Abstract
Background
As the oceans simultaneously warm, acidify and increase in P CO2, prospects for marine biota are of concern. Calcifying species may find it difficult to produce their skeleton because ocean acidification decreases calcium carbonate saturation and accompanying hypercapnia suppresses metabolism. However, this may be buffered by enhanced growth and metabolism due to warming.
Methodology/Principal Findings
We examined the interactive effects of near-future ocean warming and increased acidification/P CO2 on larval development in the tropical sea urchin Tripneustes gratilla. Larvae were reared in multifactorial experiments in flow-through conditions in all combinations of three temperature and three pH/P CO2 treatments. Experiments were placed in the setting of projected near future conditions for SE Australia, a global change hot spot. Increased acidity/P CO2 and decreased carbonate mineral saturation significantly reduced larval growth resulting in decreased skeletal length. Increased temperature (+3°C) stimulated growth, producing significantly bigger larvae across all pH/P CO2 treatments up to a thermal threshold (+6°C). Increased acidity (-0.3-0.5 pH units) and hypercapnia significantly reduced larval calcification. A +3°C warming diminished the negative effects of acidification and hypercapnia on larval growth.
Conclusions and Significance
This study of the effects of ocean warming and CO2 driven acidification on development and calcification of marine invertebrate larvae reared in experimental conditions from the outset of development (fertilization) shows the positive and negative effects of these stressors. In simultaneous exposure to stressors the dwarfing effects of acidification were dominant. Reduction in size of sea urchin larvae in a high P CO2 ocean would likely impair their performance with negative consequent effects for benthic adult populations.
Introduction
As the oceans warm and absorb increasing amounts of CO2, marine biota are faced with a suite of stressors causing major change to marine ecosystems [1]–[2]. Climate change models predict ocean warming by 4°C and a drop in pH by 0.3 to 0.5 units by ca. 2100 [3]–[4]. Ocean acidification is accompanied by a decrease in saturation of the calcium carbonate (CaCO3) minerals required to make skeletons and by increased organism P CO2 (hypercapnia) [5]–[7]. These stressors are likely to have deleterious interactive effects; increased temperature has a stimulatory effect on physiological processes (until thresholds are reached) while hypercapnia has a suppressive, narcotic effect [6]–[7]. In assessing risk to marine biota from climate change it is critical to investigate interactive effects of stressors in multifactorial experiments as this better reflects the real world scenario [1], [7].
Temperature, pH, P CO2 and CaCO3 saturation are among the most important environmental factors controlling the distribution, physiological performance, morphology and behaviour of marine invertebrates [6]–[8]. The projected reduction in CaCO3 saturation presents a major challenge to calcifiers in producing their skeletons. Fragile larval skeletons may be the weak link for persistence of some species. For benthic organisms, compromised larval performance has implications for recruitment success and persistence of adult populations [1], [9].
Despite the well known controlling influence of temperature on development and the thermal thresholds exhibited by embryos, investigation of the impacts of climate change on marine life histories has largely focussed on ocean acidification as the sole stressor [10]–[11]. The potential for interactive effects of ocean warming and CO2 driven acidification on larval development remains largely unexplored. Single stressor studies of P CO2 induced acidification show impaired development in echinoderm and mollusc larvae reared in the acidified/elevated P CO2 conditions projected for 2100 [13]–[17]. In the single study of interactive effects of ocean warming and acidification/P CO2 on early development, echinoid cleavage stage embryos and gastrulae were most affected by temperature [18]. Decreased pH (adjusted with mineral acid) and increased temperature both exert a negative effect on calcification in oyster larvae [19]. For post-larval and juvenile calcifiers transplanted to laboratory mesocosms or adults resident near CO2 vents, acidification and warming both exert negative effects with increased temperature of greatest concern [20]–[22].
We investigated the interactive effect of ocean warming and acidification on the larvae of Tripneustes gratilla, a sea urchin widely distribution throughout the Indo-Pacific [23]. This species is ecologically important, especially in sea grass habitats and is a food source with good potential for aquaculture [23]–[27]. The interactive effects of climate change stressors were investigated in T. gratilla reared in near future conditions in embryos fertilised in experimental conditions. Fertilization in this species is robust to near-future ocean warming and acidification [28]. We focused on the larval stage because it produces a fragile calcite skeleton, and because this life stage has a planktonic period of days or weeks in the water column where seawater chemistry and temperature have a major impact on development. Echinoplutei produce calcite rods that support their body, and function in swimming and feeding. Arm length, and thereby calcite rod growth, has a direct influence on the efficiency of larval feeding and on vulnerability to predation [29]–[30]. Temperature has a major influence on development in shortening the planktonic period, an effect that decreases predation pressure and also alters connectivity between populations [31]–[32].
Our experiments were placed in a climate and regionally relevant setting for the SE Australia climate change hot spot (warming: +3–6°C; acidification: −0.3–0.5 pH units) [4], [33]. Due to changes in ocean circulation, this region is warming considerably faster than the global average [33]. Fertilisation in T. gratilla and other echinoids is robust to climate change stressors [28], [34]–[35] and larval survival in this species decreases at pH 7.0 [15]. We predicted a) that development would be facilitated by warming up to a threshold; b) that skeletogenesis would be impaired by increased acidification/P CO2 and, c) due to temperature enhancement of metabolic processes, increased temperature would counter the negative effects of decreased calcite and aragonite saturation on skeletogenesis.
