Abstract
Ion gradients across intracellular membranes contribute to the physicochemical environment inside compartments. CLC anion transport proteins that localise to intracellular organelles are anion-proton exchangers involved in anion sequestration or vesicular acidification. By homology, the only CLC protein of Saccharomyces cerevisiae, Gef1, belongs to this family of intracellular exchangers. Gef1 localises to the late Golgi and prevacuole and is essential in conditions of iron limitation. In the absence of Gef1, a multicopper oxidase involved in iron uptake, Fet3, fails to acquire copper ion cofactors. The precise role of the exchanger in this physiological context is unknown. Here, we show that the Gef1-containing compartment is adjusted to a more alkaline pH under iron limitation. This depends on the antiport function of Gef1, because an uncoupled mutant of Gef1 (E230A) results in the acidification of the lumen and fails to support Fet3 maturation. Furthermore, we found that Gef1 antiport activity correlates with marked effects on cellular glutathione homeostasis, raising the possibility that the effect of Gef1 on Fet3 copper loading is related to the control of compartmental glutathione concentration or redox status. Mutational inactivation of a conserved ATP-binding site in the cytosolic cystathione β-synthetase domain of Gef1 (D732A) suggests that Gef1 activity is regulated by energy metabolism.
Keywords: CLC proteins, Copper metabolism, Glutathione homeostasis, Intracellular pH homeostasis, Secondary active transporters
Introduction
CLC proteins are anion-conducting membrane proteins found in all kingdoms (Jentsch, 2008). In higher eukaryotes, members of the CLC family fulfil different physiological functions when found at the cell surface or in the membranes of intracellular compartments. Recently, substantial progress has been made in obtaining high-resolution structures of CLC proteins by X-ray crystallography (Dutzler, 2007; Dutzler et al., 2002; Meyer et al., 2007). Combined with data from in vitro experiments using purified and reconstituted CLC proteins in lipid bilayers (Accardi and Miller, 2004), these structures have revolutionised our understanding of the CLC class of anion transport proteins: it is now clear that the prokaryotic CLC proteins function as electrogenic chloride-proton antiporters (Miller, 2006). This is in contrast to the founding member of the family, CLC-0 from Torpedo (Jentsch et al., 1990), as well as mammalian plasma membrane CLCs, all of which act as true chloride-ion channels (Jentsch, 2008). Subsequent work on mammalian and plant CLC homologues localised to intracellular compartments (such as endosomes or the plant vacuole) revealed that they function as antiporters similarly to the bacterial homologue (De Angeli et al., 2006; Graves et al., 2008; Picollo and Pusch, 2005; Scheel et al., 2005; Zdebik et al., 2008). In fact, the example of the Arabidopsis vacuolar nitrate-proton exchanger ClC-a illustrates how secondary active transport mediated by the transporter is exploited to store nitrate in the plant vacuole (De Angeli et al., 2006). For other intracellular CLC transporters, the physiological role of secondary active transport remains poorly understood.
The yeast Saccharomyces cerevisiae possesses only one CLC homologue, Gef1. Since the discovery of GEF1 as a gene required for growth on media where survival depends on proper mitochondrial function (Greene et al., 1993), the work on Gef1 has shed light on its role in cellular iron and copper metabolism (Davis-Kaplan et al., 1998; Gaxiola et al., 1998; Schwappach et al., 1998). It seems possible that this physiological role extends to higher eukaryotes, although the relationship between intracellular CLCs and metal metabolism has not yet been systematically studied in mammals or plants. Potentially, the fact that metal metabolism is very tightly regulated in multicellular organisms makes it more difficult to expose effects of single CLC proteins on cellular metal handling. Furthermore, organellar CLC function might be more redundant in mammalian or plant cells because of the presence of several intracellular CLC proteins. Nevertheless, two reports indicate that mammalian ClC-4 might affect cellular copper or iron handling (Mohammad-Panah et al., 2009; Wang and Weinman, 2004). Irrespective of a putative universal role for CLC proteins in metal handling, the study of Gef1 provides a unique opportunity to probe the relationship between CLC transport function and physiological consequences at the whole-cell level.
The progress in structural and mechanistic understanding of CLC proteins has pinpointed some key determinants of CLC function: when mutated, a glutamate located on the extracellular side of the chloride-conducting pore affects rectification and gating in the channel members of the family, and it uncouples CLC transporters such that they become non-rectifying chloride-conducting channels when mutated (Accardi and Miller, 2004; Picollo and Pusch, 2005; Scheel et al., 2005). Hence this highly conserved glutamate (E230 in Gef1) has been dubbed the ‘gating glutamate’ (Zdebik et al., 2008). A second glutamate is characteristic of the antiporter homologues of the family and hence has been termed ‘proton glutamate’ (Accardi et al., 2005; Zdebik et al., 2008). Because the proton glutamate is present in Gef1 (E287) and because of the high homology between Gef1 and mammalian ClC-4 and ClC-5 (which have been shown to be chloride-proton antiporters (Picollo and Pusch, 2005; Scheel et al., 2005; Zdebik et al., 2008), Gef1 is likely to act as an antiporter. The cytosolic C-terminus of eukaryotic CLC proteins contains two copies of the cystathione β-synthetase (CBS) domain found in a variety of proteins (Bateman, 1997). A recent structure of the CBS domains from ClC-5 revealed the presence of an ATP-binding site (Meyer et al., 2007), which is conserved in Gef1. The functional consequences of ATP binding have been studied for ClC-5 and Arabidopsis ClC-a (De Angeli et al., 2009; Zifarelli and Pusch, 2009). Although both reports suggest nucleotide regulation of CLC activity, the studies reach opposing mechanistic conclusions, with nucleotides stimulating ClC-5 function and inhibiting ClC-a.
The requirement for Gef1 under growth conditions that involve respiration and hence mitochondrial activity has been traced to deficient iron uptake into Δgef1 strains (Davis-Kaplan et al., 1998; Gaxiola et al., 1998). High-affinity iron uptake at the cell surface involves Fet3, a multicopper oxidase that is coupled directly to the actual iron transporter (Askwith et al., 1994; Askwith and Kaplan, 1997). Fet3 is homologous to mammalian ceruloplasmin, a soluble multicopper oxidase present in serum, and to laccase, a soluble multicopper oxidase contributing to the virulence of pathogenic fungi such as Cryptococcus neoformans (Zhu and Williamson, 2003). The copper-containing active centre of the Fet3 oxidase is formed in the late Golgi and prevacuolar compartment where Gef1 also accumulates in the steady state (Davis-Kaplan et al., 1998; Gaxiola et al., 1998; Schwappach et al., 1998; Yuan et al., 1997; Yuan et al., 1995). In this compartment, the P-type copper ATPase Ccc2 moves copper ions into the lumen where they are then incorporated into apo-Fet3. In the absence of Gef1, Fet3 accumulates in the apo form, which can be copper-loaded in vitro, demonstrating that this is the affected step in Fet3 maturation (Davis-Kaplan et al., 1998). The mechanistic role of Gef1 in Fet3 copper loading has been addressed by two different hypotheses: the first focuses on the electrical properties of chloride as a counter-ion that balances the movement of copper cations into the compartment (Gaxiola et al., 1998). In the second model, chloride entering the compartment via Gef1 acts as a cofactor to the actual copper-loading process by keeping apo-Fet3 in a permissive conformation (Davis-Kaplan et al., 1998). Neither model has been tested in the context that Gef1 is likely to be an anion-proton exchanger, which would add the pH gradient across the organellar membrane as an important and so-far undetermined parameter in the process.
