Abstract
Although women with gestational diabetes mellitus (GDM) are advised to incorporate physical activity into their lifestyle in order to reduce their risk of developing type 2 diabetes, it is recognized that new mothers face barriers to postpartum exercise. Thus, we sought to determine whether, following the diagnosis of GDM, women indeed alter their postpartum physical activity patterns, as compared to their peers without GDM. In this prospective observational cohort study, we assessed the physical activity patterns of 238 Caucasian women (58 with GDM, 180 without GDM) in the year prior to pregnancy and in the year following delivery, using the Baecke questionnaire, which evaluates the following 3 domains of physical activity: work, sport activity, and non-sport leisure-time activity. Prior to diagnosis with GDM, women reported lower pre-gravid sport (p=0.010) and leisure-time activity (p=0.013), compared to their peers without GDM. By 1-year postpartum, however, there were no longer significant differences between the GDM and non-GDM groups in either sport or leisure-time activity (p=0.078 and p=0.957, respectively). In particular, women with GDM significantly increased their leisure-time activity over the first year postpartum (F=10.1,p=0.002), whereas the non-GDM group did not (F=0.00,p=0.984). Indeed, on multiple linear regression analysis, GDM independently predicted an increase in leisure-time activity between 1-year pre-gravid and 1-year postpartum (t=2.55,p=0.012). Furthermore, this significant relationship persisted even after adjustment for the finding of pre-diabetes/diabetes at 3-months postpartum (t=2.83,p=0.005). In conclusion, women with GDM successfully increased their leisure-time activity in the first year postpartum, reflecting an element of lifestyle change following this diagnosis.
Keywords: Gestational diabetes, postpartum, physical activity, lifestyle
INTRODUCTION
The diagnosis of gestational diabetes mellitus (GDM) is an important life event that identifies a population of young women at high risk of developing type 2 diabetes (T2DM) in the years following the index pregnancy (1). Accordingly, women who are found to have GDM are advised to adopt healthy lifestyle practices targeting weight control, an approach that has been shown to reduce the risk of progression to T2DM in high-risk individuals, including women with a history of GDM (2,3). Typically, the recommended lifestyle modifications include the institution of healthy eating habits and regular physical activity. In women with GDM, the significance of these recommendations is further underscored by the fact that the population in question is one of young women of childbearing age, in whom the prevention of diabetes will have considerable implications for the patients, their children and society in general.
Recently, a series of studies have noted that there are several potential barriers to physical activity in the postpartum period, including (i) a lack of familial and social supports for new mothers, (ii) variations in risk perception and exercise beliefs, and (iii) disparities in access to healthcare (4–10). In light of these barriers, it is important to determine whether women with GDM are actually able to make any change in their postpartum physical activity. Thus, to address this question in a systematic fashion, we sought to compare physical activity patterns in the first year postpartum to those in the year prior to pregnancy, in women with and without GDM.
RESEARCH DESIGN AND METHODS
This analysis was conducted in the context of an ongoing observational study of early events in the natural history of T2DM, in which a cohort of women recruited at the time of antepartum GDM screening is undergoing longitudinal metabolic characterization in pregnancy and the postpartum period, as previously described in detail (11,12). Standard obstetrical practice at our institution involves universal screening for GDM in all pregnant women at 24–28 weeks’ gestation by a glucose challenge test (GCT), followed by referral for a diagnostic oral glucose tolerance test (OGTT), if the GCT result is abnormal. In the study, healthy pregnant women are recruited from a single hospital either prior to or just after their GCT, with the latter approach having the effect of enriching the study population for women with GDM, as previously noted (11). Regardless of the GCT result, all study participants then undergo a 3-hour 100g OGTT for determination of glucose tolerance status in pregnancy. At 3-months postpartum and 1-year postpartum, participants return to the clinical investigation unit for re-assessment that includes evaluation of glucose tolerance by 2-hour 75g OGTT. The study protocol was approved by the Mount Sinai Hospital Research Ethics Board and all participants have given written informed consent. By Sept. 2007, 308 women had completed the pregnancy OGTT and the 3-months and 12-month postpartum visits. The current analysis was restricted to women of Caucasian descent (n=238), in order to limit the potential confounding effects of previously-demonstrated ethnic differences in access to care post-GDM and ethno-cultural differences in beliefs and practices regarding exercise in women (8,13).
