Abstract
We tested whetherArtemia abd-A could repress limbs in Drosophila embryos, and found that although abd-A transcripts were produced, ABD-A protein was not. Similarly, developing Artemiaepidermal cells showed expression of abd-A transcripts without accumulation of ABD-A protein. This finding in Artemia reveals a new variation in Hox gene function that is associated with morphological evolution. In this case, a HOX protein expression pattern is completely absent during early development, although the HOX protein is expressed at later stages in the central nervous system in a "homeotic-like" pattern. The combination of an absence of ABD-A protein expression in the Artemia limb primordia and the weak repressive function of Artemia UBX protein on the limb promoting gene Dll are likely to be two reasons why homonomous limbs develop throughout the entire Artemia trunk.
Keywords: Abd-A, transcription, Hox, evolution, limb development, Distal-less
INTRODUCTION
Hox genes are transcription factors that specify regional identities along the anterior-posterior axis of both invertebrates and vertebrate embryos. In many metazoans, Hox genes are found within one or multiple clusters and the order of Hox genes on the chromosome typically correlates with the order of their anterior expression boundaries on the anterior-posterior axis (Lemons and McGinnis, 2006). In arthropods, evolutionary variations in the expression patterns and functions of Hox genes have been intensively studied (Carroll et al. 2005). This is because Hox genes were initially discovered in an arthropod model organism, Drosophila melanogaster, and because of the enormous morphological diversity in arthropods, particularly in appendage morphology. Among the Pancrustacea, (crustaceans + insects - Regier et al. 2005), the hexapod insects are at an extreme in terms of appendage modification, having lost limbs in the posterior trunk and forming limbs on only three trunk segments. At another extreme are the branchipod crustaceans with homonomous trunk segments, all bearing limbs, a state that is believed to resemble the trunk morphology of the common ancestor of insects and crustaceans (Regier et al. 2005; Vanhook and Patel, 2008; Budd and Telford, 2009). It has been proposed that the extreme modification of limb morphology seen in the insect lineage was accomplished in part by evolutionary changes in the expression patterns and functions of the posterior trunk Hox genes Ultrabithorax (Ubx) and abdominal-A (abd-A) (reviewed in Hughes and Kaufman, 2002b; Carroll et al. 2005).
Most studies on the role Hox genes have played in evolving arthropod appendage morphology have involved analyses of Hox gene expression patterns in different species (Hughes and Kaufman, 2002b, Angelini and Kaufman, 2005). For example, in a few different crustacean species, the anterior expression boundary of the HOX protein UBX correlates with the transition between segments that bear maxillipeds - feeding appendages, and those that bear limbs -locomotory appendages (Averof and Patel, 1997; Shiga et al. 2006). In the crustacean Porcellio scaber, maxilliped development in the first thoracic segment (T1) is associated with the expression of the HOX protein SCR protein in the developing T1 appendage (Abzhanov and Kaufman, 1999). A variety of evidence of this type suggests variations in Hox expression patterns have modified appendage morphology during arthopod evolution, just as variations of Hox expression patterns can modify appendage morphology during Drosophila development (Struhl, 1982; McGinnis and Krumlauf, 1992).
A few studies have found evidence that HOX protein evolution has contributed to limb repression and diversity (Ronshaugen et al. 2002, Galant and Carroll, 2002, Shiga et al., 2002). For example, when tested in Drosophila embryos, the UBX proteins of insects were found to have a stronger repressive function on the appendage-promoting gene Distal-less (Dll) than UBX proteins from a branchiopod crustacean (Artemia franciscana) or an onycophoran (Akanthokara kaputensis sp). This finding correlates with the observation that Artemia and Akanthokara develop limbs in body segments that express UBX protein. For Artemia UBX, it was suggested that a consensus Casein Kinase 2 (CK2) site (Pinna 1990) in the C-terminal region might inhibit UBX limb repressive function (Ronshaugen et al. 2002), and one goal of the current study is to test this hypothesis.
We also wished to gain additional insight into evolutionary diversity in HOX and body plan variation by studying the function of the Artemia franciscana Hox gene abd-A. It has long been proposed that the cluster of Hox genes arose from successive tandem duplications (Lewis, 1978) and it is believed that the duplication which generated Ubx and abd-A occurred prior to the evolution of a common ancestor of crustaceans and insects (Averof and Akam, 1995). In a wide variety of insects, genetic evidence and in situ analysis of expression patterns indicate that abd-A completely represses Dll and limbs (Sánchez-Herrero and Morata, 1985; Tear et al., 1990; Nagy et al., 1991; Vachon et al., 1992; Sánchez-Herrero et al., 1994; Shippy et al., 1998; Lewis et al. 2000; Angelini et al., 2005; Zhang et al., 2005). In the crustaceans Porcellio scaber (woodlouse) and Procambarus clarkii (crayfish) the early phase of abd-A expression is correlated with trunk segments that develop small, highly modified appendages, consistent with the model that ABD-A partially represses limb development in at least some crustaceans (Abzhanov and Kaufman, 2000a; Abzhanov and Kaufman, 2000b; Angelini and Kaufman, 2005). However, the crustacean Artemia develops homonomous limbs on all of its trunk segments, even though previous studies have reported that UBX and ABD-A proteins are expressed throughout the developing trunk region (Averof and Akam, 1995; Shiga et al. 2006). Based on this, it seemed possible that the Artemia ABD-A protein (like Artemia UBX) might have a reduced repressive function on Dll and allow the development of limbs.
To test the function of Artemia abd-A on limb development, we induced the expression of an Artemia abd-A coding sequence in Drosophila embryos. This crustacean version of full-length abd-A had no effect on modifying limb development, but for a completely unexpected reason. Artemia abd-A transcripts were produced in Drosophila embryos, but ABD-A protein was not. This result was paralleled when we analyzed the expression pattern of ABD-A protein in developing Artemia. During the early larval stages when Artemia limb primordia are forming, abd-A transcripts are detected at low levels, but no ABD-A protein is detected in larval trunk segments. This finding in Artemia reveals yet another variation in Hox gene function that is associated with morphological evolution. In this case, a HOX protein expression pattern is completely absent in the epidermis during early development, although the Hox protein is expressed at later stages in the central nervous system in a "homeotic-like" pattern. The combination of an absence of ABD-A protein expression in the Artemia limb primordia and the relatively weak repressive function of Artemia UBX protein on Dll are likely to be two reasons why homonomous limbs develop throughout the entire Artemia trunk.
MATERIALS AND METHODS
in vitro CK2 assays
N-terminal GST-fusion proteins of interest were expressed in E. coli and purified. in vitro CK2 kinase reactions were performed using 65 ng/µl protein, 3 U/µl CK2 (NEB, Cat. #P6010S), 3 nmols ATP and 5 µCi α-32P ATP. All of the reaction was loaded onto a 10% SDS-PAGE gel then exposed to film at −80°C. The acrylamide gel and autoradiograph were scanned and relative amounts of protein and phosphorylation were determined using ImageJ. The level of Artemia UBX phosphorylation was set at 100% and phosphorylation levels for the other proteins was calculated relative to Artemia UBX, after normalizing for the amount of protein. The percentages are an average of two separate experiments.
Simultaneous Protein detection and FISH
armadillo-Gal4 (arm-GAL4) virgin females were crossed to males containing the appropriate UAS-UBX construct. Embryo collection was performed as described in Kosman et al. (2004) with the following modifications. Washes were carried out for 5 minutes in 1 ml volumes, unless otherwise noted. Embryos were rocked in 1:1 xylene:ethanol mixture for 30 minutes. Embryos were washed twice in 100% ethanol, then twice in 100% methanol. Embryos were rehydrated in a graded methanol:H20 series (75%, 50%, 25%), then in 100% H20. Embryos were then permeabilized with 80% acetone in H20 for 10 minutes at −20°C (Nagaso et al. 2001). Permeabilized embryos were washed twice with PBT (Phosphate Buffered Saline + 0.1% Tween) and post-fixed for 25 minutes in 5% formaldehyde in PBT.
Embryos were hybridized with Digoxigenin (DIG)-labeled Dll probe and a biotin-labeled wingless (wg) probe (used for staging and orientation). The Dll probe was transcribed from a ~2 kb fragment from the 3rd Dll intron amplified from genomic DNA (using the primers 5’-GAATCTGGCGGTCAGAGAAC and 5’-ACCGAGAACATTTGGCAGTC) and cloned into the pCR II vector (Invitrogen). The resulting plasmid was cut with HindIII (NEB, Cat. # R0104S) and antisense RNA was transcribed using the T7 promoter. DIG-labeled UTP nucleotides (Roche, Cat #11277073910) were incorporated during transcription about once every 20 nucleotides. DIG haptens were detected with a sheep α-DIG antibody (Roche, Cat. # 11333089001, 1:800 dilution) and a donkey α-sheep antibody (1:400 dilution) conjugated to Alexa647 fluorophores (Invitrogen, Cat. # A-21448). Biotin haptens were detected with a mouse anti-biotin antibody (Roche, Cat. #1297597, 1:800) and a donkey anti-mouse antibody (1:400) conjugated to Alexa488 fluorophores (Invitroten, Cat. #A-21202). UBX-HA proteins were detected with a rabbit α-HA primary antibody (Invitrogen, Cat. #71–5500, 1:100) and a donkey α rabbit antibody (1:400) conjugated to Alexa555 fluorophores (Invitrogen, Cat. # A-31572). Embryos were mounted in Prolong Antifade (Invitrogen, Cat. # P-7481).
