Abstract
Intercalation of beneficial anion into inorganic host has lead to an opportunity to synthesize various combinations of new organic–inorganic nanohybrids with various potential applications; especially, for the controlled release formulation and storage purposes. Investigation on the release behavior of 2,4-dichlorophenoxyacetate (2,4-D) intercalated into the interlayer of Zn–Al-layered double hydroxide (ZAN) have been carried out using single, binary and ternary aqueous systems of chloride, carbonate and phosphate. The release behavior of the active agent 2,4-D from its double-layered hydroxide nanohybrid ZANDI was found to be of controlled manner governed by pseudo-second order kinetics. It was found that carbonate medium yielded the highest accumulated release of 2,4-D, while phosphate in combination with carbonate and/or nitrate speeds up the release rate of 2,4-D. These results indicate that it is possible to design and develop new delivery system of latex stimulant compound with controlled release property based on 2,4-D that is known as a substance to increase latex production of rubber tree,Hevea brasiliensis.
Keywords: Layered double hydroxide; 2,4-Dichlorophenoxyacetic acid; Pseudo-second order kinetics; Intercalation; Controlled release
Introduction
Nanotechnology has grown tremendously in the past few years, and the importance of this type of technology in industry and society could not be denied. This is due to the fact that this technology can contribute to almost every aspect of life, from transportation to food and from medical to agriculture. Nanotechnology can be taken as the manipulation of matter at the scale size of 1–100 nm, which promises invention of new materials; especially, nanomaterials and devices. One of the advantages of nanomaterials is that they could be designed according to a specific use. Lately, nanotechnology has been attracting much more attention due to its growing importance in industry and academia [1-3]. Significant achievements in this area of research could be referred in literatures for nanoscience and nanotechnology, which has proven to have widespread applications [4-6].
One type of nanomaterials that is subjected to intense research lately is inorganic layered material; especially, layered double hydroxide (LDH). LDH can be used as the host for the formation of organic–inorganic nanohybrid material. A variety of organic moieties can be intercalated into the LDH interlayers, which makes them extremely promising for the purposes of drug delivery and gene therapy [7,8], controlled release of plant growth regulator and herbicides [9-11], contaminants remover [12], polymer composite material with enhanced thermal stability [13] and various other applications. Research in the area of organic–inorganic nanohybrids often lead to formation of new materials with enhanced properties such as physico-mechanical, thermal, water swelling, electrical properties, etc. [14].
LDH is classified as layered anionic material formed by the positively charged layers with two or more types of metallic cations and exchangeable hydrated gallery anions. The general formula of LDH is 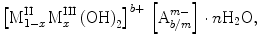 where MII represents divalent cations (Mg2+, Mn2+, Fe2+, Co2+, Cu2+, Ni2+, Zn2+, Ca2+, etc.), MIII represents trivalent cations (Al3+, Cr3+, Mn3+, Fe3+, Co3+, La3+) and Am− represents anions (CO32−, SO42−, NO3−, PO43−, Cl−) in the interlayer region [15]. The ability of LDH to undergo anion exchange process that occurs in the interlayer domain makes it flexible to incorporate or intercalate beneficial anion for the target use.
where MII represents divalent cations (Mg2+, Mn2+, Fe2+, Co2+, Cu2+, Ni2+, Zn2+, Ca2+, etc.), MIII represents trivalent cations (Al3+, Cr3+, Mn3+, Fe3+, Co3+, La3+) and Am− represents anions (CO32−, SO42−, NO3−, PO43−, Cl−) in the interlayer region [15]. The ability of LDH to undergo anion exchange process that occurs in the interlayer domain makes it flexible to incorporate or intercalate beneficial anion for the target use.