Baseline Evaluation
On the morning of the OGTT in pregnancy, anthropometric measurements of height and weight were obtained and data pertaining to pre-pregnancy weight and medical history were collected by interviewer-administered questionnaire. Pre-gravid physical activity in the year prior to pregnancy was assessed using the Baecke questionnaire, an established instrument that has been extensively validated in several populations, including women of childbearing age (14,15). This questionnaire was completed during the OGTT, with participants thus reporting on their physical activity in the year preceding the pregnancy without knowledge of whether or not they had GDM (16). The Baecke questionnaire measures the following 3 component domains of physical activity: (i) occupation-associated activity (work index); (ii) sport-related physical activity (sport index); and (iii) leisure-time activity not including sports (non-sport leisure-time activity index). The work index quantifies the exertion related to occupational activities, such as sitting, standing, lifting, and walking, as well as associated effects on the individual (eg. fatigue, perspiration). The sport index characterizes vigorous/sports activity with respect to intensity (using the updated Compendium of Physical Activities (17)), duration and frequency. The leisure-time index quantifies the exertion associated with non-sport recreational activities (such as walking and television viewing).
GDM was diagnosed on the antepartum OGTT using the National Diabetes Data Group criteria (18). At our institution, women diagnosed with GDM are referred to a specialized clinic for diabetes in pregnancy, where they are treated with glucose-lowering therapy in pregnancy (dietary and exercise counseling, +/−insulin) and counseled on the benefits of healthy lifestyle practices in the postpartum, in order to control body weight and modify their risk of progression to T2DM. The counseling is provided by a diabetes nurse and/or dietitian in the setting of a single educational class of ~150 minutes in duration. At this class, patients receive a handout that encourages postpartum physical activity for diabetes prevention. The specific text on this handout regarding postpartum physical activity is as follows: “To help decrease the risk of developing type 2 diabetes, it is important to maintain a healthy weight, eat a balanced diet and exercise regularly. Walking is a great form of exercise. Walking 30 minutes 5 days a week at a moderate pace has been shown to lower the chance of developing type 2 diabetes.”
Postpartum Evaluations
Participants returned to the clinical investigation unit at both 3-months and 1-year postpartum. On both occasions, physical examination was performed (including weight and waist measurement) and questionnaires were administered by the study nurse. Glucose tolerance status was determined by 2-hr 75g OGTT, using the criteria of the current Canadian Diabetes Association clinical practice guidelines (19). Pre-diabetes collectively refers to impaired fasting glucose (IFG), impaired glucose tolerance (IGT), and combined IFG/IGT. Postpartum glucose intolerance refers to pre-diabetes and diabetes.
At 3-months postpartum, administration of the Baecke questionnaire assessed only sport activity and leisure-time activity over the preceding 3 months (as many women would not be working at their usual occupation during that time). At 1-year postpartum, the Baecke questionnaire assessed all 3 physical activity component domains (work, sport, leisure-time) over the preceding year (i.e. the one year period since delivery). For the current analysis, in order to address the impact of the diagnosis of GDM on physical activity patterns, we focused on the differences in component domains between 1-year pre-gravid (measured at baseline visit) and 1-year postpartum (measured at the 1-year postpartum visit), since the significant lifestyle implications of caring for an infant in the first several weeks of life would likely limit the relevance of physical activity patterns during the first 3-months postpartum.
Statistical Analyses
All analyses were conducted using the Statistical Analysis System (SAS, Version 9.1, SAS Institute, Cary NC). Continuous variables were tested for normality of distribution and natural log transformations of skewed variables were used, where necessary, in subsequent analyses. In Table 1, univariate differences between the women with GDM and the women without GDM were assessed using one-way Analysis of Variance (ANOVA) for continuous variables and either χ2 or Fisher’s exact test for categorical variables. SAS Proc Mixed was used for repeated ANOVA analysis to test for differences in domains of physical activity between the GDM and non-GDM groups across two levels of time (1-year pre-gravid and 1-year postpartum). This analysis addressed whether (i) the domains changed over time within the groups and (ii) whether they changed differently over time between the groups. These models consider the effects of group, time, and the interaction between group and time. Plots of the least squares means for both groups for each domain at both timepoints are shown in Figure 1. Multiple linear regression analysis was used to determine factors independently associated with the change in leisure-time activity between 1-year pre-gravid and 1-year postpartum (Table 2). Covariates considered were age, pre-pregnancy BMI, family history of diabetes, parity, GDM, waist circumference at 3-months postpartum, and glucose intolerance at 3-months postpartum.
Table 1.
