Multidetector Computed Tomography (MDCT) is now an established modality for noninvasive cardiac imaging. Until recently, 64-slice CT provided state-of-the-art noninvasive coronary imaging. The 64 slices per gantry rotation can be achieved with either 64-detector rows, or 32-detector rows and a strategy to double the slice number by alternating the focal spot of the x-ray source. These “64-generation” scanners offered considerable advantages over earlier technology: superior spatial resolution, temporal resolution, volume coverage, and lower radiation doses for patients. Improving upon these fundamental CT parameters is also the goal of the next generation, or “post-64” era of coronary CT angiography (CTA). This review highlights improvements in the post-64 era of cardiac MDCT.
The newest technology offers significant advantages, but unlike the evolution from 4-to 64-detector row coronary CTA, current CT hardware releases are far from uniform, reflecting different approaches to image acquisition in the post-64 era. No single CT scanner offers the full portfolio of the newest features. This situation underscores the importance of understanding the properties of a cardiac CT scanner. Also, CT vendors continue to eclipse the state-of-the-art from even the recent past. Thus, it is very challenging to accumulate “current” clinical evidence. This reality is reflected in two multi-center 64-era MDCT trials, each of which focused on a single CT platform. The first of these publications,1 the Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography (ACCURACY) trial, enrolled United States subjects from predominantly nonacademic (private) centers and found a (patient based) sensitivity, specificity, and positive and negative predictive values to detect >50% or >70% stenosis of 0.95, 0.83, 0.64, and 0.99, respectively, and 0.94, 0.83, 0.48, 0.99, respectively. The second publication,2 the Coronary Artery Evaluation Using 64-Row Multidetector Computed Tomography Angiography (Core64) study was both international and included predominantly academic centers. This trial had lower test characteristics (patient based detection of >50% stenosis, sensitivity, specificity, and positive and negative predictive of 0.85, 0.90, 0.91, 0.83) but showed a similar ability of CT when compared to catheterization to identify, on the basis of obstructive coronary stenoses, patients who underwent revascularization.
The results from the Core64 study call into question the very high negative predictive value,3 a previously accepted strength of MDCT, namely the exclusion of coronary artery diseases (CAD) in low- and intermediate-risk patients with an exam that is not only much simpler to perform but also has fewer complications than coronary catheterization. With post-64 technology described below, excluding CAD is typically accomplished with radiation doses comparable to, and in many cases lower than, catheterization. The same argument also extends to emergency room imaging; CT can potentially reduce the overall cost of emergency room stays for chest pain patients4 However, even before the results of multicenter trials can be fully appreciated in the literature, the practice of coronary CT has changed. Specifically, patients in both multicenter studies were scanned with retrospective ECG gating; the current trend in coronary CTA is prospective ECG gating that delivers a lower radiation dose but has fewer phases within the R-R interval to assess the coronary arteries (Fig. 1). Thus, the performance of CT cited by each trial must be now confirmed for lower dose, prospectively ECG gated, image acquisitions. The differences and tradeoffs between retrospective and prospective gating are detailed below.
Fig. 1.
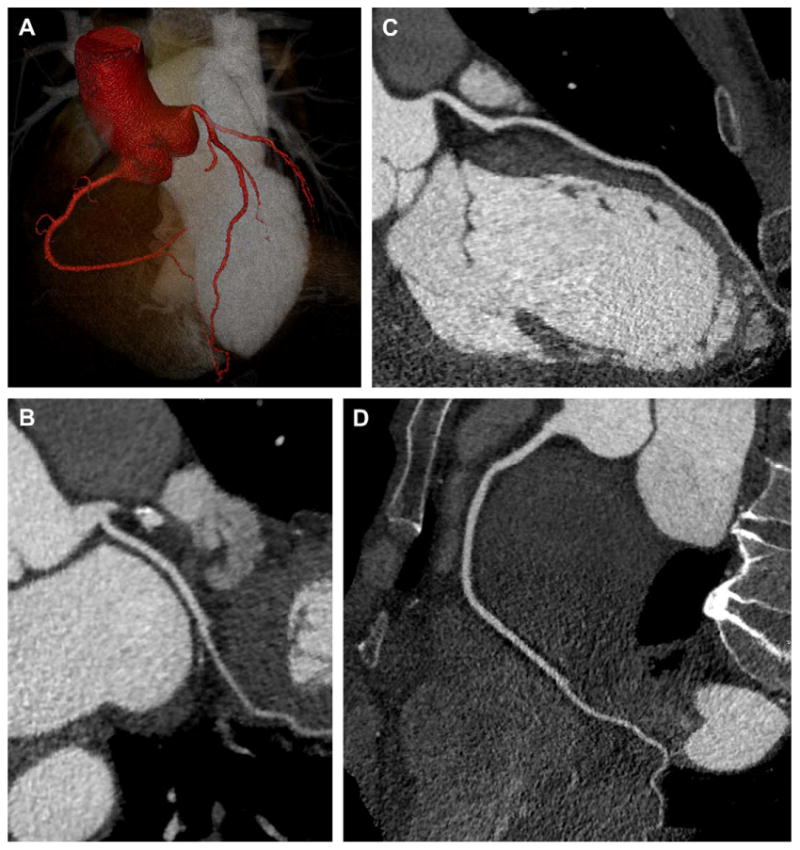
48-year-old woman with atypical chest pain and nonspecific ECG findings. Single heartbeat coronary imaging was performed using 320-slice CT (Toshiba AquilionOne, Tochigi-ken, Japan) with 100 kV, 400 mAs, 350 millisecond gantry rotation, and 80 mL of iopamidol 370 mg I/mL (Isovue-370, Bracco Diagnostics, Princeton, NJ). The use of prospective ECG gating (phase window width = 10%) and low kV (patient weight <170 pounds) enabled a 2.6 mSv acquisition. (A) 3D volume rendering (Vitrea fx, Vital Images, Minnetonka, MN) of the aortic root, major coronary arteries, and left ventricular cavity. (B, C, D) Curved multiplanar reformatted images of the normal coronary arteries.
