Abstract
We report the optical activation of erbium coated silicon nanowires (Er–SiNWs) grown with the assist of platinum (Pt) and gold (Au), respectively. The NWs were grown on Si substrates by using a chemical vapor transport process using SiCl4 and ErCl4 as precursors. Pt as well as Au worked successfully as vapor–liquid–solid (VLS) catalysts for growing SiNWs with diameters of ~100 nm and length of several micrometers, respectively. The SiNWs have core–shell structures where the Er-crystalline layer is sandwiched between silica layers. Photoluminescence spectra analyses showed the optical activity of SiNWs from both Pt and Au. A stronger Er3+ luminescence of 1,534 nm was observed from the SiNWs with Pt at room- and low-temperature (25 K) using the 488- and/or 477-nm line of an Ar laser that may be due to the uniform incorporation of more Er ions into NWs with the exclusion of the formation of catalyst-induced deep levels in the band-gap. Pt would be used as a VLS catalyst for high performance optically active Er–SiNWs.
Keywords: Si nanowires, Erbium, Luminescence, Platinum catalyst
Introduction
Interest lies in developing an efficient silicon (Si)-based light emitting material that enables the integration of photonics with Si technology. In this regard, studies have demonstrated some approaches to achieve luminescence from Si [1,2]. For example, Si nanocrystals (Si-ncs) in Si-rich Si-oxide (SRSO) emit visible luminescence and suggest the possibility of their use as a Si-based optoelectronic device. In particular, the erbium (Er) doping of Si has attracted attention as it can allow the realization of a Si-based light emitter in the technologically important 1.5 µm range [2,3]. Excellent optical properties were obtained by using SRSO, which consists of nano-cluster Si (nc-Si) embedded inside a SiO2 matrix [4-7]. However, the isolation of nc-Si inside the SiO2 matrix makes current injection into SRSO difficult. Thus, SRSO-based light emitting diodes (LEDs) generally require either very high voltage or very thin SRSO layers that can limit the light output [8-10].
Such problems may be solved by using Si nanowires (SiNWs) instead [10]. NWs offer thermodynamically stable features and have ultra high aspect ratio with single crystalline, thereby possessing a number of advantages over thin films with respect to Si-based photonic devices [9-11]. A large surface area to active activation volume ratio is another advantage of NWs as a better light emitter at the wavelength of 1.54 μm. Our previous studies have shown that the optical activation of SiNWs is feasible by surface-coating with Er-doped silica [10] or the formation of Si/Er2Si2O7/SiO2 core–shell NWs in an in-situ mode [11]. Optical characterization indicates that these Er–SiNWs have potential as a new material platform for Si-based photonics.
The optically active Er–SiNWs in the previous studies were grown by a vapor–liquid–solid (VLS) mechanism with the assist of Au as a catalyst. In fact, Au has been exclusively used as VLS catalyst for SiNWs. Meanwhile, recent studies indicated that Au atoms could remain in the NWs [12,13]. Unfortunately, Au is deep-level impurities in Si, and, thus, may harm the optical properties of Er–SiNWs [14,15]. Herein, we report on the optical activation of Er–SiNWs grown with Pt as a VLS catalyst. We investigated Pt because it does not make deep level in the Si band-gap and, thus, will not degrade the optical properties of Er–SiNWs [15-17]. Our investigation showed that Pt works successfully as a VLS catalyst for Er–SiNWs. Pt also enables better optical properties than those of Er–SiNWs grown with Au.
Experimental Procedure
We synthesized vertically aligned Er–SiNWs on Si (111) substrates coated with Pt or Au (for comparison) as a VLS catalyst by a CVD process employing Si tetrachloride (SiCl4, Alfa, 99.999%) as the Si source [18]. Erbium trichloride (ErCl3, Aldrich, 99.9%) powder placed in quartz susceptor was inserted into the center of a quartz tube at intervals of 1 in. The substrates were also placed in the quartz tube at a distance of 1 in. from the ErCl3 powders. Carrier gas transfers the source precursor through a bubbler to the quartz reactor, and H2 is then introduced into the system at a flow rate of 20 sccm. H2 (100 sccm) and Ar (100 sccm) gas were used as diluent gases, which regulate the concentration of the mixture containing SiCl4 vapor and carrier gas. Typically, the system was heated to 900–1,000 °C and maintained for 30 min as the flow of SiCl4 then cooled to room temperature. We observed the NWs by using a scanning electron microscope (SEM) and transmission electron microscopy (TEM). The photoluminescence (PL) spectra were measured using either the 488- and/or 477-nm line of an Ar laser, a grating monochromator, a thermoelectrically cooled InGaAs diode, and the standard lock-in technique. The low-temperature measurements were made using a closed-cycle cryostat.
