Abstract
ZnO nanowires were produced using an electrospinning method and used in gas sensors for the detection of ethanol at 220 °C. This electrospinning technique allows the direct placement of ZnO nanowires during their synthesis to bridge the sensor electrodes. An excellent sensitivity of nearly 90% was obtained at a low ethanol concentration of 10 ppm, and the rest obtained at higher ethanol concentrations, up to 600 ppm, all equal to or greater than 90%.
Keywords: Zinc oxide, Electrospinning, Ethanol sensor, Sensitivity
ZnO is an interesting chemically and thermally stable n-type semiconductor with a large exciton binding energy (60 meV) and a large band gap (3.37 eV) energy. ZnO nanomaterial also appears to be the mostly studied one as it exhibits a wide variety of nanostructures such as nanowires [1], nanowalls [2-4], nanobelts [5], nanorods [6], nanosheets [7], and so on. Among its applications, ZnO nanowire is receiving greater interests for use in gas sensors for detecting, for example, ethanol. ZnO nanowires prepared by a reactive thermal deposition method were used for ethanol sensing [8]. The sensitivity, i.e., (Ra − Rg)/Ra where Ra and Rg are, respectively, the resistance of the nanowires exposed to air without and with the detecting gas, increases from ~47% to ~98% while the ethanol concentration increases from 1 ppm to 200 ppm at a high temperature of 300 °C. However, at lower temperatures of 200 °C and 250 °C, the sensitivities drop significantly to ~67% and ~86%, respectively. The response time and recovery time are 10 s and 55 s, respectively, at a ethanol concentration of 200 ppm and a temperature of 300 °C. Ethanol sensors based on ZnO nanowires prepared using a self-catalyzed vapor–liquid–solid (VLS) method exhibit a sensitivity that increases from 18% to 61% while the ethanol concentration increases from 50 ppm to 1500 ppm at a high temperature of 300 °C [9]. However, the response time of the ZnO nanowires to the ethanol is not clear. ZnO nanowires fabricated using a hydrothermal method show an ethanol sensitivity of 92% at an ethanol concentration of 100 ppm at 330 °C [10]. ZnO nanobelts prepared by RF sputter-deposition method were also used for ethanol sensing [5]. The sensitivity to ethanol increases from 86% to 96% while the ethanol concentration increases from 50 ppm to 1000 ppm at a temperature of 220 °C. However, the response–recovery characteristics of the ZnO nanobelt-based ethanol sensors were not reported. Common to these reports is that for the fabrication of gas sensors, the as-synthesized ZnO nanowires or nanorods must be removed from a substrate and/or be transferred into a solution, and then dispersed randomly onto the sensor devices. This “pick-and-place” technique raises a concern of incompatibility with the Si processing [11]. However, by using the electrospuning process for the synthesis of nanowires, the “pick” step is eliminated as it allows a direct placement of nanowires onto a sensor chip.
In the past few decades, the electrospinning process was developed for the fabrication of nanowires. Electrospinning represents a very simple, versatile, and low cost method for large-scale fabrication of nanowires. This technique has been successfully used to produce various polymeric, inorganic, and hybrid nanowires or nanowires [12-14]. Considerable efforts have also been made to fabricate functional nanodevices using the electrospun nanowires [15,16]. However, it is only recently that a few reports have shown the synthesis of ZnO nanowires using the electrospinning process [1,17-19]. Moreover, there has been no report showing the use of electrospun ZnO nanowires as gas sensor, except a very recent one in which electrospun ZnO nanowires were used as a photoelectric gas sensor for the detection of oxygen under the illumination of a 500 W Xe lamp [20]. However, the need of a Xe lamp limits its applications.