Metal acquisition by metalloproteins is a delicate step of protein biogenesis (Waldron et al., 2009). Different proteins compete for the same metals, which are often tightly bound by metallochaperones to avoid dangerous unspecific reactions. At the same time, the incorporation of the wrong metals into a given protein has to be avoided. In the cytosol, copper is tightly bound to metallochaperones such as Atx1 (Pufahl et al., 1997) and kinetic factors determine the transfer of copper from one protein to another. For example, Atx1 directly interacts with the copper pump Ccc2 that will then translocate copper to the lumen of the late Golgi and prevacuole. How copper is handled in the lumen of this compartment is currently unknown. In addition to metallochaperones, glutathione (GSH) has been shown to be an important small molecule ligand for copper (Freedman et al., 1989). At physiological pH, glutathione is an anion (Pirie and Goodwin Pinhey, 1929) that would be affected by any effects on membrane potential that Gef1-mediated electrogenic exchange might have. Although the transporters responsible for GSH transport across many membranes in the secretory pathway are unknown, several studies on intracellular GSH transport report a tight connection with organellar pH (Staleva et al., 2002). Therefore, we have not only investigated the effect of Gef1 on compartmental pH, but also tested whether Gef1 function affects cellular GSH homeostasis.
Our main finding is that the pH gradient across the relevant intracellular membrane was adjusted upon challenge by an iron chelator. The lumen that was normally slightly acidified with respect to the cytosol became more alkaline. This change depended on the presence of the gating glutamate in Gef1 and correlated with copper loading onto Fet3. At the same time, the gating glutamate of Gef1 contributed to glutathione homeostasis under iron starvation, because its absence led to marked differences in cellular GSH handling as shown by indicators of GSH conjugation and response of the GSH redox potential to an acute H2O2 challenge.
Results
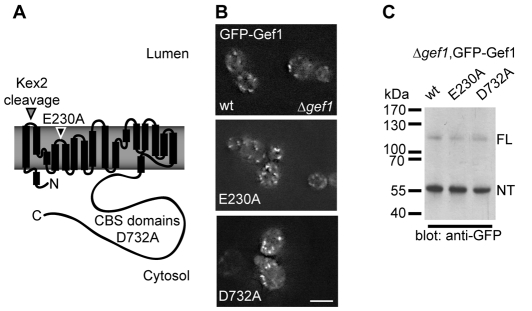
To test the physiological relevance of coupled anion transport and investigate a putative role of ATP binding to the CBS domains, we introduced two mutations to Gef1 (Fig. 1A) that have been well characterised in other CLC homologues such as ClC-4 and ClC-5, two closely related mammalian antiporters (Meyer et al., 2007; Picollo and Pusch, 2005; Scheel et al., 2005; Zdebik et al., 2008; Zifarelli and Pusch, 2009). Unlike mammalian ClC-4 and ClC-5, Gef1 cannot be functionally expressed in heterologous expression systems (our unpublished results). Hence, based on results with other homologues, we have predicted that mutating the highly conserved gating glutamate E230 to alanine leads to uncoupled chloride transport. Likewise we tested the physiological effects of mutating aspartate D732, a key residue in the conserved ATP-binding site, under the assumption that it causes loss of ATP binding as observed in ClC-5 (Meyer et al., 2007). In the context of an N-terminal green fluorescent protein (GFP) fusion protein Gef1 E230A or D732A gave rise to a fluorescence pattern indistinguishable from the wild-type (wt) constructs as assessed by live-cell imaging in a Δgef1 deletion strain (Fig. 1B). Gef1 is known to accumulate in the late Golgi and prevacuolar compartments (Gaxiola et al., 1999; Gaxiola et al., 1998; Schwappach et al., 1998). Passage through the late Golgi can be monitored by proteolytic cleavage of Gef1 that is performed by the Kex2 protease (Wachter and Schwappach, 2005). Both Gef1 E230A and D732A gave rise to a major N-terminal cleavage product and a smaller amount of full-length Gef1 when cellular Gef1 protein species were analysed by western blotting (Fig. 1C). We conclude that both mutant constructs undergo normal transport to the late Golgi and prevacuolar compartment in which Gef1 is known to reside, suggesting that the proteins are not subject to ER (endoplasmic reticulum) retention or ER-dependent degradation and hence not grossly misfolded.
Fig. 1.
Structure-based mutagenesis of GEF1. (A) Scheme of the Gef1 protein based on helices present in the crystal structure of the prokaryotic homologue from E. coli (Dutzler et al., 2002). A Kex2 protease-cleavage site in the first extracellular loop is indicated. Kex2 is known to process Gef1 in the late Golgi, yielding a smaller N-terminal and a large C-terminal fragment. The position of the gating glutamate E230 is indicated. In closely related mammalian homologues such as ClC-5, mutation of the gating glutamate to alanine leads to uncoupled chloride movement through the transporter (Picollo and Pusch, 2005; Scheel et al., 2005). The C-terminus of eukaryotic CLC homologues contains cystathione β-synthetase (CBS) domains that have been shown to bind ATP in a recent crystal structure of the C-terminus of ClC-5 (Meyer et al., 2007). In ClC-5, mutation of the aspartate corresponding to Gef1 D732 to alanine abolishes ATP binding. (B) Subcellular localisation of N-terminal GFP-Gef1 fusions with the indicated mutations revealed by live-cell imaging in a Δgef1 strain. Scale bar: 5 μm. (C) Anti-GFP western blot of total cellular lysates from cells expressing the indicated GFP-Gef1 fusion proteins. ‘FL’ and ‘NT’ respectively indicate the migration position of uncleaved full-length Gef1 and the N-terminal product of the cleavage reaction.
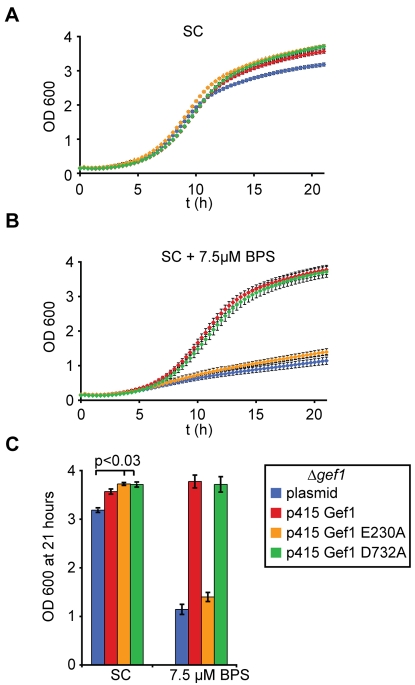
The failure to grow under iron depletion is the most drastic phenotype of a Δgef1 deletion strain, although decreased resistance to toxic cations, sensitivity to neutral pH and quality-control defects at the ER have also been reported (Gaxiola et al., 1998; Li et al., 1999; Schwappach et al., 1998). Therefore, we investigated the ability of uncoupled Gef1 E230A and the presumably ATP-binding-deficient mutant D732A to support the growth of a Δgef1 deletion strain under iron starvation (Fig. 2). Interestingly, both mutants of Gef1 resulted in a small but significant increase in fitness under normal growth conditions compared with a control transformed with empty plasmid (Fig. 2A,C; P<0.03 for either mutant). This suggests that both proteins exhibited some activity that ‘rescued’ growth in minimal medium to wt levels. Under iron depletion, however, Gef1 E230A did not increase the growth of the Δgef1 deletion strain (Fig. 2B,C). We conclude that the gating glutamate of Gef1 is required for the role of the transporter in high-affinity iron uptake and asked next whether this defect occurred at the level of Fet3 copper loading.
Fig. 2.
Mutation of the gating glutamate interferes with the physiological role of Gef1 under iron starvation. (A) Growth curves of Δgef1 strains bearing the indicated plasmids in minimal SC medium lacking leucine. n=9 (three independent colonies measured in triplicate in a 96-well plate); error bars indicate s.e. (B) The same experiment as in A with 7.5 μM iron chelator bathophenanthroline sulfonate (BPS) added to the medium. (C) Bar diagram illustrating final OD600 after 21 hours of growth for the different constructs. Error bars indicate s.e. and P value for improved growth of Gef1-variant bearing strains in minimal medium is indicated.