Intercalation that involves insertion or incorporation of beneficial agent has gained overwhelming interests lately due to its unique physicochemical properties. The research on new and improved properties of intercalation product appears to be very interesting, because it gives rise to an almost unlimited set of new compounds, the so-called nanohybrid materials with a large spectrum of known and unknown properties [16-20]. Various types of intercalation method could be adopted such as anion exchange of a precursor LDH, direct synthesis by co-precipitation, rehydration of a calcined LDH precursor and thermal reaction [21]. One of the beneficial agents that can be intercalated into LDH is agrochemical; for example, 2,4-dichlorophenoxyacetic acid (2,4-D).
2,4-Dichlorophenoxyacetic acid is widely used in agriculture sector. It is a systemic hormone-type selective herbicide [22], where at low concentration it can act as an auxin analogue, promoting plant growth but lethal to plants at high concentrations. Therefore, 2,4-D is also used as an herbicide against broad-leafed and woody plants [23-25]. It was also reported that 2,4-D can be used as latex stimulant for Hevea Brasiliensis[26], but the use of 2,4-D was later partially discontinued due to the introduction of an ethylene producing compound into the market [27]. Concern on agrochemicals contamination in the environment has recently risen due to the potential hazards. As an example, 2,4-D can easily be transferred into water body due to its high solubility [28] and entering the human and animal food chains, and finally causing serious health problems. Formation of such intercalated compound or controlled release formulation of agrochemicals is one of the methods to solve this problem.
Apart from LDHs, many other matrices can also be used as the hosts for controlled release formulations. Previous works show that nanoporous, silicified phospholipids and stimuli–responsive magnetic nanoparticles can also be used as the hosts for glycolic acid and 4-diamino-6-mercaptopyrimidine, respectively [29,30]. It was found that both the hosts and the intercalated guests play important role in determining the controlled release property of the resulting controlled release formulations.
Here, we describe the synthesis and the controlled release property of 2,4-D, a latex stimulant agent, in which the 2,4-D is intercalated into Zn–Al-LDH for the formation of the nanohybrid. The release was studied using single, binary and ternary systems. To our knowledge, no controlled release study of 2,4-D from its LDH nanohybrid in various aqueous media has intensively been carried out. The Zn–Al–2,4-D nanohybrid material is expected to inherit the same property of 2,4-D, which is to affect the physiological process of rubber plant in order to improve the quality and to increase the latex yield, but the release of 2,4-D is in a controlled manner. Further understanding of the role of controlled release behavior of 2,4-D on the latex output from the rubber tree could lead to the application of 2,4-D in the form of slow release formulation. It is hoped that the associated process is safe and environmentally friendly as the 2,4-D is not exposed directly to the user and the environment, and, therefore, could prevent the associated problems.
Materials and Methods
Synthesis of LDH and the Nanohybrid
All chemicals were used as received, and deionised distilled water was used throughout this work. The formation of both Zn–Al-LDH (ZAN) and Zn–Al–2,4-D nanohybrid (ZANDI) was carried out by spontaneous self-assembly method. For the formation of ZAN, the mother liquor solution consisting of Zn(NO3)2and Al(NO3)3was set at Zn to Al molar ratio,R = 4, and the pH was brought to 10 by drop-wise addition of 2 M NaOH. The same method was adopted to synthesize the nanohybrid ZANDI, but 0.16 M 2,4-D was alternately added with the 2 M NaOH. During the addition, the solution was stirred under nitrogen atmosphere to avoid contamination from atmospheric carbon dioxide. The resulting slurry was aged for 18 h with continuous agitation. The ZAN and ZANDI formed were cooled, centrifuged and washed several times, dried and kept in sample bottles for further use and characterizations.
Characterization
Powder X-ray diffraction (PXRD) patterns of the samples were obtained using filtered CuKαradiation in a Shimadzu Diffractometer, D-600. Fourier transform infrared (FTIR) spectra were recorded by a Perkin–Elmer 1750 spectrophotometer. KBr pallet of 1% sample was used to obtain the FTIR spectra. The elemental analyses were done using a CHNS-932 (LECO) and the Inductively Couple Plasma Atomic Emission Spectrometry (ICP-AES), with a Perkin–Elmer Spectrophotometer model Optima 2000DV under standard condition. The surface morphology of the samples was observed with a scanning electron microscope (SEM), Philips XL30 ESEM.
