Abstract
One-step lateral flow test is recommended as the first line screening of syphilis for primary healthcare settings in developing countries. However, it generally shows low sensitivity. We describe here the development of a novel fluorescent POC (Point Of Care) test method to be used for screening for syphilis. The method was designed to combine the rapidness of lateral flow test and sensitiveness of fluorescent method. 50 syphilis-positive specimens and 50 healthy specimens conformed by Treponema pallidum particle agglutination (TPPA) were tested with Quantum Dot-labeled and colloidal gold-labeled lateral flow test strips, respectively. The results showed that both sensitivity and specificity of the quantum dots–based method reached up to 100% (95% confidence interval [CI], 91–100%), while those of the colloidal gold-based method were 82% (95% CI, 68–91%) and 100% (95% CI, 91–100%), respectively. In addition, the naked-eye detection limit of quantum dot–based method could achieve 2 ng/ml of anti-TP47 polyclonal antibodies purified by affinity chromatography with TP47 antigen, which was tenfold higher than that of colloidal gold–based method. In conclusion, the quantum dots were found to be suitable for labels of lateral flow test strip. Its ease of use, sensitiveness and low cost make it well-suited for population-based on-the-site syphilis screening.
Keywords: Syphilis, Lateral flow, Diagnostics, Quantum dots, Nanomaterial
Introduction
Lateral flow test, one of the most common Point-Of-Care (POC) test formats, is well-known via home pregnancy test strips and widely applied into qualitative or semi-quantitative detection of numerous analytes including antibodies, viruses, bacteria, nucleic acid, antibiotics, specific biomarkers, and even pesticide [1-7]. The lateral flow test was first introduced as a popular platform for rapid test in late 1980s. Since then, it has been developing rapidly and playing an important role in POC test market. Traditional lateral flow tests use colloidal gold, dye, or latex bead conjugates to generate visual signal [7]. Compared with core laboratory test formats, lateral flow test has clear advantages in ease of use, rapid result and portability, making it well-suited for operation at the site of patient care [8].
As reported previously, although rapid POC test was less sensitive than laboratory-based tests, it could facilitate the treatment of those patients infected with sexually transmitted infections (STIs) better than laboratory-based tests [9]. It is well known that STIs are very common in developed and developing countries. Sexually transmitted diseases are regarded as one of the first ten causes of unpleasant diseases in young male adults in developing countries and the second major cause of unpleasant diseases in young female adults [10]. Especially syphilis brought approximate 12 million new cases and the majority occurred in developing countries with limited laboratory services [9]. Therefore, the cheap and rapid POC tests may be taken as the first line screening formats for syphilis. Because the inherent limitation of traditional gold-based lateral flow test strips causes its limited sensitivity, researchers have been working on different tags, including enzyme, silver enhancement, chemiluminescent tags or fluorescent tags, in hopes of increasing sensitivity [11].
Quantum dots have been subject to intensive investigations due to their unique photoluminescence properties and potential application prospect [12]. So far, several methods have been developed to synthesize water-soluble quantum dots for use in biological relevant studies [13]. For example, quantum dots have been used successfully in cellular imaging [14,15], immunoassays [16,17], biosensor [18] and optical barcoding [19]. Quantum dots are also used to study the interaction between protein molecules or detect the course of signal transduction in live cells by Fluorescence Resonance Energy Transfer (FRET) [20,21]. Quantum dots provide a new functional platform for bioanalytical sciences and biomedical engineering.
Herein, we describe the development and evaluation of a novel QDs-based lateral flow test for the rapid screening of syphilis, which is economical, easy to use and retains the high sensitivity of fluorescent dyes. Furthermore, this novel fluorescent tag has incomparable advantages in terms of size-dependent fluorescence, broad absorption spectra and narrow emission spectra, excellent photostability over traditional organic dyes, and has great potential application in clinical disease quick diagnosis.