To this end, we prepared total membrane protein from a Δfet3 deletion strain and a Δgef1 deletion strain lacking or expressing the different forms of Gef1 and resolved the solubilised proteins on a clear native gel (Fig. 3A). After electrophoresis, an in-gel assay of Fet3 oxidase activity was performed, using the chromogenic substrate para-phenylendiamine (PPD) (Davis-Kaplan et al., 1998; Yuan et al., 1995). The Δfet3 deletion strain still exhibited some multicopper oxidase activity that could be due to Fet5, a homologous protein localised to the vacuolar membrane (Spizzo et al., 1997). Consistent with a general copper-loading defect, multicopper oxidase activity of either Fet3 or Fet5 was absent in the Δgef1 deletion strain, as well as in the presence of Gef1 E230A (Fig. 3A). To exclude the trivial explanation that Fet3 was not expressed under the conditions where we failed to observe oxidase activity, we C-terminally tagged Fet3 with GFP by homologous recombination into the genomic locus (Fig. 3B). When grown under iron starvation, Δgef1 cells transformed with plasmid alone or plasmid encoding Gef1 E230A expressed a lot more Fet3GFP than a wt strain, or a Δgef1 deletion strain transformed with a plasmid encoding Gef1, as demonstrated by western blot (Fig. 3B). This suggests that the gating glutamate of Gef1 was required for survival in iron-limiting medium because Gef1 E230A failed to adjust the physicochemistry of the compartment to a state compatible with copper loading of Fet3. Consistent with this hypothesis, we observed an upregulation of N-terminally tagged Gef1 upon iron depletion (Fig. 3C) when tagged GEF1 was expressed under the control of the endogenous promoter (Wachter and Schwappach, 2005). The degree of Kex2 cleavage (as indicated by the relative abundance of full-length protein and the N-terminal cleavage product) did not change drastically when cells grew in low-iron medium, suggesting that Gef1 distributed similarly along the secretory pathway and into the prevacuole.
Fig. 3.
The uncoupling mutation E230A in Gef1 abolishes maturation of multicopper oxidases. (A) Total membrane protein isolated from the indicated strains resolved on a clear native gel and incubated with PPD to reveal multicopper oxidase activity (upper panel). After the in-gel assay, the gel was stained with Coomassie Blue to demonstrate equal loading of all lanes. Purified human ceruloplasmin was loaded as a positive control for multicopper oxidase activity. (B) Fet3GFP expression analysed in the indicated strains to verify expression of Fet3 in the strains lacking Gef1 antiporter activity. (C) 4PC-Gef1 expressed under the control of the endogenous GEF1 promoter to monitor physiological changes of Gef1 expression under iron starvation. Expression was analysed by western blotting of total cell lysates.
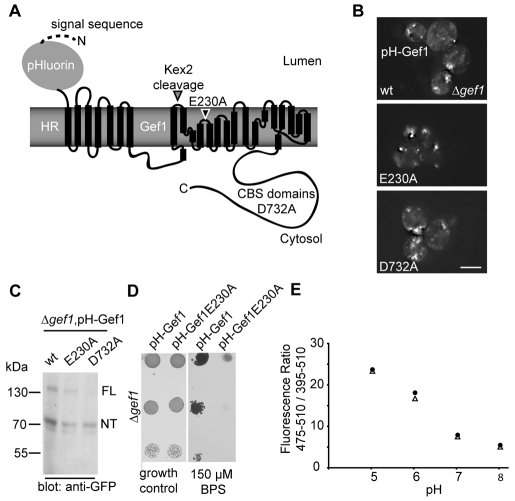
One important parameter in the further dissection of Gef1 function is compartmental pH, because it might drive Gef1 antiport activity, or change as a result of this activity. Thus we aimed to monitor the pH gradient across the relevant compartmental membrane by assessing cytosolic and luminal pH in untreated cells and those challenged by iron depletion (Figs 4 and 5). To achieve this, we constructed a Gef1 fusion protein that exposes a pH-sensitive GFP, ratiometric pHluorin (Miesenbock et al., 1998), to the luminal side. As the N-terminus of Gef1 is cytosolic, we used a signal sequence that initiated the translocation of pHluorin and the archeal membrane protein halorhodopsin (HR) as a spacer to achieve the desired topology of our fusion construct (pH-Gef1, Fig. 4A). Although HR acts as light-driven chloride pump in halobacteria, this function requires integration of a retinol cofactor, which is not synthesised in yeast. When expressed alone, HR had no discernable effect on the growth of a Δgef1 deletion strain and did not complement the growth defect under iron starvation (data not shown). The pH-Gef1 fusion protein was constructed with wt Gef1 as well as E230A and D732A to investigate the effect of either variant on compartmental pH. Again, all three fusion constructs localised similarly, as assessed by GFP imaging (Fig. 4B) and western blotting, demonstrating cleavage by the Kex2 protease (Fig. 4C). pH-Gef1 complemented the growth defect on low-iron medium, whereas the corresponding construct bearing the E230A mutation did not, as demonstrated by a plate growth assay (Fig. 4D), a result that was consistent with the growth assay using Gef1 and its variants (Fig. 2B,C). Together, this demonstrates that pH-Gef1 reached the site of Gef1 function in this phenotype. Finally, we calibrated the luminal pH-Gef1 probe and cytosolic pHluorin by incubation of digitonin-permeabilised cells in buffers of known pH (Fig. 4E). We conclude that the pH-Gef1 probe was suited to obtain pH values for the lumen of the Gef1-containing compartment.
Fig. 4.
Methodology to determine the pH gradient across the Gef1-containing compartment. (A) Scheme of the Gef1 fusion protein used as a probe to assess pH inside the Gef1-containing compartment. The signal sequence of invertase aids translocation of the pH-sensitive GFP pHluorin to the lumen. The archeal membrane protein halorhodopsin serves to adapt the luminal pHluorin to the cytosolic N-terminus of Gef1. All other details as indicated in Fig. 1A. (B) Subcellular localisation of N-terminal pHluorin-HR-Gef1 fusions with the indicated mutations revealed by live-cell imaging in a Δgef1 strain. Scale bar: 5 μm (C) Anti-GFP western blot of total cellular lysates from cells expressing the indicated pHluorin-HR-Gef1 fusion proteins. ‘FL’ and ‘NT’ indicate the migration position of uncleaved full-length Gef1 and the N-terminal product of the cleavage reaction, respectively. (D) The fusion protein used as a luminal pH probe complements the growth defect of a Δgef1 strain on low-iron medium. Serial dilutions of cultures grown in SC medium were spotted on a SC plate without (‘growth control’) or with 150 μM of the iron chelator BPS as indicated. (E) Calibration curves of the cytosolic (triangles) and luminal (circles) pHluorin probes. Background-corrected fluorescence ratios of both probes are reported as a function of pH after digitonin treatment in the indicated buffer.
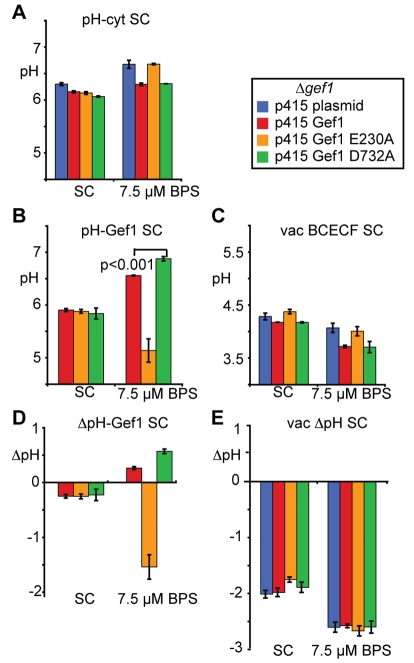
Fig. 5.