In addition to coronary imaging, cardiac CT enables visualization of the myocardium, the cardiac chambers, the location and patency of coronary bypass grafts, and significant extracardiac disease. Retrospective ECG gating also enables the assessment of cardiac function and valves. Although the high negative predictive value for CAD is now generally accepted, there are other very promising applications with less validation: plaque characterization, the evaluation of myocardial ischemia (perfusion) and scar (myocardial delayed enhancement), and the assessment of coronary artery endothelial shear stress.
The reader who is studying rapidly emerging data should be cautious. Because of its great potential in cardiac diagnoses, the early interpretation of the post-64 era has included speculation, invited by the environment of new technology. Investigators are faced with the challenge of critically evaluating the technology and determining best practice standards for their patients. For imagers and administrators who are acquiring new MDCT technology, it can be challenging to identify and analyze the data. In the authors' experience, the best way to do this is to focus on the physical principles common to cardiac CT. This article has a subsection devoted to each parameter. In addition, the authors individually describe how the choice of one parameter influences other parameters.
This article includes current perspectives from November 2008, before the hardware releases expected at the annual meeting of the Radiological Society of North America (RSNA). In this review, the most recent CT scanner to receive FDA approval is the Siemens Definition AS+ that achieves 128 slices per gantry rotation obtained with 64-detector rows and an x-ray CT source focal spot that alternates between two positions to double the number of slices with respect to the number of detector rows. As discussed in detail in the temporal resolution section, the Siemens Definition (dual-source) 64-slice (32-detector rows × two focal spot positions) scanner features a two x-ray tube and two detector configuration to reduce the temporal resolution to 83 milliseconds.5 Only days before the Definition AS+ announcement, the General Electric LightSpeed 750HD received FDA approval. Like the AS+, the LightSpeed 750HD is very new, and there are no peer-review publications to date. However, this technology has a proposed improved spatial resolution that will be evaluated in the peer-review literature. This scanner will use the “step and shoot” technique for prospective ECG gating that was introduced with the earlier General Electric LightSpeed VCT64. In November 2007 at the annual meeting of the RSNA, Toshiba introduced the AquilionOne with 320-detector rows and whole heart coverage. This scanner achieves single heartbeat cardiac imaging, including lower-dose imaging with prospective gating.6 Because the entire cardiac volume is imaged at one time-point, this technology potentially enables myocardial perfusion CT at a single state of iodinated contrast opacification. At RSNA 2007, Philips announced the FDA approval and release of the Brilliance iCT with a 270-millisecond rotation time and 256 slices (128-detector rows × two focal spot positions).
This review of the post-64 era is divided into four parts: spatial resolution; temporal resolution; cranio–caudal volume coverage; and patient radiation. Because coronary imaging is most robustly performed with catheter-based angiography, each part describes particular technologies with catheterization standards in mind. The authors present a set of ideal features of CT, or “what you want” from the perspective of a cardiac imager. The hardware and associated software advances are then framed in the format of “what you can get,” keeping in mind that the substantial improvements are still under evaluation and cannot fulfill all expectations.
Spatial Resolution
What You Want: 0.1 mm and What You Get: 0.35–0.5 mm
The CT spatial resolution refers to the ability to separate two structures and is scientifically determined by measuring the ability of a CT system to separate line pairs.7 There is no systemic comparison of the true spatial resolution capabilities between CT scanners, either in the 64 or post-64 era. The voxel size has become the surrogate parameter for spatial resolution; it is determined by the field of view (FOV), the image matrix, and the section thickness.8 Because CT applications, including cardiac applications, have demanded characterization of small anatomic structures as well as image post-processing in multiple planes, the spatial resolution has dramatically improved. Even before the 64-era technology matured, most CT image reconstruction algorithms included isotropic voxels. That is, the CT voxels in the x, y, and z dimensions are cubic with equal lengths along each side, for example 0.35 mm3. This practice is now standard as it facilitates image post-processing.
Because normal coronary arteries have a lumen diameter of approximately 3 mm, a CT scanner with 0.35 mm3 isotropic voxels will depict the coronary lumen on roughly 9 voxels. Thus, CT can spatially resolve a normal coronary lumen and depict disease, the fundamental basis of coronary CTA. However, the resolution is generally insufficient to perform quantitative assessments of coronary stenosis with high confidence. For example, volume averaging over a single voxel can alter the quantification of stenosis by more than 10%. Thus, stenosis grading should be limited to categories such as <50%, 50%–70%, and >70%. In some practices the categorization is limited to less than 50% versus 50% or greater. The spatial resolution also explains why, to date, the greatest benefit of coronary CTA has been its high negative predictive value. Because of the spatial resolution of cardiac CT, the evaluation of normalcy is simpler and can be made with greater confidence than the assessment of the percentage stenosis.
Typical fluoroscopy tubes have a spatial resolution is approximately 0.16 mm,9 and thus a normal coronary artery is depicted on roughly 18 pixels. Another important difference is that fluoroscopic images are projections (two-dimensional [2D]), not volumetric as in CT. On one hand, fluoroscopy images do not suffer from volume averaging artifacts inherent to slice thickness. However, CT volumes have a distinct advantage in that lesions can be evaluated from any perspective.
The spatial resolution of sonography is largely determined by the transducer frequency. IntraVascular UltraSound (IVUS) performed with coronary catheterization has frequencies between 20–50 MHz, yielding an axial spatial resolution of 0.15 mm.10 In addition to the superior spatial resolution, IVUS has good coronary artery contrast resolution, ie, the ability to resolve acoustic differences between calcified, noncalcified, fibrous, mixed, lipid-rich core, and necrotic content plaque. The limitation of IVUS relates to its invasive nature, the large catheter sizes with high rates of complication (3% chance of coronary vasospasm and up to 1% chance of coronary rupture). Also, dense calcification can severely limit the transmission of the high frequency sound waves and thus distort IVUS images.