Results and Discussion
Figure 1a, 1b shows an SEM image of SiNWs grown on the substrate. The NWs vertically grew with the diameter ranging from 80 to 150 nm and lengths of ~10 µm, respectively. It is known that the epitaxial relationship between the substrate and NWs attributes to the vertical growth of NWs [19] and, thus, both catalysts should yield epitaxial interfaces between the NWs and substrate. The inset images show the liquid globules at the tips of the NWs, indicating the success of both metals as a VLS catalyst [18].
Figure 1.
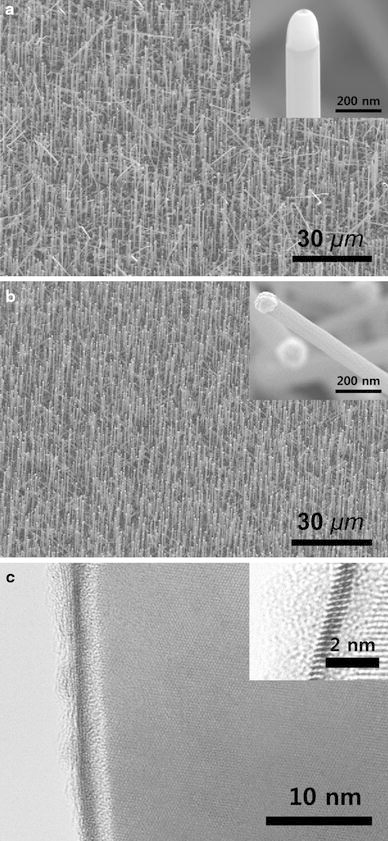
SEM images of Er–SiNWs grown with a Pt- and b Au-catalyst.Inset shows the alloy globule at the tip of Er–SiNWs. c TEM image of an individual Er–SiNW grown with Pt, showing the core–shell structure with shell layer conducted with <2 nm Er-rich crystalline layer within amorphous SiOx layer.Inset shows HR-TEM image of SiNWs showing the single crystalline nature of Er–SiNWs with Er-crystalline layers with 1–2 nm within amorphous SiOx shell
Figure 1c is a typical TEM image of an individual Er–SiNW grown with Pt. The outer sheath of the NWs consists of a trilayer with nanometer-thin shells. An energy dispersive spectroscopy (EDS) analysis of the surface region indicates the presence of Er, Si, and O in the surface layer, and a high-resolution TEM image of the surface region shows the presence of a single-crystalline layer sandwiched between nanometer-thin amorphous silica shells (Fig. 1c). This core–shell structure is the same as the structures of the Er–SiNWs with the Au catalyst that we have reported [11]. It indicates that both Pt and Au catalyst work as catalysts for core–shell structure Er–SiNWs. The formation of core–shell structures in a one-step fabrication process implies that the heterostructure is formed via a self organization process by a spontaneous phase separation in the Er–Si–O alloy system. As suggested in our previous study, the formation of the core–shell seems driven by the different solubility of Er between liquid catalyst and solid NWs. In the VLS mechanism, both Si and Er are supplied to the catalyst in supersaturation. Since Er and Si have solubility to Pt or Au [20,21], respectively, Er can precipitate at the interface between the catalyst and the growing NWs. However, Er has an extremely low solubility in Si due to large atomic size and is thus segregated to the surface in the Er-crystalline phase [22].
Figure 2 shows the PL spectra of the Er–SiNWs array measured at room temperature using the 488- and/or 477-nm line of an Ar laser. The 488-nm line is directly absorbed by Er3+ ion, and the 477-nm line is absorbed only by SiNWs and not directly by Er3+ ion. Figure 2a shows the PL spectra of the Er–SiNWs using the 488-nm line. Both NWs emitted a spectrum centered at 1,534 nm. This indicates that Er3+ ions are successfully incorporated into the NWs, as proposed by previous studies [6-11]. The intensity from NWs with Pt was approximately two times higher than that of Au. The intensity of the luminescence spectrum from Er3+ ions primary depends on the Er content under characterization. As shown in Fig. 1, the density of Er–SiNWs with Pt (1.89 × 107/cm2) was lower than that of NWs with Au (2.56 × 107/cm2). Therefore, the stronger intensity in Er–SiNWs with Pt may be due to the incorporation of more Er ions into NWs. In fact, the core–shell structure of both Er–SiNWs is almost the same as c.a. 1 nm of Er-layers. Thus, the amount of Er incorporated into individual NWs would be almost the same. Meanwhile, we found in the course of characterization that the Er–SiNWs samples with Pt showed the more homogeneous optical activity through the area under investigation. This implies that Pt produced Er–SiNWs more uniformly over the substrate and is ascribed to the stronger intensities.