In this article, we demonstrate the use of electrospun ZnO nanowires in silicon-based gas sensors for the detection of ethanol with very high sensitivities. Gel of zinc acetate/polyvinyl alcohol (PVA) was used as the precursor. The precursor was loaded into a syringe, which was connected to a high-voltage power supply and served as the positive electrode. The negative electrode was an aluminum foil where chips having interdigitated electrodes were placed. The interdigitated electrode area is 1 mm × 1 mm and the distance between two adjacent interdigitated electrodes is 70 μm. Fixing chips on the surface of the aluminum foil allows the direct placement of electrospun polymeric nanowires onto its surface, leading to the bridging of two electrodes by the subsequently formed ZnO nanowires. For the electrospinning, an electric field of 15 kV was applied. The resulting electrospun polymeric nanowires were subjected to a calcination process without or with the chips at 600 °C to form inorganic ZnO nanowires. The calcination time ranged from 1 h to 7 h. The morphology of the obtained nanowires was examined using scanning electron microscopy (SEM). X-ray diffraction analysis was carried out to determine the crystalline structures of the nanowires. Cathodoluminescence (CL) spectroscopy analysis was also performed at the liquid nitrogen temperature. The gas sensing characteristics were measured in a cylindrical chamber. The chamber has an inlet port connected to a gas inlet valve and outlet port connected to an air pump. Sensors were connected to an outside multimeter to monitor the resistance changes.
Figure 1a shows a general view of the ZnO nanowires obtained. The nanowires are abundant and have uniform diameter. The average diameter ranges from 220 ± 15 nm to 90 ± 10 nm and decreases with the calcination time. For the nanowires obtained at a calcination time ≤5 h, the surfaces are smooth as shown in Fig. 1b for ones obtained at the calcination time of 5 h. The average diameter is 120 nm ± 10 nm. When the calcination time exceeds 5 h, surface cracks were observed as shown in Fig. 1c for ones obtained at a calcination time of 7 h. The average diameter is 90 nm ± 10 nm. The difference in the diameter and the existence of the cracks have an important consequence on the sensitivity as will be discussed later. It was also observed that the existence of such surface cracks does not affect the crystallinity and the CL emission. XRD analysis indicates that all the nanowires are polycrystalline and the crystallinity becomes better, i.e., the long-range order is improved, with the calcination time. This is illustrated in Fig. 2. The nanowires calcined for a time <5 h exhibit broadened diffraction peaks, especially those appearing at 2θ >~47°. These diffraction peaks are weak or even insignificant and also the nanowires contain amorphous structures left from the precursor, which may be seen, as shown by the lower spectral line in Fig. 2 for a 1-h sample. The intensity of the diffraction peaks increases and the peak widths decrease with the calcination time. The nanowires calcined for a time equal to or greater than 5 h shows sharp diffraction peaks as shown by the upper spectral line in Fig. 2 for a 5-h sample. It appears that a minimum of 5 h is required for complete calcination. In the mean time, according to the CL spectroscopy analysis, well-calcined ZnO nanowires exhibit a strong UV emission at 368 nm and a very insignificant emission or nearly none at 465 nm, as shown in Fig. 3. The UV emission is related to the near-band edge emission. The physical origin of the very weak emission at 486 nm, as shown in the insert, is not clearly understood at this stage. It can be attributed to either the presence of impurities or native defects [21], surface defects [22], and oxygen vacancies [23]. The CL characteristics indicate that the well-calcined ZnO nanowires exhibit a good structure quality.
Figure 1.