Quantification of Dll transcripts and UBX protein concentration
Mid stage-11 embryos were selected and scanned using a Leica TCS SP2 AOBS confocal microscope at non-saturating intensity levels, using identical instrument settings for all embryos. Limb fields were scanned deep enough to capture all Dll mRNA signal (~15 um). Where applicable, UBX-HA antibody signal was also detected. Dll transcription levels were determined as follows: Dll image stacks were collapsed to create single images. The anterior portions of each limb field were cropped and quantified by determining total fluorescent intensity. Eight limb fields were scored to determine the final average value. UBX protein levels were determined as follows: HA stacks were collapsed to obtain merged images and were cropped to the same dimensions as the Dll images. Average fluorescent intensity was determined using Volocity 4.0 (Improvision) by normalizing total fluorescence to stack thickness.
Assaying Keilin’s organ repression
Cloning of abd-A cDNA from Artemia was carried out as described in Ronshaugen et al. 2002. The coding sequence (Genbank accession #GQ141056) was cloned into the pUAST vector with the addition of a Kozak sequence at the 5’ end, encompassing the start codon to ensure translation initiation and sequences encoding a hemagglutinin (HA) tag at the 3’ end to allow for detection of the transgene product. Art UBX(1–7) and Art UBX(7) mutations were introduced using PCR. These constructs were injected into w1118 embryos and stably integrated via P element transformation. Multiple transgenic lines of each construct were obtained and homozygous lines established. Ectopic expression was induced using the GAL4/UAS system (Brand and Perrimon., 1993) by crossing the UAS-lines to flies carrying arm-GAL4. Use of this driver allowed for proper timing of expression starting at stage 9, prior to expression of endogeous Dll, to assay effects on limb formation (Sánchez-Herrero et al., 1994). To determine the levels of protein expression, 4–8 hour embryos collected at 25°C were fixed for 20 min at RT. For Ubx constructs, a standard line ectopically expressing Drosophila UBX at 83%±6% of endogenous levels (Tour et al., 2005) as used.
To establish a standard line for the abd-A transgenes, embryos from lines ectopically expressing Drosophila ABD-A and wild type embryos (progeny of w1118 crossed to arm-GAL4) were stained with an α Drosophila ABD-A mouse monoclonal antibody, mAbDMabd-A subclone 6A8.12 (Kellerman, et al., 1990) at 1:500. Regions corresponding to the ventrolateral position of Dll expression of stage 11 embryos (determined morphologically) were measured for average luminosity in abdominal segments 2 and 3 for wild type and in all three thoracic segments for ectopic abd-A lines. Three Drosophila abd-A lines with an average luminosity near endogenous levels were identified.
For quantification of all other ectopically expressed abd-A lines, a Drosophila abd-A line was used as the standard (98%±11%). Ectopic proteins were subsequently detected using a rat α HA antibody (clone 3F10, Roche, Cat. # 11867423001). The average luminosity in regions of the thoracic segments corresponding to the ventrolateral position of Dll expression in stage 11 embryos was calculated and reported as % endogenous protein expression.
To determine Keilin’s organ repressive ability, cuticles were collected and cleared for phenotypic analysis (Wieschaus and Nüsslein-Volhard, 1986). For each line, all three thoracic segments of 35 cuticles (210 possible Keilin’s organs) were scored for presence or absence of Keilin’s organs.
Hatch assays
Ten to 20 males of the UAS-transgenic line were crossed to 20–25 virgin females carrying the arm-GAL4 driver. Progeny from the crosses were collected for 24 hours and counted. After aging for 24 hours, unhatched embryos were counted to determine the number of embryos hatched. This number was normalized relative to a cross of w1118 to arm-GAL4 carried out in parallel. All available lines of each truncation were tested for viability, along with all available lines carrying the full-length Artemia abd-A and w1118 as a control.
Artemia husbandry
Artemia franciscana cysts (San Francisco Bay Brand) were hatched (day 1) in 1 L 15% Instant Ocean (Aquarium Systems, Cat. # SS3-50) with continuous light and aeration overnight. Temperature of the culture was maintained between 28°C to 32°C. Early development progressed at about one molt per day under these conditions until stage L4. For L1 to L4 mixed stage collections, 0.3 g dehydrated cysts were hatched on day 1, an additional 0.2 g cysts added on days 2 and 3, 0.1 g cysts added on day 4. Live animals were collected on day 5. To grow Artemia to late larval stages, 250 µl of diluted Tahitian Blend algal paste (Brine Shrimp Direct) were added every other day starting on day 3. Animals were collected and fixed once they developed to the desired stage.
Antibodies
An α-Artemia ABD-A antibody was made against a GST-fusion of the N-terminal domain, up to and including the YPWM motif of Artemia ABD-A (Pocono Rabbit Farm and Laboratory Inc.). The rabbit polyclonal sera were affinity purified through a Quickpure column (Sterogene, Cat. # QP01-01) and tested for specificity on Drosophila embryos at 1:200 dilution. The 4F11 α-EN antibody (Patel et al., 1989) was used at 1:30 dilution. The FP6.87 antibody (Kelsh et al, 1994) was concentrated 4–5 fold using Centricon filter units (Millipore), then used at 1:2 dilution. α-Drosophila ABD-A (Kellerman et al., 1990) was used at 1:400. Rat α-HA was used at 1:500 dilution (Roche, Cat. # 11867423001).
Artemia immunohistochemistry
Artemia were fixed based on protocols supplied by Nipam Patel (personal communications) with modifications. 0.2 g of live L1–L4 Artemia or 400–500 µl late-stage animals were fixed in 33 ml 0.1 M PIPES, 2 mM EGTA, 1 mM MgSO4, 3.4% Formaldehyde (ULTRAPURE ampules, Polysciences, Cat. #18814) for 5 minutes. Tween-20 was added to 0.02% and fixation continued for another 3 minutes. The Artemia were subsequently dehydrated using a stepwise transfer into methanol and stored at −20°C until ready to proceed with the immunodetection. Antibody staining was carried out as described for Drosophila embryos in Kosman, et al. (2004) with the addition of a sonication step prior to blocking. Sonications were carried out using a Branson Sonifier 150 at a maximum output of 5W in 40 ml PBT. Total sonication time was stage dependent. 100 µl of L1 to L4 stage Artemia were sonicated for a total of 16 seconds, seven to eight L9–L10 stage animals for 50 seconds and seven to eight L11 to L12 stage animals for 62 seconds. Two second bursts of sonication were followed by inversion of the sample several times to mix the animals thoroughly between bursts until the total sonication time was achieved. For detection of nuclei to aid in staging, DAPI was added to the mounting media. Animals were mounted ventral side up in 2.5% w/v DABCO (Sigma #D-2522), 50 mM Tris pH 8.0, 90% glycerol. Images were obtained using a Leica TCS SP2 AOBS confocal microscope. Though dilutions worked out for staining Drosophila embryos using the 4F11 antibodies and FP6.87 antibodies also worked well in Artemia, for Artemia stains, the rabbit α-Artemia ABD-A was used at the higher concentration of 1:100 to ensure that the inability to detect protein during early stages was not due to insufficient levels of antibodies.
In situ hybridization
For Artemia in situ hybridizations, probes to the first 731 bp of coding sequence for Artemia EN and to the complete coding sequence of Artemia Ubx and abd-A were prepared as described in Kosman et al. (2004). Artemia were fixed in the same manner as for immunohistochemistry, then hydrated using a MeOH:PBT series and sonicated as previously described. in situ hybridizations were carried out based on Drosophila protocols above with the following modifications: Artemia were not treated with xylenes, Protease K treatment was carried out for 2 minutes at a final concentration of 5 µg/ml (Grace Boekhoff-Falk, personal communications), the transfer to hybridization solution prior to the prehybridization step was carried out with reagents pre-heated to 55°C and included an extra five minute wash with hybridization solution at 55°C. The hybridization solution was modified with an addition of SDS to a final concentration of 1% as suggested by N. Patel (personal communications). Probes were hybridized for 19–19.5 hours at 55°C. After hybridization, animals were transferred to PBT using a graded hybridization solution/PBT series pre-heated to 55°C. Subsequent washes and antibody incubations were carried out at room temperature or 4°C if carried out overnight. Detection of probes required tyramide amplification (TSA Plus, Perkin Elmer, Cat. # NEL744001KT, NEL741001KT) at 1:75 for 15 minutes at RT with occasional mixing. Tyramides were resusupended in water instead of DMSO. DIG probes were detected with Cy3 tyramide and FITC probes with FITC tyramide using sequential tyramide reactions (Kosman et al. 2004) and a post-hybridization fixation in 1% formaldehyde for 5 minutes followed by two rinses and three 5 minute washes with PBT was added prior to antibody detection. Mounting was carried out as described above for immunohistochemistry. Images were obtained using a Leica TCS SP2 AOBS confocal microscope and deconvolved using the AutoDeblur software (MediaCybernetics).
Drosophila embryo in situ hybridizations and immunohistochemistry were carried out as described in Kosman et al. (2004).
RT-PCR and Quantitative PCR
L4 stage Artemia were collected by hatching 0.5 g cysts and on day 2 collecting L1 nauplii as previously described. The L1 nauplii were transferred to fresh 15% Instant Ocean then light and aeration were continued until day 5. On day 5, animals were collected by taking advantage of their positive photo-taxic behavior. In this manner, ~98% of the animals collected were stage L4 larvae.