Characteristics of study participants, stratified by presence (n=58) or absence (n=180) of GDM, on assessment on 3 occasions: (i) prior to antepartum OGTT, (ii) at 3-months postpartum, and (iii) at 1-year postpartum
| Non-GDM | GDM | ||
|---|---|---|---|
| (n=180) | (n=58) | p | |
| Baseline Characteristics | |||
| Age (yrs) | 34.4 [4.2] | 33.9 [4.0] | 0.444 |
| Weeks’ gestation at OGTT (wks) | 30.0 [28 – 32] | 29.0 [27 – 31] | 0.041 |
| Family history of DM (%) | 46.1 | 55.2 | 0.231 |
| Parity: | 0.679 | ||
| Nulliparous (%) | 48.5 | 45.3 | |
| One or more (%) | 51.5 | 54.7 | |
| Smoking exposure: | 0.189 | ||
| Never (%) | 63.9 | 72.4 | |
| Remote (%) | 35.0 | 25.9 | |
| Current (%) | 1.1 | 1.7 | |
| Pre-pregnancy BMI (kg/m2) | 23.6 [21.5–27.5] | 25.3 [22.3–30.3] | 0.040 |
| Pre-gravid physical actitivity: | |||
| Work index | 2.3 [1.9–2.8] | 2.4 [2.0–2.9] | 0.472 |
| Sport index | 2.3 [1.8–3.0] | 2.0 [1.5–2.5] | 0.010 |
| Leisure-time index | 3.0 [2.8 – 3.3] | 3.0 [2.5–3.3] | 0.013 |
| Working outside of home (%) | 92.2 | 91.4 | 0.837 |
| 3-months Postpartum | |||
| Months postpartum | 3.1 [2.9–3.6] | 3.0 [2.9–3.4] | 0.505 |
| BMI (kg/m2) | 25.9 [23.4–30.0] | 27.0 [23.7–31.0] | 0.177 |
| Waist circumference (cm) | 84.5 [79.0–93.0] | 89.7 [81.0–96.5] | 0.043 |
| Physical activity: | |||
| Sport index | 2.0 [1.8–2.5] | 1.8 [1.5–2.0] | 0.016 |
| Leisure-time index | 3.3 [2.8–3.3] | 2.8 [2.5–3.0] | 0.001 |
| 1-year Postpartum | |||
| Months postpartum | 12.2 [11.6–13.1] | 12.1 [11.6–13.0] | 0.398 |
| BMI (kg/m2) | 24.6 [22.0–28.5] | 26.7 [22.5–30.5] | 0.137 |
| Waist circumference (cm) | 84.0 [77.2–91.4] | 87.1 [80.0–94.5] | 0.070 |
| Physical actitivity: | |||
| Work index | 3.0 [2.5–3.4] | 3.1 [2.6–3.5] | 0.302 |
| Sport index | 2.5 [1.8–3.0] | 2.0 [1.5–2.6] | 0.078 |
| Leisure-time index | 3.3 [2.8–3.5] | 3.3 [2.8–3.5] | 0.957 |
| Working outside of home (%) | 40.8 | 29.3 | 0.119 |
Data are presented as median followed by interquartile range, with the exception of (i) age (presented as mean followed by standard deviation) and (ii) family history of DM, parity, smoking exposure, and working outside of home (presented as percentages).
p-values refer to overall differences between the groups as derived from ANOVA analysis for continuous variables (with log transformations applied to skewed variables) or χ2 test for categorical variables.
