Abstract
Transverse surface plasmon resonances (SPR) in Au–Ag and Ag–Au core–shell structure nanowires have been investigated by means of quasi-static theory. There are two kinds of SPR bands resulting from the outer surface of wall metal and the interface between core and wall metals, respectively. The SPR corresponding to the interface, which is similar to that of alloy particle, decreases and shifts obviously with increasing the wall thickness. However, the SPR corresponding to the outer surface, which is similar to that of pure metal particle, increases and shifts slightly with increasing the wall thickness. A mechanism based on oscillatory surface electrons under coulombic attraction is developed to illuminate the shift fashion of SPR from bimetallic core–shell interface. The net charges and extra coulombic force in metallic wall affect the SPR energy and the shift fashion.
Keywords: Surface plasmon resonance (SPR), Interface, Bimetallic, Core–shell structure, Nanowires
Introduction
The optical properties of bimetallic Au–Ag nanoparticles are currently of considerable interest and the subject of a significant literature due to the potential uses of the tunable light absorption, scattering and local field enhancement in the UV-visible region [1,2]. There are two types of bimetallic nanoparticles: alloy particles (particles with a homogeneous distribution of two kinds of metals) and core–shell structure particles (particles with heterogeneous arrangement of two kinds of metals leading to core–shell structure) [3]. The composition or shell thickness dependent surface plasmon resonance (SPR) absorption spectra of both core–shell type and alloy model Au–Ag nanoparticles have already been studied experimentally and theoretically [3-6].
As we know, only one SPR peak occurs at around 520 nm for pure Au spherical nanoparticles [7]. Similarly, only one SPR peak occurs at around 400 nm for pure Ag spherical nanoparticles [8]. In bimetallic Au–Ag nanoparticles, the SPR band depends on the composition and the distribution of the two metals [9]. For alloy type particles, the two kinds of metals are homogeneously distributed over the whole volume on an atomic scale, so there is still only one SPR peak located between those of pure Au and Ag nanoparticles. This SPR in alloy type Au–Ag bimetallic nanoparticles may shift from 400 nm to 520 nm linearly by changing the molar fraction of Au from 0 to 100% [10].
For core–shell structure particles, one of the two metals constitutes the core of the structure, and the other one the external shell, the SPR become complex because of the interface between core and shell. A direct approach to determining the interface is to see the core–shell structure by transmission electron microscopy (TEM), because a boundary between Ag and Au elements can be distinguished by bright and dark contrast in the TEM imaging [2]. In the core–shell bimetallic Au–Ag nanoparticles, two SPR bands can be observed [11]. Due to the two interacting metals at the interface, SPR peak positions for both silver and gold shift [3]. For example, when Au nanoparticle was coated by an Ag shell, a blue shift for the original pure gold peak was observed. However, with increasing the amount of silver, the Ag shell thickness grows resulting in a red-shift of the SPR maximum for the silver fraction [3]. At last, the Au–Ag core–shell structure bimetallic colloids show only one SPR at around 402 nm, which can be attributed to the SPR of silver particles alone [12]. The question arises for the origin and the shift fashion of the two SPR bands. In the report of Hodak et al. [13], it is concluded that two types of collective electron oscillations occur. However, the mechanism and physical picture of these two kinds of SPR, especially the mechanism of collective electron oscillation at the interface, have not been studied in great detail so far. In this paper, we propose to investigate the origin of the SPR at the interface of Au–Ag core–shell structure bimetallic nanowire by calculating the wall thickness dependent absorption spectra. The mechanism based on oscillatory surface electrons under coulombic attraction has been used to illuminate the shift fashion of SPR bands that were observed experimentally and calculated theoretically.