Results
Normal Development
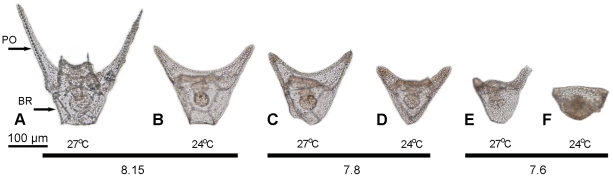
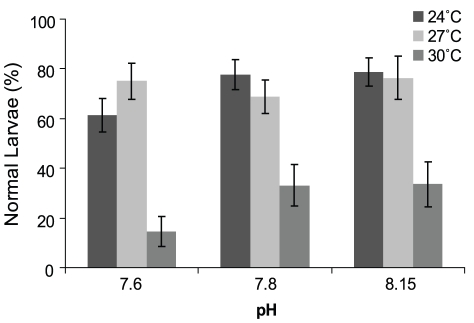
The range of morphology of T. gratilla seen in the treatments on day 5 is shown in Fig. 1. There was a significant effect of temperature and pH/P CO2, on the percentage of normal larvae (temp: p = <0.003; pH: p = 0.04; Table 1, Fig. 2). The upper warming, +6 (30°C) approached the thermal tolerance of development (<30% normal larvae). The percentage of normal larvae was highest (>85%) in the control pH/P CO2 and 24°C and 27°C treatments. At 24°C and 27°C, the percentage of normal larvae was >60% across all treatments. The slight decrease in normal development at 27°C pH 7.8 (Fig. 2) was not statistically significant (Table 1). Egg source was significant (p = <0.0001), but did not interact with the other factors.
Figure 1. Tripneustes gratilla larvae reared for 5 days in 3 pH and 2 temperature treatments.
A-B. Control pH 8.15, largest larvae were from +3°C (27°C) treatments. PO, post oral arms; BR, body rod. C-D. pH 7.8. E-F. pH 7.6. With increased acidity/P CO2 larval size decreased and there was an increase in abnormal development.
Table 1. ANOVA of percentage normal Tripneustes gratilla larvae reared in temperature (temp) and pH/P CO2 (as fixed factors) treatments, with egg source (female) as a random factor, and Tukey-Kramer post-hoc tests (TK).
| Source | df | MS | F | p | TK |
| Temp* | 2 | 3.595 | 35.3 | 0.0029 | (24, 27) >30 |
| pH* | 2 | 0.230 | 8.0 | 0.0402 | (8.15, 7.8) (7.8, 7.6), 8.15 >7.6 |
| temp × pH | 4 | 0.126 | 2.2 | 0.1598 | |
| Female* | 2 | 1.348 | 30.6 | <0.0001 | |
| Temp × female | 4 | 0.101 | 2.3 | 0.0691 | |
| pH × female | 4 | 0.028 | 0.7 | 0.6243 | |
| temp × pH × female | 8 | 0.057 | 1.3 | 0.2595 | |
| Residual | 54 | 0.044 | |||
| Total | 80 |
*Significant, p<0.05; df, degrees of freedom; MS, mean square; n = 3 replicates for each of 3 females.
Figure 2. Percentage of normal T. gratilla larvae.
Percentage of normal T. gratilla larvae in nine treatments (3 pH×3 temperature levels) in the larvae from 3 females. See Table 4 for P CO2, Ωcalcite and Ωaragonite conditions.
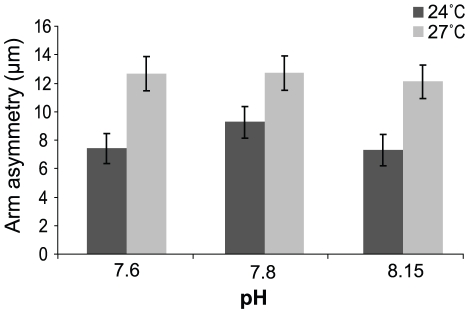
The mean difference in PO arm length (larval asymmetry) differed among the 24°C and 27°C treatments (p = 0.001; Table 2, Fig. 3). Asymmetry was most marked in the faster growing larvae reared at +3°C (27°C). Arm asymmetry was not significantly affected by pH/P CO2 (p = 0.545; Table 2). There was no interaction between factors.
Table 2. ANOVA on difference in PO arm length (asymmetry) data for Tripneustes gratilla larvae reared in temperature (temp) and pH/P CO2 (as fixed factors) treatments.
| Source | df | MS | F | p |
| pH | 2 | 0.4 | 0.6 | 0.545 |
| Temp* | 1 | 0.9 | 17.1 | 0.001 |
| pH × temp | 2 | 0.02 | 0.4 | 0.687 |
| Residual | 12 | 0.05 | ||
| Total | 17 |
*Significant, p<0.05; df, degrees of freedom; MS, mean square; n = 3 from the means of 35 larvae per female.
Figure 3. Mean arm asymmetry in T. gratilla larvae.
Mean arm asymmetry in T. gratilla larvae in six treatments (3 pH ×2 temperature levels) in 35 larvae from each of 3 females (n = 3, ±SE). See Table 4 for P CO2, Ωcalcite and Ωaragonite conditions.