A sample of each set of collected animals was taken and the developmental stage confirmed under a dissecting scope. The collected animals were flash frozen in liquid Nitrogen and stored in 50 mg aliquots at −80°C. The following were all carried out per the manufacturer’s instructions: 1++) RNA was extracted using 1ml Trizol reagent (Invitrogen, Cat. #15596-018) per 50 mg L4 Artemia. 2++) PolyA RNA was purified using the Oligotex mRNA kit (Qiagen, Cat. #70042) then treated with DNase (Ambion, Catalog # AM2222). 3++) cDNA were generated using the Ambion RETROscript kit (Catalog #, AM1710) and 4++) treated with RNase A (Ambion, Catalog # AM2270).
RT-PCR amplification of the resulting cDNA was carried out using the following primers for amplification of Artemia abd-A: 5’-CCCAAATGGTTGTCCTCG-3’ and 5’-GTCCATCATTCCATCAGGTG-3’ and for amplification of Artemia Ubx: 5’-ATGAATTCGTATTTTGAACAGAATGG-3’ and 5’-AAGCTTTTCATCTTTTTCATCGTCACT-3’. Cycling conditions used were 95°C for 5 min followed by 35 cycles at 94°C for 30s, 50°C (abd-A) or 55°C (Ubx) for 30s, 72°C for 1 min then 72°C for 5 min.
Quantitative PCR was carried out using the ABI Prism 7000 System using 5 ul cDNA, 2.5 ul each of 600 nM primers and 10 ul Power Sybr Green PCR Master Mix (ABI, Catalog #4367659) on six 10−1 serial dilutions of cDNA template using the default cycling conditions to generate a standard curve. The dissociation protocol was run to determine if more than one product was generated. Primers used for amplification of Artemia abd-A were 5’-CGTCTATGGCTACAGCAGCA-3’ and 5’-TTCGAAGGGTCATTTGAAGC-3’ and for Artemia α-tubulin, the primers used were 5’-GAAAGTACGTGGCCTGCTG-3’ and 5’-GCATTGACGTCTTTTGGTACG-3’-3’. Reactions were carried out in duplicate.
RESULTS
The Serine within the Artemia UBX C-terminal CK2 consensus sequence is a major in vitro CK2 phosphorylation site
In insects, the Hox gene Ubx is expressed in the anterior abdominal primordia of embryos, where it partially or completely represses limb development. In developing Artemia, Ubx transcripts and protein are expressed throughout the trunk, but Dll is still expressed in limb buds and large limbs develop. Ronshaugen et al. (2002) found that Serine (Ser) and Threonine (Thr) residues in the C-terminus of Artemia Ubx partially inhibited its limb repressive function. One of these Ser residues is in a consensus phosphorylation site for Casein Kinase 2 (CK2). It seemed plausible that the consensus CK2 site in Artemia UBX might inhibit its limb repressive function, as mutation of four CK2 sites in the Drosophila Hox protein ANTP changed it into a limb repressor in embryos (Jaffe et al., 1997). The Artemia UBX protein has a single consensus CK2 site in C-terminal sequences, but whether this site is an actual substrate for CK2, and whether it is necessary or sufficient to inhibit Artemia UBX limb repressive function, has not been tested.
CK2 kinase assays were performed to determine if Artemia UBX is actually phosphorylated within the C-terminus consensus CK2 site. The substrates and controls in the kinase assays were GST-UBX fusion protein variants that were produced in E. coli. These included fusion proteins with wild type Drosophila UBX-1a, wild type Artemia UBX, and two mutant versions of Artemia UBX with Ala substitutions in C-terminal Ser/Thr residues (Figure 1).
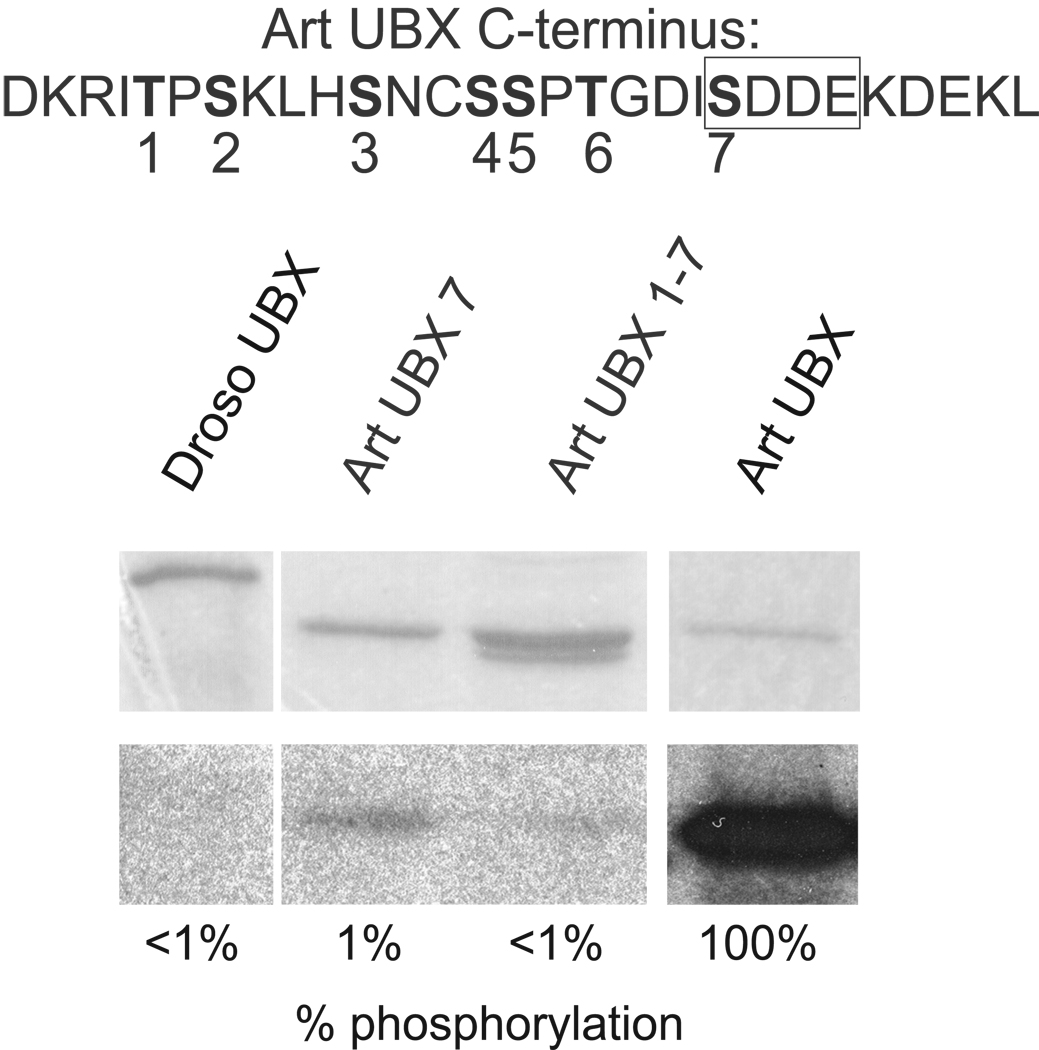
Figure 1. in vitro CK2 phosphorylation occurs mainly at the consensus CK2 site within the C-terminus of Artemia UBX.
Sequence of the C-terminus of Artemia UBX is shown at the top with the Serines/Threonines in bold type and numbered. The CK2 consensus site is boxed. GST-fusion protein constructs were purified and in vitro phosphorylation reactions carried out. GST-Art UBX was strongly phosphorylated by CK2 and the amount of phosphorylation set as the standard at 100%. CK2 phosphorylation for all other proteins was calculated relative to GST-Art UBX. There was no detectable phosphorylation of GST-Droso UBX by CK2. GST-Art UBX with all Serines and Threonines mutated to Alanine (Art UBX 1–7) showed almost no phosphorylation by CK2 in vitro and GST-Art UBX with mutation of the Serine within the CK2 consensus site to Alanine (Art UBX 7) showed a similar level of reduced phosphorylation.
The levels of CK2 phosphorylation for the UBX proteins was determined by scanning autoradiographed gels that were loaded with kinase reactions (Materials and Methods), then compared to Artemia UBX (set at 100%), after normalizing by the amount of protein per reaction. The percentage of phosphorylation in Figure 1 is the average from two separate experiments. GST alone was not phosphorylated by CK2 (data not shown). Artemia UBX but not Drosophila UBX was strongly phosphorylated by CK2 (Figure 1). Mutation of only the Ser within the CK2 consensus site (Art UBX 7, Figure 1) abolished almost all phosphorylation of Artemia UBX. Mutation of additional Ser/Thr residues in the C-terminus showed only an additional modest reduction of phosphorylation levels (Art UBX 1–7, Figure 1).
To test whether the Ser in the CK2 site of Artemia UBX inhibited limb repressive function, we compared the effects of ectopic expression in Drosophila embryos of the proteins we tested in the in vitro CK2 assays. The UAS-GAL4 system (Brand and Perrimon, 1993) was used to express the UBX proteins at or near endogenous levels, and the effects of the proteins on limb development were assayed by quantifying the levels of Dll transcripts in the first thoracic segment (T1) of stage 11 Drosophila embryos (Figure 2). The average level of Dll transcript signal in wild type embryos was set to 100%.