Release Study of 2,4-D into Aqueous Solutions
The release of 2,4-D from the nanohybrid into the release media was accomplished using various aqueous solutions: chloride, carbonate and phosphate and the combination of them by adding about 0.34 g of ZANDI into a 500 ml of the aqueous solution. The accumulated amount of 2,4-D released into the solution was measured at preset time at λmax = 283.1 nm using a Thermo Corporation, Helios α uv spectrophotometer. Data were automatically collected every 10 min, stored and analyzed.
Results and Discussion
Characterizations of the Sample
Figure 1 shows PXRD patterns of ZAN and its nanohybrid ZANDI. As shown in the figure, the basal spacing of ZAN, which contains nitrate as the counter anion in the interlayer was recorded to be 8.9 Å. The insertion of 2,4-D occurred in the interlayer, resulting in the expansion of basal spacing from 8.9 to 20.1 Å. Previous study on the intercalation of 2,4-D into various LDH systems showed slightly different d-spacing, as the value reported is very much depending on the parameters used for the synthesis [9,31-34]. The increase in basal spacing indicated that the interlayer has been expanded in order to accommodate the 2,4-D moiety, which is bigger in size compared to the nitrate as the counter anion in the LDH. We found that at the optimum condition in which a well-ordered layered nanohybrid could be synthesized is at pH 10 by using 0.16 M 2,4-D with Zn to Al molar ratio of 4.
Figure 1.
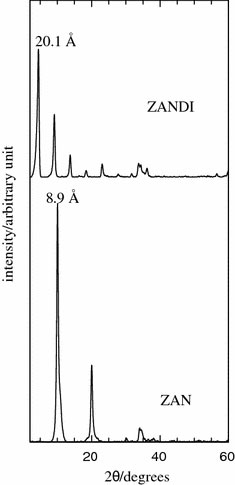
PXRD patterns of Zn–Al-LDH (ZAN) and its nanohybrid with 2,4-D (ZANDI)
Figure 2 shows the FTIR spectra of ZAN, ZANDI and 2,4-D. The insertion of 2,4-D into the interlayer of ZAN was confirmed by the FTIR spectrum, which is complementary to that of PXRD results. The FTIR spectra of ZANDI obviously show combination features of the FTIR spectra of ZAN the parent material and 2,4-D the guest anion. For ZANDI, a band at 3,438 cm−1 corresponds to the OH internal hydrogen bond, while a band at 1,614 cm−1 corresponds to the carboxylate ion and this band overlapped with the deformation vibration of water molecules in the interlayer domain. The presence of 2,4-D functional groups could be observed in ZANDI as shown by the presence of C = C bond vibrations of the aromatic ring that can be observed at 1,486 cm−1, while the antisymmetric and symmetric vibrations of C–O–C appeared at 1,286 cm−1 and 1,068 cm−1, respectively. A band at 868 cm−1 corresponds to C–Cl vibration, while the C–H deformation vibration of benzenic group out of plane appeared at 768 cm−1 and 804 cm−1[32]. The other two bands that appeared at 620 and 428 cm−1 can be attributed to the Al–OH and Zn–Al–OH bonding vibrations, respectively. Band at 1,384 cm−1 in ZAN is not present in the FTIR spectrum of ZANDI, which implies that the nitrate anions were totally replaced by 2,4-D anions.
Figure 2.
