entire piece of excised muscle (Brinkmann Polytron; Brinkmann Instruments, Rexdale, Ontario, Canada) for 15 s followed by sonication (Branson Sonifier 250) for 20 pulses in ice-cold conditions. After centrifugation at 7500 g for 10 min at 4°C, aliquots of supernatant were stored at −80°C before assays to determine protein concentration and citrate synthase activity.
Citrate synthase activity assay
Supernatant of muscle homogenates was evaluated for citrate synthase activity utilizing the method described by Srere (44). Briefly, as citrate synthase activity catalyzes the condensation reaction of acetyl CoA and oxaloacetic acid to yield citrate, the exposed thiol group on CoA-SH reacted with 5,5′-dithiobis-(2-nitrobenzoic acid) to form 5-thio-2 nitrobenzoic acid, which produced a yellow product detectable at 412 nm using a Molecular Devices (Sunnyvale, CA); SpectraMax M5. Supernatants of muscle homogenates were evaluated for protein concentration using a commercially available kit from Pierce® (Thermo Fisher Scientific, Rockford, IL) designed to follow the bicinchoninic acid method originally described by Smith with bovine serum albumin as the standard. Citrate synthase activity was expressed in nanomoles per minute per milligram of protein. The coefficient of variation for the assay was <5%.
Statistical analyses
Cancer outcomes were evaluated by regression chi-square test if independence (incidence) and Poisson regression (tumor count) (23). Group differences in citrate synthase activity and body weight were evaluated by analysis of variance. The associations between citrate synthase activity and the cancer outcomes were evaluated using logistic regression for incidence and Poisson regression for tumor count. Regression models were used to evaluate whether the effects of wheel running on carcinogenic response are mediated through effects on citrate synthase activity (24). Specifically, if citrate synthase activity mediates treatment effect, the magnitude of the treatment effect will be greatly reduced when the measure of citrate synthase activity is included as a covariate in the regression of cancer response on treatment group, and citrate synthase activity will be a significant predictor of the response (25). The potential for citrate synthase activity as a discriminator of fitness was evaluated graphically, whereas the relationship between citrate synthase activity and meters run was estimated by linear regression. Analyses were done in SAS 9.2 (Sas Institute, Cary, NC). Figures were created using R (A Language and Environment for Statistical Computing, R Foundation for Statistical Computing, Vienna, Austria, 2009).
Results
The carcinogenic response observed in sedentary control rats versus wheel runners is shown in Table I and is similar to that reported in the parent studies (20,21). Both cancer incidence and cancer multiplicity were reduced by wheel running in comparison with the sedentary control, consistent with the hypothesis that higher aerobic capacity is associated with a diminished carcinogenic response in this model system. Overall, the relative risk for cancer was 1.33 in the sedentary control group in comparison with animals that were given access to an activity wheel (95% confidence interval 1.11, 1.60), an effect similar to that reported in a number of meta analyses of the epidemiological evidence (14,15,26). Also, shown in Table I are the effects of treatment on final body weights. Average body weights varied <5% between sedentary controls and animals that ran. While this difference was statistically significant, it is below the level that has been shown to have a detectable effect on tumor occurrence in this model system (27).
Table I.
Effect of wheel running on citrate synthase activity and the carcinogenic response
| Treatment | Type | N | Incidence % (N) | Average AC/rat ± SEM | citrate synthase activity ± SEM | Final body weight (g) ± SEM |
| Wheel running | Motorized wheel | 27 | 70 (19) | 1.63 ± 0.30 | 373.4 ± 24.0 | 199.2 ± 3.0 |
| Free wheel | 23 | 74 (17) | 1.96 ± 0.30 | 329.1 ± 10.8 | 188.7 ± 1.9 | |
| Sedentary control | None | 50 | 96 (48) | 2.84 ± 0.27 | 275.6 ± 9.0 | 204.9 ± 2.6 |
| PA compared with SC | P value | 0.0006 | 0.0006 | <0.0001 | 0.0008 | |
| Motorized wheel compared with free wheel | P value | 0.78 | 0.39 | 0.26 | 0.02 | |
| Motorized wheel compared with SC | P value | 0.002 | 0.0013 | <0.0001 | 0.13 | |
| Free wheel compared with SC | P value | 0.007 | 0.03 | 0.004 | <0.0001 |
Differences were tested by chi-square test for independence of rows and columns (incidence), Poisson regression (tumor count) and one-way analysis of variance (bodyweight and the log transformation of citrate synthase activity); AC, adenocarcinoma; SEM, standard error of the mean; PA, physical activity; SC, sedentary control.
