Abstract
Porous alumina film on aluminum with gel-like pore wall was prepared by a two-step anodization of aluminum, and the corresponding gel-like porous film was etched in diluted NaOH solution to produce alumina nanowires in the form of densely packed alignment. The resultant alumina nanowires were reacted with NH3and evaporated aluminum at an elevated temperature to be converted into densely packed aluminum nitride (AlN) nanowires. The AlN nanowires have a diameter of 15–20 nm larger than that of the alumina nanowires due to the supplement of the additional evaporated aluminum. The results suggest that it might be possible to prepare other aluminum compound nanowires through similar process.
Keywords: Aluminum nitride, Alumina, Nanowire, Chemical converting, Porous film
Introduction
In past few decades, aluminum nitride (AlN) has attracted considerable interests because of its exceptional mechanical, thermal, electrical, and optical properties [1-4]. For example, its good thermal conductivity, low dielectric constant, and high electrical resistance as well as thermal expansion coefficient matching to that of silicon offer an application in semiconductor devices as passivation and dielectric layers, and electric substrates [5-10]. Also, as one of wide band-gap semiconductors, aluminum nitride has a promising candidate for optoelectronic and field-emission materials [11,12]. Furthermore, AlN fibers and nanowires are considered to be able to optimize these properties and applications. To date, AlN nanowires have been synthesized by some methods including silica-assisted catalytic growth [13], dc-arc plasma process [14], extended vapor–liquid–solid growth technique [15], and chemical vapor deposition method [16]. However, the developed methods seem to have technologic and economic limits for mass-produced AlN nanowires. Here, we demonstrate a low-cost approach for fabricating densely packed AlN nanowires via a chemical conversion of Al2O3 nanowires produced by a chemical etching of the porous anodic alumina film.
Experimental Details
In our work, the fabrication of AlN nanowires mainly involves three steps: preparation of porous anodic alumina film, formation of Al2O3 nanowires, and conversion of the nanowires into AlN nanowires. First, the porous anodic alumina film was prepared by a two-step anodization of aluminum as described elsewhere [17,18]. Briefly, the cleaned and electropolished high-purity aluminum sheet (99.999%) was anodized in oxalic acid at 40 V for 4–6 h to form porous alumina layer on the aluminum surface. After the alumina layer was removed in a mixture of phosphoric acid (6% wt) and chromic acid (1.8% wt), the Al sheet was again anodized under identical conditions to those of the first anodization for 30 min, which results in forming porous alumina film on the aluminum surface. Then, the as-prepared porous film was etched in diluted NaOH solution to produce Al2O3 nanowires. Finally, the chemical conversion of the alumina nanowires into AlN nanowires was carried out at 1,300–1,400 °C for 2 h in ammonia atmosphere with a flow of ~150 SCCM. The samples were observed by field-emission scanning electron microscopy (FE-SEM; JEOL JSM-6700F). XRD measurement was taken using a Rigaku D/MAX-2500 X-ray diffractometer with CuKα incident radiation.
Results and Discussion
Figure 1 shows FE-SEM image of porous alumina film on aluminum. The film exhibits an almost perfect, hexagonal closely packed, cylindrical pore arrangement with a uniform diameter and a pore interval. Its average pore diameter and interval are ~60 and 120 nm, respectively. Significantly, a simple chemical etching of the porous alumina film in NaOH solution can result in splitting of the pore wall and give alumina nanowires with densely packed alignment, as shown in Fig. 2. The nanowires have a diameter scale of 5–10 nm. Formation of the densely packed alumina nanowires can be attributed to the peculiar pore wall structure of the porous film. Thompson et al. [19,20] thought that the pore wall is mainly composed of gel-like alumina. The chemical etching of porous alumina film can relax colloidal alumina wires, and thus produce the densely packed nanowires [21].
Figure 1.
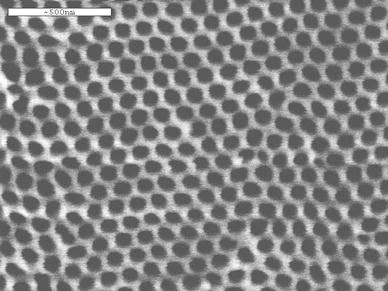
FE-SEM image of porous alumina film with ordered pore structure formed at 40 V in 0.3 mol L−3H2C2O4.Scale bar500 nm
Figure 2.