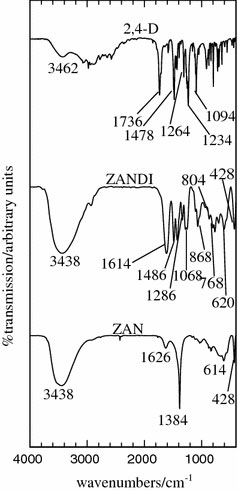
FTIR spectra of Zn–Al-LDH (ZAN) and its nanohybrid with 2,4-D (ZANDI) and 2,4-D
Elemental analysis shows that the final Zn to Al molar ratio Rf for ZAN and ZANDI is 3.8 and 4.0, respectively. The initial molar ratio of Zn/Al mother liquor Ri was 4. This shows that the Zn to Al molar ratio of the product was adjusted accordingly to counter the anionic charge of the guest so that the resulting LDH or its nanohybrid rendered the neutral charge [34]. The CHNS results show that ZAN contains 2.8% nitrogen. This is in agreement with the presence of a strong, sharp band at 1,384 cm−1, which is due to the nitrate group in the FTIR spectrum of ZAN, shown in Fig. 2. CHNS analyses for ZANDI shows the absence of nitrogen content, which further supports the FTIR spectrum, indicating complete replacement of nitrate by 2,4-D. The content of carbon in ZANDI is 14.7%, and this is expected due to the intercalated 2,4-D into the interlayer, which is equivalent to 33.9% loading of 2,4-D in the nanohybrid. The summary of elemental analysis is given in Table 1.
Table 1.
Basal spacing and elemental analysis of Zn–Al-LDH (ZAN) and its nanohybrid with 2,4-D (ZANDI), the rate constants and correlation coefficients obtained from pseudo-second order fitting of the release of 2,4-D into single, binary and ternary aqueous systems
| Sample | Basal spacing (Å) | Zn/Al ratio | (N)/C (%) | 2,4-D (% w/w)a | ||||
|---|---|---|---|---|---|---|---|---|
| ZAN | 8.9 | 3.8 | (2.8) | – | ||||
| ZANDI | 20.1 | 4.0 | 14.7 | 33.9 |
| Aqueous solution (0.05 M) | Maximum release (%) | Maximum release time (min) | Zeroth order | First order | Parabolic diffusion | Pseudo-second order | ||
|---|---|---|---|---|---|---|---|---|
| r2 | r2 | k(mg L s−1)b | t½(min)c | |||||
| Cl | 25 | 4,273 | 0.779 | 0.811 | 0.914 | 0.996 | 0.000027 | 498 |
| CO3 | 99 | 3,828 | 0.372 | 0.966 | 0.529 | 1.000 | 0.000033 | 107 |
| PO4 | 93 | 701 | 0.427 | 0.511 | 0.616 | 0.997 | 0.000083 | 45 |
| Cl-CO3 | 80 | 1,744 | 0.623 | 0.697 | 0.797 | 0.990 | 0.000024 | 167 |
| Cl-PO4 | 88 | 840 | 0.378 | 0.445 | 0.567 | 0.999 | 0.000068 | 58 |
| CO3-PO4 | 90 | 725 | 0.539 | 0.586 | 0.715 | 0.986 | 0.000035 | 104 |
| Cl-CO3-PO4 | 88 | 270 | 0.564 | 0.586 | 0.774 | 0.982 | 0.00004 | 91 |
aEstimated from CHNS analysis based on pure 2,4-D
b,cEstimated using pseudo-second order kinetic model
aEstimated from CHNS analysis based on pure 2,4-D
b,cEstimated using pseudo-second order kinetic model
The surface morphology of ZAN and ZANDI is shown in Fig. 3a, b, respectively. The micrographs were obtained using a scanning electron microscope at 5000× magnifications. The SEM images for both ZAN and ZANDI show agglomerates of nonporous, flaky structure, but the latter shows less compact and fluffy granular structure. This structure is believed to influence the release profiles of 2,4-D from its nanohybrid, as the surface morphology plays a role in determining the surface area and in turn exposure to the incoming anion that get in contact and finally ion exchanged with.
Figure 3.