Materials and Methods
Clinical Samples
Informed consent was obtained from patients, and this study was approved by the Medical Ethics Committee of No.261 Hospital of PLA. Human experimentation guidelines of the No.261 Hospital of PLA were followed in the conduct of clinical research. Fifty TPPA-positive serum samples collected from September 2007 to May 2008 in Center of Biological Diagnosis and Therapy of No.261 Hospital of PLA, China, were selected for this study. The gold standard we selected for a ‘‘true positive sample’’ was defined as a sample with a TPPA titer of 1: 80 or above and a positive Fluorescent Treponemal Antibody Absorption result (FTA-Abs). The negative control group consisted of 50 healthy TPPA-negative and FTS-Abs negative sera from volunteer blood donors. All of the samples were collected in an anonymous manner, and the exact clinical status or drug usage of the patients was unknown. After being collected, the samples were kept at −70°C refrigerator until further use.
Materials
Thioglycolic acid (TGA), sodium borohydride and carbodiimide hydrochloride (EDC), chloroauric acid, Staphylococcal Protein A (SPA) are purchased from Sigma (Sigma. USA) and were used without further treatment or purification. The nitrocellulose membrane was purchased from Amersham. Human immunoglobulin G (IgG) and TP15, TP17, TP47 recombinant syphilis antigen, as well as bovine serum albumin (BSA), were purchased from Linchao Biotechnology (Linchao. China). All synthetic preparations and measurements were carried out in Millipore water as solvent with 18 MΩ/cm or less. All other analytically purified reagents were purchased domestically.
Preparation of Quantum Dots
About 5 mmol of CdCl2·5H2O was dissolved in 110 mL of water, and 12 mM of TGA was added under stirring. The pH of resultant solution was adjusted to 11 by dropwise addition of 1 M NaOH solution. The solution was then transferred into a three-necked flask and deaerated by N2 bubbling for 30 min. Under stirring, 2.5 mM of oxygen-free NaHTe solution, which was freshly prepared from tellurium powder and NaBH4 (molar rate of 1: 2) in water at 0°C, was injected into the three-necked flask. After reflux at 100°C for 4 h, the 3.5 nm diameters TGA-capped CdTe QDs emitted with a maximum wavelength around 610 nm were synthesized. The obtained QDs were purified by precipitation in ethanol and redispersed in PBS. Absorbance spectrum and photoluminescence (PL) spectrum were used to characterize the fluorescent properties of QDs; they were measured in 1-cm quartz in air at room temperature using a LabTech UV2000 ultraviolet spectrophotometer and Perkin Elmer LS 55 spectrofluorimeter. The size determination of QDs was performed using a transmission electron microscope (TEM, JEM2010, at 200 kV). Its size distributions were obtained by measuring QDs of the TEM image which had more than 300 nanoparticles.
Preparation of Colloidal Gold
About 15 nm monodisperse colloidal gold particles were prepared according to the method described before by the authors [22]. Briefly, 50 mL (0.2 mg/mL) chloroauric acid was heated to boiling point, and then 1.2 mL (10 mg/mL) sodium citrate was added. Boiling lasted 5 min until it became dark red in color. After cooling down to room temperature, 20 μL of colloidal gold were used for the analysis of transmission electron microscope. Its size distributions were measured in the same way as mentioned in QDs section.
Conjugation of SPA with QDs and Colloidal Gold
Firstly, 5 mL of 3 mg/mL QDs and 5 mL of 0.05, 0.1, 0.2, 0.4, 0.8 mg/mL SPA were mixed, respectively, and then 0.5 mL 1 mg/mL EDC were added and blended by vortex. The resulting solution reacted at room temperature for 3 h with continuous mixing and then at 4°C for 24 h. BSA was added into the solution at a concentration of 5 mg/mL and incubated at room temperature for 3 h. The QDs-labeled SPAs were then centrifuged at 15,000 g for 30 min and the supernatant was discarded. A volume of 10.5 mL PBST [Phosphate-Buffered Saline (PBS); 0.2 mg/mL KCl, 1.44 mg/mL Na2HPO4, 0.24 mg/mL KH2PO4, 8 mg/mL NaCl, pH 7.4] with 0.5% tween-20 (v/v) was used to resuspend and wash QDs-labeled SPA by centrifugation at 15,000 g for three times. Finally, the QDs-labeled SPA were dispersed in 105 mL PBST and kept at 4°C until use.