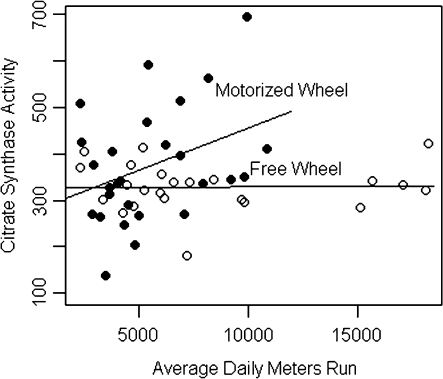
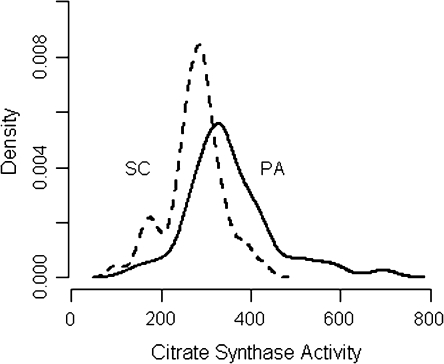
Citrate synthase activity was greater in wheel runners than in sedentary control animals, as expected. To better understand how the different characteristics of running for a food reward in either a non-motorized free wheel or a motorized activity wheel affected aerobic capacity measured as skeletal muscle citrate synthase activity, enzymatic activity was plotted against the average daily distance that each animal ran. Animals in the free wheel group ran farther than rats in the motorized wheel group (8213 ± 1056 m/day versus 5544 ± 477 m/day, P = 0.03); however, as shown in Figure 1, citrate synthase activity was positively correlated with distance run in the motorized wheel but not the free wheel wheels. As shown in Figure 2 and Table II, the intrinsic aerobic capacity reflected by muscle citrate synthase activity in this out-bred population of sedentary rats was variable and overlapped considerably with the citrate synthase activity induced by wheel running. Nonetheless, enzymatic activity was highest in the motorized wheel group, a finding consistent with our hypothesis that the motorized wheel would provide more intense running. However, citrate synthase activity was more variable in the motorized wheel group while enzymatic activity tended to be lower and less variable in the free wheel group. Consistent with the stepped effect of sedentary versus free wheel versus motorized wheel on citrate synthase activity, cancer incidence and multiplicity were reduced in both wheel running groups relative to sedentary control; the differences between free wheel and motorized wheel in both cancer incidence and cancer multiplicity were not statistically significant.
Fig. 1.
Citrate synthase activity by daily meters run by wheel-running group. Slopes estimated by ordinary least squares regression: 0.018 ± 0.007, P = 0.02 (motorized wheel); 0.00 ± 0.004, P = 0.95 (free wheel).
Fig. 2.
Probability density of citrate synthase activity by treatment. While the means are statistically different, the overlap of the distributions suggests poor discrimination of wheel running physical activity (PA) by citrate synthase activity; SC, sedentary control. A thorough discussion of the implications of this result can be found in Ware (28).
Table II.
Effect of Wheel running types on running duration and citrate synthase activity
| Treatment | Wheel running type | N | Distance run meters per rat ± SEM | Citrate synthase activity U/mg protein ± SEM |
| Wheel running | Motorized wheel | 27 | 5544 ± 477 | 373.4 ± 24.0 |
| Wheel running | Free wheel | 23 | 8213 ± 1056 | 329.1 ± 10.8 |
| Sedentary control | None | 50 | N/A | 275.6 ± 9.0 |
| Wheel running compared with control | P value | N/A | <0.0001 | |
| Motorized wheel compared with free wheel | P value | 0.03 | 0.26 | |
| Motorized wheel compared with control | P value | N/A | <0.0001 | |
| Free wheel compared with control | P value | N/A | 0.004 |
Differences were tested by appropriate contrasts in a one-way analysis of variance. Both meters run and citrate synthase activity were log transformed for analysis; N/A, Not applicable.