The pH gradient of the Gef1-containing compartment reverses upon iron limitation. (A) Steady-state pH measurements of the cytosol in a Δgef1 strain expressing the indicated constructs and the cytosolic pHluorin probe. Cells were grown in minimal SC medium lacking uracil and leucine. n=4-5 for A to C; error bars indicate s.e.; differences between the strains in SC and under iron starvation are significant (P<0.02). (B) Steady-state pH measurements of the Golgi and prevacuolar lumen in a Δgef1 strain expressing the indicated constructs and the pH-HR-Gef1 pHluorin probe. Growth and statistics as in A. (C) Steady-state pH measurements of the vacuolar lumen in a Δgef1 strain expressing the indicated constructs as determined by BCECF fluorescence measurements. Growth and statistics as in A. Differences between the strains in SC and under iron starvation are significant (P<0.02). (D) pH gradients across the membrane of the Gef1-containing compartment as calculated from values obtained in A and B. (E) pH gradients across the membrane of the vacuole as calculated from values obtained in A and C.
Steady-state pH measurements of the cytosol, the Gef1-containing compartment and the vacuole were then performed with Δgef1 deletion strains expressing the different Gef1 variants (Fig. 5). The vacuole was included in the measurements because of its important role in pH and metal homeostasis and because its luminal pH was accessible by staining with the pH-sensitive ratiometric dye BCECF (Ali et al., 2004). Under normal growth conditions in minimal medium, cytosolic pH (Fig. 5A) in a Δgef1 deletion strain was 6.3. Expression of any Gef1 variant resulted in a cytosolic pH close to 6 (differences to the deletion strain are statistically significant with P<0.02). At the same time, the Gef1-containing compartment was slightly acidic with respect to the cytosol and the vacuolar lumen substantially more acidic (Fig. 5B,C,D,E), as expected for these acidifying compartments from the literature (Klionsky et al., 1992; Manolson et al., 1994; Martinez-Munoz and Kane, 2008; Plant et al., 1999; Rothman et al., 1989). Challenge with the iron chelator bathophenanthroline sulfonate (BPS) affected pH homeostasis of all three compartments monitored: in the presence of Gef1, the cytosol became slightly more neutral, the Gef1-containing compartment substantially more neutral and the vacuole more acidic (Fig. 5A,B,C). Although the data shown in Fig. 5 represent cells that have grown in the low-iron medium for 21 hours, more neutral pH values were determined for the cytosol and the Gef1-containing compartment as early as 1 hour after transfer to low-iron medium (wt Gef1; data not shown). For all three compartments, significant differences between strains lacking Gef1 or harbouring Gef1 E230A and those expressing either wt Gef1 or the D732A variant were observed (P<0.02) but only in the case of the Gef1-containing compartment did this lead to a change in the ΔpH across the intracellular membrane (Fig. 5D). By contrast, the ΔpH across the vacuolar membrane was maintained at a difference of 2.6 pH units more acidic with respect to the cytosol under conditions of iron starvation (Fig. 5E). We conclude that the failure to copper-load Fet3 (Fig. 3) and to grow under iron starvation in the presence of Gef1 E230A (Fig. 2) correlates specifically with a lack of late Golgi and prevacuolar alkalinisation under these conditions (Fig. 5B,D). Instead, we observed an inappropriate acidification of the compartment in the presence of Gef1 E230A. This is consistent with the idea that Gef1 activity directly causes a change in ΔpH or that Gef1 coupling with the activity of another transporter (see Discussion) results in the observed net change in ΔpH.
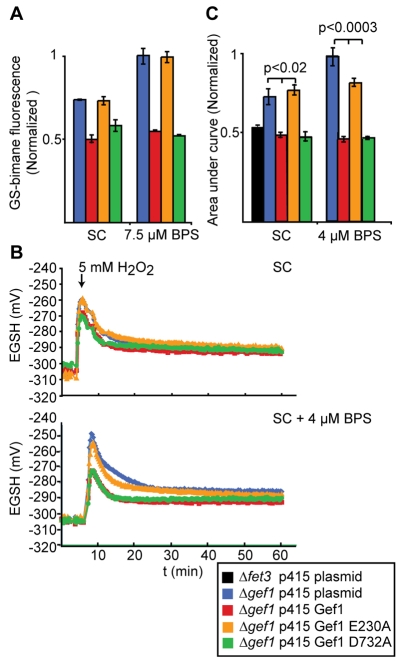
There is a conceptual gap between copper transport into the lumen by Ccc2 and copper incorporation into Fet3 that could potentially be closed by GSH acting as a luminal copper chelator. The lumen-specific glutathione concentration is currently an inaccessible parameter for technical reasons. However, any significant redistribution of reduced and/or oxidised glutathione across intracellular membranes is likely to affect general glutathione homeostasis, including cytosolic functions. As a first step to investigate a putative connection between Gef1 activity and GSH homeostasis, we asked whether the pool of reduced GSH available for the detoxification of an electrophilic xenobiotic is influenced by Gef1 expression and function. To this end, we used monochlorobimane (MCB), a membrane-permeant, non-fluorescent compound that is conjugated to GSH by glutathione S-transferase to generate the intensely fluorescent bimane S-conjugate (Staleva et al., 2002). Remarkably, cells lacking Gef1 or expressing Gef1 E230A exhibited increased bimane conjugation (Fig. 6A), probably indicative of higher GSH levels under xenobiotic stress. We then investigated the influence of mild iron starvation. In cells expressing Gef1 or Gef1 D732A, iron starvation did not affect bimane conjugation. However, in cells lacking Gef1 or E230, the increase in bimane conjugation became much more pronounced (Fig. 6A). Thus, iron starvation induced higher GSH levels specifically in those cells lacking Gef1 antiport activity. In conclusion, it appears that Gef1 antiport activity contributes to the global balancing of the glutathione system, especially under conditions of iron deficiency.
Fig. 6.
The uncoupling mutation in Gef1 decreases the capacity of the cytosol to maintain a reduced EGSH upon oxidative challenge. (A) Staining of Δgef1 strain expressing the indicated constructs with monochlorobimane (MCB) after growth in minimal SC medium lacking uracil and leucine and 7.5 μM BPS where indicated. The accumulation of MCB-GS conjugates was determined spectrophotometrically (n=3, error bars depict s.e.). (B) EGSH as a function of time in cells expressing the indicated construct and the cytosolic Grx1-roGFP2 probe. Cells were grown in minimal SC medium lacking uracil and leucine. At the time indicated, 5 mM H2O2 was added to the cell suspension. Lower panel shows response from cells grown in the presence of 4 μM BPS. (C) Area under the time course from the time of oxidative challenge (t=0) to 30 minutes after treatment calculated from data in B and corresponding data for the indicated constructs. n=3; error bars indicate s.e.
Because the bimane conjugation experiments suggested a significant influence of Gef1 on the cytosolic glutathione pool, we also examined the cytosolic glutathione redox potential (EGSH) using Grx1-roGFP2 (Gutscher et al., 2008), a genetically encoded fluorescent probe. We expressed Grx1-roGFP2 in the cytosol to obtain compartment-specific, quantitative and time-resolved measurements of EGSH. The absence of Gef1, or mutations resulting in E230A or D732A, did not have a significant influence on EGSH in the steady state. However, when challenged with a single bolus of H2O2, cells lacking Gef1 or expressing Gef1 E230A showed enhanced oxidation and delayed reductive recovery (Fig. 6B,C). Growth of the cells under mild iron starvation strongly enhanced this effect to highly significant levels (Fig. 6B,C). In conclusion, it appears that without Gef1 antiport activity, cytosolic glutathione is more susceptible to oxidation and more refractory to reductive recovery. Importantly, this was not the case for a Δfet3 deletion strain (Fig. 6C; black bar on the left, P<0.03 for a difference to the plasmid control Δgef1 strain and P<0.002 for a difference to the strain expressing Gef1 E230A), which shares the high-affinity iron-uptake defect observed for a Δgef1 deletion strain. This finding is consistent with the idea that the changes in glutathione redox homeostasis are upstream and not downstream of deficient Fet3 copper loading observed for strains lacking Gef1 antiporter activity.