So, “what do you want?” The spatial resolution of CT would be greatly enhanced if it rivaled catheterization, because a significant limitation in MDCT today is the inability to quantify coronary stenosis. Consider an example of 0.1 mm3 voxels, recognizing that such a dramatic “improvement” is unrealistic with current technology. With respect to spatial resolution alone, the images would be comparable or superior to coronary catheterization; the lumen of a normal coronary artery would be spanned by 30 voxels, and advanced techniques for measurement of stenoses would become far more robust. However, voxels of this size would present difficulties related to image storage and manipulation with current image post-processing systems. Assuming that the acquisition spans 160 mm in the craniocaudal (z-axis) direction, image reconstruction at a single phase of the R-R interval would include 1600 images. Five reconstructions, currently considered routine, would absorb the memory space of 8000 high-definition images and would overwhelm current data transfer and storage systems.
The signal to noise ratio (SNR) is another important parameter intimately related to spatial resolution. The SNR refers to the amount of signal, or information that can be used for interpretation, divided by the image noise. The noise contains no information; it is akin to an imperfect canvas on which the image data, or signal, is painted. In fact, the image noise is calculated by making Hounsfield unit measurements in air. In general, CT enjoys a healthy SNR, particularly when compared to MRI where low signal can represent a limiting step for both cardiac and noncardiac applications. However, as the CT slice thickness decreases, so does the amount of tissue per slice and hence the signal. This same principle is important to recognize when imaging larger patients who have less signal per unit of photon flux because larger patients have a higher total x-ray CT attenuation. The first step to image this population is to maximize the x-ray CT tube current. However, a significantly higher SNR data set, ie, less noisy images, can be obtained when images are reconstructed at double the slice thickness, for example 0.8 mm instead of 0.4 mm.
It should also be noted that thinner slices are desirable with preserved volume coverage per gantry rotation. The benefits of large volume coverage are discussed in detail later in this article. Thinner slices unchanged in number would result in smaller z-axis coverage per gantry rotation. In turn, this would increase scan time and could compromise image quality by the introduction of breathing artifact. Moreover, longer scan times are generally associated with higher doses of radiation to patients.
Another way to improve the apparent spatial resolution is to increase coronary artery diameter, thus increasing the number of voxels spanning each artery. This is the basis of the near-universal practice of coronary vasodilatation before the CT acquisition. Patients with a contraindication to sublingual nitroglycerine, the agent typically used, can be imaged without it.
Temporal Resolution
What You Want: Less Than 30 ms and What You Get: 83–175 ms
The temporal resolution refers to the time needed to acquire a complete data set for image reconstruction. Superior temporal resolution improves the ability to freeze cardiac motion. Unlike static CT applications, the heart is beating and the gantry of the CT scanner is chasing it. This can result in cardiac motion artifact that compromises image quality. Specifically, severe motion artifact in only a single coronary segment can decrease confidence in rendering a negative examination to the point where additional imaging, typically catheterization, is needed.
The temporal resolution of a single x-ray CT tube and detector system is one half (180 degrees) of the gantry rotation time because image reconstruction requires approximately 180 degrees. The gantry rotation time is the time needed for a complete 360-degree revolution. Paralleling the rapid evolution of multidetector technology have been improved gantry rotation speeds. At the time when each CT vendor reached 64 slices per gantry rotation, the gantry rotation times reached 400 milliseconds or less and are now as low as 270 milliseconds with the Philips Brilliance iCT (Fig. 2).
Fig. 2.
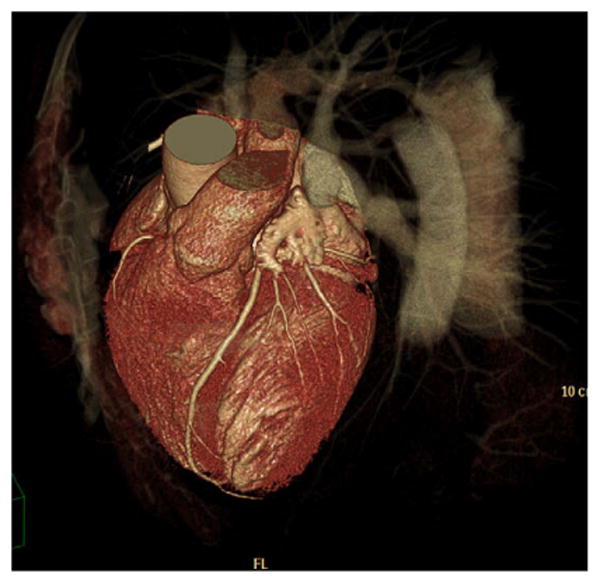
3D volume rendered image of a 62-year-old man with chest pain. CT was requested and excluded CAD. The patient was imaged with 256-slice CT, 128 detector rows and an alternating focal spot (Philips Brilliance iCT, Best, The Netherlands). With a scan time of 4 sec, 13 cm of craniocaudal coverage was achieved. (Courtesy of Nathan Peled, Carmel Medical Center, Haifa, Israel.)
Conventional angiography uses fluoroscopy with acquisition on the order of 30 frames per second. Thus, the temporal resolution (expressed in units of time for image acquisition) is approximately 33 milliseconds, adequate for motion-free cardiac imaging. As an analogy, the temporal resolution of a movie at a theater is 42 milliseconds, or 24 frames per second. At the temporal resolution of all CT applications, motion artifact can be detected, and, in some cases, this problem represents a significant limitation to image quality. Motion artifacts generally become more severe at higher heart rates because CT is unable to “freeze” cardiac motion.11 Echocardiography has a temporal resolution of between 33–66 milliseconds (15–30 frames per second), effectively freezing cardiac motion, even at high heart rates.12
Because motion artifact can compromise CTA studies, there has been substantial effort to improve temporal resolution and effective temporal resolution (defined below). In addition to speeding up the gantry rotation, the temporal resolution can be improved with the introduction of a second x-ray CT source with an independent detector system. Dual-source CT features two x-ray CT sources and detector systems located 90 degrees from one another.5 Consequently, using both x-ray CT tubes, the temporal resolution is halved from 165 milliseconds (330 milliseconds gantry rotation) to 83 milliseconds, because only one quarter of a gantry rotation is needed for reconstruction (Fig. 3).
Fig. 3.