Figure 2.
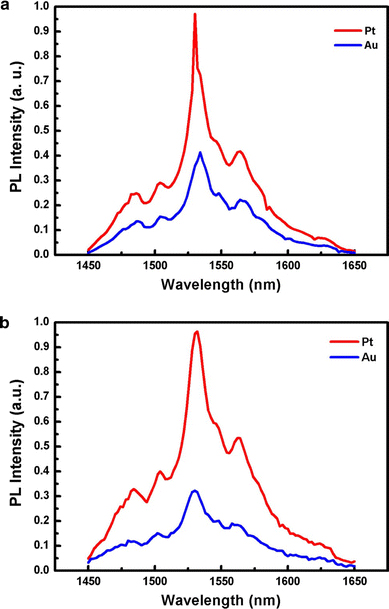
The PL spectra of the Er–SiNWs array with Pt and Au, respectively, measured at room temperature using the a 488- and/or b 477-nm line of an Ar laser
Figure 2b shows the PL spectra using the 477-nm line. A luminescence appeared centered at 1,530 nm from the SiNWs, indicating an energy transfer from the SiNWs to the Er3+ ions in both cases [10]. In the case of 477-nm line, the intensity of the PL spectrum from the NWs with Pt is approximately three times stronger than with Au. This implies that, in addition to the incorporation of more Er ions into NWs, a more efficient energy transfer occurs in Er–SiNWs grown with Pt. The results shown in Fig. 2, thus, indicate that Pt enables the preparation of optically more active Er–SiNWs by incorporating Er3+ ions uniformly and more efficient energy transfer from NWs to Er3+ ions.
It is difficult to clearly address why more Er ions are uniformly incorporated into SiNWs with Pt than that with Au because it happens in a complex VLS process. Three phases, two interfaces (that is, vapor–liquid and liquid–solid interfaces), and chemical reactions are involved [23] in the VLS process. In terms of kinetics, it consists of four main steps: (1) mass-transport in the gas phase, (2) chemical reaction on the vapor–liquid interface, (3) diffusion in the liquid phase, and (4) incorporation of atoms in a crystal lattice [18,23-25]. Nevertheless, an explanation may be possible. According to the kinetics of the VLS process [23] and our previous study on the formation of Er shells on the SiNWs [11], the mechanism of incorporation of Er into the NWs could be schemed as dissolving Er into liquid catalyst and precipitates on the surface of NWs [11,22]. In this mechanism, the amount of Er3+ ions incorporated into NWs may relate to the difference of solubility of Er for liquid Si in catalyst compare to that for solid Si in NWs. Meanwhile, Si-rich globule was formed when using the Pt catalyst, while an Au-rich globule was formed when using the Au catalyst [18]. This compositional difference may be the differential solubility of Si in Pt and Au. Specifically, the solubility of Si in Au is 18.6 at.% [9], whereas in Pt it is as high as 67 at.% [26] at their eutectic points. Therefore, it is possible that the Pt catalyst under a liquid state has a Si-rich composition. Since the incorporation of Er into NWs through catalyst is related to the difference in solubility of Er between liquid and solid Si in catalyst and NWs, respectively [11], Pt catalyst that can dissolve the more Si as compare to Au catalyst could incorporate the more Er into SiNWs.
Meanwhile, the more efficient energy transfer may relate to the contamination of the catalyst component in the SiNWs. Recent studies have indicated that Au can be incorporated into NWs in the course of VLS growth [12,13] and, thus, Pt would do. Figure 3 illustrates the energy transfer mechanism for the activation of Er3+ ions in the Er–SiNWs by 477-nm line. Figure 3a illustrates the energy transfer from SiNWs to Er3+ ion without trap levels in the NWs. The SiNWs absorbs the photon energy from 477-nm line, and the electrons excite from the valence band (VB) to the conduction band (CB). Then, the recombination of the electrons in the CB with holes in the VB yields emissions of ~0.8 eV. Since the 0.8 eV energy couples well with the 4I13/2 level of the Er, energy transfers to the Er ions and excites it. Since Pt does not form deep level in the Si band-gap, it does not hinder the energy transfer illustrated in Fig. 3a, even if it exists in the NWs. In fact, Pt forms trap level at Ev +0.03 eV and Ec −0.23 eV a donor and /or acceptor level in Si [17]; however, it does not hinder the energy transfer to activate the Er+3 ions. On the other hand, Au forms two deep trap levels in Si. The two Au-related trap levels at energies of Ev +0.35 eV and Ev +0.53 eV were identified as a donor and an acceptor level, respectively [14]. As illustrated in Fig. 3b, the trap levels absorb the energy that should transfer from Si to Er+3 ions and would degrade the optical properties.