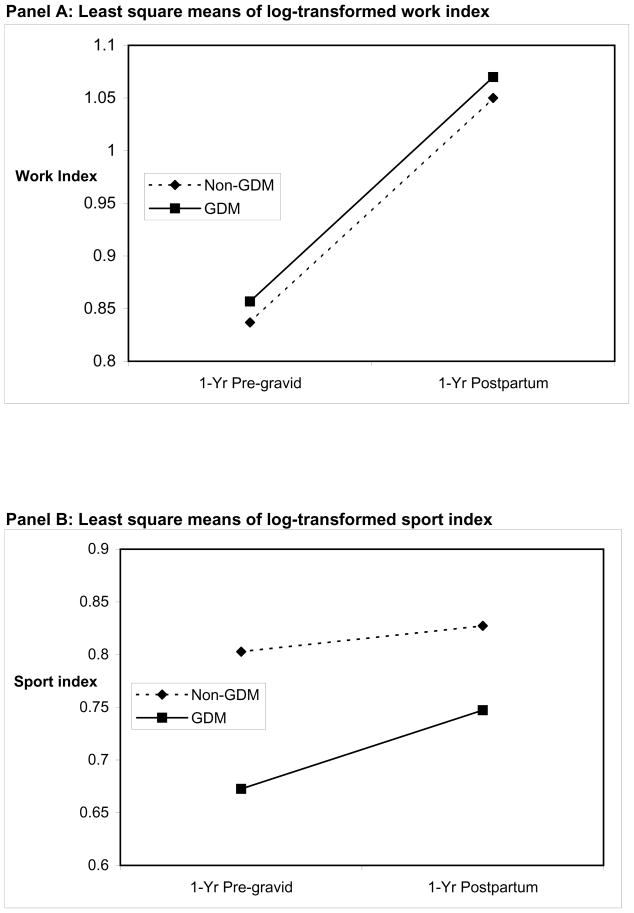
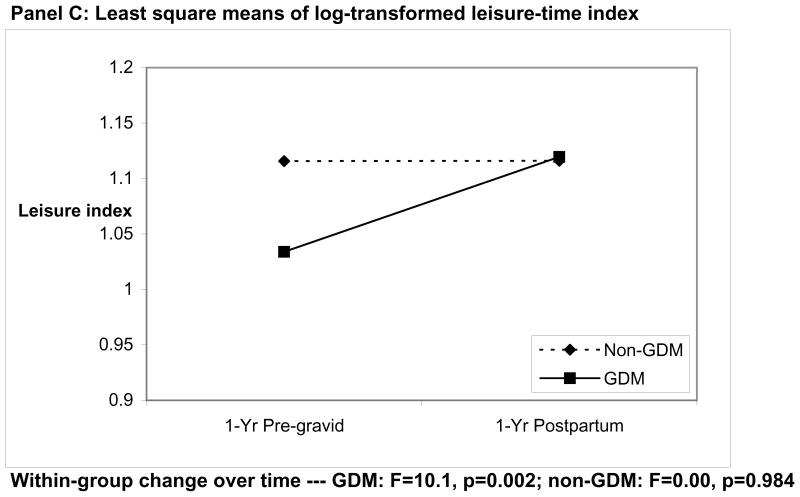
Figure 1.
Plots of change between 1-year pre-gravid and 1-year postpartum in least square means of log-transformed work index (Panel A), sport index (Panel B), and leisure-time index (Panel C), in women with and without GDM.
Table 2.
Multiple linear regression analyses of the (dependent variable) change in leisure-time activity between 1-year pre-gravid and 1-year postpartum: (Model A) adjusted for age, pre-pregnancy BMI, family history of DM, parity and GDM; (Model B) adjusted for covariates in Model A + waist circumference at 3-months postpartum; (Model C) adjusted for covariates in model A + glucose intolerance at 3-months postpartum
| Model A: | ||||
|---|---|---|---|---|
| Variable |
Parameter Estimate |
Standard Error |
t value |
p |
| Age | −0.00014 | 0.00917 | −0.02 | 0.988 |
| Pre-pregnancy BMI | 0.00144 | 0.00719 | 0.20 | 0.842 |
| Family history of DM | 0.07646 | 0.07481 | 1.02 | 0.308 |
| Parity | −0.22640 | 0.07639 | −2.96 | 0.003 |
| GDM | 0.22679 | 0.08904 | 2.55 | 0.012 |
| Model B: | ||||
|
Variable |
Parameter Estimate |
Standard Error |
t value |
p |
| Age | −0.00709 | 0.00949 | −0.75 | 0.456 |
| Pre-pregnancy BMI | −0.00160 | 0.00986 | −0.16 | 0.871 |
| Family history of DM | 0.03206 | 0.07843 | 0.41 | 0.683 |
| Parity | −0.22209 | 0.07863 | −2.82 | 0.005 |
| GDM | 0.22397 | 0.09025 | 2.48 | 0.014 |
| Waist circumference at 3-months | 0.00155 | 0.00444 | 0.35 | 0.727 |
| Model C: | ||||
|
Variable |
Parameter Estimate |
Standard Error |
t value |
p |
| Age | −0.00735 | 0.00941 | −0.78 | 0.436 |
| Pre-pregnancy BMI | −0.00028 | 0.00732 | −0.04 | 0.970 |
| Family history of DM | 0.03788 | 0.07666 | 0.49 | 0.622 |
| Parity | −0.21388 | 0.07835 | −2.73 | 0.007 |
| GDM | 0.26561 | 0.09392 | 2.83 | 0.005 |
| Glucose intolerance at 3-months | −0.17153 | 0.11131 | −1.54 | 0.125 |
RESULTS
Characteristics of Participants at Baseline, 3-months Postpartum and 1-year Postpartum
Table 1 shows the demographic and clinical characteristics of the study participants, stratified according to the presence (n=58) or absence (n=180) of GDM. The antepartum OGTT was performed slightly later in the non-GDM group compared to the GDM group (median 30.0 vs 29.0 weeks’ gestation, p=0.041). Otherwise, there were no significant differences between the groups with respect to age, family history of diabetes, parity or smoking exposure. The women without GDM had lower pre-pregnancy BMI than their peers with GDM (mean 23.6 vs 25.3 kg/m2, p=0.040). Furthermore, for the 1-year period prior to the pregnancy, the GDM group reported lower sport activity and non-sport leisure-time activity than the non-GDM group (p=0.010 and p=0.013, respectively) (Note that, for leisure-time activity, although the median was the same in both groups, the respective interquartile ranges indicate that this measure was higher in the non-GDM group). Pre-gravid work index was not significantly different between the groups.