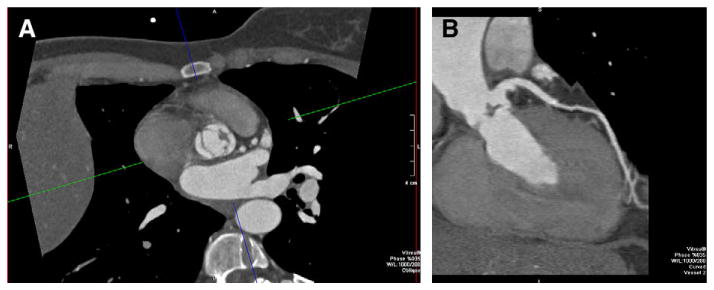
55-year-old man with abnormal aortic valve motion by echocardiography. CT was requested to exclude CAD. The patient's heart rate (>100 beats per minute at the time of acquisition) could not be safely lowered because of hypotension. (A) Retrospectively ECG gated dual-source cardiac CT (Siemens Definition, Erlangen, Germany) with 83 milliseconds temporal resolution depicted the vegetation of the aortic valve, as noted on the axial images. (B) Curved multiplanar reformatted image of the LAD also shows the vegetation in addition to demonstrating a normal LAD.
In general, better temporal resolution improves image quality, decreasing the dependence on heart rate control with beta-blockade. The relationship between image quality, heart rate, and temporal resolution achieved by CT are areas of active investigation, with several investigators testing the hypothesis that routine cardiac CT can be performed without beta-blockade13 and with reports that beta-blockers are not needed.14 The benefits of scanning without heart-rate control include: simplified logistics; the ability to scan patients with a contraindication to beta-blockers; and increasing the number of patients scanned per day because of less patient preparation. However, heart-rate control can still be beneficial by reducing beat-to-beat variability; in dual-source CT, data is acquired over multiple R-R intervals. The stack of cardiac slabs, with each slab obtained from one heartbeat, are then visualized together. Thus, variability between heartbeats can degrade image quality.
To date there is no established heart rate “cut-off” at which dual-source CT can confidently image; establishing such a limit is challenging. Instead of focusing on imaging without beta-blockade, one can capitalize on dual-source CT by combining the superior temporal resolution with heart-rate control to better eliminate cardiac motion from the list of variables that can compromise an acquisition.
All cardiac CT acquisitions use ECG gating to mitigate motion artifact. Thus, the discussion of temporal resolution inherently includes the two forms of ECG gating: retrospective and prospective. During retrospective gating, the image and ECG data are acquired throughout the cardiac cycle. Therefore, image reconstruction can be performed at any cardiac phase. This was briefly noted in the introduction; all current MDCT data from multicenter trials have been acquired with retrospective gating. Moreover, when retrospective gating is used, the cardiac cycle can be displayed as a cine loop in any projection, enabling myocardial function and valve evaluation. In prospective gating, the scanner uses the ECG to plan the timing of image acquisition and exposes the patient to radiation only during a preselected temporal window of the cardiac cycle. To date, the majority of clinical experience with prospective gating has been acquired with a 64 × 0.625 mm detector row (General Electric LightSpeed VCT64, Waukesha, WI) step-and-shoot technique (Fig. 4). In a typical coronary CTA case, imaging will occur over seven heartbeats. Four beats will be used for the prospective data acquisition (the “shoot”) and the intervening three beats will be used to move the patient into the updated position within the gantry (the “step”).
Fig. 4.
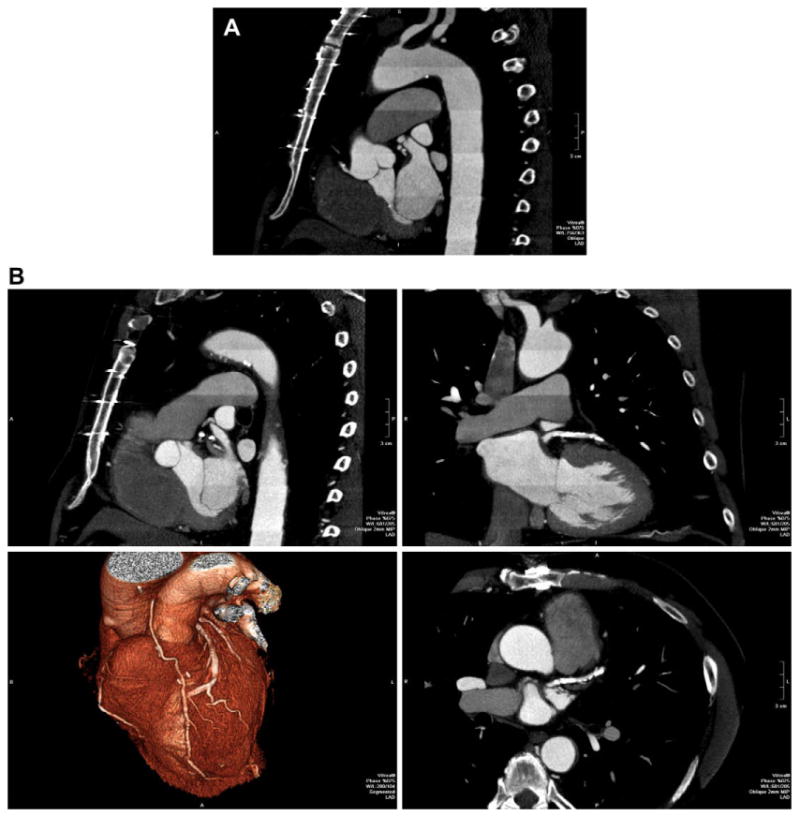
72-year-old man status post coronary artery bypass grafting. Because of the large craniocaudal field of view, imaging was performed over 13 heartbeats, 7 beats of data acquisition plus 6 move the patient within the gantry. This “step and shoot” technique significantly decreases the radiation exposure when compared to retrospective ECG gating. (A) Left anterior oblique reformation over the entire z-axis FOV shows high image quality. Enhancement pattern in the aorta demonstrates the individual “steps” as described. (B) Multiplanar reformatted images as well as 3D volume rendering (lower left) focused on the heavily calcified left anterior descending.
The preselected temporal window of the cardiac cycle, or “phase window”15 can be described as a lower and upper bound of the cardiac phases, eg, 65%–75% of the R-R. Alternatively, it can also be described as a “center” phase and a surrounding “pad,” eg, 100 milliseconds centered on 75% of the R-R interval.16 By eliminating patient exposure for the majority of the R-R interval, prospective gating significantly reduces radiation exposure and is now preferred in patients who do not present with a clinical indication for evaluating cardiac function.