Modeling
We choose a long nanowire as our model, because gold nanowire will become a kind of widely used element of optoelectronic nano-devices. Furthermore, noble metallic nanowires and nanotubes have already been synthesized experimentally [14-16]. In our studies, the bimetallic composite model consists of a metallic wire core of radius r1 coated by a metallic wall of thickness r2 − r1, as shown in Fig. 1. The dielectric functions of the core, wall, and embedding medium are ε1, ε2 and ε3, respectively. It is important to note that ε1 and ε2 have real and imaginary frequency-dependent components and can be expressed as [17]
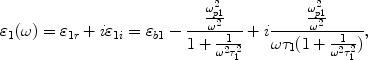 |
(1) |
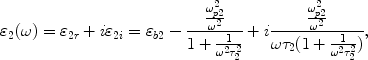 |
(2) |
Figure 1.
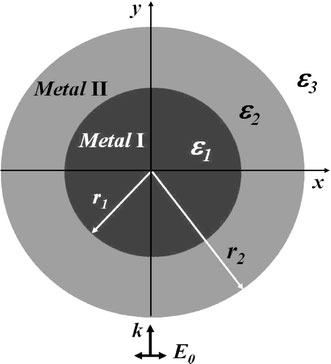
Core–shell structure bimetallic nanowire geometry. ε1, ε2, ε3are the dielectric functions for the metallic core, metallic wall and embedding regions, respectively;r1denotes the core radius andr2denotes the wall radius
where εb1 and εb2 are dielectric function of bulk metal of core and wall which are due to inter-band transition and varies with frequency. ωp1 and ωp2 denote the plasmon frequency of the bulk metal of core and wall, τ1 and τ2 are size limit relaxation time of metallic nanowire and nanowall, and ω is the frequency of electromagnetic wave.
In our analysis, the radius of bimetallic nanowire changes from 10 to 25 nm, which is much smaller than the light wavelength (300–800 nm). So the quasi-static approximation can be employed in this calculation [9]. In this theory, the spatial variation of the electromagnetic field is neglected while the temporal dependence is preserved. Therefore, the metallic particle is subjected to an almost uniform field and oscillates like a dipole with polarization proportional to the incident electric field [17,18]. Now we consider one bimetallic nanowire illuminated by the light of wavelength λ. This incident light travels in the forward y direction and the nanowire to be infinitely extended perpendicular to x–y plane. When the external light-induced electric field,  , travels in the forward y direction and polarized in x axis direction, the solution for the local electric field in the bimetallic nanowire could be derived from Laplace’s equation [19]. When r=r2, ϕ = 0 (here ϕ is the included angle of incident field makes with the position vector
, travels in the forward y direction and polarized in x axis direction, the solution for the local electric field in the bimetallic nanowire could be derived from Laplace’s equation [19]. When r=r2, ϕ = 0 (here ϕ is the included angle of incident field makes with the position vector  ), the corresponding electric field is
), the corresponding electric field is
| (3) |
the corresponding polarizability is
| (4) |
finally, we can obtain the absorption cross section by using scattering theory [18],
| (5) |
Although the length of the nanowire in this calculation is infinite, the absorption cross sections per unit length of the nanowire can be finite [19,20]. So we refer to such an absorption cross section per unit length as simply an absorption cross section in this study.
Results and Discussion
The calculated absorption spectra from transverse SPR of Ag–Au core–shell structure nanowire are shown in Fig. 2. Here, the core radius r1 is fixed at 10 nm, whereas the wall thickness r2 − r1 changes from 0 to 15 nm. When Au wall thickness is 0 nm, the only peak at 410 nm has been ascribed to the transverse SPR of pure Ag nanowire. With increasing the Au wall thickness, the other peak takes place at around 530 nm and red shifts slightly. This peak at 530 nm resulted from the transverse SPR of out surface of Au wall, the red shift is ascribed to the increasing size of Au wall. Meanwhile, the SPR peak at 410 nm red shifts obviously with increasing the Au wall thickness. All these spectral characters are in agreement with the experimental results [21].
Figure 2.