Larval Growth
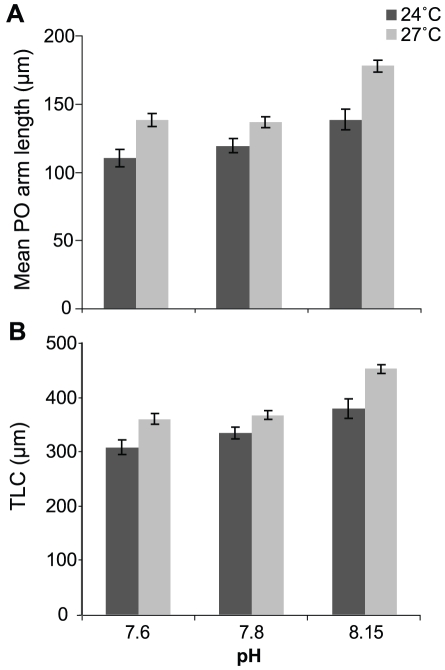
Five-day echinoplutei had well developed PO arms and these were the longest skeletal element (Fig. 1). Arm length significantly increased with temperature and decreased in acidified conditions (temp: p = <0.0001; pH: p = <0.0001; Table 3, Fig. 1,4). The PO arms were longer in the +3°C (27°C) treatment than in controls (24°C), 178.1 µm (SD = 3.9, n = 35) and 138.7 µm (SD = 2.6, n = 35), respectively. Larvae reared at pH 7.6 and pH 7.8 had smaller PO arms when compared with those reared at control pH across both temperature treatments (TK 7.6 = 7.8<8.15; Table 3, Fig. 1). However a +3°C warming diminished the negative effects of low pH/high P CO2. This is seen in the similar PO arm length of larvae reared at 27°C/pH 7.6 and 27°C/pH 7.8 and those reared in control temperature and pH (Fig. 4). As TLC is largely comprised of the PO arms, this measure followed a similar pattern (temp: p = 0.0001; pH: p = <0.0001; Table 3; Fig. 4). There was no interaction between temperature and pH (Table 3).
Table 3. ANOVA of mean post oral arm length (PO) and total length of calcite rod (TLC) data for Tripneustes gratilla larvae reared in temperature (temp) and pH/P CO2 (as fixed factors) treatments, and Tukey-Kramer post-hoc tests (TK).
| Parameter | Source | df | MS | F | p | TK |
| PO | pH* | 2 | 2105 | 28 | <0.0001 | 8.15>(7.8, 7.6) |
| Temp* | 1 | 3621 | 48 | <0.0001 | ||
| pH × temp | 2 | 183 | 2.4 | 0.1297 | ||
| Residual | 12 | 75 | ||||
| Total | 17 | |||||
| TLC | pH* | 2 | 11136 | 27 | <0.0001 | 8.15>(7.8, 7.6) |
| Temp* | 1 | 12448 | 30 | 0.0001 | ||
| pH × temp | 2 | 624 | 1.5 | 0.2623 | ||
| Residual | 12 | 416 | ||||
| Total | 17 |
*Significant, p<0.05; df, degrees of freedom; MS, mean square; n = 3 from the means of 35 larvae per female.
Figure 4. Postoral arm and total calcite rod length in T. gratilla larvae.
A. Mean post oral (PO) arm length and B. total length of calcite rods (TLC) of T. gratilla larvae in six treatments (3 pH ×2 temperature levels) in 35 larvae from each of 3 females (n = 3, ±SE). See Table 4 for P CO2, Ωcalcite and Ωaragonite conditions.
Discussion
In this first study of the effects of simultaneous exposure to warming and CO2 driven acidification on calcification in marine invertebrate larvae reared in experimental conditions from fertilization, we show the positive and negative effects of these stressors. Larval growth in T. gratilla was positively correlated with increased temperature across all pH treatments until the thermal threshold was breached, supporting our first prediction. In contrast, larval growth was negatively correlated with increased acidity/P CO2 and decreased calcite and aragonite saturation, resulting in smaller larvae, supporting prediction two. Warming countered to some extent the negative effects of acidification, providing some support for prediction three.
Temperature is considered to be the primary environmental factor controlling the physiology, phenology, planktonic larval duration and biogeography of marine invertebrates [31]–[32], [36]–[37]. The response of T. gratilla larvae to increased temperature reflected the typical pattern seen in echinoids and other invertebrates with a balance between facilitation at certain levels of warming and failure at upper thermal limits [32], [37]–[41]. Temperature is well known to control the pace of development in marine larvae. A +3°C warming resulted in faster growth and increased size in T. gratilla larvae.
Developmental thermotolerance varies greatly between echinoids with a +4°C warming above ambient approaching the thermal limit of many species [34]. Embryogenesis in tropical species such as T. gratilla and Echinometra spp. is more robust to thermal increase [37], [38], [41]. For T. gratilla, 30°C approximates the lethal threshold for development in both high and low latitude populations [41, this study]. This thermotolerance will facilitate persistence of T. gratilla and possible poleward spread from its southern limit in Australia where mean SST are not expected to go beyond ∼28°C by 2100, but does not bode well for tropical populations where SSTs will continue to exceed 30°C [4], [33].
A +3°C warming enhanced larval growth of T. gratilla across all pH/P CO2 treatments, to some extent buffering the negative effects of these factors. Larvae reared at low pH had significantly shorter PO arms than those reared in control pH suggesting suppressed calcification, as in a previous study [15]. This reduction in size is likely due to hypercapnic suppression of metabolism causing delayed development and decreased availability of CaCO3 for skeletogenesis. Decreased biomineralisation in response to near-future (ca. 2100) acidification is reported for other echinoid larvae [13], [14], [17].