A narrower phase window lowers patient radiation dose by shortening the patient exposure time. Initial evidence also suggests that patients with a lower heart rate have a wider range of phases without motion artifact,15 arguing that beta-blockade will reduce the overall patient radiation dose by increasing the confidence that high quality images will be achieved with a narrow phase window.
Multisegment reconstruction decreases the effective temporal resolution by using CT data from more than one R-R interval to reconstruct a volume.17 For example, consider a 64 × 0.5 mm detector configuration with 350 milliseconds–gantry rotation. In single-segment reconstruction, 3.2 cm cranio–caudal slabs are reconstructed with 175 milliseconds temporal resolution from one R-R interval. In two-segment reconstruction, the same slab is reconstructed from CT data over two R-R intervals. Thus, only half the data is required per R-R interval, and the effective temporal resolution is reduced to 87.5 (175/2) milliseconds. This strategy decreases motion artifact but has drawbacks. In particular, patients receive more radiation because the exposure time is longer, and as the number of segments increases, so does the length of the exam.
In general, there are two ways to mitigate cardiac motion. The first strategy is to scan faster than the heat beats. The second is to slow the heart rate. Heat rate control can be achieved by using oral or IV beta-blockade, both of which have a very high safety profile,18 particularly when large doses are avoided. Many large practices, including that of the authors, have no record of an adverse event. In the authors' practice, all patients without a contraindication receive beta-blockade. The contraindications include: symptomatic hypotension or bradycardia, severe decompensated heart failure, severe chronic obstructive lung disease, and severe peripheral arterial disease. Between 5 and 15 mg of metoprolol tartrate IV typically suffices to lower the heart rate below 65 beats per minute. A first dose of 5 mg can be followed with a second and third dose at 5 min intervals. In patients who are orally premedicated, additional IV beta-blockers can also be used with vital sign monitoring. Oral beta-blockers alone can also be used with a dose that increases in proportion to the patient's heart rate. This protocol is perceived to be safer than intravenous protcols. Efficiency is improved if additional time for IV beta-blockade can be avoided, although the heart rate monitoring required is identical.
Cardiac Coverage Per Gantry Rotation
What You Want: Single Gantry Rotation Whole Heart Coverage and What You Get: 1.9 cm–16 cm
Multidector CT expanded the volume coverage per gantry rotation and revolutionized the imaging of many body parts, including the heart. Although the speed of the CT acquisition was enhanced by faster gantry rotation times, the most dramatic advances came from the doubling and continuous re-doubling from single detector slip ring CT to 2-, to 4-, to 8-, and eventually to 64-detector row CT.
Along with the increase in number of detectors, the term “multislice” was introduced and, to a certain extent, was adopted. “Multislice CT” is simpler to say and to write when compared to “multidetector row CT,” but there are differences with respect to volume coverage per unit time. As noted in the introduction, an alternating x-ray CT source focal spot doubles the number of slices with respect to the number of detector rows. However, it does not double the volume coverage. As an example, a 128 × 2 × 0.625 mm system (128 detector rows, 2 focal spot positions, and 0.625 mm detector rows) will yield 256 (128 × 2) slices per gantry rotation while the z-axis coverage is 80 mm (128 × 0.625 mm).
The first advantage of greater volume coverage is a faster overall CT acquisition. A more rapid acquisition decreases the probability of an irregular cardiac rhythm, typically a premature ventricular contraction, that can severely compromise image quality. A second advantage is the ability to capture the state of iodinated contrast media at a single point in time. The largest volume coverage to date is 320-detector row CT (Toshiba AquilonOne Dynamic Volume CT) that features a 320 × 0.5 mm configuration, or 16 cm of craniocaudal coverage per gantry rotation. The result is single R-R, or single heartbeat cardiac imaging.
Three-hundred and twenty detector row cardiac CT eliminates “stair-step” artifacts inherent in other forms of cardiac CT in which cardiac subvolumes over multiple gantry rotations are subsequently stacked to create a cardiac volume. In addition, the 16 cm maximum single gantry rotation cranio–caudal coverage decreases the overall exam time to less than one second, and subsecond cardiac CT is expected to reduce artifacts. This timeframe also potentially reduces radiation exposure by avoiding the oversampling related to helical scanning.
In addition to the ability to perform single heartbeat coronary imaging at doses comparable to diagnostic catheterization, wide area detector coverage enables imaging at multiple points where each entire volume is acquired in a single R-R interval. A craniocaudal field of view that spans the entire heart gives the potential for single heartbeat myocardial perfusion CT, considered to be one of the most important potential applications of post-64 CT technology. An exam that includes accurate perfusion data could, in theory, supplant other technologies such as nuclear imaging and MRI. Myocardial perfusion CT highlights some of the differences between post-64 era technologies. In order to detect ischemia, CT perfusion must be performed under pharmacologic stress, increasing the heart rate. From this perspective alone, dual-source CT would be well suited for perfusion CT since the temporal resolution is 83 milliseconds. However, dual-source CT has relatively poor volume coverage per gantry rotation (1.9 cm) in comparison with 320-detector row CT (16.0 cm). Thus, dual-source CT perfusion would acquire the cardiac volume over approximately eight heartbeats, and the iodinated contrast opacification would be different for each slab. It is likely that accurate delineation of either small or subtle perfusion defects will require that images of the entire myocardial volume are obtained at a single point of iodinated contrast opacification, ie, using 320-detector row CT. However, this scanner has a temporal resolution of 350 milliseconds and thus two-segment reconstruction will be required for the majority of patients so that an effective temporal resolution of 87.5 milliseconds can be achieved (Fig. 5). Because the patient will be exposed over two heartbeats instead of one, the radiation will be higher than for single heartbeat imaging. Cardiac CT dose is the topic of the next section.
Fig. 5.
