Abstract
Ultrafine WO3 nanoparticles were synthesized by nanocasting route, using mesoporous SiO2 as a template. BET measurements showed a specific surface area of 700 m 2/gr for synthesized SiO2, while after impregnation and template removal, this area was reduced to 43 m 2/gr for WO3 nanoparticles. HRTEM results showed single crystalline nanoparticles with average particle size of about 5 nm possessing a monoclinic structure, which is the favorite crystal structure for gas sensing applications. Gas sensor was fabricated by deposition of WO3 nanoparticles between electrodes via low frequency AC electrophoretic deposition. Gas sensing measurements showed that this material has a high sensitivity to very low concentrations of NO2 at 250°C and 300°C.
Keywords: WO3, Nanocasting, Low frequency AC electrophoretic deposition, NO2 gas sensor
Introduction
Semiconductor gas sensors offer good advantages with respect to other gas sensor devices due to their simple implementation, low cost and good reliability for real-time control systems [1-3]. Among them, ZnO, WO3 and SnO2 have widely been studied [4]. Tungsten oxide (WO3) is a wide band gap, n-type semiconductor that has interesting physical and chemical properties [5-9]. WO3 is a good photochromic, electrochromic, thermochromic and catalytic material [9-12].
Gas sensing is a surface effect. As gas adsorption occurs at surface level, an increase in the active surface area of the semiconductor oxide would enhance the properties of materials used as gas sensors [4,13-15]. Nanostructured mesoporous materials have been widely studied in the development of gas sensing and catalytic systems, due to their large, controllable pore size and high surface area [1,13,16-18]. Nanocasting is a novel methodology for preparing nanoparticles by using mesoporous materials as a template to accommodate different oxides. In this synthesis pathway, the surface and voids of a preformed mesoporous solid are coated with desired precursors. The subsequent mineralization of these precursors and removal of the former solid templates may lead to mesostructures with other compositions and crystalline frameworks [1,13,19].
While using in chemical sensors, the sensing material should be aligned and connected between the electrode structures, so that the change of their properties is converted into electrical signal, when exposed to the measurand. Several approaches have been reported in the literature for placement of the nanomaterials between the electrodes, but most of them do not offer selective settlement of particles or need expensive apparatus and high vacuum [20]. Using electric field is one of the most versatile techniques known till date for selective and inexpensive assembly of nanoparticles [21].
In this research, we applied a nanocasting route for synthesis of ultrafine WO3 nanoparticles. In the first step, mesoporous silica (SBA-15 structure) was prepared using a soft template, and in the second step, this material was used as a hard template in nanocasting of WO3 nanoparticles. Also, alternating electric field was used to manipulate the sensing material between electrodes, in order to fabricate gas sensor.
Experimental
Mesoporous SiO2 was synthesized, using bottom-up method. Polyethylene oxide–polypropylene oxide block copolymer was used as soft template and tetraethyl orthosilicate (TEOS) as silicate source. In the first step, 6 gr of the copolymer, 180 cc distilled water and 30 gr HCl (35% wt) were mixed together, and the solution was stirred for 6 h at 35°C. In the second step, TEOS was added to this acidic solution and stirred for 24 h at the same temperature and then heated at 100°C for another 24 h as a hydrothermal treatment. Small volume of NH3 was added in order to increase the PH up to 7. Then, the product was dried at room temperature in air atmosphere and calcined at 550°C for 4 h. The solid product of this process is mesoporous SiO2, which will be used as nanotemplate in production of WO3 nanoparticles.
The synthesis of WO3 nanoparticles was carried out as follows: equal amounts of phosphotungstic acid hydrate and mesoporous SiO2 were dissolved in ethanol, stirred vigorously for 1 h, dried at room temperature at air atmosphere and heated at 570°C for 5 h. The SiO2 hard template was removed by HF (2% wt), and the product was collected by centrifugation. The product was washed both with water and ethanol for three times and dried at room temperature.
For sensor fabrication, WO3 nanopowders were deposited on an alumina substrate between electrodes, via low frequency AC electrophoretic deposition [21-23]. For this purpose, a suspension of 1 gr/l WO3 nanoparticles in acetone was prepared and ultrasonicated for 15 min to become stable and homogenous. Deposition was done by applying a voltage of 25 v and a frequency of 1 KHz. The sensor was calcined for 1 h at 58°C in an electric furnace and air atmosphere.