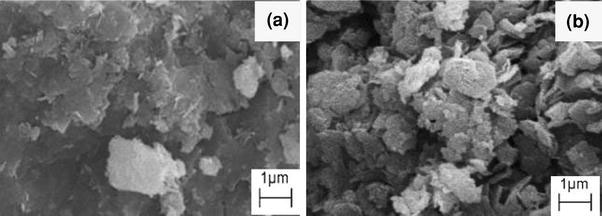
SEM micrograph of Zn–Al-LDH (ZAN) and its nanohybrid with 2,4-D (ZANDI)
Controlled Release of 2,4-D into Aqueous Media
The release of 2,4-D from the nanohybrid interlamellae into various single, binary and ternary systems using 0.05 M NaCl, 0.05 M Na2CO3and 0.05 M Na3PO4have been conducted. The release profiles are shown in Fig. 4. The effect of various media systems on the release of 2,4-D were evaluated according to the maximum accumulated release and can be written as follows;
Figure 4.
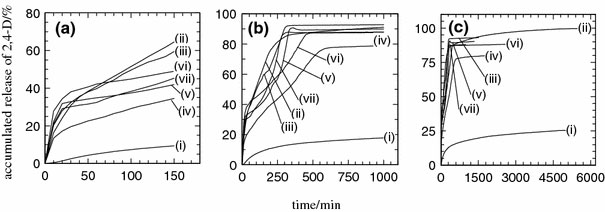
Release profiles of 2,4-D from the interlamellae of ZANDI, the nanohybrid into various aqueous solution systems containing single, binary and ternary anions of chloride, carbonate and phosphate at 0–150 min (a), at 0–1,000 min (b) and at various release times (c), chloride (i), carbonate (ii), phosphate (iii), chloride–carbonate (iv), carbonate–phosphate (v), chloride–phosphate (iv) and chloride–carbonate–phosphate (vii)
1. Carbonate > phosphate > chloride for single anion system.
2. Carbonate–phosphate > chloride–phosphate > chloride–carbonate for binary anions system.
3. Carbonate > phosphate > carbonate–phosphate > chloride–carbonate-phosphate > chloride–phosphate > chloride–carbonate > chloride for the all single, binary and tertiary systems.
In the single system release media, it could be observed that carbonate dominated the accumulated release percentage at 99% compared to phosphate and chloride with a value of 93 and 25%, respectively. Carbonate is known to have the strongest affinity toward the interlayer of layered double hydroxides [35]. As is shown in Fig. 4, 2,4-D is almost fully replaced by CO32−, resulting in the highest accumulated release among the media studied. The maximum release time shows that 2,4-D is replaced by PO43− at 701 min followed by CO32− at 3,828 min and Cl− at 4,273 min. It is worth to note that even though CO32− shows the highest accumulated release (Table 1), the replacement of 2,4-D by CO32− was found to be slower when compared to PO43− as mentioned earlier. This could be due to the fact that CO32− anion undergoes single hydrolysis process that might have resulted in less ionic interaction for the replacement of 2,4-D to occur rapidly compared to the PO43− anion [36].
In binary system release media, the highest accumulated release of 2,4-D was found in the carbonate–phosphate release medium with 90% accumulated release followed by the chloride–phosphate and chloride–carbonate with release of 88 and 80%, respectively. It was found that whenever PO43− anion is present in the release media, the release rate will be faster, and the accumulated release of 2,4-D will be higher. This could be due to the multiple hydrolysis of phosphate, leaving only the tertiary PO43− to compete in the ion exchange process that finally speeds up the replacement process of 2,4-D in the interlayer [36]. From the maximum release time data, carbonate–phosphate was found to replace the 2,4-D anion at 725 min followed by chloride–phosphate at 840 min and chloride–carbonate at 1,744 min.
For ternary anions system of chloride–carbonate–phosphate, 88% of 2,4-D was found to be released at 270 min, which is the fastest maximum release time among all of the release media used in this study. However, the existence of chloride in the release medium decreases the accumulated percentage release of 2,4-D, which could be due to the low ion exchange affinity of chloride toward the interlayer of the inorganic interlamellae [37,38].