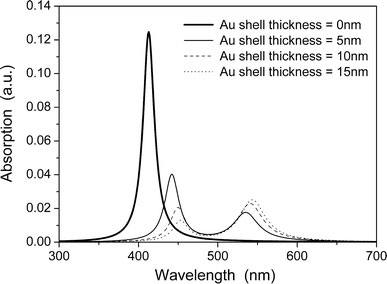
Calculated light absorption spectra of Ag–Au core–shell structure nanowires with varying wall thickness, the core radiusr1 = 10 nm
In order to find the effect of wall thickness on the shift of SPR peaks, we also plot the absorption cross section as a function of wavelength and gold wall thickness, as shown in Fig. 3a. It is obvious that the increasing Au wall thickness leads to the longer wavelength peak from Au wall get intense and red shift slightly, and leads to the shorter wavelength peak decrease in intensity and red shift nonlinearly. The similar decrease and red shift of the shorter wavelength peak has also been observed experimentally in Ag core/Au shell nanoparticles by Steinbruck et al. [3] and Lee et al. [21]. In this Ag–Au core–shell structure nanowire, the longer wavelength SPR peak gets intense very slowly with increasing the wall thickness. Therefore, as Au formed a thin wall layer (< 4 nm) on the Ag core wire, the surface plasmon absorption band showed only one peak, which is in agreement with the experimental result [6]. However, the shorter wavelength SPR peak of interface red shifts and gets weak rapidly with increasing the Au wall thickness within a few nanometers. Therefore, when the Au wall is too thick, the shorter wavelength SPR peak from interface becomes very weak. So the absorption spectrum has only one distinct peak from out surface at longer wavelength, which is in agreement with the experimental result [3,6,12]. That is why it is seldom to observe two SPR band in Ag–Au core–shell structure bimetallic nanoparticles [21]. Another reason is the suggestion that Au and Ag formed alloy on the surface [6].
Figure 3.
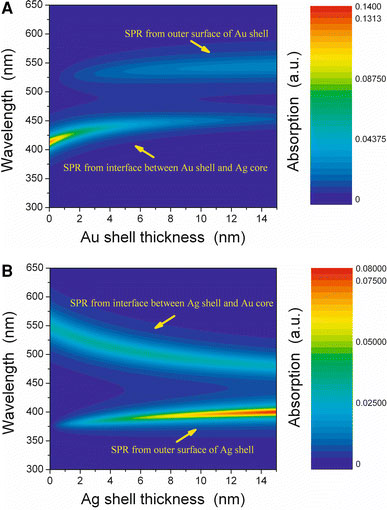
aAbsorption cross section versus wavelength in Ag–Au core–shell structure nanowires with different gold wall thickness;babsorption cross section versus wavelength in Au–Ag core–shell structure nanowires with different silver wall thickness. The core radius is 10 nm
In order to investigate the origin of the shorter wavelength SPR peak of gold coated silver nanowire, we also calculated the absorption spectra of silver coated gold nanowire (i.e. Au–Ag core–shell structure nanowire), as shown in Fig. 3b. For Au–Ag core–shell structure nanowire, we also observed two absorption peaks in the visible region. However, increasing the Ag wall thickness leads to the longer wavelength peak blue shift distinctly and nonlinearly (the shift fashion is similar to that of shorter wavelength peak in Ag–Au core–shell structure nanowire), and leads to the shorter wavelength peak red shift slightly (the shift fashion is similar to that of longer wavelength peak in Ag–Au core–shell structure nanowire). These spectral characters have also been observed experimentally in Au core/Ag shell bimetallic nanoparticles by Pande et al. [12,13].