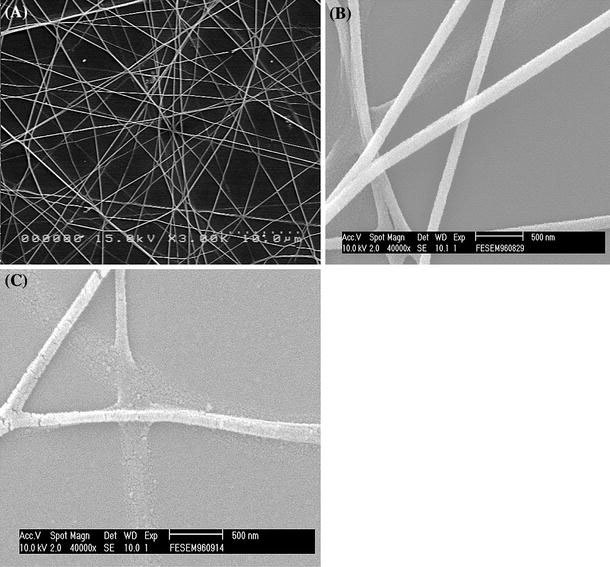
aA general view of electrospun ZnO nanowires. ZnO nanowires obtained at calcination times ofb5 h andc7 h
Figure 2.
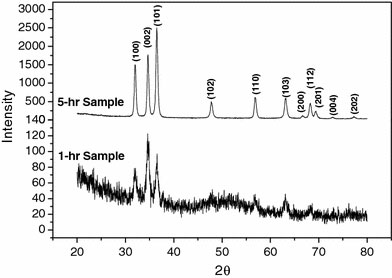
XRD spectrum of ZnO nanowires obtained at 1 and 5 h of calcination
Figure 3.
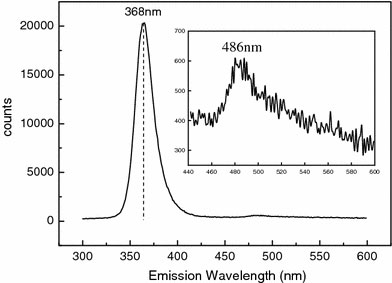
CL spectrum of ZnO nanowires obtained at a calcination temperature and time of 600 °C and 5 h, respectively
As mentioned above, chips having interdigitated electrodes were attached onto the aluminum electrode to allow direct placement electrospun nanowires. The chips were subjected to 5-h and 7-h periods of calcination. Figure 4 shows ZnO nanowires bridging the sensor electrodes. Normally, more or less straight nanowires cross sensor electrodes when the pick-and-place method is used in conjunction with other nanowire synthesis techniques. As shown in Fig. 4, curved ZnO nanowires bridge the electrodes, providing a higher surface area for better sensitivities as shown below. The sensor was found to exhibit a wide detecting range, from 600 ppm to as low as 10 ppm at a low temperature of 220 °C. Figure 5a shows the sensitivities of 5-h-calcined ZnO nanowire sensor at this ethanol concentration range. The sensitivities were determined to be 32, 73, 80, 85, and 89% at ethanol concentrations of 10, 160, 240, 400, and 600 ppm, respectively. The response time and recovery time are approximately 16 s and 25 s, respectively. The response time is defined as the time required for the resistance to reach 90% of the equilibrium value following an injection of the test gas, and the recovery time is the time necessary for the resistance to return to 10% above original resistance in air after the removal of the test gas [10]. Furthermore, the sensitivity can be further increased by using 7-h-calcined ZnO nanowires, as shown in Fig. 5b. Now the sensitivities are 89, 90, 92, 93, and 94% at ethanol concentrations of 10, 160, 240, 400, and 600 ppm, respectively. Sensitivity was determined from measured resistances, Ra and Rg, each of which is an average of five data points. The measured resistance are very consistent such that the standard deviations are typically less than 0.5%. This makes the sensitivities distinguishable. It is noteworthy that the sensitivity at the lowest ethanol concentration of 10 ppm is nearly 90%, and the rest are all ≥90%. In fact, it was found that the sensitivity increases with reduced nanowire diameter. The enhanced sensitivity is attributed to the increased specific surface area (area/volume) and improved crystallinity, both of which increase with reduced nanowire diameter. In addition to the enhanced sensitivity, the 7-h-calcined ZnO nanowire sensor also exhibits a similar response time of 19 s. However, the recovery time was found to be several times higher. This is also attributed to the existence of the surface cracks as shown in Fig. 1c. It is believed that the cracks easily absorb the ethanol, which is then trapped inside the cracks, and therefore takes time to be desorbed. For comparison, it was found that depending on the ethanol concentration, the sensitivities are in general better than or at least comparable to the previously reported values as noted above. At the same sensing temperature of 220 °C, we have obtained sensitivities of 89 and 93% at 10 and 400 ppm, respectively; while in Ref. [5], the sensitivities are 86 and 95% at 50 and 500 ppm, respectively. At 300 °C, the sensitivities reported in Ref. [8] are 93% at 50 ppm. In Ref. [9], the sensitivities obtained 300 °C, i.e., from 18 to 61% at 50–1500 ppm, are all lower than that obtained in this study. In Ref. [10], the sensitivity obtained at an even higher temperature of 330 °C is 92% for 100 ppm methanol.