From this study, the accumulated release of 2,4-D into various aqueous systems under our experimental condition shows that the release rate of 2,4-D is mainly dominated by phosphate ion when it is combined with other anions. The release rate was found to be faster when PO43−anion is present in the release medium. In single ion release media, carbonate was found to dominate the accumulated release of 2,4-D.
Release Kinetics
It was reported that the release of organic moieties from the interlayer of LDH could be controlled by either the dissolution of LDH [9,39] or diffusion through LDH [40]. Kinetic study of the release behavior of 2,4-D was further elucidated by fitting the data to four selected models: zeroth [41], first [42], pseudo-second order kinetics [43] and parabolic diffusion [44]. The data of the 2,4-D released were fitted to the kinetic models at the full release periods for each of the release medium in order to understand the release behavior of 2,4-D into various aqueous solutions, and their binary and ternary combinations. The obtained parameters from the fitting (Fig. 5) are given in Table 1.
Figure 5.
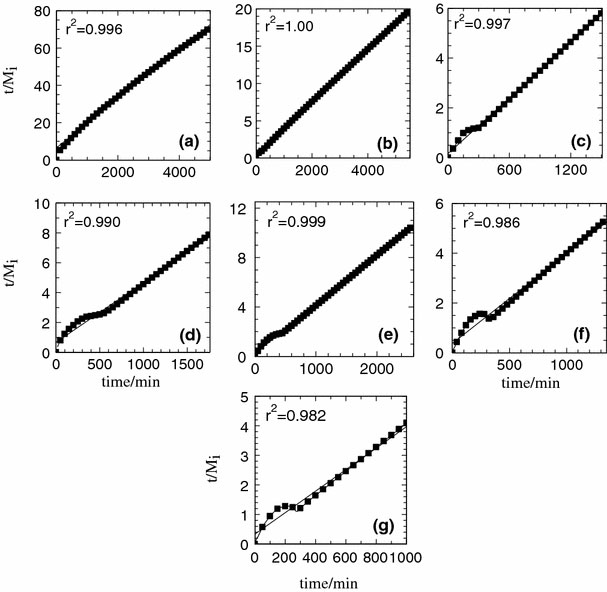
Fitting the data of the release of 2,4-D from the interlamellae of ZANDI, the nanohybrid into various aqueous solutions systems containing single, binary and ternary anions: chloride (a), carbonate (b), phosphate (c), chloride–carbonate (d), chloride–phosphate (e), carbonate–phosphate (f) and chloride–carbonate–phosphate (g) using pseudo-second order kinetic model
The kinetic models used in the fitting are given as follows:
| (1) |
| (2) |
| (3) |
| (4) |
wherexrepresents the percentage release of 2,4-D at the timet,Cis a constant,Mtrepresents the concentration of 2,4-D at the timet,Mfrepresents the final concentration of 2,4-D andkis a rate constant, and att=0,MtisMi, the initial concentration of 2,4-D.
By comparing the correlation coefficient,r2values obtained from the fitting, it is clear that the release profile of 2,4-D from the nanohybrid is governed by the pseudo-second order kinetics. Thet1/2values of pseudo-second order show that PO43−anion accelerates the ion exchange of 2,4-D with the lowestt1/2value at 45 min followed by CO32−at 107 min and Cl−at 498 min. Combination of PO43−with Cl−as the incoming anions in the release media resulted int1/2value of 58 min, which could be due to the less competition between PO43−anion and the Cl−anion. In the ternary release medium, the presence of CO32−that could be competing with PO43−anion to replace the 2,4-D anion resulted in higher value oft1/2at 91 min. This shows that the affinity of the anion toward the interlayer of Zn–Al-LDH, and the degree of competition between the anions to replace the 2,4-D anion play a role in determining thet1/2values.