The as-prepared colloidal gold was regulated to pH 8.0 with 0.1 mol/L K2CO3. Under magnetic stirring, the final concentration of 12.5, 25, 50, 100, 200 μg/mL SPA was added, respectively. After 10 min, BSA was added to obtain a concentration of 10 mg/mL. The resultant mixture was centrifuged at 2,500 g for 5 min. The supernatant was transferred to a new centrifuge tube and centrifuged at 12,000 g for 20 min. The precipitate was dissolved by PBS containing 5 mg/mL BSA and then stored at 4°C.
Preparation of Lateral Flow Test Strips
The mixture of TP15, TP17 and TP47 recombinant syphilis antigen was dispensed on the nitrocellulose membrane with Biodot XYZ3000 dispenser at concentration of 1 mg/mL of each as test line. IgG was dispensed at concentration of 0.5 mg/mL as control line. After drying for 30 min, the nitrocellulose membrane was blocked with PBS containing 1% BSA (w/v). The Colloidal gold conjugates were printed on conjugate pad at optimized concentration described later and dried at room temperature. Absorbent pad, nitrocellulose membrane, conjugate pad and sample pad were pasted on the laminating card in order with proper overlapping distance. Then the whole card was cut into final test strips of 4 mm in width with guillotine cutter. The preparation of QD-based test strips was slightly different from colloidal gold–based test strips in their lamination (Fig. 1).
Figure 1.
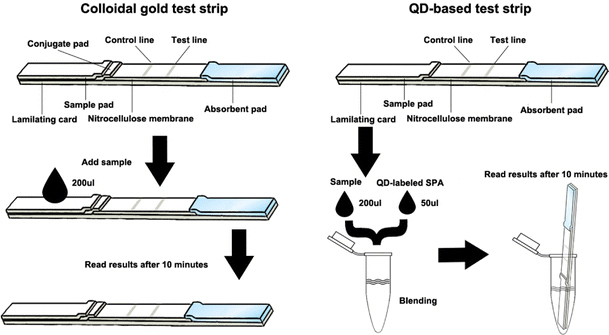
The schematic drawing of QD-based lateral flow test strips
Optimization of Labeling with QDs and Colloidal Gold
The control line of test strips was used to determine the optimum amount of labeling. For the optimization of labeling amount of SPA, QDs and colloidal gold labeling different concentration of SPA were used. The colloidal gold test strips were inserted into 1.5-mL test tubes containing 200 μL PBS and the coloring of control line was observed after 10 min. The QD-based test strips were inserted into 1.5 mL test tubes containing 200 μL of QD-labeled SPA solution and the coloring of control line was observed after 10 min. A slight difference was that the results of QDs-based test strips were observed under the portable ultraviolet lamp.
Limit of Detection
The anti-TP47 polyclonal antibodies purified by affinity chromatography with TP47 antigen were a kind gift from associate Prof. Ding Li. Upon receipt, the polyclonal antibodies were quantified by absorbance at 280 nm and diluted tenfold serially from 2 μg/mL to 0.2 ng/mL. Those polyclonal antibodies were used to determine the naked-eye limit of detection of both colloidal gold-based and QDs-based test strips.
Test of Clinical Samples
The test group and negative control group were tested with colloidal gold–based and QDs-based test strips, respectively, and the performance of the two labels was analyzed with Statistics Package for Social Sciences (SPSS) software (SPSS Inc, Chicago, USA).
Results and Discussion
The diameter and size distribution of as-prepared colloidal gold and CdTe QDs synthesized in this work were determined by TEM. A representative example was presented in Fig. 2. The mean diameter of colloidal gold is about 15 nm and that of QDs is about 3.5 nm (Fig. 3). The absorbance spectrum, fluorescence spectrum and images of QDs before and after the coupling with SPA under UV irradiation is shown in Fig. 4. It is showed that the QDs could be excited effectively under ultraviolet band and their maximum fluorescence wavelength was at around 610 nm before conjugation and shifted to around 596 nm after conjugation.