Figure 3.
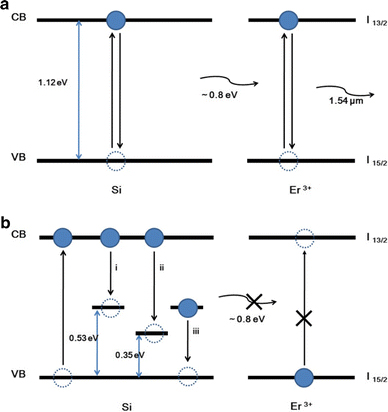
Schematic of the energy transfer mechanisms of Er–SiNWs a without deep level and b with deep level possibly induced by Au
Figure 4 shows the PL spectrum of the Er–SiNWs measured at 25 K using the 488-nm line of an Ar laser. A typical Er3+ luminescence centering around 1,534 nm was observed. The spectral width of observable peaks is limited by the system resolution, which was 1.5 nm. It is noted that some additional luminescent peaks located at 1,530, 1,534, 1,542, 1,547, 1,561, and 1,563 nm, respectively, appear for the Er–SiNWs with Pt. As the intra-4f transition of rare earth ions are forbidden transitions that are allowed because of parity mixing by the crystal field, such sharp, well-defined peaks as seen in Fig. 4 indicate that the Er3+ ions are located in well-defined sites in a crystalline environment [22], as shown in Fig. 1c.
Figure 4.
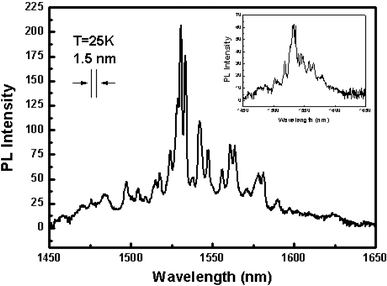
The PL spectrum of the Er–SiNWs array grown with Pt catalyst measured at 25 K using the 488-nm line of an Ar laser. The inset shows the PL spectrum of the Er–SiNWs grown with Au catalyst
Conclusion
In summary, we vertically grew Er–SiNWs with the assistance of Pt as a VLS catalyst. The Er–SiNWs showed stronger luminescence at 1,530 nm wavelength at low- and room-temperature than did the Er–SiNWs with Au. The outcomes indicate that Pt is an excellent candidate as a VLS catalyst for optically more active Er–SiNWs. It was noted that SiNWs in most cases are prepared with the assistance of Au as a VLS catalyst, which may be harmful for their electrical as well as optical properties. Therefore, our results suggest that Pt can be used for SiNWs with improved electrical as well as optical properties.
Acknowledgments
This research was supported by a grant from the National Research Laboratory program (R0A-2007-000-20075-0), Pioneer research program (2009-008-1529) for converging technology through the Korea Science and Engineering Foundation funded by the Ministry of Education, Science & Technology and the IT R&D program of MKE/KEIT [2008-F-023-01].
References
- Ennen H, Schneider J, Pomrenke G, Axman A. Appl. 1983. p. 943. COI number [1:CAS:528:DyaL3sXmt1WlsLw%3D]; Bibcode number [1983ApPhL..43..943E] [DOI]
- Ren L, Jeung WY, Choi HJ. Appl. 2007. p. 8885. COI number [1:CAS:528:DC%2BD2sXpt1Srt7k%3D]; Bibcode number [2007ApSS..253.8885R] [DOI]
- Jalali B, Fathpour S. J. 2006. p. 4600. COI number [1:CAS:528:DC%2BD2sXitV2hs7w%3D]; Bibcode number [2006JLwT...24.4600J] [DOI]
- Kenyon AJ, Trwoga PF, Federighi M, Pitt CW. J. Phys. Condens. Matter. 1994. p. L319. Bibcode number [1994JPCM....6L.319K] [DOI]
- Fujii M, Yoshida M, Kanazawa Y, Hayashi S, Yamamoto K. Appl. 1997. p. 1198. COI number [1:CAS:528:DyaK2sXlsl2qs7o%3D]; Bibcode number [1997ApPhL..71.1198F] [DOI]
- Shin JH, Kim MJ, Seo SY, Lee C. Appl. 1998. p. 1092. COI number [1:CAS:528:DyaK1cXhtFOkur4%3D]; Bibcode number [1998ApPhL..72.1092S] [DOI]