Conclusions
Pure phase nanohybrid compound in which 2,4-D is intercalated into Zn–Al-LDH was successfully synthesized at Zn to Al initial molar ratio 4, using 0.16 M 2,4-D by drop-wise addition of NaOH to bring the solution to pH 10. Expansion of basal spacing from 8.9 Å in the Zn–Al–LDH to 20.1 Å in the nanohybrid indicates that 2,4-D was successfully intercalated into the interlayer of Zn–Al-LDH. Both FTIR and elemental analysis further supported the intercalation episode of 2,4-D in the resulting nanohybrid. Single anion release medium of carbonate was found to yield the highest release percentage of 2,4-D at 99%. In the binary and ternary release media, the presence of phosphate anion speeds up the release rate. The data of the release of 2,4-D from its nanohybrid compound showed that the release of 2,4-D is governed by the pseudo-second order kinetics. This study shows that the release rate and amount of 2,4-D could be tailor-made using co-anions to tune the release properties.
Acknowledgments
The support of the research by MOHE under FRGS no. 02-11-08-615FR is gratefully acknowledged. AMJ thanks UPM for PASCA Siswazah Scheme studentships.
References
- M.C. Roco, J. Nanopart. Res. 5(3–4), 181–189 (2003)
- M.C. Roco, MRS Bull. 28(6), 416–417 (2003)
- M.C. Roco, W.S. Bainbridge, J. Nanopart. Res. 7(1), 1–13 (2005)
- Yan C, Xue D. Adv. 2008. pp. 1055–1058. COI number [1:CAS:528:DC%2BD1cXlt1WmtL0%3D] [DOI]
- Liu J, Xue D. Adv. 2008. pp. 2622–2627. COI number [1:CAS:528:DC%2BD1cXptVOrsLc%3D]; Bibcode number [2005JMatR..20.2622L] [DOI]
- C. Yan, J. Liu, F. Liu, J. Wu, K. Gao, D. Xue, Nanoscale Res. Lett. 3, 473–480 (2008) [DOI] [PMC free article] [PubMed]
- H. Jung, H. Mi-Kim, Y.B. Choy, S.J. Hwang, J.H. Choy, Appl. Clay Sci. 40(1–4), 99–107 (2008)
- Yang QZ, Yang J, Zhang CK. Int. 2006. pp. 148–152. COI number [1:CAS:528:DC%2BD28XhtFKhsLrN] [DOI] [PubMed]
- M.Z. Hussein, Z. Zainal, A.H. Yahaya, D.W.V. Foo, J. Control. Release 82(2–3), 417–427 (2002) [DOI] [PubMed]
- Hussein MZ, Zainal Z, Yahaya AH, Kian LH. Sci. 2005. pp. 956–962. [DOI]
- Hussein MZ, Sarijo SH, Yahaya AH, Zainal Z. J. 2007. pp. 2852–2862. [DOI] [PubMed]
- Lv L, He J, Wei M, Evans DG, Duan X. J. 2006. pp. 119–128. [DOI] [PubMed]
- H. Acharya, S.K. Srivastava, A. Bhowmick, Nanoscale Res. Lett. 2, 1–5 (2007)
- A. Ganguly, A.K. Bhowmick, Nanoscale Res. Lett. 3, 36–44 (2008) [DOI] [PMC free article] [PubMed]
- F. Cavani, F. Trifiro, A. Vaccari, Catal. Today 11(2), 173–301 (1991)
- L. Obalova, K. Jiratova, F. Kovanda, K. Pacultova, Z. Lacny, Z. Mikulova, App. Catal. B Environ. 60, 297–305 (2005)
- Q.Z. Yang, J. Yang, C.K. Zhang, Int. J. Pharm. 326, 148–152 (2006) [DOI] [PubMed]