Increased ocean acidity, hypercapnia and decreased carbonate mineral saturation are inextricably linked and are all likely to exert negative effects on larvae. This may be through direct pH effects on metabolic systems such as those involved with calcite precipitation (eg. carbonic anhydrase) and cellular protection (eg. heat shock proteins) and direct hypercapnic suppression of metabolism [6], [7], [17], [42]–[44]. Although calcite and aragonite remained saturated in our treatments (Ωcalcite 1.6–5.9, Ωaragonite 1.1–4.0), they decreased markedly at low pH, with aragonite approaching minimal levels. At Ω <1, seawater becomes corrosive causing dissolution and impaired skeleton deposition in larval and adult sea urchins [8], [44].
Calcification in sea urchin larvae occurs internally, under a different chemical environment than surrounding seawater through an amorphous phase of CaCO3 that would dissolve if exposed to low pH seawater [45], [46]. Echinoderms and other invertebrates adjust internal pH through accumulation of bicarbonate ions [42], which would alter internal calcite conditions. Some echinoderms and other benthic calcifiers may be able to maintain an alkaline environment at the internal mineralization site despite reduced external pH [46]. What is poorly understood is how the availability of carbonate ions in the ocean affects this process.
The effects of ocean acidification on marine calcifiers vary among phyla, species, life history stages and latitudes/habitats. With regard to the pelagic life stage, some larvae show deleterious effects of near future P CO2 driven acidity, while others, even closely related species are more robust [16], [34], [47]. Embryos may be most vulnerable to warming while larvae that survive early mortality bottlenecks may be more affected by acidification [13], [15], [18], [34], [47]. Regional settings for projected change are also a crucial consideration. Cold high latitude waters will become carbonate under-saturated first and so high latitude calcifiers may be most vulnerable to ocean acidification [5]. However, a recent study showed that Antarctic echinoplutei were less affected by acidification than temperate and tropical counterparts [15].
Despite the pervasive effect of ocean warming on development, this factor is rarely considered in studies of climate change impacts. Larval performance may differ in experiments when temperature is brought into the mix of factors assessed. For regions with significant warming such as SE Australia, temperature is the most immediate and contemporary climate change stressor. Many progeny will not reach the calcified larval stage in a warm ocean, regardless of pH/P CO2 changes [18]. Our data for T. gratilla were from embryos fertilised and reared in experimental conditions to the larval stage. The larvae were from the subset of survivors available for measurement. With regard to comparisons between climate change stressor studies, some studies translocate embryos fertilised in present day conditions to experimental treatments and others rear embryos from the outset in experimental conditions [34]. Experimental outcomes may differ between these approaches, the latter being more realistic.
While our results clearly showed the effects of climate change stressors on larval development, egg source also exerted a significant influence. We did not set out to test maternal effects, but it is important to be cognisant of this factor in considering the larval responses. Maternal provisioning influences larval tolerance and ecological outcomes for invertebrate larvae [48]–[49].
With respect to the benthic life phase of marine calcifiers, numerous studies investigate the response of juveniles or adults sourced from field collections or aquaculture translocated from present day to acidified conditions [21]–[22], [44], [46], [50]–[53]. These studies show varied responses, decreased calcification in some species, no change in others and increased calcification in others. A study of arm regeneration in an ophiuroid showed increased calcification at low pH [53]. Simultaneous exposure to warming and acidification resulted in increased growth in juvenile sea stars [52]. The contrasting responses among species are likely to be due to differences in calcifying systems [46] and the environmental history of the organisms prior to being placed in treatments.
We focussed on the pelagic life phase because this is the crucial dispersal stage and is considered to be most vulnerable to environmental perturbations [47]. The thin arm rods of echinoderm plutei are essential for feeding, swimming and protection from predation and feeding success is related to arm length [29]–[30]. Smaller larvae with a longer planktonic duration are more vulnerable to predation in a changing ocean, decreasing chances of survival and recruitment [54]. Projected near future ocean change may result in a major bottleneck for marine life histories with negative flow on effects for the integrity of benthic populations and communities [1], [2], [9]. Calcifying taxa across many phyla play important roles in marine ecosystem function as bioturbators and keystone species and, on a larger scale, biocalcification plays a critical role in the carbon cycle [55]. Negative impacts on calcifiers in a changing ocean have far-reaching implications for biodiversity and ocean health.
Materials and Methods
Specimen collection and spawning
Tripneustes gratilla, collected near Coffs Harbour, New South Wales (30°12.5'S. 153°16.1'E.), were maintained in flow-through aquaria (∼3500 L) at ambient temperature (∼24°C). They were induced to spawn by injection of 2–3 ml 0.5 M KCl. The eggs of three females were spawned into 500 ml beakers of filtered seawater (FSW 0.2 µm). Sperm were collected dry using pipettes. Before use, the eggs were checked for shape and integrity and sperm were checked for motility. The eggs of each female were fertilised by sperm from multiple males. Each experiment was undertaken with independent sources of gametes with replication based on the three females.