Figure 4.
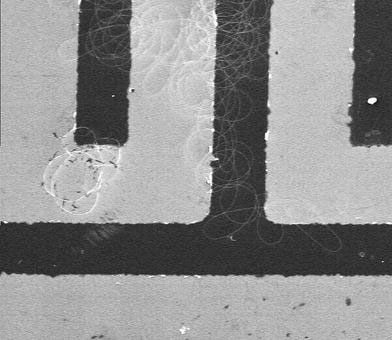
ZnO nanowires on the electrodes (dark area)
Figure 5.
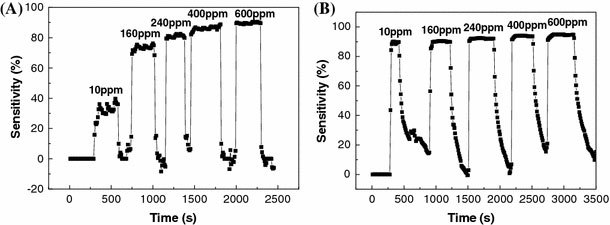
Sensitivities of thea5-h calcined andb7-h calcined ZnO nanowires gas sensors measured at ethanol concentrations ranging form 10 ppm to 600 ppm
We have fabricated electrospun polycrystalline ZnO nanowires directly placed onto interdigitated electrodes to form gas sensors for the detection of ethanol. Sensors exhibiting very high sensitivities, and fast response time and recovery time were demonstrated. The average nanowire diameter ranges from 220 ± 15 nm to 90 ± 10 nm and decreases with the calcination time. The ZnO nanowires exhibit a strong UV emission at 368 nm and a very insignificant emission or nearly none at 465 nm. For the detection of ethanol at 220 °C, an excellent sensitivity of nearly 90% was obtained at a low ethanol concentration of 10 ppm and the rest obtained at higher ethanol concentrations, up to 600 ppm, are all ≥90%. Fast response time and recovery times of 16 s and 25 s, were also obtained.
Acknowledgments
This work was supported by the National Science Council in Taiwan under grant No. 97-2120-M-006-001 and the Top University Program at the National Cheng Kung University in Taiwan under R048/D97-3360.
References
- Yang X, Shao C, Guan H, Li X, Gong J. Inorg. 2004. pp. 176–178. COI number [1:CAS:528:DC%2BD2cXoslChsw%3D%3D] [DOI]
- Heo YW, Norton DP, Tien LC, Kwon Y, Kang BS, Ren F, Pearton SJ, LaRoche JR. Mater. 2004. p. 1. [DOI]
- Wu GS, Xie T, Yuan XY, Li Y, Yang L, Xiao YH, Zhang LD. Solid State Commun. 2005. p. 485. COI number [1:CAS:528:DC%2BD2MXjtF2gsr8%3D]; Bibcode number [2005SSCom.134..485W] [DOI]
- Zhang Y, Yu K, Ouyang S, Zhu Z. Mater. 2006. p. 522. COI number [1:CAS:528:DC%2BD2MXht1Ois7fF] [DOI]
- Choopun S, Hongsith N, Mangkorntong P, Mangkorntong N. Physica E. 2007. pp. 53–56. COI number [1:CAS:528:DC%2BD2sXmvVGhsLw%3D]; Bibcode number [2007PhyE...39...53C] [DOI]
- Gao T, Wang TH. Appl. Phys. A. 2005. pp. 1451–1454. COI number [1:CAS:528:DC%2BD2MXisFWitrY%3D]; Bibcode number [2005ApPhA..80.1451G] [DOI]
- Chen SJ, Liu YC, Shao CL, Mu R, Lu YM, Zhang JY, Shen DZ, Fan XW. Adv. 2005. p. 8.