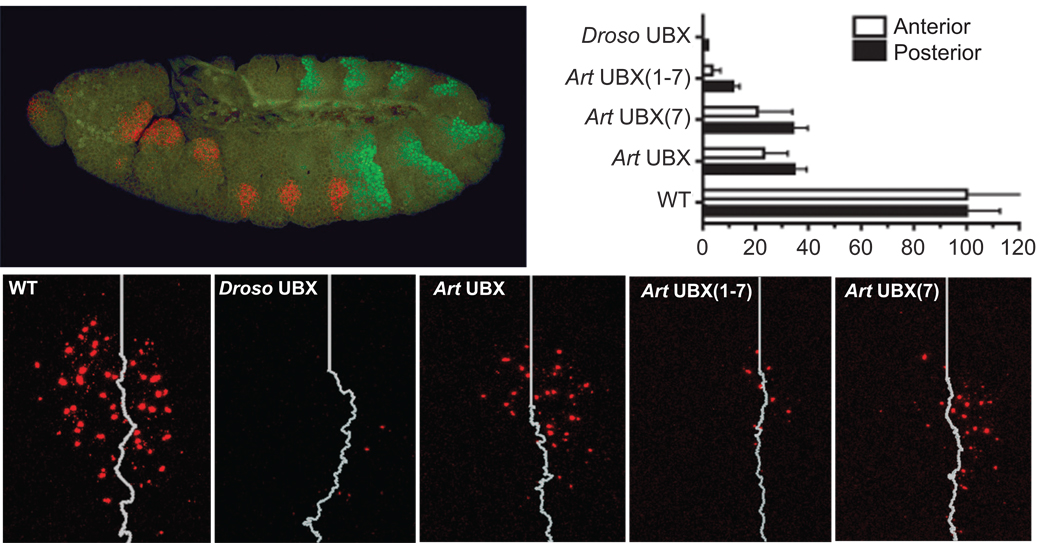
Figure 2. Artemia UBX with all Serine/Threonine sites within the C-terminus mutated to Alanine represses Dll expression in Drosophila embryos.
Wild type (WT) stage 11 embryos express Dll transcript (red) encompassing encompassing a stripe of wg expression that demarcates the anterior field from the posterior field. Abd-A protein staining is shown in green. The white line marks the separation of the anterior and posterior fields of Dll expression (as determined by wg expression, data not shown). Stage 11 embryos ubiquitously expressing Drosophila UBX, Artemia UBX or Artemia UBX with Serine/Threonine to Alanine mutations in the C-terminus show varied abilities to repress Dll expression. Mutation of all Serines/Threonines within the C-terminus of Artemia UBX, Art UBX(1–7), results in strong repression of Dll. While mutation of the serine within the CK2 consensus site only, Art UBX(7), results in a level of Dll expression comparable to wild type Artemia UBX.
Drosophila UBX completely repressed Dll in the anterior compartment of T1, with only occasional transcript signals detected in the posterior compartment. Wild type Artemia UBX repressed Dll to 20%–40% of wild type levels, with a stronger repressive effect in the anterior compartment of T1 as compared to the posterior compartment (Figure 2). When all C-terminal Ser/Thr residues were mutated to Ala, the repressive effect of Artemia UBX on Dll was increased significantly, close to the levels observed for Drosophila UBX. However, mutation of only the Ser within the CK2 consensus site (Art UBX 7) did not increase Artemia UBX repressive function. We conclude that although Ser and Thr residues in the C-terminus of Artemia UBX reduce its ability to repress Dll transcription, the Ser residue in the CK2 site is not crucial for this reduction of repressive ability in embryos.
Artemia ABD-A protein expression is not produced in Drosophila embryos unless the C-terminus is truncated
While the reduced repressive function of Artemia UBX on Dll expression can help to explain how limbs develop in the Artemia trunk, whether Artemia ABD-A also has a similar reduced repressive function was unknown. Averof and Akam, (1995) suggested that Artemia ABD-A protein was present in limb-bearing trunk segments, but this was largely based on the use of a monoclonal antibody, FP6.87, which detects a conserved epitope shared by both UBX and ABD-A (Kelsh et al., 1994; Averof and Akam 1995). The previous study left open many possibilities, including that Artemia ABD-A protein might be produced at levels so low that limb development was not repressed, or that Artemia ABD-A protein function had evolved to have reduced limb repressive ability.
To test the embryonic/larval limb repressive ability of Artemia ABD-A, we constructed transgenic Drosophila lines carrying an Artemia abd-A full-length protein coding sequence fused to a hemagglutinin tag (HA) (Figure 3A), under the control of the GAL4-UAS system. To our surprise, after inducing expression of an Artemia ABD-A-HA cDNA in early embryos, the larvae develop into viable and fertile adults with normal limbs. To test whether the transgenic strains with the inducible Artemia cDNAs produced abd-A messenger RNA, we carried out in situ hybridizations using an antisense probe to the entire ABD-A coding sequence. Ubiquitous high levels of cytoplasmic transcripts were detected in lines containing the full-length Artemia ABD-A-HA fusion (Figure 3B). We next tested whether Artemia ABD-A protein was being produced in transgenic embryos using an α-HA antibody and immunofluorescence assays. No Artemia ABD-A-HA protein was detected during embryogenesis, in any tissues, including the central nervous system (Figure 3B). We concluded that either the ABD-A mRNAs were not being translated into protein, or that the resulting proteins were highly unstable.
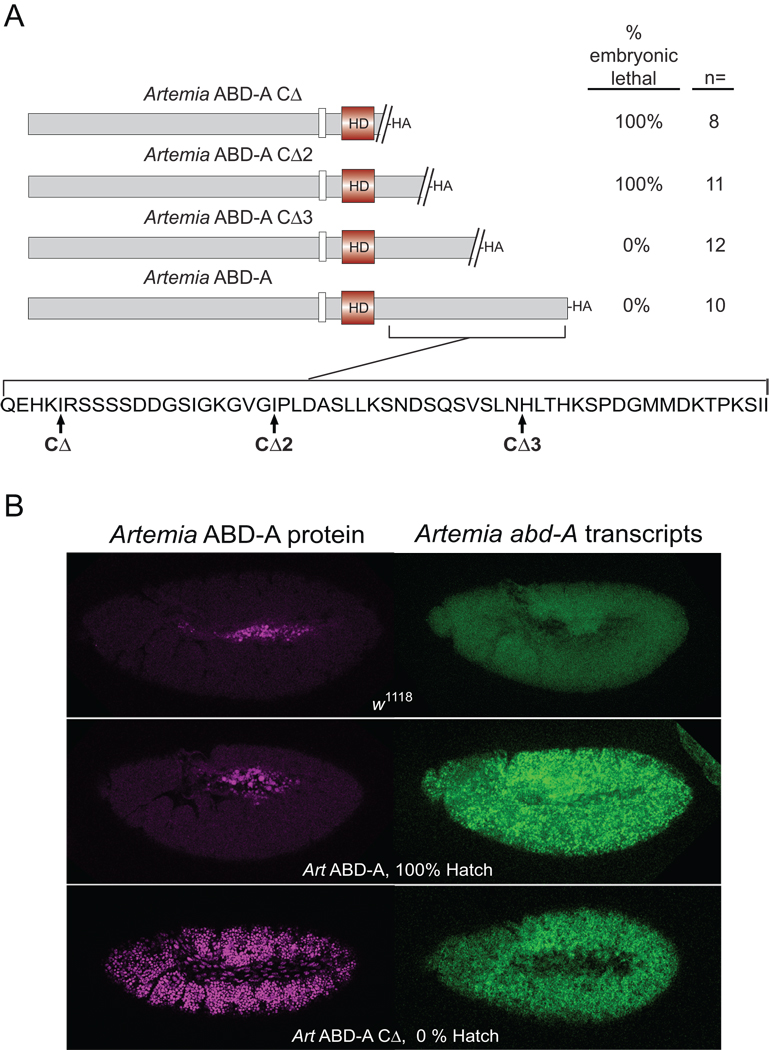
Figure 3. Protein expression of Artemia ABD-A cannot be induced in Drosophila embryos unless C-terminal sequences are deleted.
(A) Hatch assays of various Artemia ABD-A truncations. The truncation point for each transgene is shown at the bottom of the panel. Hatch percentages were calculated relative to w1118. Ubiquitous expression of Artemia ABD-A protein showed the expected embryonic lethal phenotype only for truncated proteins Artemia ABD-A CΔ and Artemia ABD-A CΔ2. (B) Drosophila embryos induced to ubiquitously express Artemia abd-A transgenes were tested for the presence of transcript and protein. The top panel shows the negative control of embryos from arm-GAL4 crossed to w1118. Transcripts were detected in embryos expressing both full-length and truncated ABD-A, but protein was only detected for embryos expressing the truncated abd-A transgene.
The Artemia ABD-A-HA coding region was cloned into an expression vector with the open reading frame flanked with a 5' UTR from Drosophila hsp-70 and a 3' UTR from SV40. Protein expression from similar expression constructs containing numerous coding regions have been induced in Drosophila embryos, both in our lab and others. We decided to test whether the Artemia ABD-A coding sequences contained regions that prevented protein accumulation in Drosophila embryos.
A deletion of Artemia ABD-A C-terminal protein coding sequences identified a region that prevents translation of the abd-A transcripts or accumulation of ABD-A proteins. This deletion mutant, ABD-ACΔ produced abundant amounts of both Artemia ABD-A transcripts and proteins in Drosophila embryos (Figure 3B), and resulted in embryonic lethality (Figure 3A). A test of smaller deletion mutants suggested that the proposed translational inhibition or protein instability signal resides in the 18 amino acid coding sequence between the CΔ2 and CΔ3 endpoints (Figure 3A).