- Irrera A, Pacifici D, Miritello M, Franzo G, Priolo F, Iacona F, Sanfilippo D, Di Stefano G, Fallica PG. Appl. 2002. p. 1866. COI number [1:CAS:528:DC%2BD38Xms1ehtL0%3D]; Bibcode number [2002ApPhL..81.1866I] [DOI]
- Polman A, Hoven GN, Custer JS, Shin JH, Sema R, Alkemade PFA. J. 1995. p. 1256. COI number [1:CAS:528:DyaK2MXjt12ru7g%3D]; Bibcode number [1995JAP....77.1256P] [DOI]
- Suh K, Shin JH, Seo SJ, Bae BS. Appl. 2006. p. 223102. Bibcode number [2006ApPhL..89v3102S] [DOI]
- Suh K, Shin JH, Park OH, Bae BS, Lee JC, Choi HJ. Appl. 2005. p. 053101. Bibcode number [2005ApPhL..86e3101S] [DOI]
- Choi HJ, Shin JH, Suh K, Seong HK, Han HC, Lee JC. Nano Lett. 2005. p. 2432. COI number [1:CAS:528:DC%2BD2MXht1SmtrrP]; Bibcode number [2005NanoL...5.2432C] [DOI] [PubMed]
- Hannon JB, Kodambaka S, Ross FM, Thomp RM. Nature. 2006. p. 69. COI number [1:CAS:528:DC%2BD28XhvFajtLo%3D]; Bibcode number [2006Natur.440...69H] [DOI] [PubMed]
- Oh SH, Benthem K, Molina SI, Borisevich AY, Luo W, Werner P, Zakharov ND, Kumar D, Pantelides ST, Pennycook SJ. Nano Lett. 2008. p. 1016. COI number [1:CAS:528:DC%2BD1cXjtlSgsLg%3D]; Bibcode number [2008NanoL...8.1016O] [DOI] [PubMed]
- Will NF, Hofmann K, Schulz M. Appl. Phys. A. 1986. p. 107. Bibcode number [1986ApPhA..41..107W] [DOI]
- Garnett EC, Liang W, Yang P. Adv. 2007. p. 2946. COI number [1:CAS:528:DC%2BD2sXht1eisrrF] [DOI]
- Sekhar PK, Sambandam SN, Sood DK, Bhansali S. Nanotechnology. 2006. p. 4606. COI number [1:CAS:528:DC%2BD28XhtFKjsbfI]; Bibcode number [2006Nanot..17.4606S] [DOI] [PubMed]
- Evwaraye AO, Sun E. J. 1976. p. 3172. COI number [1:CAS:528:DyaE28XltlGkt7k%3D]; Bibcode number [1976JAP....47.3172E] [DOI]
- Jeong H, Park TE, Seong HK, Kim M, Kim U, Choi HJ. Chem. 2009. p. 331. COI number [1:CAS:528:DC%2BD1cXhsFCjsr7I]; Bibcode number [2009CPL...467..331J] [DOI]
- Huang MH, Mao S, Feick H, Yan H, Wu Y, Kind H, Weber E, Russo R, Yang P. Science. 2001. p. 1897. COI number [1:CAS:528:DC%2BD3MXksVaqsb0%3D]; Bibcode number [2001Sci...292.1897H] [DOI] [PubMed]
- Okamoto H. J. 2007. p. 486. COI number [1:CAS:528:DC%2BD2sXhtlSktbvP] [DOI]
- Predel B. Numerical Data and Functional Relationships in Science and Technology. 5th edn. Springer, Berlin; 1995. [Google Scholar]
- Tang YS, Heasman KC, Gillin WP, Sealy BJ. Appl. 1989. p. 432. COI number [1:CAS:528:DyaL1MXmtVCnur4%3D]; Bibcode number [1989ApPhL..55..432T] [DOI]
- Givargizov EI. J. Cryst. Growth. 1975. p. 20. COI number [1:CAS:528:DyaE28Xot1KmtQ%3D%3D]; Bibcode number [1975JCrGr..31...20G] [DOI]
- Kamins TI, Williams RS, Basile DP, Hesjedal T, Harris JS. J. 2001. p. 1008. COI number [1:CAS:528:DC%2BD3MXmvVyn]; Bibcode number [2001JAP....89.1008K] [DOI]
- Kikkawa J, Ohno Y, Takeda S. Appl. 2005. p. 123109. Bibcode number [2005ApPhL..86l3109K] [DOI]
- Massalski T, Murray J, Bennett L, Baker H. Binary Alloy Phase Diagrams. American Society for Materials, Florida; 1986. [Google Scholar]