Figure 2.
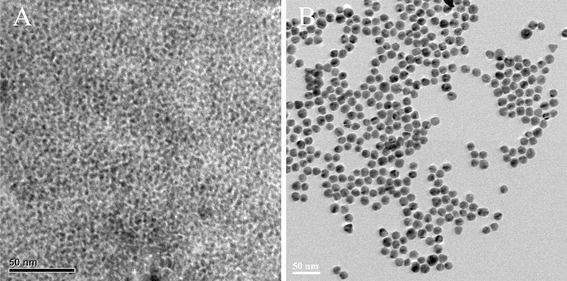
Characterization of QDs (a) and colloidal gold (b) by transmission electron microscopy
Figure 3.
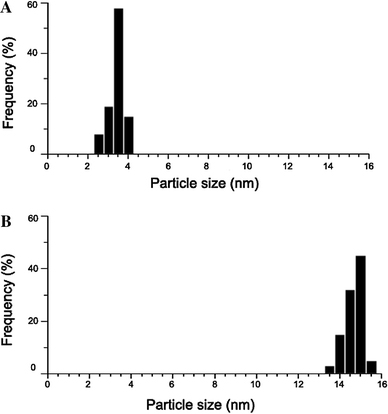
Size distributions of QDs (a) and colloidal gold (b)
Figure 4.
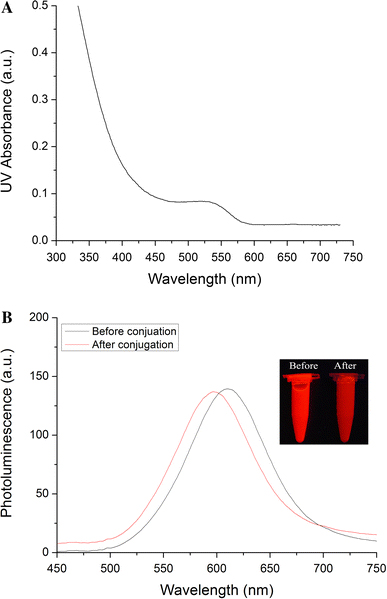
UV–vis absorption spectra (a) and PL spectra (b) of QDs
According to the coloring of colloidal gold–based test strips, the concentration of 50 μg/mL presented the strongest red control line. Under or above this concentration would decrease the coloring. Therefore, 50 μg/mL was the best concentration for labeling of colloidal gold. Similarly, QDs-capped SPA prepared with 0.2 mg/mL exhibited the strongest fluorescence intensity and thus this concentration was determined as best one for labeling of QDs. In this work, the naked-eye limit of detection of colloidal gold–based lateral flow test strips could only reach 20 ng/mL of anti-TP47 polyclonal antibody solution, whereas that of QDs-based test strips could easily reach 2 ng/mL of anti-TP47 polyclonal antibody solution, tenfold higher than that of colloidal gold test strips.
Out of the 50 TPPA-positive samples, 9 samples were false negative in the colloidal gold–based lateral flow test strips, resulting in an overall sensitivity of 82%, with a 95% confidence interval (CI) (scored by Wilson’s method [23]) of 68–91% (Table 1). The TPPA titer of 9 false negative samples was below or equal to 1: 160. However, its specificity was 100% (95% CI, 91–100%). Both sensitivity and specificity of QDs-based test strips were 100% (95% CI, 91–100%). The typical test results were shown in Fig. 5.
Table 1.
A comparison of colloidal gold- and QDs-based test
| TPPA | Validity (95% CI) | ||||
|---|---|---|---|---|---|
| Positive | Negative | Total | |||
| Colloidal gold–based test | Positive | 41 | 0 | 41 | Sensitivity 82% (68–91) |
| Negative | 9 | 50 | 59 | Specificity 100% (91–100) | |
| QDs-based test | Positive | 50 | 0 | 50 | Sensitivity 100% (91–100) |
| Negative | 0 | 50 | 50 | Specificity 100% (91–100) | |
Note: CI confidence interval (by Wilson’s method)
Figure 5.