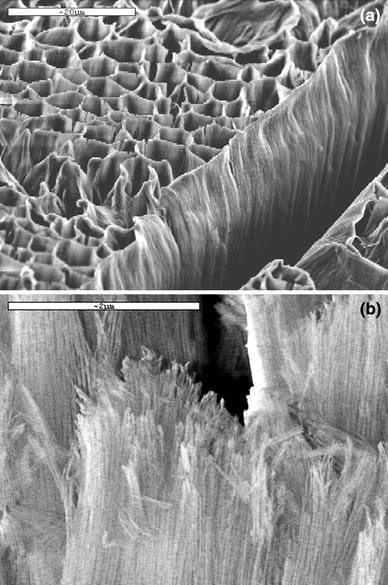
FE-SEM images of densely packed alumina nanowires produced by chemical etching of the porous alumina film in diluted NaOH solution:alowmagnification;bhighmagnification.Scale bara20 μm andb2 μm
Figure 3shows a typical FE-SEM image of the above-described nanowires converted at 1,300–1,400 °C in ammonia atmosphere. It can be seen from the figure that the converted nanowires are still with closely packed arrangement, but their morphology is obviously different from that of the starting alumina ones. It is noted that the converted nanowires have a diameter of 15–20 nm, which is obviously larger than that of the unconverted ones. Figure 4gives the corresponding XRD spectrum of the converted nanowires. The XRD result shows that the converted nanowires are mainly hexagonal wurtzite phase of AlN (Ref: PDF card of No. 25-1133) but a small quantity of α-Al2O3phase. This indicates that most of the alumina nanowires have been converted into the AlN nanowires during heat treatment.
Figure 3.

FE-SEM image of close-packed AlN nanowires obtained by chemical conversion of the alumina nanowires shown in Fig. 2.Scale bar2 μm
Figure 4.
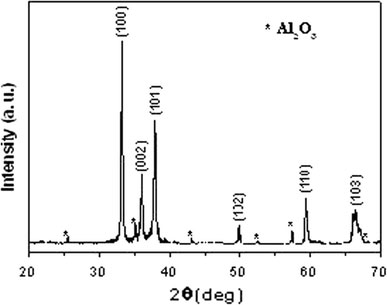
XRD pattern of the close-packed AlN nanowires obtained by chemical conversion of the alumina nanowires shown in Fig. 2
It is well known that aluminum is a volatile metal when heated to an elevated temperature. Obviously, the underlying aluminum of the closed-packed alumina nanowires should also be evaporated at the converting temperatures of 1,300–1,400 °C. And the evaporated aluminum can react with alumina nanowires, and may result in the formation of highly-active Al2O nanowires, it is expressed as follows:
| (a) |
And then the following reaction can take place:
| (b) |
As a result, the Al2O3nanowires are chemically converted into the AlN nanowires. Furthermore, the converting reaction from Al2O3to AlN nanowires is involved with the supplement of the additional evaporated aluminum, and thus gives a larger diameter of the AlN nanowires than that of the Al2O3ones.
Summary and Conclusions
We have developed a convenient method for the preparation of densely packed AlN nanowires based on a new strategy. In this strategy, the porous anodic alumina film on aluminum is etched into the alumina nanowires, and then the alumina nanowires are chemically converted into the AlN ones. In principle, the successful preparation of the aluminum nitride nanowires suggests that the strategy could be applied to prepare a range of aluminum compound nanowires (i.e., sulfide, carbide, chloride, etc.), which is of great fundamental and practical significance.
Contributor Information
Zhi-Hao Yuan, Email: zhyuan@tjut.edu.cn.
Da-Jian Wang, Email: dajian@tjut.edu.cn.
Acknowledgments
The authors would like to thank Prof. S. S. Fan, Prof. Y. D. Li, Prof. L. J. Bie, and Mr. Y. C. Sun for their help and valuable discussions. This work was supported by the National Natural Science Foundation of China under Grant No. 20671070, the Key Project of Chinese Ministry of Education under Grant No. 207008, the Science and Technology Programs of Tianjin (China) under Grant No. 06YFGZGX02900 and 06TXTJJC14602, and Tianjin Key Subject for Materials Physics and Chemistry.