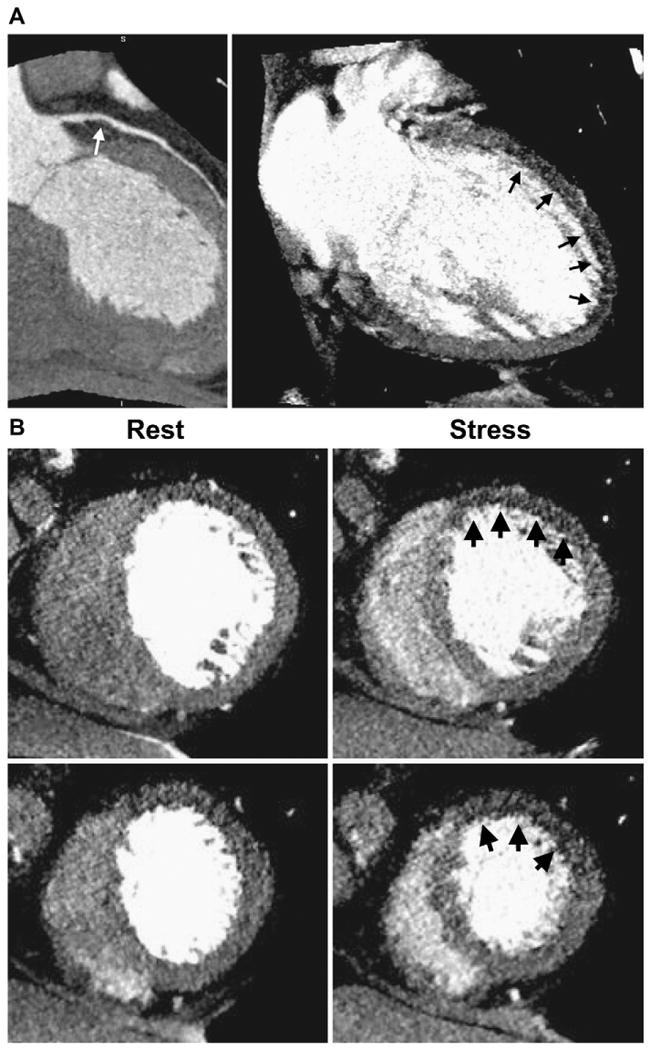
57-year-old woman with history hypertension and hyperlipidemia complaining of atypical chest pain. The patient underwent 320-detector row CT (Toshiba AquilionOne, Tochigi-ken, Japan) coronary CTA plus perfusion imaging. (A) CTA (left) followed by adenosine stress CT perfusion imaging (right). CTA shows a noncalcified plaque (white arrow) causing a moderate stenosis in the proximal LAD. Stress perfusion imaging (right) shows a subendocardial perfusion deficit in the anterior and apical walls (black arrows). (B) Short axis views reformatted with 3-mm slice thickness. Rest images (left) demonstrate very mild hypoattenuation in the anterior wall. Stress CT perfusion images (right) show perfusion deficit in the anterior wall at the level of the mid and distal left ventricle. (Courtesy of Richard T. George and Joao AC. Lima, Johns Hopkins University, Division of Cardiology.)
Myocardial perfusion CT is still experimental and will require first small and then larger validation studies before it could become a routine clinical examination. However, the potential for CT is noteworthy, as it is theoretically possible to use a single bolus of contrast media to acquire anatomic coronary images, followed by myocardial perfusion to detect regions of ischemia, and then myocardial delayed enhancement (MDE) images for the assessment of myocardial scar. MDE images are typically acquired 5–10 min after contrast injection; this acquisition does not require precise timing because iodine remains for some time in the extracellular space surrounding myocardial scar.
Cardiac CT Dosimetry
What You Want: The Minimum Effective Dose to Answer a Specific Clinical Question. What You Get: Dose Estimates, Retrospective vs. Prospective Gating, and ECG Dose Modulation
Medical radiation accounts for a large proportion of the total population exposure. CT is a major contributor; every patient referred for CT requires an evaluation of medical necessity versus radiation risk.19,20 Because of ECG gating and subsequent image oversampling, cardiac CT is a high radiation dose exam. Image oversampling is discussed below. Although substantial improvements have been made in the last two years, many practices deliver cardiac CT doses on the order of 20 mSv, with reports up to 40 mSv.21 To place this into a medical imaging perspective, some cardiac CT scans deliver a dose more than 50 times higher than screening mammography.
Some consequences of radiation have an established threshold dose, such as inducing a skin burn.22 This would require multiple scans over a short period of time. The main concern is induction of a radiation induced fatal neoplasm where cumulative dose is considered to increase risk. The relatively high doses from cardiac CT underscore the need for the imager to understand and be able to estimate dose.
For the radiation levels of diagnostic imaging, there is no data to confirm or refute a radiation “threshold,” that is, a level of cumulative effective dose at which the risk of a radiation induced fatal neoplasm is zero. Because data is so scarce, mathematical models are used to estimate risk. These models extrapolate from high-level exposures encountered by Japanese nuclear bomb survivors to much lower levels of medical radiation. Most models use a linear fit to estimate risk and have no threshold. That is, models assume that the risk is linearly proportional to the dose, and risk is present even for the smallest dose. To continue the comparison between the cardiac CT and the mammography patients, both individuals would be at risk for a radiation induced fatal neoplasm (no threshold), and for every one death induced by mammography, 50 people would die from cardiac CT. Because our best estimates are so rough, and because the risk is so low, it is challenging for medical personnel to consider medical radiation risks on a per patient basis. There is also a large potential for miscommunication. However, the cumulative radiation risk to the entire population must be considered, and cardiac CT is appropriately moving towards lower dose acquisitions.
CT has specific parameters to describe radiation dose: the computed tomography dose index (CTDI) and dose length product (DLP). The CTDI uses a 100 mm–long ion chamber and 150 mm–long, 320 mm–diameter cylindrical phantom to measure the radiation dose normalized by the beam width in milliGrays (mGy). Current CTDI estimates also weight the average of the center and peripheral contributions and helical pitch or axial scan spacing to dose. This is referred to as CDTIvol. Although the phantom should somehow reflect the attenuation of a human body, the CTDI is not used to make a statement regarding an individual patient's dose but rather as an index of radiation from CT scanning. In contrast to parameters such as the tube current, CTDI values reflect delivered dose because parameters such as the scanner geometry and filtration are considered. In practical terms, the CDTI can be used to compare scanning platforms and individual protocols. The CTDIvol is available from the CT console of all major manufacturers.