Discussion
The presence of just one CLC protein in yeast, Gef1, has allowed us to perform an in vivo analysis of the physiological activity of this protein. Our most important finding is the result that specific physiological conditions, e.g. iron starvation, lead to an adaptation of the pH gradient across the membrane of the Gef1-containing late Golgi and prevacuolar compartment (Fig. 5): although the compartment is kept more acidic with respect to the cytosol under standard growth conditions, the gradient is depleted or even reversed upon challenge with an iron chelator in the growth medium – a case of vesicular alkalinisation.
Because of the presence of several other transporters and pumps it is impossible to interpret this finding directly in terms of the direction of proton transport via Gef1. One speculation is that Gef1 moves protons out of the compartment resulting in alkalinisation. The alternative speculation reverses a recent argument about compartmental acidification: because of the stoichiometry of two Cl− ions transported per one proton, e.g. because of the electrogenicity of CLC-mediated exchange, it has been argued that proton removal from a compartment by Gef1 could still result in a net acidification if some other transport system pumps protons (Jentsch, 2007). If this logic is reversed, the alkalinisation observed here could be consistent with Gef1 moving protons into the compartment, thereby exporting net negative charge and hence decreasing acidification by a different transport system.
Although we can only speculate about the mechanistic basis for the observed alkalinisation, the process was strictly correlated with a number of other observations. First, only strains capable of the change in compartmental ΔpH were also able to load copper on Fet3 and to grow under iron starvation (Figs 2 and 3). When the lumen was strongly acidified in the presence of Gef1 E230A, the compartment was unable to support Fet3 maturation and high-affinity iron uptake. This is consistent with the idea that the observed ΔpH brings about specific physicochemical conditions required for copper loading. This could be luminal pH itself, because the solubility of metals is known to be strongly pH dependent. However, as solubility increases with a more acidic pH, the argument seems of limited plausibility. In fact, copper and iron depletion is thought to be the main cause of growth defects observed in an alkaline environment (Serrano et al., 2004). Second, depending on the currently unknown direction of Gef1 exchanger activity, the observed results would be consistent with the previously suggested role of chloride ions in the actual loading process (Davis-Kaplan et al., 1998). If Gef1 moved protons out and chloride ions into the compartment, this would be consistent with the observed alkalinisation and would lead to an accumulation of chloride that is otherwise present at very low concentrations inside yeast cells (Jennings and Cui, 2008). A third possibility is that transport of either protons or chloride affects movement of GSH and/or GSSG across the relevant membrane. This could be due to either the pH dependence or the electrogenicity of glutathione transport, which is too poorly understood to exclude either of these alternatives.
Because of the missing link between copper import via Ccc2 and copper incorporation into Fet3 in the lumen of the Gef1-containing compartment, we have used two assays to gain insight into any possible relationship between Gef1 activity and GSH homeostasis (Fig. 6). Gef1 antiport activity was a relevant parameter in both assays of stress-related glutathione function, electrophile detoxification and cytosolic H2O2 scavenging. Thus, Gef1 antiport activity might help to maintain a pattern of glutathione movement between cellular compartments that is crucial for redox homeostasis and stress resistance throughout the cell. It is conceivable that the increased levels of GSH observed by the bimane assay reflect an adaptive response to defective Gef1-dependent glutathione transport. In any case, our data show that Gef1 strongly affects cellular GSH homeostasis in line with a general role for ion transport processes at the prevacuole and vacuole that has been revealed by systematic analysis of the process (Perrone et al., 2005). Therefore, it seems plausible to further test the hypothesis that GSH takes part in the chaperoning of copper in the Fet3-loading compartment and that its availability and/or redox state is controlled directly or indirectly by the exchanger activity of Gef1.
We have compared Gef1 D732A with wt Gef1 in its ability to control the ΔpH of the late Golgi and prevacuole and in its effects on growth under iron starvation, Fet3 copper loading and glutathione homeostasis. Although the mutant behaves qualitatively similarly to the wt protein and supports Fet3 copper loading and growth under iron starvation there was a quantitatively significant difference in the alkalinisation of the Gef1-containing compartment (Fig. 5B,D; P<0.001): the reversal of acidification was more pronounced in the presence of Gef1 D732A compared with wt Gef1. Hence our data are consistent with a regulatory function of the conserved ATP-binding site in Gef1. Interestingly, iron starvation leads to major remodelling of carbohydrate metabolism, with a shift away from oxidative phosphorylation (Puig et al., 2008). Therefore, we speculate that falling ATP levels under iron starvation activate the Gef1 exchanger to prepare the physicochemical environment of the late Golgi and prevacuole for Fet3 maturation. Once high-affinity iron uptake is in place, rising ATP concentrations might downregulate Gef1 activity. In this view, the overshoot in late Golgi and prevacuolar alkalinisation (Fig. 5B) of the presumably ATP-binding deficient Gef1 D732A would be consistent with the results presented by De Angeli and colleagues (De Angeli et al., 2009), who report a reversible inhibition of Arabidopsis CLC-a by ATP. In conclusion, our in vivo investigation of Gef1 antiporter activity under iron starvation suggests that the activity of CLC anion transport proteins is subject to metabolic regulation that results in an adaptation of compartmental function.
Materials and Methods
Yeast strain and growth conditions
S. cerevisiae strains used in this report were all based on the Δgef1::KanR or Δfet3::KanR deletion strain obtained from the EUROSCARF collection in the BY4742 mating type α background (Accession number: Y16838, http://web.uni-frankfurt.de/fb15/mikro/euroscarf), only Fig. 3B,C used the corresponding mating type a (BY4741). Yeast strains were grown in synthetic complete (SC) medium with defined dropouts, and where indicated 4-20 μM bathophenanthroline sulfonate (BPS) was added. Methionine was eliminated from the growth medium to enhance expression from plasmids containing the MET25 promoter when α (MET15) strains were used (Mumberg et al., 1994). Genomic integration of a C-terminal GFP tag into the FET3 locus was performed as described by Sheff and Thorn (Sheff and Thorn, 2004) using plasmid pKT128. To test the endogenous expression of N-terminally 4PC-tagged Gef1, a URA3-marked integrating vector constructed by Wächter and Schwappach (Wächter and Schwappach, 2005) was used.
Molecular biology and general yeast methodology
Published molecular biology and yeast protocols were followed (Ausubel et al., 1997). Ratiometric pHluorin (Miesenbock et al., 1998), pH-Gef1 and Grx1-roGFP (Gutscher et al., 2008) were cloned into the yeast expression vector p416MET25 (Mumberg et al., 1994). For the pH-Gef1 construct, the signal sequence of invertase (Suc1) was fused to the open reading frames encoding halorhodopsin (Bamberg et al., 1993) and Gef1. The open reading frame encoding Gef1 and the indicated mutants was cloned into p415MET25 obtained by crossing p416MET25 with pRS415 (Sikorski and Hieter, 1989). All constructs were fully verified by sequencing. Total cell lysates for western blotting analysis were prepared as described (Yaffe and Schatz, 1984). Anti-GFP and anti-Pdi1 primary antibodies (kind gifts from Martin Lowe, University of Manchester, UK and Maya Schuldiner, Weizmann Institute, Israel) were diluted 1:2000 and the respective anti-rabbit and anti-sheep (Invitrogen) secondary antibodies 1:5000. Mouse anti-PC antibody (Roche) was used at 250 ng/ml; anti-mouse secondary antibody was diluted 1:5000.
Live cell imaging
Live imaging of yeast cells was performed at room temperature in SC medium using a DeltaVision restoration microscope equipped with a 100×/0.35-1.5 Uplan Apo objective and a GFP filter set (Chroma 86006). The images were collected with a Coolsnap HQ camera (Photometrics).