Truncated Artemia ABD-A represses Keilin’s organs in Drosophila embryos and is capable of homeotic function
The ability of ABD-ACΔ the stable version of Artemia ABD-A, to specify abdominal segment morphology and repress larval limb development was assayed in Drosophila embryos. The vestigial Drosophila larval limbs are called Keilin’s organs, and they develop from clusters of embryonic neuroectodermal cells that express Dll (Cohen and Jürgens, 1989). Artemia ABD-ACΔ was capable of completely repressing Keilin’s organs when expressed at levels near endogenous Drosophila ABD-A levels (Figure 4A). However, when Artemia ABD-ACΔ protein expression levels fell even 20% below endogenous Drosophila ABD-A levels, its limb repressive ability was reduced. When Artemia ABD-ACΔ protein was expressed at ~35% of endogenous Drosophila ABD-A levels, limb repressive function was nearly absent. However, Drosophila ABD-A did not display a concentration sensitivity with regard to limb repression. In ectopic expression assays, Keilin’s organs are fully repressed even when Drosophila ABD-A protein is expressed at ~40% of endogenous levels (Figure 4A).
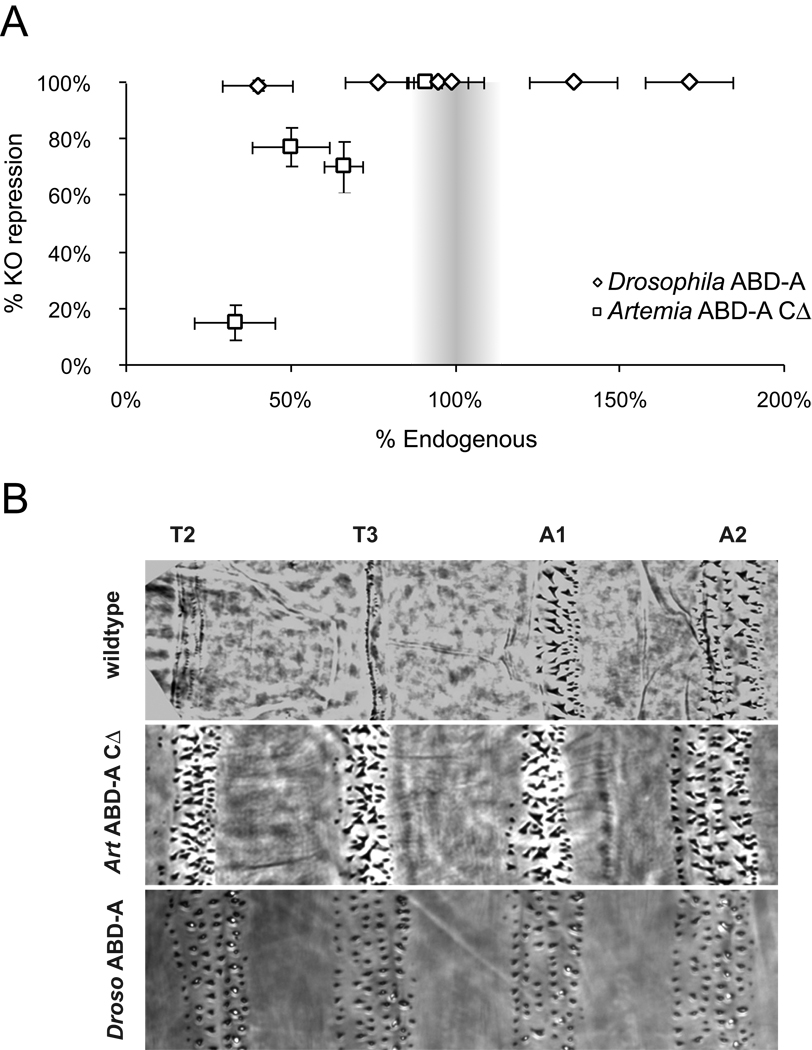
Figure 4. Truncated Artemia ABD-A represses Keilin’s organs and transforms segments toward an abdominal identity in Drosophila embryos.
(A) Drosophila ABD-A can completely repress Keilin’s organs even when ectopically expressed at ~40% of endogenous levels. Artemia ABD-ACΔ can repress 100% of Keilin’s organs when ectopic expression is near endogenous levels, but shows reduced ability to repress Keilin’s organs at lower protein concentrations. (B) When ubiquitously expressed in Drosophila embryos, Artemia ABD-ACΔ transforms thoracic denticle belts towards an abdominal A1-like identity while Drosophila ABD-A transforms denticle belts towards abdominal A2-4 identities.
Cuticles of Drosophila embryos ectopically expressing Artemia ABD-ACΔ and ranging in protein expression from 30% to 90% of endogenous levels were analyzed in parallel with the standard line ectopically expressing Drosophila ABD-A at 100% of endogenous levels. In cuticles from all Artemia ABD-ACΔ lines analyzed, we observed the same phenotypes described for ectopic expression of Drosophila ABD-A, such as head involution defects, formation of denticle belts in the head and repression of the denticle "beard" characteristic of segment T1 (data not shown). However, in constrast to ectopic Drosophila ABD-A, which transforms thoracic and abdominal segments towards A2-4 segment identities (Sánchez-Herrero et al., 1994), Artemia ABD-ACΔ transformed thoracic denticle belts toward abdominal segment 1 (A1-like) identities (Figure 4B).
Trunk development and gene expression in Artemia franciscana
Finding no accumulation of full length Artemia ABD-A protein in Drosophila embryos led us to wonder if a similar phenomenon occurred in developing Artemia. Artemia embryogenesis begins within the reproductive tract of fertilized females. Under permissive conditions, embryogenesis progresses and results in the live birth of first instar larvae, also known as L1 nauplii, into the surrounding sea water. In the L1 stage, the anterior head structures have already begun differentiation, but the gnathal and trunk region grow and differentiate in an anterior-posterior gradient of development during the 14 larval stages (Schredhardt, 1987). New segments develop from a growth zone that resides at the posterior end of the trunk. Artemia develops 11 trunk segments each bearing a pair of limbs. Trunk segmentation begins with the formation of segmentation furrows followed by limb bud formation (Figure 5A). The developing limbs undergo articulation, at which point the limbs are known as thoracopods. After articulation, the thoracopods differentiate so that within each limb several podites develop and setae form and elongate. Artemia are staged by the number of developing thoracopods and by the degree of limb differentiation (Schredhardt, 1987).
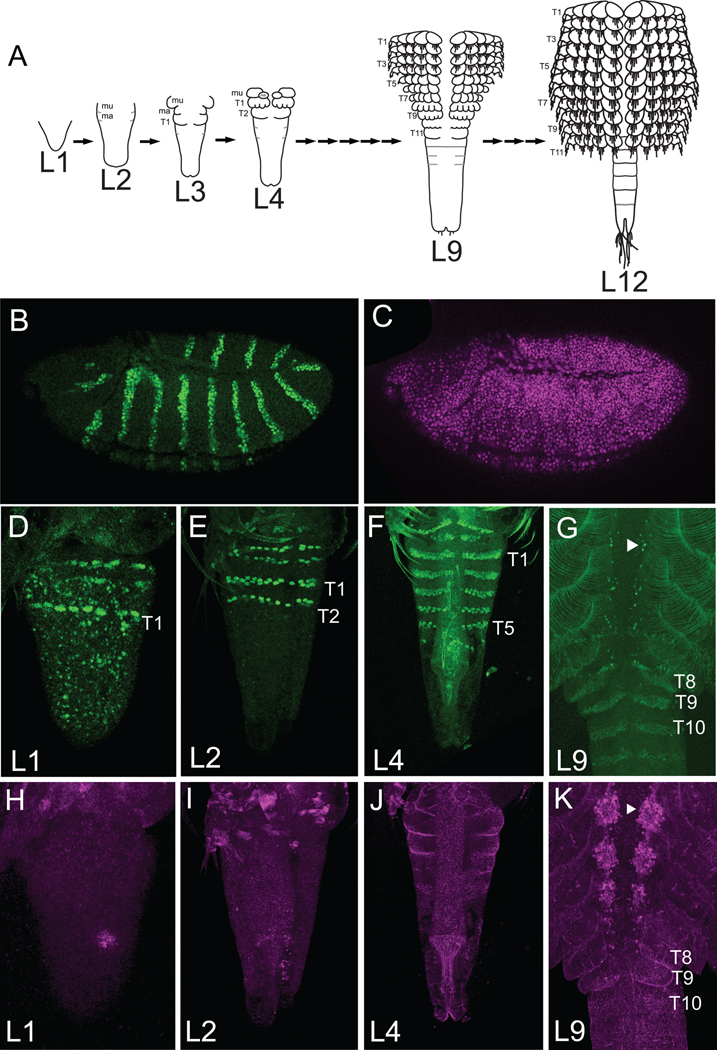
Figure 5. EN and ABD-A protein expression in Artemia.