For each egg source ca. 2000 eggs (∼20 eggs ml−1) were placed in rearing containers (100 ml), three for each temperature-pH treatment (see below), in flow-through experimental FSW (flow rate ca. 0.13 ml sec−1, 300–400 turnovers day−1) for 20 minutes prior to the introduction of sperm. The containers had a window cut from each side as an overflow and a 45 µm mesh set back from the overflow to retain eggs. The number of sperm required to achieve a sperm to egg ratio of ca. 1000:1, was determined through haemocytometer counts. The sperm was briefly activated (1–2 sec) in experimental FSW prior to addition to containers holding eggs. The flow-through system was turned off (5 min) during fertilisation and was then turned back on to remove excess sperm. This fertilisation procedure was repeated in separate experiments with the eggs of the three females.
The embryos were reared in experimental conditions to the 5 day echinopluteus stage and were not fed to avoid the potentially confounding influence of algal introduction. Tripneustes gratilla embryos have substantial maternal energetic reserves with a long (8 day +) facultative feeding period during which development proceeds in the absence of exogenous food [49]. We chose the 5 day endpoint because by this stage the larvae have well developed arms for measurement and are not nutritionally limited.
Experimental treatments and rearing
The embryos were reared in experimental flow-through FSW in three temperature (control = 24°C, +3°C, +6°C) and three pH (control = 8.15, −0.3, −0.5 pH units) levels in all combinations with three containers of embryos per treatment. Experimental pH was adjusted using an automatic CO2 injection system. Two pH controllers (Tunze), set at pH 7.6 and pH 7.8, were attached to two header tanks (60 L). The controllers, pH probes, solenoid valves and gas cylinders were connected in series and injected pure CO2 gas into the header when required, where it was dissolved using a vortex mixing device (Red Sea). The header tanks were continuously bubbled with air to aid mixing and to maintain dissolved oxygen (DO) >90%. A constant volume was maintained in the headers using a float valve. A control header was bubbled with air only. This water was fed into sub-header tanks (20 L) where it was warmed to the required temperature, +3°C (27°C) and +6°C (30°C), using aquarium heaters or unmanipulated for the ambient control. Seawater was delivered to rearing containers using irrigation drip valves. Temperature, pHNBS, DO and salinity at the level of the experimental containers with developing embryos and larvae were measured daily with a WTW multiprobe. Filtered ambient in flow water was not manipulated and had a mean temperature 23.51°C (SE = 0.04, n = 10, Range 23.3–23.8°C) and mean pH 8.13 (SE = 0.004, n = 10, Range pH 8.08–8.16). The experimental water conditions measured at the level of the rearing containers remained stable (+3°C: Mean 26.13°C, SE = 0.14, Range 25.6–26.6°C; +6°C: Mean 30.35°C, SE = 0.25, Range 29.1–31.9°C) (pH −0.3 units: Mean 7.8, SE = 0.006, Range pH 7.76–7.87; pH −0.5 units: Mean 7.61, SE = 0.004, Range pH 7.58–7.65). Total alkalinity (TA  = 2427.1, SE = 5.2, n = 4) was determined by potentiometric titration (CSIRO, Hobart). Experimental P
CO2 and calcite and aragonite saturation values (Table 4) were determined from TA, pHNBS and salinity data using CO2SYS [56]. Data for both carbonate minerals were calculated because the saturation state of echinoderm magnesian calcite may be close to that of aragonite [57].
= 2427.1, SE = 5.2, n = 4) was determined by potentiometric titration (CSIRO, Hobart). Experimental P
CO2 and calcite and aragonite saturation values (Table 4) were determined from TA, pHNBS and salinity data using CO2SYS [56]. Data for both carbonate minerals were calculated because the saturation state of echinoderm magnesian calcite may be close to that of aragonite [57].
Table 4. Temperature (T), pH, P CO2, and calcium carbonate saturation conditions in the nine experimental treatments.
| T | 24°C | 27°C | 30°C | ||||||
| pH | 8.15 | 7.8 | 7.6 | 8.15 | 7.8 | 7.6 | 8.15 | 7.8 | 7.6 |
| PCO2 | 448 (3) | 1142 (8) | 1886 (13) | 455 (3) | 1169 (8) | 1938 (14) | 460 (3) | 1196 (9) | 1990 (14) |
| Ωcalcite | 5.1 (0.00) | 2.6 (0.02) | 1.7 (0.02) | 5.5 (0.05) | 2.8 (0.03) | 1.9 (0.20) | 5.9 (0.05) | 3.1 (0.03) | 2.0 (0.02) |
| Ωaragonite | 3.4 (0.03) | 1.7 (0.02) | 1.1 (0.01) | 3.7 (0.03) | 1.9 (0.02) | 1.2 (0.01) | 4.0 (0.04) | 2.1 (0.02) | 1.4 (0.01) |
Mean (SEM) n = 4
Development
Specimens from each rearing container (n = 100–200, where available) were placed in 1.5 ml tubes containing 10% formaldehyde-FSW for 10 min, followed by rinse in 70% ETOH in FSW. The first 30 specimens removed randomly from each tube were examined microscopically to score the percentage of normal development. Thus there were three replicate data points per female for this analysis (i.e. 3 containers X 3 temp X 3 pH). Normal larvae were defined as echinoplutei with two arms and a trapezoidal/triangular body (Fig. 1), including larvae with minimal asymmetry (i.e. one arm <30% longer than the other). Abnormal specimens included larvae with marked asymmetry (one arm ≥30% larger than the other), armless arrested larvae and arrested embryos (Fig. 1).