Fet3 oxidase assay
Cells were grown to an OD600 of 0.8 per ml in minimal medium containing the indicated concentration of BPS, harvested by centrifugation (total of 160 OD600 units) and broken mechanically using glass beads in breaking buffer (25 mM Tris-HCl, pH 7.5, 150 mM NaCl, 5% glycerol, 1 mM dithiotreitol, 1 mM ascorbate, 1 mM PMSF, fungal protease inhibitors; Sigma). The lysate was cleared by low-speed centrifugation and total cellular membranes were harvested by centrifugation at 100,000 g. Membranes were washed in membrane buffer (25 mM Tris-HCl, pH 7.5, 50 mM NaCl, 50 mM 6-aminocaproic acid, 5% glycerol, 1 mM ascorbate, fungal protease inhibitor; Sigma) and then resuspended in 200 μl membrane buffer. Total membrane protein was determined and membranes containing 100 μg protein were pelleted and solubilised in membrane buffer containing 2% digitonin for 30 minutes on ice. Insoluble material was pelleted at full speed in a tabletop centrifuge, 0.5% glycerol and 0.1 % ponceau-S was added before loading onto a clear native gel (Invitrogen). 0.05% sodium deoxycholate and 0.02% dodecyl maltoside was added to the cathode buffer to improve resolution. Gels were directly incubated in 100 mM sodium acetate pH 5.5 containing 0.5 mg/ml para-phenylendiamin (PPD), as described (Davis-Kaplan et al., 1998; Yuan et al., 1997).
Physiological measurements and optical methods
Yeast growth assays were performed in a Biotek synergy HT microplate reader using Gen5 software and Costar 3596 flat-bottom microplates. Yeast cultures were inoculated from individual colonies into 200 μl overnight pre-cultures at 30°C. Triplicate precultures were made for each yeast strain tested. Inoculations for the 21 hour growth assay were made at an OD600 of 0.1, and yeast from the precultures were inoculated into triplicate wells with a final volume of 200 μl (total of nine replicates per strain). Yeast cells were grown at 30°C for 21 hours with one second of shaking on ‘low’ setting every 20 minutes immediately before OD600 readings were taken. The microplate OD600 values were converted to standard pathlength OD600 readings by an empirical calibration of our microplate reader (Warringer, 2003). Only pathlength-corrected OD600 data is presented in this report.
Intracellular pH measurements using ratiometric pHluorin were performed with cells within 2 weeks of transformation. The Δgef1 strain was transformed with p416MET25 pHluorin and either p415MET25 encoding Gef1, Gef1 E230A or D732A for the cytosolic pH determination, or p416MET25 pH-Gef1, p416MET25 pH-Gef1E230A, p416MET25 pH-Gef1 D732A and p415MET25 encoding Gef1, Gef1 E230A or D732A, respectively, for the Golgi and prevacuolar lumen pH determination. Additionally, parallel treatments were performed on yeast transformed with empty p416MET25 and empty p415MET25 plasmids, which were used to determine the contribution of yeast autofluorescence to the pHluorin spectral measurements. For the pHluorin experiments, yeast strains were grown at 30°C in 20 ml overnight precultures, then inoculated at 0.1 OD600 into Falcon tubes containing 10 ml medium. These yeast cultures were grown for 21 hours at 30°C with shaking. The cells were then centrifuged for 5 minutes at 1000 g, the supernatant discarded and the remaining cells were resuspended into a TPP 92697 U-bottom microplate. The microplate was spun for 5 minutes at 10 g to form a loose cell pellet within each well. Optical measurements were performed using a Biotek synergy HT microplate reader configured to both excite (395/10 nm, 475/10 nm) and detect (510/10 nm) fluorescence from the bottom of the plate. Background-corrected fluorescence ratios of pHluorin were converted to pH using a calibration curve. The pHluorin calibration curve was constructed by growing and treating yeast similarly as described above, but 1 hour before microplate reading, the cells were centrifuged and resuspended in 0.16% digitonin and 100 mM of buffer appropriate for achieving a pH of 5, 6, 7 or 8. After background-correcting the fluorescence, a calibration curve was constructed to relate the fluorescence ratio of both the cytosolic and Golgi pHlourin to the pH.
Vacuolar pH measurements were performed using 2′,7′-bis-(2-Carboxyethyl)-5(6)-carboxyfluorescein acetoxymethyl ester (BCECF). To calibrate the pH-dependent fluorescence of BCECF, 10 ml yeast culture was grown overnight to stationary phase and then centrifuged and resuspended in 100 μl of 100 mM buffer at an appropriate pH. Final concentrations of 50 μM BCECF and 50 μM FCCP were added to the cell solution, and then incubated at 30°C for 30 minutes with mild shaking. The cells were then centrifuged, washed twice in appropriate buffer, then resuspended into a TPP 92697 U-bottom microplate and centrifuged for 5 minutes at 10 g to form a loose cell pellet. BCECF fluorescence was determined using a Biotek synergy HT microplate reader configured to both excite (485/20 nm) and detect (528/20 nm, 645/40 nm) fluorescence (Duan et al., 2007) from the bottom of the plate. As a control for yeast autofluorescence, cells were treated in parallel, without any BCECF addition. The spectral reading of these mock-treated cells was subtracted from the BCECF-treated cells. For the experimental BCECF-treated cells, 20 ml overnight cultures were grown of Δgef1 yeast expressing a p415MET25 plasmid encoding Gef1, Gef1 E230A or D732A or an empty plasmid. These cells were then inoculated into four 10 ml cultures of appropriate medium at 0.1 OD600 and grown for 20 hours. The cultures were then centrifuged for 5 minutes at 1000 g, the supernatant discarded, and resuspended in 100 μl of fresh medium containing 50 μM BCECF. For each plasmid and media combination, one 10 ml culture did not receive any BCECF and was the control for yeast autofluorescence. The cells were incubated for 30 minutes at 30°C, then 5 ml fresh medium was added to the culture tubes and the cells were again centrifuged for 5 minutes at 1000 g. The cell pellets were resuspended in 100 μl fresh medium into a TPP 92697 U-bottom microplate and centrifuged and spectrally analysed as described above.
Monochlorobimane (MCB) staining was performed as a measure of GSH conjugation to electrophilic xenobiotics. Overnight precultures of Δgef1 strain transformed with a relevant p415MET25 plasmid were used to inoculate 10 ml cultures at OD600 0.1. After 19 hours, a final concentration of 0.15 mM of MCB was added to the 10 ml cultures and they were thoroughly mixed. After 21 total hours of growth, the cells were centrifuged and collected into a microplate as described above. The MCB fluorescence was both excited (395/10 nm) and detected (485/20 nm) from the bottom of the microplate using a Biotek synergy HT microplate reader. One 10 ml culture of each yeast strain for each growth condition had no MCB added, and it was used as a control for determination of the background fluorescence (Nair et al., 1991).
For measurement of cytosolic glutathione response to H2O2, BY4742 Δgef1 cells were transformed with both p415MET25 empty plasmid or p415MET25 encoding Gef1, Gef1E230A or Gef1 D732A and p416MET25 Grx1-roGFP2 or p416 empty plasmid which was used for background subtraction. The BY4742 Δfet3 strain was doubly transformed with both, p415MET25 empty plasmid and either p416MET25 empty plasmid or p416MET25 Grx1-roGFP2. Cells were grown in minimal selective medium, in 10 ml precultures for 24 hours at 25°C. Precultures were used to inoculate 10 ml fresh medium to a final OD600 of 0.1, which was grown for 16 hours at 25°C to yield late-exponential-phase cultures. For each measurement 1.5 OD600 units of cells were harvested by centrifugation at 1000 g for 5 minutes, and resuspended in 200 μl of 100 mM MES, pH 6.0, with the inclusion of either 20 mM diamide or 100 mM DTT for oxidised and reduced controls, respectively. Samples were transferred to a flat-bottomed 96-well plate, which was spun at 20 g for 5 minutes. Measurements were performed using a BMG Labtech FLUOstar Omega plate-reader, using excitation wavelengths of (390/10 nm) and (480/10 nm) and recording emission at (520/10 nm) from the bottom of the plate. A glutathione response was initiated by the injection of H2O2 to a final concentration of 5 mM. Background corrected emission at 520 nm from both excitation wavelengths was used to calculate the degree of probe oxidation (OxDroGFP2) according to equation 1 (Meyer and Dick, 2010):
| (1) |
The corresponding intracellular probe redox potential EroGFP2 was derived from the Nernst equation (equation 2):
| (2) |
Finally, given Grx1-mediated equilibration between the glutathione and roGFP2 redox pairs, EGSH was taken to equal EroGFP2. It should be noted that the calculation of actual redox potentials is based on the standard redox potential of roGFP2, which assumes pH 7. Area under the curve from 0-30 minutes was calculated with GraphPad Prism5 software using a baseline of −300 mV.