There was a clear effect of wheel running on aerobic capacity measured by citrate synthase activity, as shown in Table II. Given the effect of wheel running on both citrate synthase activity and the carcinogenic response, we explored the possibility that the effect of wheel running is mediated by citrate synthase activity. A logistic regression of cancer incidence on citrate synthase activity provided no evidence of an association between citrate synthase activity and cancer incidence (P = 0.78). Similarly, although cancer multiplicity is significantly different across treatment groups (Table I, P < 0.0001) and wheel running increased citrate synthase activity significantly (Table II, P < 0.0001), there was no significant association between citrate synthase activity and cancer multiplicity (P = 0.38, data not shown). A preliminary test of the hypothesis that citrate synthase activity mediates the protective effect of wheel running on the carcinogenic response corroborated the negative results of the regression analyses (supplementary data and Table 1 are available at Carcinogenesis Online).
Discussion
Higher intensity physical activity is generally associated with increased aerobic capacity and there appears to be an inverse association between physical activity intensity and breast cancer risk (14,15,26). However, to our knowledge, the relationship between aerobic capacity and the development of breast cancer has not been investigated in either human observational studies or animal experiments although breast cancer associated mortality has been reported to be reduced in women with high cardiovascular fitness (4). While treadmill-based exercise training protocols that increase VO2max have been reported to reduce incidence and multiplicity in an experimental model for breast cancer, the VO2max of individual animals in those experiments were not reported, hence precluding the evaluation of the relationship between aerobic capacity and the carcinogenic response (17,18,29). Similarly, while a number of reports of the effects of voluntary wheel running on mammary carcinogenesis have been published (30–32), there are no data available on how wheel running affects any measure of aerobic capacity and in turn whether aerobic capacity is associated with the carcinogenic response, although the need for this information has been noted in a recent review and analysis of preclinical studies in this field (19).
As defined in (1) and discussed further in (33), aerobic capacity reflects the functional ability of an organism to perform work. Since the mitochondrial density of skeletal muscle is one of the recognized component of the complex array of determinants that constitute aerobic capacity, skeletal muscle mitochondrial density was evaluated via the measurement of citrate synthase. Animals that ran in an activity wheel were protected against the development of mammary cancer during the post initiation stage of mammary carcinogenesis, and on average, the animals that ran had higher muscle citrate synthase activity. This provides direct evidence that running on an activity wheel improved aerobic capacity measured as citrate synthase activity. However, citrate synthase activity was quite variable, even in sedentary control animals, a finding consistent with a number of reports of exercise training effects on citrate synthase activity in human participants (reviewed in ref. 9), and as shown in Figure 2, there was considerable overlap between the citrate synthase activity in sedentary controls and animals that ran; consequently, it would not be a useful discriminator between those who are physically fit and those who are not. Nonetheless, consistent with our expectation that running in a motorized wheel at 40 m/min would be a more intense activity and induce a higher aerobic capacity than running in a non-motorized wheel, the average citrate synthase activity was highest in animals that voluntarily ran in the motorized wheel (Table II). Citrate synthase activity was highly variable in the motorized wheel group and consequently, its enzymatic activity was not statistically different from the levels of citrate synthase activity induced in rats that ran on the non-motorized wheel. Citrate synthase activity was significantly associated with running duration on the motorized wheel but not on the free wheel, despite the greater range of distance run by the free wheel animals; this interaction highlights intensity of activity as a distinct factor with clear implications for the modification of exercise regimens. These findings underscore the complexity of the effects of physical activity on aerobic capacity and the limitations of citrate synthase activity as a sole index for its assessment. While effects of wheel running on the carcinogenic response and on citrate synthase activity were consistent with the hypothesis that aerobic capacity mediates the carcinogenic response, there was no evidence of a direct effect of muscle citrate synthase activity on carcinogenesis, highlighting the limitations of a single biochemical measure in evaluating the relationship between two complex processes, aerobic capacity and carcinogenesis. Such information is useful to current deliberations about the design of a clinical trial to investigate the effects of a physical activity intervention on either breast cancer risk in women at elevated risk for the disease or on disease-free interval in breast cancer survivors (34). While it might be presumed that a muscle biopsy taken to evaluate citrate synthase activity could serve as a gold standard for assessing training effects and improvements in aerobic capacity, the data provided herein, which in essence were derived from a muscle biopsy, do not support the usefulness of that approach.