Larval growth
Larval growth was documented in an image analysis study of photographs of larvae reared at 24°C and 27°C. The 30°C treatments were excluded due to high mortality and insufficient larvae to measure. Haphazardly selected plutei positioned flat to the plane of focus were photographed using a digital camera mounted on a compound microscope. For each female 35 larvae (taken across the three rearing containers) from each treatment were measured using Image J (NIH, USA). Thus a total of 630 larvae were used (3 females ×35 larvae ×3 pH×2 temperatures). For each larva the length of the two post oral (PO) arms body rods (BR) were measured. The mean length of the two PO arms was determined and the difference in their length was calculated as a measure of arm asymmetry. Total length of calcite rods (TLC), determined as the sum of all skeletal elements was used as a proxy for biocalcification.
Statistics
For the percentage of normal development data where larvae from three separate rearing containers were scored per female a three factor ANOVA with pH and temperature as fixed orthogonal factors and egg source as a random factor was used. Percentage data were arcsine transformed prior to analysis. Homogeneity of variance was checked using Cochran’s test. For the data on difference in PO arm lengths (arm asymmetry), PO length and TLC where a single mean data point derived from 35 larvae sourced from across 3 rearing containers was determined, a two factor ANOVA with pH and temperature as fixed factors was used. The raw data on arm asymmetry was heterogeneous and was ln(x) transformed prior to analysis to meet the assumptions of ANOVA. Normality was confirmed by plotting residuals against normal distributions. Where treatments differed, Tukey-Kramer (TK) post-hoc tests were conducted to detect differences amongst means. For the mixed model ANOVA on the percentage normal data we ran the TK test using the interaction term MS and the residual MS and note that the results were identical. All statistics were carried out using NCSS 2007 (V 17).
Acknowledgments
The reviewers are thanked for helpful comments.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: The study was funded by the Australian Research Council. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Contribution #, Institute for Conservation Biology and # from Sydney Institute of Marine Science.
References
- 1.Przeslawski R, Ahyong S, Byrne M, Worheide G, Hutchings P. Beyond corals and fish: the effects of climate change on non-coral benthic invertebrates of tropical reefs. Glob Ch Biol. 2008;14:2773–2795. [Google Scholar]
- 2.Brierley AS, Kingsford MJ. Impacts of climate change on marine organisms and ecosystems. Curr Biol. 2009;19:602–614. doi: 10.1016/j.cub.2009.05.046. [DOI] [PubMed] [Google Scholar]
- 3.Caldeira K, Wickett ME. Anthropogenic carbon and ocean pH. Nature. 2003;425:365. doi: 10.1038/425365a. [DOI] [PubMed] [Google Scholar]
- 4.IPCC (Intergovernmental Panel on Climate Change) Cambridge: Cambridge University press; 2007. The fourth assessment report of the IPCC. [Google Scholar]
- 5.Feely RA, Sabine CL, Lee K, Berelson W, Kleypas J, et al. Impact of anthropogenic CO2 on the CaCO3 system in the oceans. Science. 2004;305:362–366. doi: 10.1126/science.1097329. [DOI] [PubMed] [Google Scholar]
- 6.Pörtner H-O. Ecosystem effects of ocean acidification in times of ocean warming: a physiologist’s view. Mar Ecol Prog Ser. 2008;373:203–217. [Google Scholar]
- 7.Widdicombe S, Spicer JI. Predicting the impact of ocean acidification on benthic biodiversity: what can animal physiology tell us? J Exp Mar Biol Ecol. 2008;366:187–197. [Google Scholar]
- 8.Doney SC, Fabry VJ, Feely RA, Kleypas JA. Ocean acidification: the other CO2 problem. Ann Rev Mar Sci. 2009;1:169–192. doi: 10.1146/annurev.marine.010908.163834. [DOI] [PubMed] [Google Scholar]
- 9.Uthicke S, Schaffelke B, Byrne M. A boom-bust phylum? Ecological and evolutionary consequences of density variations in echinoderms. Ecol Mon. 2009;79:3–24. [Google Scholar]
- 10.Dupont S, Orega-Martίnez O, Thorndyke M. Impact of near-future ocean acidification on echinoderms. Ecotoxicology. 2010;19:449–462. doi: 10.1007/s10646-010-0463-6. [DOI] [PubMed] [Google Scholar]
- 11.Hendriks IE, Duarte CM, Álvarez M. Vulnerability of marine biodiversity to ocean acidification: a meta-analysis. Est Coast Shelf Sci. 2010;86:157–164. [Google Scholar]
- 12.Lawrence JM, Agatsuma Y. Ecology of Tripneustes. In: Lawrence JM, editor. Edible sea urchins: biology and ecology. Amsterdam: Elsevier; 2007. pp. 499–520. [Google Scholar]
- 13.Kurihara H, Shirayama Y. Effects of increased atmospheric CO2 on sea urchin early development. Mar Ecol Prog Ser. 2004;274:161–169. [Google Scholar]