Synthesized powders were characterized by X-ray diffraction (XRD, Siemens D500, cu Kα radiation) and transmission electron microscopy (HRTEM, JEOL2000 and TEM, Phillips). Nitrogen adsorption–desorption measurements were done using Gemini2375 instrument to determine the Brunauer–Emmett–Teller (BET) surface area. Response of the sensors toward various concentrations of NO2 was measured using a normal set-up. The gas response was calculated as Ra/Rg, where Ra and Rg represent samples’ resistances without and with the presence of gas, respectively.
Results and Discussion
TEM image of mesoporous SiO2 and HRTEM image of WO3 nanoparticles are shown in Fig. 1a and 1b, respectively. The results revealed that WO3 replica was constructed by hexagonally packed nanoparticle array in the two-dimensional SBA-15 structure of SiO2. These nanoparticles were randomly oriented in the mesostructured framework and rather uniform in diameter (∼5 nm) due to the confined growth in the channels of the mesoporous silica template. The d spacing has been measured to be 3.8 Å, which is in accordance with (001) planes in monoclinic WO3 crystal structure.
Figure 1.
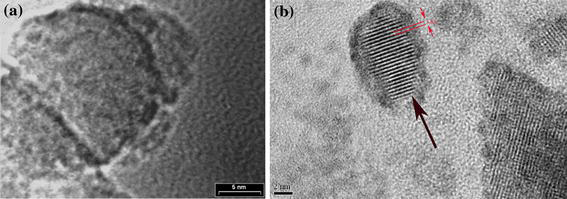
a TEM image of mesoporous SiO2; b HRTEM image of a single WO3 nanoparticle
BET measurements showed a surface area of about 700 m 2/gr for mesoporous SiO2 template that was reduced to 43 m 2/gr for WO3 nanoparticles.
XRD patterns before and after removing the SiO2 template are shown in Fig. 2, indicating that WO3 is fully crystalline with monoclinic crystal structure, which is in a good agreement with TEM results. The results show that SiO2 peaks were removed after washing with HF and pure WO3 has remained.
Figure 2.
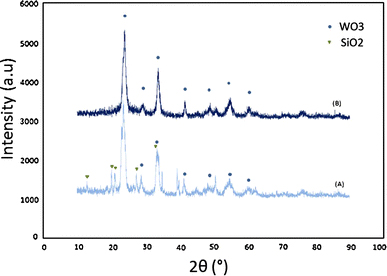
XRD pattern of synthesized WO3a before and b after removing the silicate template
In AC electric field, the nanomaterials align in a highly uniform way and orientate along the electric field direction by the electrophoresis force. As it can be seen from the SEM images in Fig. 3a and 3b, nanoparticles assemble along the direction of electric field, connect end to end and bridge the electrode gap. Based on the SEM images, AC electrophoresis has the potential for the precise manipulation of nanoparticles, which would be useful in making micro/nano electronic devices.
Figure 3.
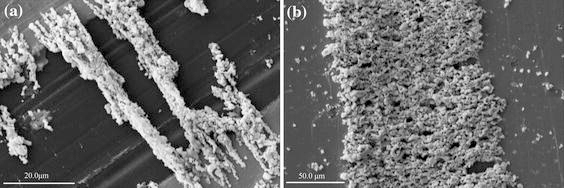
SEM image of WO3 nanoparticles between the electrodes
Optical microscopy image of fabricated sensor via AC electrophoretic deposition is shown in Fig. 4. Obviously, WO3 particles have connected the electrodes, by bridging over them as a result of AC electrophoresis forces.
Figure 4.
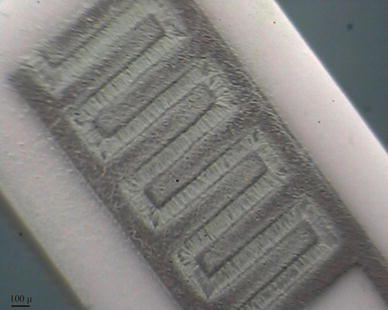
Optical microscopy image of fabricated gas sensor, the particles have connected the electrodes together
The sensing properties of fabricated sensor exposed to dilute NO2 were investigated in a dynamic mode switching from air to the specific concentration of the target gas and again back to the base air in each cycle. Figure 5a and 5b show the response transients and sensitivities of synthesized WO3 nanoparticles at 200°C. Sharp response of the sensor to NO2(Fig. 5a) is an indication of sensor quality and is a direct consequence of extremely small particle size and high specific surface area. The sensor needed a rather long time to reach its original level after switching back to the air. Sensitivity values (defined as the ratio of sensor resistance in target gas over its resistance in base air) presented in Fig. 5b follow a line presenting the device as a trustable NO2 sensor.
Figure 5.