Acknowledgments
We would like to thank Boyan Bonev, Yin Cai, and Michael Heymann for help with initial experiments, Anne Clancy and Jutta Metz for superb technical assistance. Our colleagues Eric Arakel, Neil Bulleid, Chris Grant and Matthias Seedorf have provided valuable support and comments throughout the project and we thank Andrew Smith for critical reading of the manuscript. We are grateful to Georg Nagel and James Rothman for sharing HR and pHluorin-encoding plasmids respectively and Heiner Schirmer for purified ceruloplasmin. Microscopy was performed in the FLS Bioimaging Facility. BS holds a Wellcome Senior Research Fellowship that funded herself and N.A.B. B.M. was an EMBO short-term fellow in Heidelberg. Deposited in PMC for release after 6 months.
References
- Accardi A., Miller C. (2004). Secondary active transport mediated by a prokaryotic homologue of ClC Cl− channels. Nature 427, 803-807 [DOI] [PubMed] [Google Scholar]
- Accardi A., Walden M., Nguitragool W., Jayaram H., Williams C., Miller C. (2005). Separate ion pathways in a Cl-/H+ exchanger. J. Gen. Physiol. 126, 563-570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali R., Brett C. L., Mukherjee S., Rao R. (2004). Inhibition of sodium/proton exchange by a Rab-GTPase-activating protein regulates endosomal traffic in yeast. J. Biol. Chem. 279, 4498-4506 [DOI] [PubMed] [Google Scholar]
- Askwith C., Kaplan J. (1997). An oxidase-permease-based iron transport system in Schizosaccharomyces pombe and its expression in Saccharomyces cerevisiae. J. Biol. Chem. 272, 401-405 [DOI] [PubMed] [Google Scholar]
- Askwith C., Eide D., Van Ho A., Bernard P. S., Li L., Davis-Kaplan S., Sipe D. M., Kaplan J. (1994). The FET3 gene of S. cerevisiae encodes a multicopper oxidase required for ferrous iron uptake. Cell 76, 403-410 [DOI] [PubMed] [Google Scholar]
- Ausubel F. M., Brent R., Kingston R. E., Moore D. D., Seidman J. G., Smith J. A., Struhl K. (1997). Current Protocols in Molecular Biology New York: Greene Publishing Associates and Wiley-Interscience; [Google Scholar]
- Bamberg E., Tittor J., Oesterhelt D. (1993). Light-driven proton or chloride pumping by halorhodopsin. Proc. Natl. Acad. Sci. USA 90, 639-643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman A. (1997). The structure of a domain common to archaebacteria and the homocystinuria disease protein. Trends Biochem. Sci. 22, 12-13 [DOI] [PubMed] [Google Scholar]
- Davis-Kaplan S. R., Askwith C. C., Bengtzen A. C., Radisky D., Kaplan J. (1998). Chloride is an allosteric effector of copper assembly for the yeast multicopper oxidase Fet3p: an unexpected role for intracellular chloride channels. Proc. Natl. Acad. Sci. USA 95, 13641-13645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Angeli A., Monachello D., Ephritikhine G., Frachisse J. M., Thomine S., Gambale F., Barbier-Brygoo H. (2006). The nitrate/proton antiporter AtCLCa mediates nitrate accumulation in plant vacuoles. Nature 442, 939-942 [DOI] [PubMed] [Google Scholar]
- De Angeli A., Moran O., Wege S., Filleur S., Ephritikhine G., Thomine S., Barbier-Brygoo H., Gambale F. (2009). ATP binding to the C terminus of the Arabidopsis thaliana nitrate/proton antiporter, AtCLCa, regulates nitrate transport into plant vacuoles. J. Biol. Chem. 284, 26526-26532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan X. G., Yang A. F., Gao F., Zhang S. L., Zhang J. R. (2007). Heterologous expression of vacuolar H(+)-PPase enhances the electrochemical gradient across the vacuolar membrane and improves tobacco cell salt tolerance. Protoplasma 232, 87-95 [DOI] [PubMed] [Google Scholar]
- Dutzler R. (2007). A structural perspective on ClC channel and transporter function. FEBS Lett. 581, 2839-2844 [DOI] [PubMed] [Google Scholar]
- Dutzler R., Campbell E. B., Cadene M., Chait B. T., MacKinnon R. (2002). X-ray structure of a ClC chloride channel at 3.0 A reveals the molecular basis of anion selectivity. Nature 415, 287-294 [DOI] [PubMed] [Google Scholar]
- Freedman J. H., Ciriolo M. R., Peisach J. (1989). The role of glutathione in copper metabolism and toxicity. J. Biol. Chem. 264, 5598-5605 [PubMed] [Google Scholar]
- Gaxiola R. A., Yuan D. S., Klausner R. D., Fink G. R. (1998). The yeast CLC chloride channel functions in cation homeostasis. Proc. Natl. Acad. Sci. USA 95, 4046-4050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaxiola R. A., Rao R., Sherman A., Grisafi P., Alper S. L., Fink G. R. (1999). The Arabidopsis thaliana proton transporters, AtNhx1 and Avp1, can function in cation detoxification in yeast. Proc. Natl. Acad. Sci. USA 96, 1480-1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves A. R., Curran P. K., Smith C. L., Mindell J. A. (2008). The Cl−/H+ antiporter ClC-7 is the primary chloride permeation pathway in lysosomes. Nature 453, 788-792 [DOI] [PubMed] [Google Scholar]
- Greene J. R., Brown N. H., DiDomenico B. J., Kaplan J., Eide D. J. (1993). The GEF1 gene of Saccharomyces cerevisiae encodes an integral membrane protein; mutations in which have effects on respiration and iron-limited growth. Mol. Gen. Genet. 241, 542-553 [DOI] [PubMed] [Google Scholar]
- Gutscher M., Pauleau A. L., Marty L., Brach T., Wabnitz G. H., Samstag Y., Meyer A. J., Dick T. P. (2008). Real-time imaging of the intracellular glutathione redox potential. Nat. Methods 5, 553-559 [DOI] [PubMed] [Google Scholar]
- Jennings M. L., Cui J. (2008). Chloride homeostasis in Saccharomyces cerevisiae: high affinity influx, V-ATPase-dependent sequestration, and identification of a candidate Cl-sensor. J. Gen. Physiol. 131, 379-391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch T. J. (2007). Chloride and the endosomal-lysosomal pathway: emerging roles of CLC chloride transporters. J. Physiol. 578, 633-640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch T. J. (2008). CLC chloride channels and transporters: from genes to protein structure, pathology and physiology. Crit. Rev. Biochem. Mol. Biol. 43, 3-36 [DOI] [PubMed] [Google Scholar]
- Jentsch T. J., Steinmeyer K., Schwarz G. (1990). Primary structure of Torpedo marmorata chloride channel isolated by expression cloning in Xenopus oocytes. Nature 348, 510-514 [DOI] [PubMed] [Google Scholar]
- Klionsky D. J., Nelson H., Nelson N., Yaver D. S. (1992). Mutations in the yeast vacuolar ATPase result in the mislocalization of vacuolar proteins. J. Exp. Biol. 172, 83-92 [DOI] [PubMed] [Google Scholar]
- Li Y., Kane T., Tipper C., Spatrick P., Jenness D. D. (1999). Yeast mutants affecting possible quality control of plasma membrane proteins. Mol. Cell. Biol. 19, 3588-3599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolson M. F., Wu B., Proteau D., Taillon B. E., Roberts B. T., Hoyt M. A., Jones E. W. (1994). STV1 gene encodes functional homologue of 95-kDa yeast vacuolar H(+)-ATPase subunit Vph1p. J. Biol. Chem. 269, 14064-14074 [PubMed] [Google Scholar]