- 14.Dupont S, Havenhand J, Thorndyke W, Peck L, Thorndyke M. Near-future level of CO2-driven ocean acidification radically affects larval survival and development in the brittlestar Ophiothrix fragilis. Mar Ecol Prog Ser. 2008;373:285–294. [Google Scholar]
- 15.Clark D, Lamare M, Barker M. Response of sea urchin larvae (Echinodermata: Echinoidea) to reduced seawater pH: a comparison among tropical, temperate, and a polar species. Mar Biol. 2009;156:1125–1137. [Google Scholar]
- 16.Miller AW, Reynolds AC, Sobrino C, Riedel GF. Shellfish face uncertain futures in high pCO2 world: influence of acidification in oyster larvae calcification and growth in estuaries. PLoS ONE. 2009;4:108. doi: 10.1371/journal.pone.0005661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Donnell MJ, Todgham AE, Sewell MA, LaTisha MH, Ruggiero K, et al. Ocean acidification alters skeletogenesis and gene expression in larval sea urchins. Mar Ecol Prog Ser. 2010;398:157–171. [Google Scholar]
- 18.Byrne M, Ho M, Selvakumaraswamy P, Nguyen HD, Dworjanyn SA, et al. Temperature, but not pH, compromises sea urchin fertilisation and early development under near-future climate change scenarios. Proc R Soc B. 2009;276:1884–1889. doi: 10.1098/rspb.2008.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parker LM, Ross PM, O’Connor WA. The effect of ocean acidification and temperature on the fertilization and embryonic development of the Sydney rock oyster Saccostrea glomerata (Gould 1850). Glob Change Biol. 2009;15:2123–2136. [Google Scholar]
- 20.Hall-Spencer JM, Rodolfo-Metalpa R, Martin S, Ransome E, Fine M, Turner SM, Rowley SJ, Tedesco D, Buia M-C. Volcanic carbon dioxide vents reveal ecosystem effects of ocean acidification. Nature. 2008;454:96–99. doi: 10.1038/nature07051. [DOI] [PubMed] [Google Scholar]
- 21.Findlay HS, Kendall MA, Spicer JI, Widdicombe S. Post-larval development of two intertidal barnacles at elevated CO2 and temperature. Mar Biol. 2010;157:725–735. [Google Scholar]
- 22.Rodolfo-Metalpa R, Lombardi C, Cocito S, Hall-Spencer JM, Gambi MC. Effects of ocean acidification and high temperatures on the bryozoan Myriapora truncata at natural CO2 vents. Marine Ecology. 2010 DOI: 10.1111/j.1439-0485.2009.00354.x. [Google Scholar]
- 23.Lawrence JM, Agatsuma Y. Lawrence JM, editor. Ecology of Tripneustes. The biology and ecology of edible urchins. 2007. pp. 499–520. Elsevier Science, Amsterdam.
- 24.Koike I, Mukai H, Nojima S. The role of the sea urchin Tripneustes gratilla (Linnaeus), in decomposition and nutrient cycling in a tropical sea grass bed. Ecol Res. 1987;2:19–29. [Google Scholar]
- 25.Juinio-Menez MA, Macawaris N, Bangi H. Community-based sea urchin (Tripneustes gratilla) grow-out culture as a resource management tool. Can Spec Pub Fish Aquat Sci. 1998;125:393–399. [Google Scholar]
- 26.Dworjanyn SA, Pirozzi I, Liu W. The effect of the addition of algae feeding stimulants to artificial diets for the sea urchin Tripneustes gratilla. Aquaculture. 2007;273:624–633. [Google Scholar]
- 27.Unsworth RKF, Cullen LC, Pretty JN, Smith DJ, Bell JJ. Economic and subsistence values of the standing stocks of seagrass fisheries: Potential benefits of no-fishing maring protected area management. Ocean and Coastal Management. 2010;53:218–224. [Google Scholar]
- 28.Byrne M, Soars NA, Ho MA, Wong E, McElroy D, et al. Fertilization in a suite of coastal marine invertebrates from SE Australia is robust to near-future ocean warming and acidification. Mar Biol (in press) 2010 DOI: 10.1007/s00227-010-1474-9. [Google Scholar]
- 29.Allen JD. Size-specific predation on marine invertebrate larvae. Biol Bull. 2008;214:42–49. doi: 10.2307/25066658. [DOI] [PubMed] [Google Scholar]
- 30.Soars N, Prowse TAA, Byrne M. Overview of phenotypic plasticity in echinoid larvae, ‘Echinopluteus transversus’ type vs. typical echinoplutei. Mar Ecol Prog Ser. 2009;383:113–125. [Google Scholar]
- 31.O'Connor MI, Bruno JF, Gaines SD, Halpern BS, Lester SE, et al. Temperature control of larval dispersal and the implications for marine ecology, evolution, and conservation. Proc Natl Acad Sci U S A. 2007;104:1266–1271. doi: 10.1073/pnas.0603422104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Byrne M, Selvakumaraswamy P, Ho MA, Woolsey E, Nguyen HD. Sea urchin development in a global change hot spot, potential for southerly migration of thermotolerant propagules. Deep Sea Res II (in press) 2010 [Google Scholar]
- 33.Poloczanska ES, Babcock RC, Butler A, Hobday AJ, Hoegh-Guldberg O, et al. Climate change and Australian marine life. Ocean Mar Biol Ann Rev. 2007;45:407–478. [Google Scholar]
- 34.Byrne M. Impact of climate change stressors on marine invertebrate life histories with a focus on the Mollusca and Echinodermata. In: Yu J, Henderson-Sellers A, editors. Climate alert: Climate change monitoring and strategy. Sydney: University of Sydney Press; 2010. pp. 142–185. [Google Scholar]