EN is in green and ABD-A is in magenta. Anterior is left for Drosophila, up for Artemia this and subsequent figures. (A) Trunk development in selected Artemia larval stages. The gnathal segments are shown in the L2 and L3 stages, otherwise head structures are not represented in these schematics. After the first molt, segmentation begins in the two gnathal segments (mu and ma). Additional thoracic segments emanate from the posterior growth zone, producing an anterior-posterior gradient of development with anterior segments at a more advanced stage of differentiation than the posterior segments. Thoracomers, trunk segments with limb buds, mark the start of limb formation (e.g. T1 of L3 stage Artemia or T11 of L9 stage Artemia). As limb development proceeds, articulation begins and the trunk segments are now known as thoracopods (e.g. T1 of L4 stage or T10 of L9 stage). The thoracopods undergo elongation and further differentiation so that several podites form for each limb and setae develop and elongate. (B,C) L1 to L9 mixed stages of Artemia were stained with Drosophila embryos ectopically expressing Artemia ABD-ACΔ. Detection of EN protein (B) and Artemia ABD-ACΔ protein (C) in Drosophila embryos at stage 11 are shown. In Artemia, EN stripes (which mark the posterior of each segment) are detected in early stages (D,E,F). Panels G and K shows the midline of a stage L9 Artemia. By the L9 stage (G), EN protein is also detected in a few cells in the neuromeres of each segment (arrowhead). ABD-A protein is not expressed to detectable levels in early stages (H,I,J) but is clearly expressed in neuromeres at stage L9 (K, arrowhead). In the L9 stage (G,K) limbs of trunk segments anterior to T6 are fully differentiated. T6 thoracopods have almost finished differentiation. T8 thoracopods have started articulation (vertical furrows visible in panel K) and T9 thoracomers are just about to initiate articulation. ABD-A protein expression in neuromeres have initiated in a few cells in the T8 trunk segment and within all the neuromere cells in the fully differentiated trunk segments from T6 to T1. mu: maxillulary segment, ma: maxillary segment
ABD-A protein is detected in the trunk CNS but not limb primoridia of developing Artemia
To test if ABD-A protein accumulates in the trunk epidermis of early stage Artemia prior to and during limb differentiation, we developed and affinity purified polyclonal antibodies to the N-terminal region of Artemia ABD-A. The specificity of these antibodies was tested on Drosophila embryos ectopically expressing various HOX proteins. The antibodies detected Artemia ABD-A specifically and did not cross-react with Artemia UBX, Drosophila ABD-A or Drosophila UBX proteins (data not shown).
Double stains using α ENGRAILED antibody (α-EN; Patel et al., 1989) plus α-Artemia ABD-A (Figure 5) and double stains with α-UBX/ABD-A (FP6.87) plus α-Artemia ABD-A were carried out on various stages of Artemia larvae (See Figure S1 in supplementaty material). Because of the difficulty in permeabilizing Artemia larvae, which have a cuticular exoskeleton, a variable fraction in each experiment exhibited antibody staining. The segmental staining pattern of EN was used as a larval stage marker and a positive control for antibody permeability of the Artemia larvae. Early stage Artemia nauplii (L1 to L4), and stage 11 Drosophila positive control embryos ubiquitously expressing truncated Artemia ABD-A (Artemia ABD-ACΔ, were processed together after separate fixation methods (Methods). Since ABD-A protein expression accumulates in the neuromeres of late stage Artemia (Averof and Akam, 1995), L9 to L12 stage Artemia were also stained as a positive control with the α Artemia ABD-A antibodies (Figure 5K). As a negative control, Artemia larvae were stained with an α Drosophila ABD-A antibodies (data not shown).
EN and Artemia ABD-ACΔ proteins were both detected in Drosophila control embryos induced to ubiquitously express Artemia ABDA-ACΔ (Figure 5B,C). In Artemia larvae, the EN protein expression pattern could be detected in ~ 70% of the L1 to L4 stage animals (Figure 5D,E,F). But, ABD-A protein could not be detected in any L1–L4 stage larvae in which the EN control staining was detected (Figure 5H,I,J). In late stage Artemia larvae, ABD-A expression could be detected within midline neuromeres (Averof and Akam, 1995; Figure 5K). In L9 stage animals, ABD-A neuromere expression is seen in trunk segments that have already developed thoracopods (e.g. T8 and more anterior trunk segments), but not in segments that are less differentiated (e.g. T9 and T10, Figure 5K). At this stage, EN protein expression in midline neuromeres can also be detected at roughly the same anterior-posterior position (T9, Figure 5G). Similar double stain experiments were also carried out with α Artemia ABD-A antibodies plus FP6.87 control antibody controls. In L1–L4 stage larvae where FP6.87 staining was detected throughout the trunk, a specific ABD-A signal was never detected (Figure S1).
abd-A transcripts are expressed at low levels in early Artemia epidermis
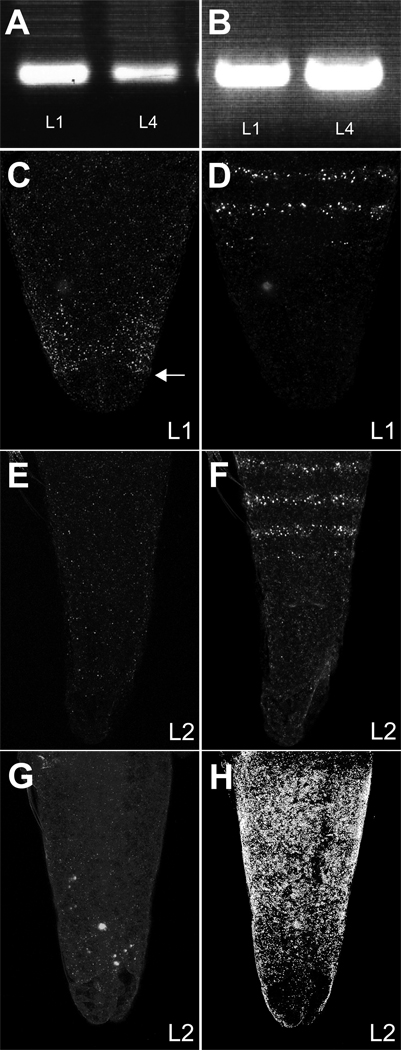
As a first test of whether abd-A transcripts were expressed in early larval stages, we carried out RT-PCR. Poly-A RNA from L1 and L4 stage Artemia larvae was purified and reverse transcribed, and PCR was carried out using primers specific to abd-A and Ubx. Single primer controls did not amplify any bands (not shown). PCR products of the expected sizes for both Ubx and abd-A were detected at both L1 and L4 stages, and DNA sequencing confirmed that the RT-PCR fragments corresponded to Artemia abd-A transcript sequence (Figure 6A,B).
Figure 6. abd-A transcript is transcribed at low levels in the Artemia trunk of early stage larvae.
RT-PCR amplified both Artemia Ubx (A) and Artemia abd-A (B) in stage L1 and L4 larvae. Double in situ hybridizations with abd-A-DIG (C,E) plus en-FITC (D,F) probes or abd-A-FITC (G) probes plus Ubx-DIG (H). The posterior boundary of the surmised growth zone is indicated by an arrow in panel C. abd-A transcript was detected in L1 larvae within the growth zone.
To determine the expression pattern of abd-A transcripts in early stage Artemia, we carried out pairwise in situ hybridizations with en, Ubx, and abd-A probes on L1 to L4 stage Artemia. Sense probe controls were also performed in parallel. As with protein detection, the efficiency of RNA detection is influenced by variable permeabilization of Artemia larval cuticle. Control RNA signals were detected in ~60% of fixed and hybridized Artemia larvae. At the L1 stage, an abd-A transcript signal could be detected within the growth zone in ~ 25% of larvae staining for en (Figure 6C,D). In stage L2 to L4 stage animals, a localized abd-A signal was not consistently detected by in situ hybridization in larvae that stained with the en probe, suggesting that abd-A transcripts were at very low levels (Figure 6E,F). Similar results were obtained with later stage larvae doubly stained for Ubx and abd-A transcripts. Ubx signal was detected in a region matching the staining pattern obtained with FP6.87 antibodies and antiserum specific for Artemia UBX (Averof and Akam, 1995; Averof and Patel, 1997; Shiga et al., 2006), but after the L1 stage a localized abd-A transcript signal was too weak to be consistently detected (Figure 6G, H).
As a further test of the presence of abd-A transcripts at L4 state, since in situ signals were not consistently detected, we carried out quantitative PCR (qPCR). Primers were made to both Artemia abd-A and a-tubulin. To ensure that the products generated were due only to amplification of transcripts, samples were treated with DNase after isolation of polyA-RNA and treated with RNase after reverse transcription. Control reactions with Water only and without reverse transcription were negative. Dissociation curves generated after the reactions were complete showed only one product for each of the experimental samples. qPCR results indicate that, considered over the entire animal, abd-A transcript is between 1000-fold and 2400-fold less abundant than a-tubulin transcript. Since the abd-A transcript is likely to be produced in only small fraction of cells that produce a-tubulin transcripts (Figure 6C), this indicates that abd-A transcripts are present at significant levels at both L1 and L4 states of development.
DISCUSSION
All segments of the Artemia trunk develop limbs despite Artemia having the trunk Hox genes Ubx and abd-A. Our results suggest two reasons why these Artemia genes fail to repress limbs during development. First, although Ubx transcripts and proteins are expressed in limb buds of the Artemia trunk (Averof and Akam, 1995, Shiga et al. 2006), Ser and Thr residues in the C-terminal region of Artemia UBX partially inhibit its repressive function on Dll (Figure 1, and Ronshaugen et al. 2002). Second, Artemia ABD-A protein is not produced at detectable levels in developing early larval limb buds or other epidermal cells, even though abd-A transcripts accumulate at low levels. We conclude that ABD-A protein does not play a role in conferring epidermal morphological identity in developing Artemia.
This study reveals a novel variation in HOX function that is associated with evolution of body patterning. In Artemia, the loss of ABD-A HOX protein function during early epidermal development is correlated with the development of a homonomous trunk, each of the segments bearing limbs. ABD-A protein does accumulate at later stages in cells that appear to be part of the central nervous system (Figure 5K, and Averof and Akam, 1995). Although there are examples of Hox genes whose expression patterns have been dramatically altered during evolution (Lohr and Pick, 2005; Hughes and Kaufman, 2002a; Hsia and McGinnis, 2003), the known examples have involved genes such as Drosophila bicoid or fushi-tarazu, which have also undergone dramatic changes in protein sequence and developmental function.