- Wan Q, Li QH, Liang YX, Wang TH. Appl. 2004. p. 3654. COI number [1:CAS:528:DC%2BD2cXjsVKksb0%3D]; Bibcode number [2004ApPhL..84.3654W] [DOI]
- Hsueh TJ, Hsu CL, Chang SJ, Chen IC. Sens. Actuators B. 2007. pp. 473–477. [DOI]
- Jiaqiang X, Yuping C, Yadong L, Jianian S. J. 2005. pp. 2919–2921. Bibcode number [2005JMatS..40.2919J] [DOI]
- Wu H, Lin D, Zhang R, Panw W. J. 2008. pp. 656–659. COI number [1:CAS:528:DC%2BD1cXitlyntbw%3D] [DOI]
- Thandavamoorthy S, Bhat GS, Tock RW, Parameswaran S. Appl. 2005. pp. 557–569. [DOI]
- Nuansing W, Ninmuang S, Jarernboon W. Mater. Sci. Eng. B. 2006. p. 147–155. [DOI]
- Sui XM, Shao CL, Liu YC. Appl. 2005. p. 117115. [DOI]
- Pinto NJ, Johnson AT, MacDiarmid AG, Mueller CH, Theofylaktos N, Robinson DC, Miranda FA. Appl. 2003. pp. 4244–4246. COI number [1:CAS:528:DC%2BD3sXovFynsL0%3D]; Bibcode number [2003ApPhL..83.4244P] [DOI]
- Liu H, Reccius CH, Craighead HG. Appl. 2005. p. 253106. Bibcode number [2005ApPhL..87y3106L] [DOI]
- Siddheswaran R, Sankar R, Ramesh Babu M, Rathnakumari M, Jayavel R. Cryst. 2006. pp. 446–449. COI number [1:CAS:528:DC%2BD28XkvVCgu7g%3D] [DOI]
- Wu H, Pan W. J. 2006. pp. 699–701. COI number [1:CAS:528:DC%2BD28XhsFWqtbg%3D] [DOI]
- Sui X, Shao C, Liu Y. Polymer. 2007. pp. 1459–1463. COI number [1:CAS:528:DC%2BD2sXitlyksbY%3D] [DOI]
- Yang M, Xie T, Peng L, Zhao Y. Appl. Phys. A. 2007. pp. 427–430. COI number [1:CAS:528:DC%2BD2sXpsFOjt74%3D]; Bibcode number [2007ApPhA..89..427Y] [DOI]
- Yuan XL, Zhang BP, Niitsuma J, Sekiguchi T. Mater. Sci. Semicond. Process. 2006. pp. 146–150. COI number [1:CAS:528:DC%2BD28Xltleltbk%3D] [DOI]
- Li D, Leung YH, Djurisic AB, Liu ZT, Xie MH, Shi SL, Appl. 2004. pp. 1601–1603. COI number [1:CAS:528:DC%2BD2cXntVKmu70%3D]; Bibcode number [2004ApPhL..85.1601L] [DOI]
- Mass J, Avella M, Jimenez J, Callahan M, Grant E. Superlattice. 2005. pp. 223–230. COI number [1:CAS:528:DC%2BD2MXht1aqtL3E]; Bibcode number [2005SuMi...38..223M] [DOI]