At three-months postpartum, the prevalence rates of pre-diabetes and diabetes were much higher in the GDM group than in the non-GDM group (pre-diabetes: 24.6% vs 8.0%; diabetes: 3.5% vs 1.2%; overall p=0.002)(These rates in the non-GDM group are likely due to the presence of women with mild gestational dysglycemia (11,12)). Waist circumference was higher in the GDM women (median 89.7 vs 84.5 cm, p=0.043), while BMI was not significantly different between the groups. The GDM group continued to report lower sport and leisure-time activity than the non-GDM groups (p=0.016 and p=0.001, respectively). By 1-year postpartum, however, there were no longer significant differences between the groups in sport (p=0.078) and non-sport leisure-time activity (p=0.957). Furthermore, the difference in waist circumference between the women with GDM and the non-GDM group also no longer reached statistical significance (median 87.1 vs 84.0 cm, p=0.070).
Changes in Domains of Physical Activity Between 1-year Pre-gravid and 1-year Postpartum
As shown by the near parallel lines in Figure 1A, the change in work index between 1-year pre-gravid and 1-year postpartum was similar in both the GDM and non-GDM groups. As such, while work index changed over time (F=98.7, p<0.0001), neither the group effect (F=0.44, p=0.508) nor the interaction between group (GDM vs non-GDM) and time was significant (F=0.0, p=0.999). In the case of sport activity, a group effect was apparent (F=5.72, p=0.018), but neither the change over time (F=3.63, p=0.058) nor the interaction of group and time (F=0.93, p=0.336) achieved significance. Thus, as shown in Figure 1B, the change in sport index between 1-year pre-gravid and 1-year postpartum was not significantly different between the groups.
In contrast to the findings for work and sport index, the change in non-sport leisure time activity was strikingly different between the groups, as readily appreciated in the departure from parallelism between the two lines in Figure 1C. Indeed, the interaction between group (GDM vs non-GDM) and time was significant (F=7.58, p=0.006), indicating that the leisure-time activity as changing over time at different rates within the two groups. Specifically, between 1-year pre-gravid and 1-year postpartum, the women with GDM significantly increased their leisure-time activity (F=10.1, p=0.002), whereas the women without GDM did not (F=0.00, p=0.984).
Determinants of Change in Leisure-Time Activity Between 1-year Pre-gravid and 1-year Postpartum
Having shown that women with GDM increased their non-sport leisure time activity in the first year postpartum, we next sought to determine if the diagnosis of GDM was independently associated with this change. On multiple linear regression analysis (Table 2 Model A), GDM indeed emerged as a positive independent determinant of the change in leisure-time activity between 1-year pre-gravid and 1-year postpartum (t=2.55, p=0.012), while parity was inversely associated with this change (t=−2.96, p=0.003). There was no significant interaction between GDM and parity (t=−0.58, p=0.565, data not shown).
We next sought to determine if the observed independent relationship between GDM and change in leisure-time activity was driven by findings at 3-months postpartum that may have motivated behavioral modification (i.e. such as overweight/obesity or glucose intolerance). In this regard, further adjustment of Model A for waist circumference at 3-months postpartum (Table 2 Model B) did not change the significant relationship between GDM and increased leisure-time activity (GDM: t=2.48, p=0.014). Even more importantly, GDM remained a positive independent predictor after further adjustment for the development of glucose intolerance (i.e. pre-diabetes or diabetes) at 3-months postpartum (t=2.83, p=0.005) (Table 2 Model C). Thus, neither central obesity nor the finding of glucose intolerance at 3-months postpartum accounted for the significant independent association between GDM and increased leisure-time activity between 1-year pre-gravid and 1-year postpartum.