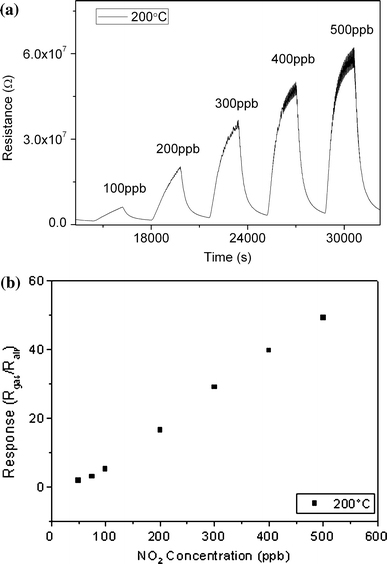
a Response transients to different concentrations of NO2 at 200°C, b corresponding sensitivities
Sensing test results prove that the synthesized nanoparticles are sensitive to low concentrations of NO2, which may be a consequence of ultrafine particle size that leads to higher active surface. Application of electric field for deposition of particles also improves the sensing properties of the fabricated gas sensor, thanks to precise placement of particles side by side and establishment of a good connection between the electrodes facilitating the charge transfer between electrodes.
Conclusions
Ultrafine WO3 nanoparticles rather uniform in diameter (∼5 nm) were synthesized efficiently by nanocasting route. AC electrophoresis was applied to deposit WO3 nanoparticles between electrodes in order to fabricate the gas sensor. The sensing measurements showed that the synthesized particles are sensitive even to very low concentrations of NO2(100 ppb), which is a result of very small particle size combined with application of proper deposition method.
References
- Rossinyol E, Arbiol J, Peiró F, Cornet A, Morante JR, Tian B, Bo T, Zhao D. Nanostructured metal oxides synthesized by hard template method for gas sensing applications. Sens. Actuators B. 2005;109:57–63. doi: 10.1016/j.snb.2005.03.016. [DOI] [Google Scholar]
- Zamani C, Shimanoe K, Yamazoe N. A new capacitive-type NO2 gas sensor combining an MIS with a solid electrolyte. Sens. Actuators B. 2005;109:216–220. doi: 10.1016/j.snb.2004.12.049. [DOI] [Google Scholar]
- Zamani C, Shimanoe K, Yamazoe N. Capacitive-type gas sensors combining silicon semiconductor and NaNO2-based solid electrolyte for NO2 detection. Sens. Actuators B. 2005;109:300–306. doi: 10.1016/j.snb.2004.12.061. [DOI] [Google Scholar]
- Srivastava V, Jain K. Highly sensitive NH3 sensor using Pt catalyze silica coating over WO3 thick films. Sens. Actuators B. 2008;133:46–52. doi: 10.1016/j.snb.2008.01.066. [DOI] [Google Scholar]
- Chen L, Tsang SC. Ag doped WO3-based powder sensor for detection of NO gas in air. Sens. Actuators B. 2003;89:68–75. doi: 10.1016/S0925-4005(02)00430-6. [DOI] [Google Scholar]
- Deepa M, Srivastava AK, Sharma SN, Govind, Shivaprasad SM. Microstructural and electrochromic properties of tungsten oxide thin films produced by surfactant mediated electrodeposition. Appl. 2008;254:2342–2352. doi: 10.1016/j.apsusc.2007.09.035. COI number [1:CAS:528:DC%2BD1cXhsVeltrs%3D]; Bibcode number [2008ApSS..254.2342D] [DOI] [Google Scholar]
- Yang B, Li H, Blackford M, Luca V. Novel low density mesoporous WO3 films prepared by electrodeposition. Curr. 2006;6:436–439. doi: 10.1016/j.cap.2005.11.035. Bibcode number [2006CAP.....6..436Y] [DOI] [Google Scholar]
- Teoh LG, Shieh J, Lai WH, Hung IM, Hon MH. Structure and optical properties of mesoporous tungsten oxide. J. 2005;396:251–254. doi: 10.1016/j.jallcom.2004.12.005. COI number [1:CAS:528:DC%2BD2MXks1Kkt7o%3D] [DOI] [Google Scholar]
- Teoh LG, Hon YM, Shieh J, Lai WH, Hon MH. Sensitivity properties of a novel NO2 gas sensor based on mesoporous WO3 thin film. Sens. Actuators B. 2003;96:219–225. doi: 10.1016/S0925-4005(03)00528-8. [DOI] [Google Scholar]
- Blo M, Carotta MC, Galliera S, Gherardi S, Giberti A, Guidi V, Malagù C, Martinelli G, Sacerdoti M, Vendemiati B, Zanni A. Synthesis of pure and loaded powders of WO3 for NO2 detection through thick film technology. Sens. Actuators B. 2004;103:213–218. doi: 10.1016/j.snb.2004.04.053. [DOI] [Google Scholar]