Recently, there has been an upsurge of interest in elucidating the role of aerobic capacity, particularly intrinsic aerobic capacity, on the occurrence of obesity and associated chronic diseases (2,8,35–40). While that work has been based on an animal model in which rats were selected for differences in intrinsic aerobic capacity based on treadmill running ability, those experiments have recently been extended to human populations with comparable findings (41). Regarding carcinogenesis, while this paper failed to find support for a relationship between a measure of aerobic capacity and risk for developing cancer, the biological plausibility of the hypothesized relationship is striking and indicates the need for a broader investigation. Specifically, a direct link has been reported to exist between aerobic capacity and ability to perform intense activity that is dependent in part on p53 mediated transcription of the synthesis of cytochrome C oxidase gene (42–45). Gene dose dependence of synthesis of cytochrome C oxidase, aerobic capacity and exercise endurance have been demonstrated with reduced electron transport activity and a severe limitation in aerobic capacity and exercise endurance in p53 null mice (46,47). Given that defects in p53 are known to occur in the majority of human cancers and the growing understanding of the role of metabolic reprogramming in the carcinogenic process (48), it seems prudent to further investigate the relationship between aerobic capacity and the development of cancer, but to do so using a more comprehensive panel of the factors that contribute to the aerobic capacity of an organism.
Supplementary material
Supplementary data and Table 1 can be found at http://carcin.oxfordjournals.org/
Funding
United States Public Health Service (U54-CA116847) from National Cancer Institute.
Supplementary Material
Acknowledgments
This work was done in partial fulfillment of the requirements of the Master of Science degree at Colorado State University by Phillip B. Mann.
Conflict of Interest Statement: None declared.
References
- 1.Caspersen CJ, et al. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100:126–131. [PMC free article] [PubMed] [Google Scholar]
- 2.Thyfault JP, et al. Rats selectively bred for low aerobic capacity have reduced hepatic mitochondrial oxidative capacity and susceptibility to hepatic steatosis and injury. J. Physiol. 2009;587:1805–1816. doi: 10.1113/jphysiol.2009.169060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Myers J, et al. Exercise capacity and mortality among men referred for exercise testing. N. Engl. J. Med. 2002;346:793–801. doi: 10.1056/NEJMoa011858. [DOI] [PubMed] [Google Scholar]
- 4.Peel JB, et al. Cardiorespiratory fitness and digestive cancer mortality: findings from the aerobics center longitudinal study. Cancer Epidemiol. Biomarkers Prev. 2009;18:1111–1117. doi: 10.1158/1055-9965.EPI-08-0846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouchard C, et al. Aerobic performance in brothers, dizygotic and monozygotic twins. Med. Sci. Sports Exerc. 1986;18:639–646. [PubMed] [Google Scholar]
- 6.Kohrt WM, et al. Effects of gender, age, and fitness level on response of VO2max to training in 60–71 yr olds. J. Appl. Physiol. 1991;71:2004–2011. doi: 10.1152/jappl.1991.71.5.2004. [DOI] [PubMed] [Google Scholar]
- 7.Bouchard C, et al. Individual differences in response to regular physical activity. Med. Sci. Sports Exerc. 2001;33:S446–S451. doi: 10.1097/00005768-200106001-00013. [DOI] [PubMed] [Google Scholar]
- 8.Wisloff U, et al. Cardiovascular risk factors emerge after artificial selection for low aerobic capacity. Science. 2005;307:418–420. doi: 10.1126/science.1108177. [DOI] [PubMed] [Google Scholar]
- 9.Leek BT, et al. Effect of acute exercise on citrate synthase activity in untrained and trained human skeletal muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001;280:R441–R447. doi: 10.1152/ajpregu.2001.280.2.R441. [DOI] [PubMed] [Google Scholar]