DISCUSSION
In this report, we demonstrate that, compared to their pre-gravid patterns, women with GDM significantly increased their non-sport leisure-time activity in the first year postpartum, unlike their peers without GDM. Indeed, the diagnosis of GDM independently predicted an increase in leisure-time activity between 1-year pre-gravid and 1-year postpartum. Moreover, this relationship was not due to findings of either central obesity or postpartum pre-diabetes/diabetes. Overall, these data suggest that, following the diagnosis of GDM, women can achieve change in their physical activity patterns in the first year postpartum, a potentially encouraging message for diabetes prevention in this high-risk patient population.
There have been limited studies to date of postpartum exercise habits in women with GDM. In a telephone survey of women with a history of GDM in the preceding 6–24 months, Smith and colleagues found that 26.5% of respondents could be classified as sedentary on the basis of their reported walking habits (5). Similarly, telephone survey data from the Behavioral Risk Factor Surveillance System (BRFSS) revealed that women with a history of GDM were more likely to report no leisure-time exertion (20), while another study using BRFSS data found no significant difference in physical activity between women with and without a history of GDM (21). Finally, whereas one mail survey completed at 11–42 months after pregnancy suggested that women with GDM didn’t change their exercise habits post-delivery (22), a separate mail survey completed by 28 women identified increased physical activity at 6-months postpartum (7). Overall, it should be noted that all of these studies were cross-sectional assessments, such that any commentary on physical activity in pregnancy was based on retrospective reporting in the postpartum (at which time the patients were aware that the pregnancy in question had been complicated by GDM).
In extending this literature, the current analysis is bolstered by 4 key strengths in study design. First of all, this study is a prospective longitudinal evaluation with assessment of both pre-gravid and postpartum physical activity patterns. Of note, the baseline questionnaire was administered during the antepartum OGTT, such that participants did not yet know whether they had GDM when reporting on their pre-gravid physical activity. Moreover, the prospective longitudinal design made it possible to demonstrate that women with GDM significantly increased their leisure-time activity in the first year postpartum, when compared to the year prior to pregnancy. A second strength of this study is that the postpartum assessment of physical activity was performed on 2 occasions (at 3- and 12-months). If the postpartum assessment were performed on a single occasion only, bias could be introduced by participants over-reporting their levels of physical activity, based on their knowledge of lifestyle recommendations for women with GDM. In the current study, however, our finding that there were significant differences in physical activity between the GDM and non-GDM groups at 3-months postpartum but not at 12-months postpartum makes it less likely that the latter result was due to over-reporting (as the same potential bias was operative at 3-months). A third strength is that this study included both women with and without GDM. Women without GDM are necessary as a comparator group, since lifestyle challenges associated with new motherhood (such as lack of time and social supports) have been identified as barriers to exercise in the postpartum (4–10). Of note, by comparing women with and without GDM, we have been able to demonstrate that the former, but not the latter, increased their leisure-time activity in the first year postpartum. Finally, use of the Baecke questionnaire made it possible to identify the specific component domain of physical activity (leisure-time activity) where women made lifestyle changes following the diagnosis of GDM.
The current findings also appear to be quite plausible. Specifically, it stands to reason that leisure-time activity (e.g. walking) would be the domain most amenable to change in the context of the first year of motherhood, when lack of time would likely preclude dramatic changes in time-intensive sporting activities. Furthermore, it appears that the main changes in leisure-time activity were made between 3- and 12-months postpartum, given that significant differences in this measure were apparent between the GDM and non-GDM groups at 3-months but had disappeared by 12-months. Again, this timing for change is reasonable, insofar as the significant demands of caring for an infant in the first several weeks of life would make the institution of increased leisure-time activities unlikely during that time period. Finally, it should be noted that the significant inverse relationship between parity and change in leisure-time activity repeatedly observed in the multiple linear regression analyses (models in Table 2) is fully consistent with previous reports, in which parenting duties and lack of support were identified as barriers to postpartum physical activity (4,7,10).