The finding that Artemia ABD-A protein is not expressed in the trunk epidermis is unexpected. In many arthropods, the expression pattern of ABD-A protein during development has been inferred by staining with an antibody that recognizes a conserved epitope in both UBX and ABD-A, and correlating this pattern with the specific expression patterns of Ubx and abd-A transcripts (Averof and Patel; 1997; Zheng et al., 1999; Abzhanov and Kaufman, 2000a; Abzhanov and Kaufman, 2000b; Blin et al., 2003). Such studies have indicated that abd-A transcripts and protein are produced in a HOX-like pattern in the posterior trunk epidermis of many insects and two crustaceans, and the abd-A expression domain is correlated with partial or complete repression of limbs (Tear et al., 1990; Nagy et al., 1991; Shippy et al, 1998; Peterson et al., 1999; Abzhanov and Kaufman, 2000a; Abzhanov and Kaufman 2000b; Hughes and Kaufman, 2002a; Zhang et al., 2005). Thus it seems likely that the absence of ABD-A protein expression in the epidermis of the developing trunk of Artemia is a derived condition in crustaceans. Whether other branchiopod crustaceans also lack ABD-A protein during epidermal development is as yet unknown.
Low levels of abd-A transcripts are detected in the epidemis in early Artemia larval stages of developing Artemia larvae. Thus it appears that the failure to accumulate abundant ABD-A protein in the developing Artemia trunk epidermis is partly mediated at the level of transcript production and/or stability. However, some regulation of abd-A at the level of translation of protein stability is apparently also occurring in early Artemia larvae since no protein is detected at any early larval stage. This is consistent with the behavior of the Artemia abd-a cDNA after its activation in Drosophila embryos. Artemia abd-A transcripts can be induced to accumulate to high levels in Drosophila embryos, but full length protein is not produced, even in the central nervous system. Our evidence indicates this is due to C-terminal sequences in the Artemia ABD-A protein coding region that either inhibit translation or result in extreme protein instability. A search for common protein motifs that promote protein instability (e.g. PEST sequences) in Artemia ABD-A C-terminal sequences did not result in any obvious matches. The lack of ABD-A protein expression in Artemia, then, appears to be the result of mechanisms occurring at both the transcriptional and translational levels.
Evolution of abd-A Hox gene expression and effects on arthropod morphology
Two previous studies have suggested that molecular variation of abd-A expression has played a role in arthropod morphological evolution. One interesting example is in cirripedes (barnacles), where at least three species appear to have lost the abd-A gene despite retaining Ubx and Abd-B (Mouchel-Vielh et al., 1998; Blin et al., 2003, Deutsch and Mouchel-Vielh, 2003). This missing abdominal Hox gene is correlated with the severe reduction or absence of abdominal segments in cirripedes. Another study involved the analysis of abd-A transcript expression patterns from two ant species with different abdominal morphologies (Niculita, 2006), finding a correlation between variations in abd-A transcript expression pattern during early development between two different ant species and variations in their abdominal segment morphologies.
Artemia UBX limb repressive function
When expressed in Drosophila embryos at levels equivalent to endogenous UBX, Ser and Thr residues in the C-terminus of Artemia UBX partially inhibit its ability to repress Dll. However, the single Ser in the Artemia UBX C-terminus that is phosphorylated by CK2 has no apparent inhibitory role. Although previous studies have shown that CK2 sites can modulate HOX function (Jaffe et al., 1997; Taghli-Lamallem et al., 2008), our results suggest that a single CK2 phosphorylation site is not sufficient to inhibit the limb repressive function of HOX proteins and multiple Ser/Thr phosphorylation sites may be required.
In this context, it is important to realize that a Hox protein can have a repressive effect on Dll and limb development without completely removing the limb appendage. It has been stated, for example, that UBX protein only evolved a limb repressive function late in insect evolution, and prior to that functioned only in modulating trunk appendage morphology (Palopoli and Patel, 1998; Lewis et al. 2000). However, one way to modulate appendage morphology is to partially repress Dll, which appears to be a function of UBX in Tribolium development (Lewis et al. 2000). It has also been shown that ANTP is likely to modulate Daphnia limb morphology by repression of Dll in a few thoracic cells (Shiga et al. 2002).
In the context of Artemia embryos, limb development in the presence of UBX is probably due to a variety of evolved mechanisms, one component of which is the reduced repressive ability of the Artemia UBX protein on Dll transcription. It is possible that Artemia UBX protein is expressed at lower levels during limb bud development than in Drosophila, as UBX repressive function, at least in Drosophila, is highly sensitive to expression levels (Tour et al. 2005). It is also possible that the Dll limb cis-regulatory sequences have evolved lower affinities for UBX or its corepressors. In addition, Drosophila Dll can be repressed by UBX only at stages prior to the stage when Dll transcription is induced in limb primordia (Castelli-Gair and Akam, 1995). This suggests that the exact timing of expression levels of HOX proteins with the potential to repress Dll, may be critical to limb number and morphology. Given that natural selection works with random genetic variations, it seems likely that evolutionary variations in many different molecules and processes in the limb development hierarchy have generated the great variation in limb shape and size found in different arthropods. In hexapod insects, this variation often reaches one extreme, that being the complete repression of Dll and limb development in the abdomen by UBX and ABD-A.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Rob White for FP6.87 antibody and Ian Duncan for α Drosophila ABD-A. We also thank Nipam Patel for advice on Artemia husbandry and immunohistochemistry and for 4F11 antibody and Grace Boekhoff-Falk for advice on Artemia in situ hybridizations.
REFERENCES
- Abzhanov A, Kaufman TC. Novel regulation of the homeotic gene Scr associated with a crustacean leg-to-maxilliped appendage transformation. Development. 1999;126:1121–1128. doi: 10.1242/dev.126.6.1121. [DOI] [PubMed] [Google Scholar]
- Abzhanov A, Kaufman TC. Crustacean (malacostracan) Hox genes and the evolution of the arthropod trunk. Development. 2000;127:2239–2249. doi: 10.1242/dev.127.11.2239. [DOI] [PubMed] [Google Scholar]
- Abzhanov A, Kaufman TC. Embryonic expression patterns of the Hox genes of the crayfish Procambarus clarkii (Crustacea, Decapoda) Evol Dev. 2000;2:271–283. doi: 10.1046/j.1525-142x.2000.00066.x. [DOI] [PubMed] [Google Scholar]
- Angelini DR, Kaufman TC. Insect appendages and comparative ontogenetics. Dev Biol. 2005;286:57–77. doi: 10.1016/j.ydbio.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Angelini DR, Liu PZ, Hughes CL, Kaufman TC. Hox gene function and interaction in the milkweed bug Oncopeltus fasciatus (Hemiptera) Dev Biol. 2005;287:440–455. doi: 10.1016/j.ydbio.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Averof M, Akam M. Hox genes and the diversification of insect and crustacean body plans. Nature. 1995;376:420–423. doi: 10.1038/376420a0. [DOI] [PubMed] [Google Scholar]
- Averof M, Patel NH. Crustacean appendage evolution associated with changes in Hox gene expression. Nature. 1997;388:682–686. doi: 10.1038/41786. [DOI] [PubMed] [Google Scholar]
- Blin M, Rabet N, Deutsch JS, Mouchel-Vielh E. Possible implication of Hox genes Abdominal-B and abdominal-A in the specification of genital and abdominal segments in cirripedes. Dev Genes Evol. 2003;213:90–96. doi: 10.1007/s00427-003-0294-z. [DOI] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Budd GE, Telford MJ. The origin and evolution of arthropods. Nature. 2009;457:812–817. doi: 10.1038/nature07890. [DOI] [PubMed] [Google Scholar]
- Carroll S, Grenier J, Weatherbee S. From DNA to Diversity: Molecular Genetics and the Evolution of Animal Design. Wiley-Blackwell; 2005. [Google Scholar]
- Castelli-Gair J, Akam M. How the Hox gene Ultrabithorax specifies two different segments: the significance of spatial and temporal regulation within metameres. Development. 1995;121:2973–2982. doi: 10.1242/dev.121.9.2973. [DOI] [PubMed] [Google Scholar]
- Cohen SM, Jürgens G. Proximal-distal pattern formation in Drosophila: cell autonomous requirement for Distal-less gene activity in limb development. EMBO J. 1989;8:2045–2055. doi: 10.1002/j.1460-2075.1989.tb03613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch JS, Mouchel-Vielh E. Hox genes and the crustacean body plan. Bioessays. 2003;25:878–887. doi: 10.1002/bies.10319. [DOI] [PubMed] [Google Scholar]