- 10.McTiernan A, et al. Recreational physical activity and the risk of breast cancer in postmenopausal women: the Women's Health Initiative Cohort Study. JAMA. 2003;290:1331–1336. doi: 10.1001/jama.290.10.1331. [DOI] [PubMed] [Google Scholar]
- 11.Thune I, et al. Physical activity and the risk of breast cancer. N. Engl. J. Med. 1997;336:1269–1275. doi: 10.1056/NEJM199705013361801. [DOI] [PubMed] [Google Scholar]
- 12.Thune I, et al. Physical activity and cancer risk: dose-response and cancer, all sites and site-specific. Med. Sci. Sports Exerc. 2001;33:S530–S550. doi: 10.1097/00005768-200106001-00025. [DOI] [PubMed] [Google Scholar]
- 13.Friedenreich CM, et al. Physical activity and breast cancer risk: impact of timing, type and dose of activity and population subgroup effects. Br. J. Sports Med. 2008;42:636–647. doi: 10.1136/bjsm.2006.029132. [DOI] [PubMed] [Google Scholar]
- 14.National Institutes of Health. Cancer Prevention and Control. Department of Health and Human Services. Center for disease control and prevention, Bethesda, MD; 2008. [Google Scholar]
- 15.Vainio H, Bianchin IF, editors. Cancer Prevention Handbook. Lyon: IARC Press; 2002. International Agency for Research on Cancer. Physical activity, weight control, and cancer. [Google Scholar]
- 16.Thompson HJ. Effects of physical activity and exercise on experimentally-induced mammary carcinogenesis. Breast Cancer Res. Treat. 1997;46:135–141. doi: 10.1023/a:1005912527064. [DOI] [PubMed] [Google Scholar]
- 17.Thompson HJ, et al. Exercise intensity dependent inhibition of 1-methyl-1-nitrosourea induced mammary carcinogenesis in female F-344 rats. Carcinogenesis. 1995;16:1783–1786. doi: 10.1093/carcin/16.8.1783. [DOI] [PubMed] [Google Scholar]
- 18.Thompson HJ. Effect of exercise intensity and duration on the induction of mammary carcinogenesis. Cancer Res. 1994;54:1960s–1963s. [PubMed] [Google Scholar]
- 19.Thompson HJ. Pre-clinical investigations of physical activity and cancer: a brief review and analysis. Carcinogenesis. 2006;27:1946–1949. doi: 10.1093/carcin/bgl117. [DOI] [PubMed] [Google Scholar]
- 20.Jiang W, et al. Effects of physical activity and restricted energy intake on chemically induced mammary carcinogenesis. Cancer Prev. Res. (Phila. Pa) 2009;2:338–344. doi: 10.1158/1940-6207.CAPR-08-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu Z, et al. Effect of nonmotorized wheel running on mammary carcinogenesis: circulating biomarkers, cellular processes, and molecular mechanisms in rats. Cancer Epidemiol. Biomarkers Prev. 2008;17:1920–1929. doi: 10.1158/1055-9965.EPI-08-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu Z, et al. Energetics and mammary carcinogenesis: effects of moderate-intensity running and energy intake on cellular processes and molecular mechanisms in rats. J. Appl. Physiol. 2009;106:911–918. doi: 10.1152/japplphysiol.91201.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCullagh P, et al. Generalized Linear Models. London: Chapman and Hall; 1989. [Google Scholar]
- 24.Baron RM, et al. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J. Pers. Soc. Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 25.Huang B, et al. Statistical assessment of mediational effects for logistic mediational models. Stat. Med. 2004;23:2713–2728. doi: 10.1002/sim.1847. [DOI] [PubMed] [Google Scholar]
- 26.World Cancer Research Fund/American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. Washington, DC: AICR; 2007. [Google Scholar]
- 27.Zhu Z, et al. Effect of caloric restriction on pre-malignant and malignant stages of mammary carcinogenesis. Carcinogenesis. 1997;18:1007–1012. doi: 10.1093/carcin/18.5.1007. [DOI] [PubMed] [Google Scholar]
- 28.Ware JH. The limitations of risk factors as prognostic tools. N. Engl. J. Med. 2006;355:2615–2617. doi: 10.1056/NEJMp068249. [DOI] [PubMed] [Google Scholar]