- Teng Y, Wu X, Zhou Q, Chen C, Zhao H, Lan M. Direct electron transfer of myoglobin in mesoporous silica KIT-6 modified on screen-printed electrode. Sens. Actuators B. 2009;142:267–272. doi: 10.1016/j.snb.2009.08.013. [DOI] [Google Scholar]
- Kanan SM, Tripp CP. Synthesis, FTIR studies and sensor properties of WO3 powders. Curr. 2007;11:19–27. doi: 10.1016/j.cossms.2007.11.001. COI number [1:CAS:528:DC%2BD1cXmvVGiu7c%3D] [DOI] [Google Scholar]
- Rossinyol E, Prim A, Pellicer E, Rodríguez J, Peiró F, Cornet A, Morante JR, Tian B, Bo T, Zhao D. Mesostructured pure and copper-catalyzed tungsten oxide for NO2 detection. Sens. Actuators B. 2007;126:18–23. doi: 10.1016/j.snb.2006.10.017. [DOI] [Google Scholar]
- Ghen GS, Liao WL, Chen ST, Su WC, Lin CK. Effects of deposition and annealing atmospheres on phase transition of tungsten oxide films grown by ultra-high-vacuum reactive sputtering. Thin Solid Films. 2005;493:301–306. doi: 10.1016/j.tsf.2005.07.309. COI number [1:CAS:528:DC%2BD2MXhtFekurvE]; Bibcode number [2005TSF...493..301G] [DOI] [Google Scholar]
- Khatko V, Gorokh G, Mozalev A, Solovei D, Llobet E, Vilanova X, Correig X. Tungsten trioxide sensing layers on highly ordered nanoporous alumina template. Sens. Actuators B. 2006;118:255–262. doi: 10.1016/j.snb.2006.04.030. [DOI] [Google Scholar]
- Zamani C, Casals O, Andreu T, Morante JR, Romano-Rodríguez A. Detection of amines with chromium-dopedWO3 mesoporous material. Sens. Actuators B. 2009;140:557–562. doi: 10.1016/j.snb.2009.04.051. [DOI] [Google Scholar]
- Abdollahzadeh Ghom S, Zamani C, Nazarpour S, Andreu T, Morante JR. Oxygen sensing with mesoporous ceria–zirconia solid solutions. Sens. Actuators B. 2009;140:216–221. doi: 10.1016/j.snb.2009.02.078. [DOI] [Google Scholar]
- Zamani C, Illa X, Abdollahzadeh-Ghom S, Morante JR, Rodríguez AR. Mesoporous silica: a suitable adsorbent for amines. Nanoscale Res. 2009. [DOI] [PMC free article] [PubMed]
- Valdés-Solís T, Fuertes AB. High-surface area inorganic compounds prepared by nanocasting techniques. Mater. 2006;41:2187–2197. doi: 10.1016/j.materresbull.2006.04.018. [DOI] [Google Scholar]
- Liu WJ, Zhang J, Wan LJ, Jiang KW, Tao BR, Li HL, Gong WL, Tang XD. Dielectrophoretic manipulation of nano-materials and its application to micro/nano-sensors. Sens. Actuators B. 2008;133:664–670. doi: 10.1016/j.snb.2008.03.032. [DOI] [Google Scholar]
- Gardeshzadeh AR, Raissi B, Marzbanrad E. Preparation of Si powder thick films by low frequency alternating electrophoretic deposition. J. 2008;43:2507–2508. doi: 10.1007/s10853-008-2519-z. COI number [1:CAS:528:DC%2BD1cXjt1WjsLw%3D]; Bibcode number [2008JMatS..43.2507G] [DOI] [Google Scholar]
- Gardeshzadeh AR, Raissi B, Marzbanrad E. Electrophoretic deposition of SnO2 nanoparticles using low frequency AC electric fields. Mater. 2008;62:1697–1699. doi: 10.1016/j.matlet.2007.09.062. COI number [1:CAS:528:DC%2BD1cXhvVektbg%3D] [DOI] [Google Scholar]
- Gardeshzadeh AR, Raissi B, Marzbanrad E, Mohebbi H. Fabrication of resistive CO as sensor based on SnO2 nanopowders via low frequency AC electrophoretic deposition. J Mater. 2009;20:127–131. doi: 10.1007/s10854-008-9652-y. COI number [1:CAS:528:DC%2BD1MXhvFCgsw%3D%3D] [DOI] [Google Scholar]