Importantly, our finding that GDM independently predicted an increase in leisure-time activity in the first year postpartum has potentially encouraging implications. Indeed, as this study was observational in nature, no specific counseling on physical activity was systematically provided to the participants, beyond the advice that they would have received through their regular clinical care. Thus, although an earlier report queried the value of preventive counseling in this patient population (23), the current data suggest that the women with GDM potentially may have increased their activity levels in response to the advice of their clinical caregivers. Moreover, their success in increasing their leisure-time activity (and in narrowing the difference in waist circumference between themselves and the non-GDM group) suggests that lifestyle change can be achieved in the postpartum, despite the many potential barriers to physical activity at that time (4–10). While it is possible that these barriers may limit changes in sporting activity more than in leisure-time habits, the current findings raise the possibility that leisure-time activity such as walking may be a reasonable target for lifestyle modification in this patient population. In this context, it should be noted that walking has previously been associated with a reduced risk of incident T2DM in women participating in the Nurses Health Study (24).
This analysis must be interpreted with recognition of certain limitations. Firstly, as this study was observational in nature, rather than a randomized clinical trial, the causal determinants of increased leisure-time activity in the GDM group (i.e. whether clinical counseling on physical activity, increased patient awareness or other) cannot be definitively ascertained. Secondly, information on pre-gravid physical activity was based on patient recall during pregnancy, although it was obtained prior to the diagnosis of GDM. Thirdly, it is possible that the study participants may not reflect the general Caucasian GDM population and may be more motivated than most to change their lifestyle patterns. Even in that event, however, their success in increasing their leisure-time activity compared to the non-GDM group participating in the same study remains an encouraging message, particularly in the absence of systematic study-associated counseling.
In summary, in this prospective observational cohort study, women with GDM significantly increased their non-sport leisure-time activity in the first year postpartum, compared to their non-GDM peers. Moreover, the diagnosis of GDM independently predicted an increase in leisure-time activity between 1-year pre-gravid and 1-year postpartum. These data thus suggest that women with GDM can successfully achieve change in their physical activity patterns in the first year postpartum, a finding which potentially bodes well for diabetes risk reduction in this high-risk patient population.
Acknowledgments
We wish to thank the Mount Sinai Hospital Department of Pathology and Laboratory Medicine and Patient Care Services. The study was supported by operating grants (MOP 67063 and 84206) from the Canadian Institutes of Health Research (CIHR). R Retnakaran is supported by a CIHR Clinical Research Initiative New Investigator Award, Canadian Diabetes Association (CDA) Clinician-Scientist incentive funding, and a University of Toronto Banting and Best Diabetes Centre New Investigator Award. AJ Hanley holds a Tier II Canada Research Chair in Diabetes Epidemiology. B Zinman holds the Sam and Judy Pencer Family Chair in Diabetes Research at Mount Sinai Hospital and University of Toronto.
Footnotes
DISCLOSURE
No conflicts of interest to disclose
References
- 1.Buchanan TA, Xiang AH. Gestational diabetes mellitus. J Clin Invest. 2005;115(3):485–491. doi: 10.1172/JCI24531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jeon CY, Lokken RP, Hu FB, van Dam RM. Physical activity of moderate intensity and risk of type 2 diabetes: a systematic review. Diabetes Care. 2007;30(3):744–752. doi: 10.2337/dc06-1842. [DOI] [PubMed] [Google Scholar]
- 3.Ratner RE, Christophi CA, Metzger BE, et al. The Diabetes Prevention Program Research Group. Prevention of diabetes in women with a history of gestational diabetes: effects of metformin and lifestyle interventions. J Clin Endocrinol Metab. 2008;93(12):4774–4779. doi: 10.1210/jc.2008-0772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim C, McEwen LN, Kieffer EC, Herman WH, Piette JD. Self-efficacy, social support, and associations with physical activity and body mass index among women with histories of gestational diabetes mellitus. Diabetes Educ. 2008;34(4):719–728. doi: 10.1177/0145721708321005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith BJ, Cheung NW, Bauman AE, Zehle K, McLean M. Postpartum physical activity and related psychosocial factors among women with recent gestational diabetes mellitus. Diabetes Care. 2005;28(11):2650–2654. doi: 10.2337/diacare.28.11.2650. [DOI] [PubMed] [Google Scholar]