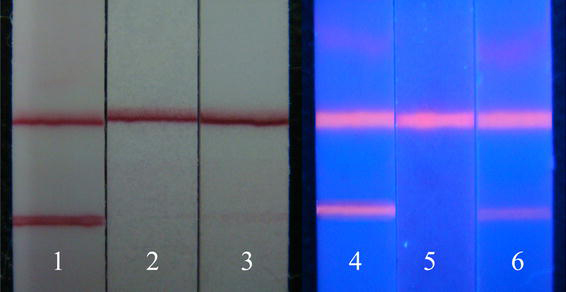
Result of detection of clinical specimens by colloidal gold and QDs. result of detection by colloidal gold: 1 positive; 2 negative; 3 weak positive; result of detection by QDs: 4 positive; 5 negative; 6 weak positive
In this study, we report a simple and sensitive QDs-based rapid detection method for syphilis antibody. Its test result was compared with that of colloidal gold–based rapid test strips. Regarding the colloidal gold–based rapid immunochromatographic test, the sensitivity of 82% (95% CI, 68–91%) was obtained in 50 known syphilis-positive sera, which is accordant to the assertion that one-step immunochromatographic rapid test was not sufficiently sensitive [24]. In particular, it is often difficult to interpret in some weakly positive sera. Owing to its ease to perform, one-step lateral flow test is still recommended for primary healthcare settings in developing countries. However, limited equipments for reserve and insufficient training in developing countries may cause the worrisomely low sensitivity. According to Zhi-qiang Chen et al., the total incidence of primary and secondary syphilis in China, 5.7 cases per 100,000 in 2005, was reported, much higher than that in most developed countries [25]. If some of those patients seek care from ill-equipped private clinical setting, the consequence of misdiagnosis caused by the low sensitivity of colloidal gold–based lateral flow test strips would be devastating. In contrast with traditional lateral flow test strips, Enzyme-Linked ImmunoSorbent Assay (ELISA) was cried up for its excellent sensitivity. But its complicated and lengthy operation makes it not suitable for the first line screening or on-site screening of syphilis. In addition, as for colloidal gold–based rapid test strips, another problem is the interference of hemolytic serum. The coloring is difficult to distinguish in some cases when hemolysis occurs. In order to resolve these problems, fluorescence or other labels have been taken into consideration [11]. However, conventional fluorescent dyes suffer from some limits including narrow excitation spectrum, poor photostability and short fluorescent lifetime, all of which greatly restrict the application of those fluorescent dyes in lateral flow test strips [26].
QDs, as a novel class of inorganic fluorophore, have attracted more and more attention and substantial progress has been made in utilization of QDs as a kind of biological label [13,17]. The efficient fluorescence and stability of QDs can improve sensitivity and prolong lifetime in its usage as a biological label. Unlike other fluorophores that have narrow excitation range, semi-conductor QDs have broad absorbance bands. This property allows the excitation of QDs at a broad wavelength range, that is, no requirement of special light source equipment with a specific excitation wavelength. Photostability is a singular advantage that QDs have. The traditional organic fluorophores bleach after only a few minutes on exposure to light, whereas QDs are extremely stable and can undergo repeated cycles of excitation and fluorescence for hours with a high level of brightness and photobleaching threshold and thus this characterization endows QDs with pretty high sensitivity [27]. As previously reported, QDs were more photostable than a number of organic dyes including Alexa 488, which is reported to be the most stable organic dye [26-29].
In our study, the as-prepared QDs had a fairly broad excitation spectrum, which meant that a cheap and portable 325-nm UV lamp could be used as effective excitation source. Besides, the blue shift occurred after the coupling of QDs with SPA and the bright red fluorescence of QDs turned dark red. The probable reason is that the carboxyl group on the surface of QDs was covalently combined with the amino group of SPA, which reduced the surface charge of QDs, decreased the polarization rate of the surrounding molecules and reduced the Stokes displacement, finally resulting in a blue shift in the emission spectra [30]. Meanwhile, the full width at half maximum (FWHM) of the QDs and QDs-labeled SPA remained constant, which suggested that no aggregation happened to the QDs in the coupling process. According to the clinical performance of the methods based on these two labels, the QDs-based test strips showed much higher sensitivity than colloidal gold–based test strips.