- Galant R, Carroll SB. Evolution of a transcriptional repression domain in an insect Hox protein. Nature. 2002;415:910–913. doi: 10.1038/nature717. [DOI] [PubMed] [Google Scholar]
- Hsia CC, McGinnis W. Evolution of transcription factor function. Curr Opin Genet Dev. 2003;13:199–206. doi: 10.1016/s0959-437x(03)00017-0. [DOI] [PubMed] [Google Scholar]
- Hughes CL, Kaufman TC. Exploring the myriapod body plan: expression patterns of the ten Hox genes in a centipede. Development. 2002;129:1225–1238. doi: 10.1242/dev.129.5.1225. [DOI] [PubMed] [Google Scholar]
- Hughes CL, Kaufman TC. Hox genes and the evolution of the arthropod body plan. Evol Dev. 2002;4:459–499. doi: 10.1046/j.1525-142x.2002.02034.x. [DOI] [PubMed] [Google Scholar]
- Jaffe L, Ryoo HD, Mann RS. A role for phosphorylation by casein kinase II in modulating Antennapedia activity in Drosophila. Genes Dev. 1997;11:1327–1340. doi: 10.1101/gad.11.10.1327. [DOI] [PubMed] [Google Scholar]
- Janssen R, Damen WG. The ten Hox genes of the millipede Glomeris marginata. Dev Genes Evol. 2006;216:451–465. doi: 10.1007/s00427-006-0092-5. [DOI] [PubMed] [Google Scholar]
- Kellerman KA, Mattson DM, Duncan I. Mutations affecting the stability of the fushi tarazu protein of Drosophila. Genes Dev. 1990;4:1936–1950. doi: 10.1101/gad.4.11.1936. [DOI] [PubMed] [Google Scholar]
- Kelsh R, Weinzierl RO, White RA, Akam M. Homeotic gene expression in the locust Schistocerca: an antibody that detects conserved epitopes in Ultrabithorax and abdominal-A proteins. Dev Genet. 1994;15:19–31. doi: 10.1002/dvg.1020150104. [DOI] [PubMed] [Google Scholar]
- Kosman D, Mizutani D, Lemons D, Cox WG, McGinnis W, Bier E. Multiplex detection of RNA expression in Drosophila embryos. Science. 2004;305:846. doi: 10.1126/science.1099247. [DOI] [PubMed] [Google Scholar]
- Lemons D, McGinnis W. Genomic evolution of Hox gene clusters. Science. 2006;313:1918–1922. doi: 10.1126/science.1132040. [DOI] [PubMed] [Google Scholar]
- Lewis EB. A gene complex controlling segmentation in Drosophila. Nature. 1978;276:565–570. doi: 10.1038/276565a0. [DOI] [PubMed] [Google Scholar]
- Lewis DL, DeCamillis M, Bennett RL. Distinct roles of the homeotic genes Ubx and abd-A in beetle embryonic abdominal appendage development. Proc Natl Acad Sci U S A. 2000;97:4504–4509. doi: 10.1073/pnas.97.9.4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohr U, Pick L. Cofactor-interaction motifs and the cooption of a homeotic Hox protein into the segmentation pathway of Drosophila melanogaster. Curr Biol. 2005;15:643–649. doi: 10.1016/j.cub.2005.02.048. [DOI] [PubMed] [Google Scholar]
- McGinnis W, Krumlauf R. Homeobox genes and axial patterning. Cell. 1992;68:283–302. doi: 10.1016/0092-8674(92)90471-n. [DOI] [PubMed] [Google Scholar]
- Mouchel-Vielh E, Rigolot C, Gibert JM, Deutsch JS. Molecules and the body plan: the Hox genes of Cirripedes (Crustacea) Mol Phylogenet Evol. 1998;9:382–389. doi: 10.1006/mpev.1998.0498. [DOI] [PubMed] [Google Scholar]
- Nagaso H, Murata T, Day N, Yokoyama KK. Simultaneous detection of RNA and protein by in situ hybridization and immunological staining. J Histochem Cytochem. 2001;49:1177–1182. doi: 10.1177/002215540104900911. [DOI] [PubMed] [Google Scholar]
- Nagy LM, Booker R, Riddiford LM. Isolation and embryonic expression of an abdominal-A-like gene from the lepidopteran, Manduca sexta. Development. 1991;112:119–129. doi: 10.1242/dev.112.1.119. [DOI] [PubMed] [Google Scholar]
- Niculita H. Expression of abdominal-A homeotic gene in ants with different abdominal morphologies. Gene Expr Patterns. 2006;6:141–145. doi: 10.1016/j.modgep.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Palopoli MF, Patel NH. Evolution of the interaction between Hox genes and a downstream target. Current Biology. 1998;8:587–590. doi: 10.1016/s0960-9822(98)70228-3. [DOI] [PubMed] [Google Scholar]
- Patel NH, Martin-Blanco E, Coleman KG, Poole SJ, Ellis MC, Kornberg TB, Goodman CS. Expression of engrailed proteins in arthropods, annelids, and chordates. Cell. 1989;58:955–968. doi: 10.1016/0092-8674(89)90947-1. [DOI] [PubMed] [Google Scholar]
- Peterson MD, Rogers BT, Popadic A, Kaufman TC. The embryonic expression pattern of labial, posterior homeotic complex genes and the teashirt homologue in an apterygote insect. Dev Genes Evol. 1999;209:77–90. doi: 10.1007/s004270050230. [DOI] [PubMed] [Google Scholar]
- Pinna LA. Casein kinase 2: an 'eminence grise' in cellular regulation? Biochim Biophys Acta. 1990;1054:267–284. doi: 10.1016/0167-4889(90)90098-x. [DOI] [PubMed] [Google Scholar]
- Regier JC, Shultz JW, Kambic RE. Pancrustacean phylogeny: hexapods are terrestrial crustaceans and maxillopods are not monophyletic. Proc Biol Sci. 2005;272:395–401. doi: 10.1098/rspb.2004.2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronshaugen M, McGinnis N, McGinnis W. Hox protein mutation and macroevolution of the insect body plan. Nature. 2002;415:914–917. doi: 10.1038/nature716. [DOI] [PubMed] [Google Scholar]
- Sanchez-Herrero E, Guerrero I, Sampedro J, Gonzalez-Reyes A. Developmental consequences of unrestricted expression of the abd-A gene of Drosophila. Mech Dev. 1994;46:153–167. doi: 10.1016/0925-4773(94)90068-x. [DOI] [PubMed] [Google Scholar]
- Sanchez-Herrero E, Vernos I, Marco R, Morata G. Genetic organization of Drosophila bithorax complex. Nature. 1985;313:108–113. doi: 10.1038/313108a0. [DOI] [PubMed] [Google Scholar]
- Schredhardt A. A scanning electron-microscope study of post-embryonic development of Artemia. In: Sorgeloos P, Bengston D, Dedeir W, Jaspers E, Wetteren E, editors. Artemia research and its applications. Universa Press; 1987. pp. 5–32. [Google Scholar]
- Shiga Y, Sagawa K, Takai R, Sakaguchi H, Yamagata H, Hayashi S. Transcriptional readthrough of Hox genes Ubx and Antp and their divergent post-transcriptional control during crustacean evolution. Evol Dev. 2006;8:407–414. doi: 10.1111/j.1525-142X.2006.00114.x. [DOI] [PubMed] [Google Scholar]
- Shiga Y, Yasumoto R, Yamagata H, Hayashi S. Evolving role of Antennapedia protein in arthropod limb patterning. Development. 2002;129:3555–3561. doi: 10.1242/dev.129.15.3555. [DOI] [PubMed] [Google Scholar]
- Shippy TD, Brown SJ, Denell RE. Molecular characterization of the Tribolium abdominal-A ortholog and implications for the products of the Drosophila gene. Dev Genes Evol. 1998;207:446–452. doi: 10.1007/s004270050135. [DOI] [PubMed] [Google Scholar]
- Struhl G. Genes controlling segmental specification in the Drosophila thorax. Proc Natl Acad Sci U S A. 1982;79:7380–7384. doi: 10.1073/pnas.79.23.7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taghli-Lamallem O, Hsia C, Ronshaugen M, McGinnis W. Context-dependent regulation of Hox protein functions by CK2 phosphorylation sites. Dev Genes Evol. 2008;218:321–332. doi: 10.1007/s00427-008-0224-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tear G, Akam M, Martinez-Arias A. Isolation of an abdominal-A gene from the locust Schistocerca gregaria and its expression during early embryogenesis. Development. 1990;110:915–925. doi: 10.1242/dev.110.3.915. [DOI] [PubMed] [Google Scholar]
- Tour E, Hittinger CT, McGinnis W. Evolutionarily conserved domains required for activation and repression functions of the Drosophila Hox protein Ultrabithorax. Development. 2005;132:5271–5281. doi: 10.1242/dev.02138. [DOI] [PubMed] [Google Scholar]
- Vachon G, Cohen B, Pfeifle C, McGuffin ME, Botas J, Cohen SM. Homeotic genes of the Bithorax complex repress limb development in the abdomen of the Drosophila embryo through the target gene Distal-less. Cell. 1992;71:437–450. doi: 10.1016/0092-8674(92)90513-c. [DOI] [PubMed] [Google Scholar]
- VanHook AM, Patel NH. Crustaceans. Curr Biol. 2008;18:R547–R550. doi: 10.1016/j.cub.2008.05.021. [DOI] [PubMed] [Google Scholar]
- Warren RW, Nagy L, Selegue J, Gates J, Carroll S. Evolution of homeotic gene regulation and function in flies and butterflies. Nature. 1994;372:458–461. doi: 10.1038/372458a0. [DOI] [PubMed] [Google Scholar]
- Wieschaus E, Nusslein-Volhard C. Looking at Embryos. In: Drosophila, a practical approach. Oxford: IRL Press; 1986. [Google Scholar]
- Zhang H, Shinmyo Y, Mito T, Miyawaki K, Sarashina I, Ohuchi H, Noji S. Expression patterns of the homeotic genes Scr, Antp, Ubx, and abd-A during embryogenesis of the cricket Gryllus bimaculatus. Gene Expr Patterns. 2005;5:491–502. doi: 10.1016/j.modgep.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Zheng Z, Khoo A, Fambrough D, Jr, Garza L, Booker R. Homeotic gene expression in the wild-type and a homeotic mutant of the moth Manduca sexta. Dev Genes Evol. 1999;209:460–472. doi: 10.1007/s004270050279. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.