- 6.Kim C, McEwen LN, Piette JD, Goewey J, Ferrara A, Walker EA. Risk perception for diabetes among women with histories of gestational diabetes mellitus. Diabetes Care. 2007;30(9):2281–2286. doi: 10.2337/dc07-0618. [DOI] [PubMed] [Google Scholar]
- 7.Symons Downs D, Ulbrecht JS. Understanding exercise beliefs and behaviors in women with gestational diabetes mellitus. Diabetes Care. 2006;29(2):236–240. doi: 10.2337/diacare.29.02.06.dc05-1262. [DOI] [PubMed] [Google Scholar]
- 8.Kim C, Sinco B, Kieffer EA. Racial and ethnic variation in access to health care, provision of health care services, and ratings of health among women with histories of gestational diabetes mellitus. Diabetes Care. 2007;30(6):1459–1465. doi: 10.2337/dc06-2523. [DOI] [PubMed] [Google Scholar]
- 9.Pereira MA, Rifas-Shiman SL, Kleinman KP, Rich-Edwards JW, Peterson KE, Gillman MW. Predictors of change in physical activity during and after pregnancy: Project Viva. Am J Prev Med. 2007;32(4):312–319. doi: 10.1016/j.amepre.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Albright C, Maddock JE, Nigg CR. Physical activity before pregnancy and following childbirth in a multiethnic sample of healthy women in Hawaii. Women Health. 2005;42(3):95–110. doi: 10.1300/j013v42n03_06. [DOI] [PubMed] [Google Scholar]
- 11.Retnakaran R, Qi Y, Sermer M, Connelly PW, Hanley AJ, Zinman B. Glucose intolerance in pregnancy and future risk of pre-diabetes or diabetes. Diabetes Care. 2008;31(10):2026–2031. doi: 10.2337/dc08-0972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Retnakaran R, Qi Y, Sermer M, Connelly PW, Zinman B, Hanley AJ. Isolated hyperglycemia at 1-hour on oral glucose tolerance test in pregnancy resembles gestational diabetes in predicting postpartum metabolic dysfunction. Diabetes Care. 2008;31(7):1275–1281. doi: 10.2337/dc08-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petersen AM, Leet TL, Brownson RC. Correlates of physical activity among pregnant women in the United States. Med Sci Sports Exerc. 2005;37(10):1748–1753. doi: 10.1249/01.mss.0000181302.97948.90. [DOI] [PubMed] [Google Scholar]
- 14.Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. American Journal of Clinical Nutrition. 1982;36:936–942. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 15.Kriska AM, Casperson CJ. A collection of physical activity questionnaires for health related research. Medicine & Science in Sports & Exercise. 1997;29(Suppl 1):S1–S203. [PubMed] [Google Scholar]
- 16.Retnakaran R, Qi Y, Sermer M, Connelly PW, Zinman B, Hanley AJ. Pre-gravid physical activity and reduced risk of glucose intolerance in pregnancy: the role of insulin sensitivity. Clin Endocrinol. 2009;70(4):615–622. doi: 10.1111/j.1365-2265.2008.03393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Medicine & Science in Sports & Exercise. 2000;32(9 Suppl):S498–504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 18.National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes. 1979;28(12):1039–1057. doi: 10.2337/diab.28.12.1039. [DOI] [PubMed] [Google Scholar]
- 19.Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. Canadian Diabetes Association 2008 clinical practice guidelines for the prevention and management of diabetes in Canada. Can J Diabetes. 2008;32(Suppl 1):S10–S13. doi: 10.1016/j.jcjd.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 20.Yun S, Kabeer NH, Zhu BP, Brownson RC. Modifiable risk factors for developing diabetes among women with previous gestational diabetes. Prev Chronic Dis. 2007;4(1):1–8. [PMC free article] [PubMed] [Google Scholar]
- 21.Kieffer EC, Sinco B, Kim C. Health behaviors among women of reproductive age with and without a history of gestational diabetes mellitus. Diabetes Care. 2006;29(8):1788–1793. doi: 10.2337/dc06-0199. [DOI] [PubMed] [Google Scholar]
- 22.Stage E, Ronneby H, Damm P. Lifestyle change after gestational diabetes. Diabetes Res Clin Pract. 2004;63(1):67–72. doi: 10.1016/j.diabres.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 23.Kim C, McEwen LN, Kerr EA, et al. Preventive counseling among women with histories of gestational diabetes mellitus. Diabetes Care. 2007;30(10):2489–2495. doi: 10.2337/dc07-0435. [DOI] [PubMed] [Google Scholar]
- 24.Hu FB, Sigal RJ, Rich-Edwards JW, et al. Walking compared with vigorous physical activity and risk of type 2 diabetes in women: a prospective study. JAMA. 1999;282(15):1433–1439. doi: 10.1001/jama.282.15.1433. [DOI] [PubMed] [Google Scholar]