- Martinez-Munoz G. A., Kane P. (2008). Vacuolar and plasma membrane proton pumps collaborate to achieve cytosolic pH homeostasis in yeast. J. Biol. Chem. 283, 20309-20319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A. J., Dick T. P. (2010). Fluorescent protein-based redox probes. Antioxid. Redox Signal [Epub ahead of print] doi:10.1089/ars.2009.2948 [DOI] [PubMed] [Google Scholar]
- Meyer S., Savaresi S., Forster I. C., Dutzler R. (2007). Nucleotide recognition by the cytoplasmic domain of the human chloride transporter ClC-5. Nat. Struct. Mol. Biol. 14, 60-67 [DOI] [PubMed] [Google Scholar]
- Miesenbock G., De Angelis D. A., Rothman J. E. (1998). Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature 394, 192-195 [DOI] [PubMed] [Google Scholar]
- Miller C. (2006). ClC chloride channels viewed through a transporter lens. Nature 440, 484-489 [DOI] [PubMed] [Google Scholar]
- Mohammad-Panah R., Wellhauser L., Steinberg B. E., Wang Y., Huan L. J., Liu X. D., Bear C. E. (2009). An essential role for ClC-4 in transferrin receptor function revealed in studies of fibroblasts derived from Clcn4-null mice. J. Cell Sci. 122, 1229-1237 [DOI] [PubMed] [Google Scholar]
- Mumberg D., Muller R., Funk M. (1994). Regulatable promoters of Saccharomyces cerevisiae: comparison of transcriptional activity and their use for heterologous expression. Nucleic Acids Res. 22, 5767-5768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair S., Singh S. V., Krishan A. (1991). Flow Cytometric monitoring of glutathione content and anthracycline retention in tumor cells. Cytometry 12, 336-342 [DOI] [PubMed] [Google Scholar]
- Perrone G. G., Grant C. M., Dawes I. W. (2005). Genetic and environmental factors influencing glutathione homeostasis in Saccharomyces cerevisiae. Mol. Biol. Cell 16, 218-230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picollo A., Pusch M. (2005). Chloride/proton antiporter activity of mammalian CLC proteins ClC-4 and ClC-5. Nature 436, 420-423 [DOI] [PubMed] [Google Scholar]
- Pirie N. W., Goodwin Pinhey K. (1929). The titration curve of glutathione. J. Biol. Chem. 84, 321-333 [Google Scholar]
- Plant P. J., Manolson M. F., Grinstein S., Demaurex N. (1999). Alternative mechanisms of vacuolar acidification in H(+)-ATPase-deficient yeast. J. Biol. Chem. 274, 37270-37279 [DOI] [PubMed] [Google Scholar]
- Pufahl R. A., Singer C. P., Peariso K. L., Lin S. J., Schmidt P. J., Fahrni C. J., Culotta V. C., Penner-Hahn J. E., O'Halloran T. V. (1997). Metal ion chaperone function of the soluble Cu(I) receptor Atx1. Science 278, 853-856 [DOI] [PubMed] [Google Scholar]
- Puig S., Vergara S. V., Thiele D. J. (2008). Cooperation of two mRNA-binding proteins drives metabolic adaptation to iron deficiency. Cell Metab. 7, 555-564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman J. H., Yamashiro C. T., Raymond C. K., Kane P. M., Stevens T. H. (1989). Acidification of the lysosome-like vacuole and the vacuolar H+-ATPase are deficient in two yeast mutants that fail to sort vacuolar proteins. J. Cell Biol. 109, 93-100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheel O., Zdebik A. A., Lourdel S., Jentsch T. J. (2005). Voltage-dependent electrogenic chloride/proton exchange by endosomal CLC proteins. Nature 436, 424-427 [DOI] [PubMed] [Google Scholar]
- Schwappach B., Stobrawa S., Hechenberger M., Steinmeyer K., Jentsch T. J. (1998). Golgi localization and functionally important domains in the NH2 and COOH terminus of the yeast CLC putative chloride channel Gef1p. J. Biol. Chem. 273, 15110-15118 [DOI] [PubMed] [Google Scholar]
- Serrano R., Bernal D., Simon E., Arino J. (2004). Copper and iron are the limiting factors for growth of the yeast Saccharomyces cerevisiae in an alkaline environment. J. Biol. Chem. 279, 19698-19704 [DOI] [PubMed] [Google Scholar]
- Sheff M. A., Thorn K. S. (2004). Optimized cassettes for fluorescent protein tagging in Saccharomyces cerevisiae. Yeast 21, 661-670 [DOI] [PubMed] [Google Scholar]
- Sikorski R. S., Hieter P. (1989). A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122, 19-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spizzo T., Byersdorfer C., Duesterhoeft S., Eide D. (1997). The yeast FET5 gene encodes a FET3-related multicopper oxidase implicated in iron transport. Mol. Gen. Genet. 256, 547-556 [DOI] [PubMed] [Google Scholar]
- Staleva L., Manga P., Orlow S. J. (2002). Pink-eyed dilution protein modulates arsenic sensitivity and intracellular glutathione metabolism. Mol. Biol. Cell 13, 4206-4220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wächter A., Schwappach B. (2005). The yeast CLC chloride channel is proteolytically processed by the furin-like protease Kex2p in the first extracellular loop. FEBS Lett. 579, 1149-1153 [DOI] [PubMed] [Google Scholar]
- Waldron K. J., Rutherford J. C., Ford D., Robinson N. J. (2009). Metalloproteins and metal sensing. Nature 460, 823-830 [DOI] [PubMed] [Google Scholar]
- Wang T., Weinman S. A. (2004). Involvement of chloride channels in hepatic copper metabolism: ClC-4 promotes copper incorporation into ceruloplasmin. Gastroenterology 126, 1157-1166 [DOI] [PubMed] [Google Scholar]
- Warringer J. B. A. (2003). Automated screening in environmental arrays allows analysis of quantitative phenotypic profiles in Saccharomyces cerevisiae. Yeast 20, 53-67 [DOI] [PubMed] [Google Scholar]
- Yaffe M. P., Schatz G. (1984). Two nuclear mutations that block mitochondrial protein import in yeast. Proc. Natl. Acad. Sci. USA 81, 4819-4823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan D. S., Stearman R., Dancis A., Dunn T., Beeler T., Klausner R. D. (1995). The Menkes/Wilson disease gene homologue in yeast provides copper to a ceruloplasmin-like oxidase required for iron uptake. Proc. Natl. Acad. Sci. USA 92, 2632-2636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan D. S., Dancis A., Klausner R. D. (1997). Restriction of copper export in Saccharomyces cerevisiae to a late Golgi or post-Golgi compartment in the secretory pathway. J. Biol. Chem. 272, 25787-25793 [DOI] [PubMed] [Google Scholar]
- Zdebik A. A., Zifarelli G., Bergsdorf E. Y., Soliani P., Scheel O., Jentsch T. J., Pusch M. (2008). Determinants of anion-proton coupling in mammalian endosomal CLC proteins. J. Biol. Chem. 283, 4219-4227 [DOI] [PubMed] [Google Scholar]
- Zhu X., Williamson P. R. (2003). A CLC-type chloride channel gene is required for laccase activity and virulence in Cryptococcus neoformans. Mol. Microbiol. 50, 1271-1281 [DOI] [PubMed] [Google Scholar]
- Zifarelli G., Pusch M. (2009). Intracellular regulation of human ClC-5 by adenine nucleotides. EMBO Rep. 10, 1111-1116 [DOI] [PMC free article] [PubMed] [Google Scholar]