References
- Du XL, Qin ML, Rauf A, Yuan ZH, Yang BH, Qu XH. Mater. Sci. Eng. A. 2008. p. 269. [DOI]
- Kume S, Yasuoka M, Lee SK, Kan A, Ogawa H, Watari K. J. 2007. p. 2976. [DOI]
- Gudiksen MS, Lauhon LJ, Wang J, Smith DC, Lieber CM. Nature. 2002. p. 617. COI number [1:CAS:528:DC%2BD38XhsVCjsL4%3D]; Bibcode number [2002Natur.415..617G] [DOI] [PubMed]
- Lei M, Yang H, Guo YF, Song B, Li PG, Tang WH. Mater. Sci. Eng. B. 2007. p. 85. COI number [1:CAS:528:DC%2BD2sXhtF2mtLfE] [DOI]
- AlShaikhi A, Srivastava GP. J. 2008. p. 083554. Bibcode number [2008JAP...103h3554A] [DOI]
- Wu CC, Chen YC, Yang CF, Su CC, Diao CC. J. 2007. p. 3839. COI number [1:CAS:528:DC%2BD2sXnsVSns7g%3D] [DOI]
- Nakamura S. Science. 1998. p. 956. COI number [1:CAS:528:DyaK1cXlsVerurc%3D] [DOI] [PubMed]
- Vurgaftman I, Meyer JR, Ram-Mohan LR. J. 2001. p. 5815. COI number [1:CAS:528:DC%2BD3MXkt1Wrt74%3D]; Bibcode number [2001JAP....89.5815V] [DOI]
- Corekci S, Ozturk MK, Akaoglu B, Cakmak M, Ozcelik S, Ozbay E. J. 2007. p. 123502. Bibcode number [2007JAP...101l3502C] [DOI]
- Zhu Q, Jiang WH, Yatsui K. J. 1999. p. 5279. COI number [1:CAS:528:DyaK1MXms1eqsbk%3D]; Bibcode number [1999JAP....86.5279Z] [DOI]
- Zanatta AR, Ribeiro CTM, Jahn U. J. 2005. p. 093514. Bibcode number [2005JAP....98i3514Z] [DOI]
- Taniyasu Y, Kasu M, Makimoto T. Appl. 2004. p. 4672. COI number [1:CAS:528:DC%2BD2cXhtVSlsb%2FJ]; Bibcode number [2004ApPhL..85.4672T] [DOI]
- Tang CC, Fan SS, de la Chapelle ML, Li P. Chem. 2001. p. 12. COI number [1:CAS:528:DC%2BD3MXhtVOjtL4%3D]; Bibcode number [2001CPL...333...12T] [DOI]
- Tondare VN, Balasubramanian C, Shende SV, Joag DS, Godbole VP, Bhoraskar SV, Bhadbhade M. Appl. 2002. p. 4813. COI number [1:CAS:528:DC%2BD38Xksleksr8%3D]; Bibcode number [2002ApPhL..80.4813T] [DOI]
- Wu Q, Hu Z, Wang XZ, Lu YN, Huo KF, Deng SZ, Xu NS, Shen B, Zhang R, Chen Y. J. 2003. p. 2004.
- Tang YB, Cong HT, Wang ZM, Cheng HM. Chem. 2005. p. 171. COI number [1:CAS:528:DC%2BD2MXht1alur7I]; Bibcode number [2005CPL...416..171T] [DOI]
- Masuda H, Sotoh M. Jpn. 1996. p. L126. COI number [1:CAS:528:DyaK28XptlyqtA%3D%3D]; Bibcode number [1996JaJAP..35L.126M] [DOI]
- Yuan ZH, Huang H, Fan SS. Appl. 2001. p. 3127. COI number [1:CAS:528:DC%2BD3MXjsVKgs7k%3D]; Bibcode number [2001ApPhL..78.3127Y] [DOI]
- Thompson GE, Furneaux RC, Richardson JA, Wood GC, Richardson JA, Goode JS. Nature. 1978. p. 433. COI number [1:CAS:528:DyaE1cXltlKhuro%3D]; Bibcode number [1978Natur.272..433T] [DOI]
- Thompson GE, Wood GC. Nature. 1981. p. 230. COI number [1:CAS:528:DyaL3MXktFCqsrY%3D]; Bibcode number [1981Natur.290..230T] [DOI]
- Yuan ZH, Huang H, Fan SS. Adv. 2002. p. 303. COI number [1:CAS:528:DC%2BD38XhvFKjsL4%3D] [DOI]