- Wang Z, Han E, Ke W. Prog. 2005. pp. 29–37. COI number [1:CAS:528:DC%2BD2MXjtlKhsbk%3D] [DOI]
- C. Rossi, A. Schoubben, M. Ricci, L. Perioli, V. Ambrogi, L. Latterini, G.G. Aloisi, A. Rossi, Int. J. Pharm. 295, 47–55 (2005) [DOI] [PubMed]
- Lee K, Nam JH, Lee JH, Lee Y, Cho SM, Jung CH, Choi HG, Chang YY, Kwon YU, Nam JD. Electrochem. 2005. pp. 113–118. COI number [1:CAS:528:DC%2BD2cXhtFaqu7zE] [DOI]
- Newman SP, Jones W. New J. 1998. pp. 105–115. COI number [1:CAS:528:DyaK1cXhslait7g%3D] [DOI]
- Farrana A, Serraa C, Sepaniakb MJ. J. Chromatogr. A. 1999. pp. 209–215. [DOI]
- Tripathy N, Routray P, Sahu G, Kumar A. Mutat. 1993. pp. 237–242. COI number [1:CAS:528:DyaK2cXis1ah] [DOI] [PubMed]
- G.L. Sinton, L.T. Erickson, S.M. Lee, Enzyme Microb. Technol. 8, 395–403 (1986)
- Willemsen RE, Hailey A. Environ. 2001. pp. 71–78. COI number [1:CAS:528:DC%2BD3MXjtF2js70%3D] [DOI] [PubMed]
- B.L. Archer, B.G. Audley, F.J. Bealing, Plast. Rubber Int. 7(3), 109–111 (1982)
- J. Auzac, in Physiology of Rubber Tree Latex Florida (CRC Press, Florida, 1989), pp. 289–293.
- Cox L, Celis R, Hermosín MC, Cornejo J. J. 2000. pp. 93–99. COI number [1:CAS:528:DyaK1MXns1Kntr4%3D] [DOI] [PubMed]
- S.H. Kang, H.S. Lee, J. Lee, S. Jeong, J. Choi, S.C. Lee, K.J. Kim, J.H. Chang, Nanoscale Res. Lett. 3, 355–360 (2008)
- S. Wang, Y. Zhou, W. Guan, B. Ding, Nanoscale Res. Lett. 3, 289–294 (2008)
- Ragavan A, Khan AI, O’Hare D. J. 2006. pp. 983–986. COI number [1:CAS:528:DC%2BD28XlsFOnsb0%3D]; Bibcode number [2006JPCS...67..983R] [DOI]
- Lakraimi MA, Legrouri A, Barroug A, Roy AD, Besse JP. J. 2000. pp. 1007–1011. COI number [1:CAS:528:DC%2BD3cXhvFGlt78%3D] [DOI]
- Y.F. Chao, Appl. Clay Sci. 40(1–4), 193–200 (2007)
- M.Z. Hussein, J.M. Amin, Z. Zainal, A.H. Yahaya, J. Nanosci. Nanotechnol. 2(2), 143–146 (2002) [DOI] [PubMed]
- S. Miyata, Clay Clay Min. 28, 50–56 (1980)
- I.N. Kugelmass, Biochemistry 38(2), 587–592 (1929)
- S. Miyata, Clay Clay Min. 31, 305–311 (1983)
- Parker LM, Milestone NB, Newman RH. Ind. 1995. pp. 1196–1202. COI number [1:CAS:528:DyaK2MXks1GktbY%3D] [DOI]
- Y. Seida, Y. Nakano, in 13th Proceedings Conference of Japan Society on Adsorption (Osaka, 1999), p. 49.
- Yang JH, Han YS, Park M, Park T, Hwang SJ, Choy JH. Chem. 2007. pp. 2679–2685. COI number [1:CAS:528:DC%2BD2sXktlChsLk%3D] [DOI]
- Costa P, Lobo JMS. Eur. 2000. pp. 123–133. [DOI]
- Wagner JG. J. 1969. pp. 1253–1257. COI number [1:CAS:528:DyaF1MXlt1yltLc%3D] [DOI] [PubMed]
- Ling L, He J, Wei M, Evans DG, Duan X. Wat. 2006. pp. 735–743. [DOI] [PubMed]
- T. Kodama, Y. Harada, M. Ueda, K.I. Shimizu, K. Shuto, S. Komarneni, Langmuir 17, 4881–4886 (2001)