- 35.Byrne M, Soars N, Selvakumaraswamy P, Dworjanyn SA, Davis AR. Sea urchin fertilisation in a warm, acidified and high pCO2 ocean across a range of sperm densities. Mar Env Res. 2010;69:234–239. doi: 10.1016/j.marenvres.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 36.Fujisawa H. Differences in temperature dependence of early development of sea urchins with different growing seasons. Biol Bull. 1989;176:96–102. [Google Scholar]
- 37.Sewell MA, Young CM. Temperature limits to fertilisation and early development in the tropical sea urchin Echinometra lucunter. J Exp Mar Biol Ecol. 1999;236:291–305. [Google Scholar]
- 38.Rupp JH. Effects of temperature on fertilisation and early cleavage of some tropical echinoderms, with emphasis on Echinometra mathaei. Mar Biol. 1973;23:183–189. [Google Scholar]
- 39.Negri AP, Marshall PA, Heyward AJ. Differing effects of thermal stress on coral fertilisation and early embryogenesis in four Indo Pacific species. Coral Reefs. 2007;26:759–763. [Google Scholar]
- 40.Whalan S, Ettinger-Epstein P, de Nys R. The effect of temperature on larval pre-settlement duration and metamorphosis for the sponge, Rhopaloeides odorabile. Coral Reefs. 2008;27:783–786. [Google Scholar]
- 41.Rahman S, Tsuchiya M, Uehara T. Effects of temperature on hatching rate, embryonic development and early larval survival of the edible sea urchin, Tripneustes gratilla. . Biologia. 2009;64:768–775. [Google Scholar]
- 42.Dubois P, Chen C-P. Calcification in echinoderms. In: Jangoux M, Lawrence JM, editors. Echinoderm Studies volume 3. Rotterdam: Balkema; 1989. pp. 109–178. [Google Scholar]
- 43.Todgham AE, Hofmann GE. Transcriptomic response of sea urchin larvae Strongylocentrotus purpuratus to CO2-driven seawater acidification. J Exp Biol. 2009;212:2579–2594. doi: 10.1242/jeb.032540. [DOI] [PubMed] [Google Scholar]
- 44.Miles H, Widdicombe S, Spicer J, Hall-Spencer J. Effects of anthropogenic seawater acidification on acid-base balance in the sea urchin Psammechinus miliaris. Mar Poll Bull. 2007;54:89–96. doi: 10.1016/j.marpolbul.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 45.Politi Y, Arad T, Klein E, Weiner S, Addadi L. Sea urchin spine calcite forms via a transient amorphous calcium carbonate phase. Science. 2004;306:1161–1164. doi: 10.1126/science.1102289. [DOI] [PubMed] [Google Scholar]
- 46.Ries JB, Cohen AL, McCorkle DC. Marine calcifiers exhibit mixed responses to CO2- induced ocean acidification. Geology. 2009;37:1131–1134. [Google Scholar]
- 47.Kurihara H. Effects of CO2-driven ocean acidification on the early development stages of invertebrates. Mar Ecol Prog Ser. 2008;373:275–284. [Google Scholar]
- 48.Marshall DJ, Keough MJ. Complex life cycles and offspring provisioning in marine invertebrates. Integr Comp Biol. 2006;46:643–651. doi: 10.1093/icb/icl013. [DOI] [PubMed] [Google Scholar]
- 49.Byrne M, Sewell MA, Prowse TAA. Nutritional ecology of sea urchin larvae: influence if endogenous and exogenous nutrition on echinopluteal growth and phenotypic plasticity in Tripneustes gratilla. . Funct Ecol. 2008;22:643–648. [Google Scholar]
- 50.Shirayama Y, Thornton H. Effect of increased atmospheric CO2 on shallow water marine benthos. J Geophys Res. 2005;C09S08 [Google Scholar]
- 51.Gazeau F, Quiblier C, Jansen JM, Gattuso JP, Middelburg JJ, et al. Impact of elevated CO2 on shellfish calcification. Geophys Res Lett. 2007;34:L07603. [Google Scholar]
- 52.Gooding RA, Harley CDG, Tang E. Elevated water temperature and carbon dioxide concentration increase the growth of a keystone echinoderm. Proc Nat Acad Sci U S A. 2009;106:9316–9321. doi: 10.1073/pnas.0811143106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wood HL, Spicer JI, Widdicombe S. Ocean acidification may increase calcification rates, but at a cost. Proc R Soc B. 2008;275:1767–1773. doi: 10.1098/rspb.2008.0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lamare MD, Barker MF. In situ estimates of larval development and mortality in the New Zealand sea urchin Evechinus chloroticus (Echinodermata: Echinoidea). Mar Ecol Prog Ser. 1999;180:197–211. [Google Scholar]
- 55.Tyrrell T. Calcium carbonate cycling in future oceans and its influence on future climates. J Plank Res. 2008;30:141–156. [Google Scholar]
- 56.Pierrot D, Lewis E, Wallace DWR. ORNL/CDIAC-105a. Oak Ridge, Tennessee: Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, U.S. Department of Energy; 2006. MS Excel Program Developed for CO2 System Calculations. [Google Scholar]
- 57.Morse JW, Arvidson RS, Lüttge A. Calcium carbonate formation and dissolution. Chem Rev. 2007;107:342–381. doi: 10.1021/cr050358j. [DOI] [PubMed] [Google Scholar]