By comparing Fig. 3a and b, we find the shorter wavelength peak at around 400 nm in Au–Ag core–shell structure nanowire and the longer wavelength peak at around 530 nm in Ag–Au core–shell structure nanowire have the same origin. Both of them come from the SPR of outer metallic wall. On the other hand, the longer wavelength peak shifting from 550 to 480 nm in Au–Ag core–shell nanowire and the shorter wavelength peak shifting from 410 to 450 nm in Ag–Au core–shell nanowire also have the same origin. Both of them come from the SPR of interface between metallic core and wall. When the metallic wire has been coated with another kind of metal, a core–shell structure comes into being and the surface of the wire has transformed into an interface between wire and wall. Due to the interacting of the two metals at the interface, the corresponding SPR peak shifts obviously with changing the ratio of wire and wall radius.
However, the SPR peaks resulting from Ag–Ag interface always shift nonlinearly with varying wall thickness, which is different from the linear shift fashion of alloy Ag–Ag nanoparticles with varying gold mole fraction [22]. This phenomenon may be illuminated by the mechanism of SPR in the bimetallic core–wall interface. As we know, the SPR of noble metallic particles is intimately related to the delocalized conduction band electrons that only weakly interact with the lattice of cations [23]. The coulombic attraction between the negative electrons and the positive metal cations takes place and serves as the restoring force for the oscillatory electrons. The frequency of SPR is equal to the intrinsic frequency of oscillatory electrons in nanoparticles, which is controlled by the bound grade of the electrons. The tightly bound the electrons are, the higher the SPR energy and the intrinsic frequency are; on the contrary, the weakly bound the electrons are, the lower the SPR energy and the longer resonance wavelength are [23]. When Ag wire is coated by an Au wall, the electrons in Ag wire may transfer into the Au wall, and the oscillatory electron cloud will suffer an attractive force from net positive charges in Au wall (Au may has more valence electrons than Ag. For instance, Au3+ has more positive charges than Ag+), as shown in Fig. 4. Therefore, the bound grade has been decreased and the SPR peak red shifts. On the contrary, when Au wire is coated with an Ag wall, the oscillatory electron cloud will suffer a repulsive force from net negative charges in Ag wall. Therefore, the bound grade has been increased and the SPR peak blue shifts. Because SPR is a surface effect and the amplitude of the oscillatory electron cloud is small and limited, so the extra coulombic attraction only arises within thin wall. The shift of SPR will get weak and stop when the wall thickness exceeds the threshold value.
Figure 4.
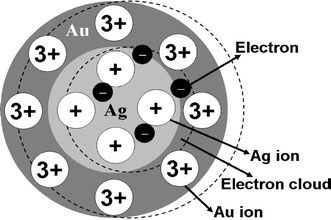
Schematic illustration of SPR from interface of Ag–Au core–shell structure nanowire
Conclusions
In conclusion, there are two kinds of transverse SPR in Au–Ag bimetallic nanowire. One is resulted from the outer surface of wall metal and is similar to that of pure metallic particle, the other is resulted from the interface between the core and wall metals and is similar to that of alloy particle. Quasi-static calculations show that, for Ag-Au core-shell structure nanowire, increasing the Au wall leads to the interface SPR red shifts obviously and decreases, whereas the outer surface SPR red shifts slightly and increases. For Au–Ag core–shell structure nanowire, increasing the Ag wall leads to the interface SPR blue shifts obviously and decreases, whereas the outer surface SPR red shifts slightly and increases. The shift fashion of SPR from bimetallic core–wall interface is different from alloy particles. The net charges and extra coulombic force in metallic wall affect the energy of SPR and leads the nonlinear shifting.
Acknowledgment
This work was supported by the National Natural Science Foundation of China under grant no. 10804091.