- 29.Thompson HJ, et al. Inhibition of mammary carcinogenesis by treadmill exercise. J. Natl Cancer Inst. 1995;87:453–455. doi: 10.1093/jnci/87.6.453. [DOI] [PubMed] [Google Scholar]
- 30.Cohen LA, et al. Voluntary exercise and experimental mammary cancer. Adv. Exp. Med. Biol. 1992;322:41–59. doi: 10.1007/978-1-4684-7953-9_5. [DOI] [PubMed] [Google Scholar]
- 31.Cohen LA, et al. Modulation of N-nitrosomethylurea induced mammary tumorigenesis by dietary fat and voluntary exercise. In Vivo. 1991;5:333–344. [PubMed] [Google Scholar]
- 32.Cohen LA, et al. Influence of dietary fat, caloric restriction, and voluntary exercise on N-nitrosomethylurea-induced mammary tumorigenesis in rats. Cancer Res. 1988;48:4276–4283. [PubMed] [Google Scholar]
- 33.Rundle A. Molecular epidemiology of physical activity and cancer. Cancer Epidemiol. Biomarkers Prev. 2005;14:227–236. [PubMed] [Google Scholar]
- 34.Ballard-Barbash R, et al. Physical activity, weight control, and breast cancer risk and survival: clinical trial rationale and design considerations. J. Natl Cancer Inst. 2009;101:630–643. doi: 10.1093/jnci/djp068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bikman BT, et al. High-fat diet induces Ikkbeta and reduces insulin sensitivity in rats with low running capacity. Int. J. Sports Med. 2009;30:631–635. doi: 10.1055/s-0029-1224174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Britton SL, et al. Animal genetic models for complex traits of physical capacity. Exerc. Sport Sci. Rev. 2001;29:7–14. doi: 10.1097/00003677-200101000-00003. [DOI] [PubMed] [Google Scholar]
- 37.Haram PM, et al. Aerobic interval training vs. continuous moderate exercise in the metabolic syndrome of rats artificially selected for low aerobic capacity. Cardiovasc. Res. 2009;81:723–732. doi: 10.1093/cvr/cvn332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koch LG, et al. Development of animal models to test the fundamental basis of gene-environment interactions. Obesity. (Silver Spring) 2008;16(suppl. 3):S28–S32. doi: 10.1038/oby.2008.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Noland RC, et al. Artificial selection for high-capacity endurance running is protective against high-fat diet-induced insulin resistance. Am. J. Physiol. Endocrinol. Metab. 2007;293:E31–E41. doi: 10.1152/ajpendo.00500.2006. [DOI] [PubMed] [Google Scholar]
- 40.Spargo FJ, et al. Dysregulation of muscle lipid metabolism in rats selectively bred for low aerobic running capacity. Am. J. Physiol. Endocrinol. Metab. 2007;292:E1631–E1636. doi: 10.1152/ajpendo.00702.2006. [DOI] [PubMed] [Google Scholar]
- 41.Novak CM, et al. Endurance capacity, not body size, determines physical activity levels: role of skeletal muscle PEPCK. PLoS One. 2009;4:e5869. doi: 10.1371/journal.pone.0005869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Finkel T, et al. The Krebs cycle meets the cell cycle: mitochondria and the G1-S transition. Proc. Natl Acad. Sci. USA. 2009;106:11825–11826. doi: 10.1073/pnas.0906430106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ma W, et al. A pivotal role for p53: balancing aerobic respiration and glycolysis. J. Bioenerg. Biomembr. 2007;39:243–246. doi: 10.1007/s10863-007-9083-0. [DOI] [PubMed] [Google Scholar]
- 44.Srere PA. Citrate Synthase. Methods Enzymol. 1969;13:3–11. [Google Scholar]
- 45.Park JY, et al. p53 improves aerobic exercise capacity and augments skeletal muscle mitochondrial DNA content. Circ. Res. 2009;105:705–712. doi: 10.1161/CIRCRESAHA.109.205310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kruse JP, et al. Modes of p53 regulation. Cell. 2009;137:609–622. doi: 10.1016/j.cell.2009.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kruse JP, et al. p53 aerobics: the major tumor suppressor fuels your workout. Cell Metab. 2006;4:1–3. doi: 10.1016/j.cmet.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 48.Vousden KH, et al. p53 and metabolism. Nat. Rev. Cancer. 2009;9:691–700. doi: 10.1038/nrc2715. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.