Despite the satisfying results, there is a need for further studies to estimate the diagnostic accuracy of the QDs-based lateral flow test strip in patients with suspected infection and with positive serologic test results, and patients with other pathologic conditions. Besides, a multi-centric study in which the same samples are evaluated would be highly valuable. Moreover, it was very difficult to take clear fluorescent photos under ultraviolet lamp by the nonprofessional digital camera we used and therefore our group is working at a kind of mini-reader with ultraviolet source and a miniaturized professional charge-coupled device (CCD). We hope to take clear fluorescent photographs of QDs-based lateral flow test strips for qualitative and quantitative analysis by this reader. With this equipment, we will be able to obtain a more accurate and objective result.
Conclusions
In conclusion, we have developed a novel fluorescent POC rapid test method to be used for screening for syphilis that is inexpensive, easy to perform, and has been demonstrated to be suitable for on-the-site syphilis screening. Its clinical performance in clinical tests could make it a promising replacement for colloidal gold–based lateral flow test strips.
Misc
The first two authors contributed equally to this article.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No.20803040 and No.20471599), Chinese 973 Project (2010CB933901), 863 Key Project (2007AA022004), New Century Excellent Talent of Ministry of Education of China(NCET-08-0350), Special Infection Diseases Key Project of China (2009ZX10004-311), Shanghai Science and Technology Fund (10XD1406100).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Laderman EI, Whitworth E, Dumaual E, Jones M, Hudak A, Hogrefe W, Carney J, Groen J. Clin. 2008. p. 159. COI number [1:CAS:528:DC%2BD1cXkvVGju7g%3D] [DOI] [PMC free article] [PubMed]
- Nielsen K, Yu WL, Lin M, Davis SA, Elmgren C, Mackenzie R, Tanha J, Li S, Dubuc G, Brown EG, Keleta L, Pasick J. J. 2007. p. 307. COI number [1:CAS:528:DC%2BD2sXhtVGktbjI] [DOI] [PubMed]
- Kuloğlu Z, Kansu A, Kirsaçlioğlu CT, Ustundag G, Aysev D, Ensari A, Kucuk NO, Girgin N. Diagn. 2008. p. 351. [DOI] [PubMed]
- Mens PF, van Amerongen A, Sawa P, Kager PA, Schalling HD. Diagn. 2008. p. 421. COI number [1:CAS:528:DC%2BD1cXoslWnsLk%3D] [DOI] [PubMed]
- Zhao Y, Zhang G, Liu Q, Teng M, Yang J, Wang J. J. 2008. p. 12138. COI number [1:CAS:528:DC%2BD1cXhtl2nsrjM] [DOI] [PubMed]
- Kikuta H, Ebihara T, Endo R, Ishiguro N, Sakata C, Ochiai S, Ishiko H, Gamo R, Sato T. Hybridoma (Larchmt) 2007. p. 17. COI number [1:CAS:528:DC%2BD2sXhvF2iu78%3D] [DOI] [PubMed]
- Wang S, Zhang C, Zhang Y. Methods Mol. 2009. p. 237. COI number [1:CAS:528:DC%2BD1MXhslWhsbc%3D] [DOI] [PubMed]
- Warsinke A. Anal. 2009. p. 1393. COI number [1:CAS:528:DC%2BD1MXktVejsQ%3D%3D] [DOI] [PubMed]
- Peeling RW. Sex. 2006. p. 425. COI number [1:STN:280:DC%2BD28jkvVCqtA%3D%3D] [DOI] [PMC free article] [PubMed]
- Da Ros CT, Schmitt Cda S. Asian J. 2008. p. 110. [DOI] [PubMed]
- Posthuma-Trumpie GA, Korf J, van Amerongen A. Anal. 2009. p. 569. COI number [1:CAS:528:DC%2BD1cXhsFWit77M] [DOI] [PubMed]
- Lu W, Ji ZQ, Pfeiffer L, West KW, Rimberg AJ. Nature. 2003. p. 422. COI number [1:CAS:528:DC%2BD3sXjvFaltLc%3D]; Bibcode number [2003Natur.423..422L] [DOI] [PubMed]
- He R, You X, Shao J, Gao F, Pan B, Cui D. Nanotechnology. 2007. p. 315601. Bibcode number [2007Nanot..18E5601H] [DOI] [PubMed]
- Li Z, Huang P, Lin J, He R, Liu B, Zhang X. J. 2010. p. 1533.