References
- Moskovits M, Srnova-Sloufova I, Vlckova B. J. 2002. p. 10436. [DOI]
- Tsuji M, Matsuo R, Jiang P, Miyamae N, Ueyama D, Nishio M, Hikino S, Kumagae H, Kamarudin KSN, Tang XL. Cryst. 2008. p. 2529. [DOI]
- Steinbruck A, Csaki A, Festag G, Fritzsche W. Plasmonics. 2006. p. 79. [DOI]
- Mulvaney P, Giersig M, Henglein A. J. 1993. p. 7061. COI number [1:CAS:528:DyaK3sXktlOgsLo%3D] [DOI]
- Han HF, Fang Y, Li ZP, Xu HX. Appl. 2008. p. 023116. Bibcode number [2008ApPhL..92b3116H] [DOI]
- Chen HM, Liu RS, Jang LY, Lee JF, Hu SF. Chem. 2006. p. 118. COI number [1:CAS:528:DC%2BD28Xis1emtLg%3D]; Bibcode number [2006CPL...421..118C] [DOI] [PubMed]
- Link S, El-Sayed MA. J. Phys. Chem. B. 1999. p. 4212. COI number [1:CAS:528:DyaK1MXivVart78%3D] [DOI]
- Kometani N, Tsubonishi M, Fujita T, Asami K, Yonezawa Y. Langmuir. 2001. p. 578. COI number [1:CAS:528:DC%2BD3MXhsVehug%3D%3D] [DOI]
- Kreibig U, Vollmer M. Optical Properties of Metal Clusters. Springer, New York; 1995. [Google Scholar]
- Link S, Wang ZL, El-Sayed MA. J. Phys. Chem. B. 1999. p. 3529. COI number [1:CAS:528:DyaK1MXitlKrtb8%3D] [DOI]
- Yang Y, Shi JL, Kawamura G, Nogami M. Scr. 2008. p. 862. COI number [1:CAS:528:DC%2BD1cXjt12qurY%3D] [DOI]
- Pande S, Ghosh SK, Praharaj S, Panigrahi S, Basu S, Jana S, Pal A, Tsukuda T, Pal T. J. Phys. Chem. C. 2007. p. 10806. COI number [1:CAS:528:DC%2BD2sXnsVCqs7g%3D] [DOI] [PubMed]
- Hodak JH, Henglein A, Giersig M, Hartland GV. J. Phys. Chem. B. 2000. p. 11708. COI number [1:CAS:528:DC%2BD3cXnvFSrtr4%3D] [DOI]
- Gong HM, Zhou ZK, Xiao S, Su XR, Wang QQ. Plasmonics. 2008. p. 59. COI number [1:CAS:528:DC%2BD1cXhtFensLvL] [DOI]
- Hendren WR, Murphy A, Evans P, Oconnor D, Wurtz GA, Zayats AV, Atkinson R, Pollard RJ. J. 2008. p. 362203. [DOI]
- Spuch-Calvar M, Pacifico J, Perez-Juste J, Liz-Marzan LM. Langmuir. 2008. p. 9675. COI number [1:CAS:528:DC%2BD1cXks1OntLY%3D] [DOI] [PubMed]
- Zhu J. Nanotechnology. 2007. p. 225702. Bibcode number [2007Nanot..18v5702Z] [DOI]
- Averitt RD, Westcott SL, Halas NJ. J. Opt. Soc. Am. B. 1999. p. 1824. COI number [1:CAS:528:DyaK1MXmtlOms78%3D]; Bibcode number [1999OSAJB..16.1824A] [DOI]
- Zhu J. Mater. Sci. Eng. A. 2007. p. 685.
- Oliva JM, Gray SK. Chem. 2003. p. 325. COI number [1:CAS:528:DC%2BD3sXnsVeltbg%3D]; Bibcode number [2003CPL...379..325O] [DOI]
- Zhang QB, Xie JP, Lee JY, Zhang JX, Boothroyd C. Small. 2008. p. 1067. COI number [1:CAS:528:DC%2BD1cXhtVehurbP] [DOI] [PubMed]
- Alqudami A, Annapoorni S, Shivaprasad SM. J. 2008. p. 1027. COI number [1:CAS:528:DC%2BD1cXnsFegtb8%3D] [DOI]
- Schwartzberg AM, Zhang JZ. J. Phys. Chem. C. 2008. p. 10323. COI number [1:CAS:528:DC%2BD1cXmvVWrurs%3D] [DOI]