The second parameter is the DLP, expressed in mGy × centimeters. The DLP is defined as the product of the CTDIvol and the z-axis FOV. Even though the DLP reflects most closely the radiation dose for a specific CT examination, it is important to keep in mind that the DLP is a function of patient size, ie, how much z-axis coverage is required to complete the CT scan.
CTDIvol and DLP values can be used to characterize the scanner; they do not represent effective dose. The effective dose, with units of milliSevert (mSv), is the weighted sum over the organ doses. Although an estimation because individual organ doses can not be measured directly, the effective dose is the only useful parameter for individual patient risk assessment. The effective dose itself includes estimation because individual organ doses can not be measured directly. A simple estimation is that the effective dose is the product of the DLP and a conversion factor, EDLP that is specific to a body region. For example, for the chest the EDLP = 0.017 mSv × mGy−1 × cm−1.
As an example, suppose a coronary CTA acquisition results in a CTDIvol = 50mGy as stated on the scanner console. If the cranio–caudal extent of the scan is 14 cm (a normal sized heart), the DLP = 50mGy × 14 cm = 700 mGy × cm. Because the heart is in the chest, the appropriate conversion factor is EDLP = 0.017 mSv × mGy−1 × cm−1, and the effective dose for this patient is 50mGy × 14 cm × 0.017mSv × mGy−1 × cm−1, or about 12 mSv. At present, there are few centers that include dosimetry in the cardiac CT report. This practice may change over time as the awareness of referring clinicians and patients continues to increase.
There are several strategies to reduce cardiac CT dose. In the “pre-64” era of coronary CTA, all acquisitions used retrospective gating, and the patient exposure was high throughout the R-R interval. This practice continued into the early stages of the 64 era, during which doses began to increase, as did awareness of the high doses. A major step in dose reduction was the introduction of retrospective ECG-dependent tube current modulation.23 This strategy reduces tube current during systole, that part of the R-R interval least often used for interpretation because of greater coronary motion. The standard tube current is used for a predetermined phase window in diastole where motion is expected to be minimized.
As noted earlier, retrospective ECG gating enables cine evaluations throughout the R-R interval. With ECG-dependent tube current modulation, the images in systole have high noise (low SNR), lending itself to an important tradeoff between dose and image quality. For some applications, such as endocardial tracings to calculate left ventricular function, a relatively lower SNR is acceptable. However, as dose is reduced by further lowering the tube current in systole, the image degradation will eventually render those images not interpretable.
With respect to dose, prospective gating can be considered to be the limit of this tradeoff. As noted earlier, in prospective gating, there is no data acquisition outside the phase window, ie, the tube current is reduced to zero in systole. Coronary calcium scoring uses prospective gating with a very narrow phase window, usually a single phase of the R-R interval. Because there is no data for most of the cardiac cycle, cine evaluations for the myocardium and valves are not available. As the phase window is narrowed, cardiac CT doses become comparable, and in many cases less than, diagnostic cardiac catheterization. However, as this tradeoff is pushed to the limits of a narrower phase window and lower dose, fewer and fewer reconstruction phases are available, and the benefit of very low dose can be negated if every available phase is degraded by motion artifact. There is evidence for single heartbeat imaging that patients with slower heart rates have more phases that contain motion-free images.15 Thus, beta-blockade will lower the population radiation dose. The phase window should be chosen carefully; there are currently no published guidelines, but lowest dose (eg, single phase) imaging should be reserved for those patients in whom there is very high diagnostic confidence of motion-free images.
The MDCT pitch is a unitless measure for helical CT that describes how much the table feeds per gantry rotation. The pitch determines the amount of overlap between gantry rotations and hence the degree of oversampling for the ECG gated acquisition. For example, for a helical acquisition with a pitch of 0.2, an individual z-axis position of the heart will provide data that is received by 5 (1/0.2) detectors per gantry rotation. The 64-era scanners include software that automatically varies the pitch as a function of the patient's heart rate to optimize image quality and dose. For 320-detector row CT, images are acquired axially and thus the pitch is not a consideration.
One of the simplest methods to lower coronary CTA radiation doses is to limit the z-axis FOV. Scanning as superior as the aortic arch or as inferior as the adrenal glands delivers unnecessary radiation. Also, a complete “x-y”, or “skin-to-skin” reconstruction must be obtained, given the high prevalence of extracardiac findings. This does not influence the radiation dose since the larger x-y reconstruction will include only the z-axis FOV acquired to evaluate the coronary arteries.
Summary
The 64-generation scanners added a noninvasive dimension to coronary imaging and have been applied to a large number of patients. Image acquisition has been labor-intensive in comparison to CT of other body parts. In addition, the high radiation levels delivered to the patient using retrospective ECG gating, even though the actual risks are poorly defined, can offset the benefits in comparison with catheterization. The comparison between coronary catheterization and CT is complex, but CT will be forced to measure up to these technical specifications. Thus, imagers and referring clinicians should have a solid understanding of spatial resolution, temporal resolution, volume coverage, and radiation dose.
Noninvasive cardiac imaging now includes the post- 64 era of coronary CTA, and the new hardware incrementally improves on these parameters. The improvement is not uniform. As the technology strives to mirror catheterization benchmarks, the CT vendors have chosen to improve individual parameters. A clear example is the introduction of dual-source and 320-detector row CT. The former has demonstrated improved temporal resolution; the latter has demonstrated whole heart coverage.
Cardiac imaging will continue to define the state-of-the-art in CT. It is safe to predict that the next generation of scanners will again change the way clinicians think about image acquisition. In the meantime, patients today can and should benefit from very high negative predictive value of coronary CTA. Those patients who require coronary imaging but have a low or intermediate probability of CAD can undergo noninvasive diagnostic coronary imaging with radiation levels comparable to catheterization. This group of patients includes uncomplicated patients today who present to the emergency room. Although these patients enjoy a simpler exam to exclude CAD, the newer applications enabled by the post-64 era will be tested.