- Wang Z, Ruan J, Cui D. Nanoscale Res. 2009. p. 593. COI number [1:CAS:528:DC%2BD1MXnt1Sktrw%3D]; Bibcode number [2009NRL.....4..593W] [DOI] [PMC free article] [PubMed]
- Yang H, Guo Q, He R, Li D, Zhang X, Bao C, Hu H, Cui D. Nanoscale Res. 2009. p. 1469. COI number [1:CAS:528:DC%2BD1MXhsFWqsbbL]; Bibcode number [2009NRL.....4.1469Y] [DOI] [PMC free article] [PubMed]
- Cui D, Pan B, Zhang H, Gao F, Wu R, Wang J, He R, Asahi T. Anal. 2008. p. 7996. COI number [1:CAS:528:DC%2BD1cXhtFKnsLbM] [DOI] [PubMed]
- Zhang X, Guo Q, Cui D. Sensors. 2009. p. 1033. COI number [1:CAS:528:DC%2BD1MXisVWgsr0%3D] [DOI] [PMC free article] [PubMed]
- Han M, Gao X, Su JZ, Nie SM. Nat. 2001. p. 631. COI number [1:CAS:528:DC%2BD3MXkvFGjtrg%3D] [DOI] [PubMed]
- Jares-Erijman EA, Jovin TM. Nat. 2003. p. 1387. COI number [1:CAS:528:DC%2BD3sXosFOksbo%3D] [DOI] [PubMed]
- Huang X, Li L, Qian H, Dong C, Ren C. Angew. 2006. p. 5140. COI number [1:CAS:528:DC%2BD28Xot12nu70%3D] [DOI] [PubMed]
- Li D, Yang H, Zhang WH, Pan H, Wen DQ, Han FC, Guo HF, Wang XM, Yan XJ. Gynecol. 2006. p. 220. [DOI] [PubMed]
- Wilson EB. J. 1927. p. 209. [DOI]
- van Dommelen L, Smismans A, Goossens VJ, Damoiseaux J, Bruggeman CA, van Tiel FH, Hoebe CJPA. Sex. 2008. p. 292. [DOI] [PubMed]
- Chen ZQ, Zhang GC, Gong XD, Lin C, Gao X, Liang GJ, Yue XL, Chen XS, Cohen MS. Lancet. 2007. p. 132. [DOI] [PMC free article] [PubMed]
- Jamiesona T, Bakhshia R, Petrova D, Pocock R, Imani M, Seifalian AM. Biomaterials. 2007. p. 4717. [DOI] [PubMed]
- Gill R, Zayats M, Willner I. Angew. 2008. p. 7602. COI number [1:CAS:528:DC%2BD1cXht1GltLzK] [DOI] [PubMed]
- Wu X, Liu H, Liu J, Haley KN, Treadway JA, Larson JP, Ge N, Peale F, Bruchez MP. Nat. 2003. p. 41. COI number [1:CAS:528:DC%2BD3sXhvVyl] [DOI] [PubMed]
- Chan WC, Nie S. Science. 1998. p. 2016. COI number [1:CAS:528:DyaK1cXmtlKnsrk%3D]; Bibcode number [1998Sci...281.2016C] [DOI] [PubMed]
- Lakowica JR. Effect of solvents on fluorescence emission spectra, in Principles of Fluorescence Spectroscopy. Plenum Press, New York; 1983. p. 187. [Google Scholar]