Footnotes
None of the authors received support for collaborating with this manuscript.
References
- 1.Budof MJ, Dowe D, Jollis J, et al. Diagnostic Performance of 64-Multidetector Row Coronary Computed Tomographic Angiography for Evaluation of Coronary Artery Stenosis in Individuals Without Known Coronary Artery Disease: Results From the Prospective Multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) Trial. J Am Coll Cardiol. 2008;52:1724–32. doi: 10.1016/j.jacc.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 2.Miller JM, Rochitte CE, Dewey M, et al. Diagnostic Performance of Coronary Angiography by 64-Row CT. N Engl J Med. 2008;359:2324–36. doi: 10.1056/NEJMoa0806576. [DOI] [PubMed] [Google Scholar]
- 3.Achenbach S. Cardiac CT: state of the art for the detection of coronary arterial stenosis. J Cardiovascular Computed Tomography. 2007;1:3–20. doi: 10.1016/j.jcct.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 4.Hoffmann U, Pena AJ, Cury RC, et al. Cardiac CT in emergency department patients with acute chest pain. Radiographics. 2006;26:963–78. doi: 10.1148/rg.264055709. discussion: 979–80. [DOI] [PubMed] [Google Scholar]
- 5.Flohr TG, McCollough CH, Bruder H, et al. First performance evaluation of a dual-source CT (DSCT) system. Eur Radiol. 2006;16:256–68. doi: 10.1007/s00330-005-2919-2. [DOI] [PubMed] [Google Scholar]
- 6.Rybicki FJ, Otero HJ, Steigner ML, et al. Initial evaluation of coronary images from 320-detector row computed tomography. Int J Cardiovasc Imaging. 2008;24:535–46. doi: 10.1007/s10554-008-9308-2. [DOI] [PubMed] [Google Scholar]
- 7.Judy PF. The line spread function and modulation transfer function of a computed tomographic scanner. Med Phys. 1976;3:233–6. doi: 10.1118/1.594283. [DOI] [PubMed] [Google Scholar]
- 8.Sprawls P. AAPM tutorial. CT image detail and noise. Radiographics. 1992;12:1041–6. doi: 10.1148/radiographics.12.5.1529128. [DOI] [PubMed] [Google Scholar]
- 9.Gershlick AH, de Belder M, Chambers J, et al. Role of non-invasive imaging in the management of coronary artery disease: an assessment of likely change over the next 10 years. A report from the British cardiovascular society working group. Heart. 2007;93:423–31. doi: 10.1136/hrt.2006.108779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nissen SE, Yock P. Intravascular ultrasound: novel pathophysiological insights and current clinical applications. Circulation. 2001;103:604–16. doi: 10.1161/01.cir.103.4.604. [DOI] [PubMed] [Google Scholar]
- 11.Dewey M, Teige F, Laule M, et al. Influence of heart rate on diagnostic accuracy and image quality of 16-slice CT coronary angiography: comparison of multisegment and halfscan reconstruction approaches. Eur Radiol. 2007;17:2829–37. doi: 10.1007/s00330-007-0685-z. [DOI] [PubMed] [Google Scholar]
- 12.Goldstein A. Overview of the physics of US. Radiographics. 1993;13:701–4. doi: 10.1148/radiographics.13.3.8316678. [DOI] [PubMed] [Google Scholar]
- 13.Ropers U, Ropers D, Pflederer T, et al. Influence of heart rate on the diagnostic accuracy of dual-source computed tomography coronary angiography. J Am Coll Cardiol. 2007;50:2393–8. doi: 10.1016/j.jacc.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 14.Achenbach S, Ropers D, Kuettner A, et al. Contrast-enhanced coronary artery visualization by dual-source computed tomography–initial experience. Eur J Radiol. 2006;57:331–5. doi: 10.1016/j.ejrad.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 15.Steigner ML, Otero HJ, Cai T, et al. Narrowing the phase window width in prospectively ECG-gated single heart beat 320-detector row coronary CT angiography. Int J Cardiovasc Imaging. 2009;25:85–90. doi: 10.1007/s10554-008-9347-8. [DOI] [PubMed] [Google Scholar]
- 16.Earls JP, Berman EL, Urban BA, et al. Prospectively gated transverse coronary CT angiography versus retrospectively gated helical technique: improved image quality and reduced radiation dose. Radiology. 2008;246:742–53. doi: 10.1148/radiol.2463070989. [DOI] [PubMed] [Google Scholar]
- 17.Halliburton SS, Stillman AE, Flohr T, et al. Do segmented reconstruction algorithms for cardiac multi-slice computed tomography improve image quality? Herz. 2003;28:20–31. doi: 10.1007/s00059-003-2445-4. [DOI] [PubMed] [Google Scholar]
- 18.Pannu HK, Alvarez W, Jr, Fishman EK. {beta}-Blockers for cardiac CT: a primer for the radiologist. Am J Roentgenol. 2006;186:S341–5. doi: 10.2214/AJR.04.1944. [DOI] [PubMed] [Google Scholar]
- 19.Brenner DJ, Hall EJ. Computed tomography–an increasing source of radiation exposure. N Engl J Med. 2007;357:2277–84. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 20.Cohnen M, Poll L, Puttmann C, et al. Radiation exposure in multi-slice CT of the heart. Rofo. 2001;173:295–9. doi: 10.1055/s-2001-12490. [DOI] [PubMed] [Google Scholar]
- 21.Paul JF, Abada HT. Strategies for reduction of radiation dose in cardiac multislice CT. Eur Radiol. 2007;17:2028–37. doi: 10.1007/s00330-007-0584-3. [DOI] [PubMed] [Google Scholar]
- 22.McNitt-Gray MF. AAPM/RSNA physics tutorial for residents: topics in CT. Radiation dose in CT. Radiographics. 2002;22:1541–53. doi: 10.1148/rg.226025128. [DOI] [PubMed] [Google Scholar]
- 23.Husmann L, Valenta I, Gaemperli O, et al. Feasibility of low-dose coronary CT angiography: first experience with prospective ECG-gating. Eur Heart J. 2008;29:191–7. doi: 10.1093/eurheartj/ehm613. [DOI] [PubMed] [Google Scholar]


