Abstract
The bacterial action of gentamicin and that of a mixture of gentamicin and 15-nm colloidal-gold particles onEscherichia coliK12 was examined by the agar-well-diffusion method, enumeration of colony-forming units, and turbidimetry. Addition of gentamicin to colloidal gold changed the gold color and extinction spectrum. Within the experimental errors, there were no significant differences in antibacterial activity between pure gentamicin and its mixture with gold nanoparticles (NPs). Atomic absorption spectroscopy showed that upon application of the gentamicin-particle mixture, there were no gold NPs in the zone of bacterial-growth suppression in agar. Yet, free NPs diffused into the agar. These facts are in conflict with the earlier findings indicating an enhancement of the bacterial activity of similar gentamicin–gold nanoparticle mixtures. The possible causes for these discrepancies are discussed, and the suggestion is made that a necessary condition for enhancement of antibacterial activity is the preparation of stable conjugates of NPs coated with the antibiotic molecules.
Keywords: Colloidal-gold nanoparticles, Gentamicin, Drug delivery, Antibacterial activity, Agar-well-diffusion method, Minimum inhibitory concentration, Maximum tolerant concentration, Atomic absorption spectroscopy
Introduction
Over the recent decade, gold nanoparticles (NPs) [1-3] have attracted significant interest as a novel platform for various applications such as nanobiotechnology and biomedicine [4-7] because of convenient surface bioconjugation [8] with molecular probes and remarkable plasmon-resonant optical properties [9]. Recently published examples include applications of NPs to biosensorics [10], genomics [11,12], clinical chemistry [13], immunoassays [14], immune response enhancement [15], detection and control of microorganisms [16], optical imaging of biological cells (including cancer cell imaging with resonance scattering [17,18], optical coherence tomography [19], two-photon luminescence [20], and photoacoustic [21,22] techniques), cancer cell photothermolysis [23,24], and targeted delivery of drugs or genetic and immunological substances [25-29]. In particular, there is great interest in the development of nanoparticle-based vectors that decrease the toxicity of free drugs and ensure targeted delivery directly to tumor cells [30-33]. Gold NPs have been used for delivery of not only antitumor agents, but also insulin [34], tocopherol [35], and other drugs [16,29].
Conjugates of gold NPs with antibiotics and antibodies also have been used for selective photothermal killing of protozoa and bacteria [36-38]. In regard to antibacterial activity, Williams et al. [39] showed that gold NPs themselves do not affect bacterial growth or functional activity, whereas conjugates of vancomycin to gold NPs decrease the number of growing bacterial cells [37]. Gu et al. [40] synthesized stable gold NPs covered with vancomycin and showed significant enhancement of antibacterial activity for this conjugate, in comparison with the activity of the free antibiotic. A similar result was reported for ciprofloxacin conjugated with Au/SiO2 core/shell NPs [41].
In contrast to gold NPs, silver NPs may exhibit antibacterial activity [42]. Furthermore, silver NPs were shown to enhance the antibacterial activity of penicillin G, amoxicillin, erythromycin, clindamycin, and vancomycin against Staphylococcus aureus and Escherichia coli[43]. Similar conclusions were reported on the antibacterial activity of silver and gold NPs stabilized with hyperbranched poly(amidoamine), containing terminal dimethylamine groups [44].
It should be emphasized that in the above-cited studies [37,40,41], the authors used NPs functionalized with antibiotics by physical or chemical adsorption. Compared with bare NPs, stable conjugates exhibited small changes in the absorption spectra. For the naked eye, the conjugated sols retained their red color, typical of colloidal-gold sols.
In 2007, four papers have been published [45-48], reporting the use of blue aggregated mixtures of drugs and GNPs, rather than of stable red conjugates. Such a color change and transmission electron microscopy (TEM) images unambiguously indicated NP aggregation [49]. The drugs used were aminoglycoside antibiotics (streptomycin, gentamicin, kanamycin, and neomycin), quinolones (ciprofloxacin, gatifloxacin, and norfloxacin), ampicillin (a penicillin antibiotic), and 5-fluorouracil (an antimetabolite of nucleic metabolism). The preparations obtained by the authors were tested for antibacterial activity toward gram-positive (S. aureusMicrococcus luteus) and gram-negative (E. coliPseudomonas aeruginosa) microorganisms, and they also were examined for antifungal activity toward Aspergillus fumigatus and Aspergillus niger. The basic experimental tests for the determination of antibacterial activity were the disk diffusion method [45,46,48] and the agar-well-diffusion method [47]. Depending on the antibiotic used, increase in the activity of the antibiotic–colloidal-gold mixture ranged from 12 to 40%, as compared with the activities of the native drugs. From those data, the authors concluded that the antibacterial activities of the antibiotics were enhanced through the use of gold NPs [45-48].
However, as noted by the authors themselves [43-48], the question of the mechanisms governing possible enhancement of the antibacterial action of drugs or polymers remains unanswered. Whereas several hypotheses have been raised for aggregatively stable NP–antibiotic conjugates [40], the enhancement mechanism for aggregated NP–antibiotic mixtures—if it exists at all—is absolutely incomprehensible, at least when the activity of preparations is assessed by the agar-well-diffusion method. First, no gold NPs have been shown to be present in the agar zone of bacterial-growth inhibition. Antibiotic addition to an NP suspension leads to NP aggregation, readily detectable with extinction spectra and with TEM images. The question now arises, can particle aggregates diffuse into agar at all? Let us suggest for a moment that diffusion is impossible. In that case, the question of enhancement of antibacterial action loses its meaning altogether. Here, therefore, we decided to examine the antibacterial activity of an NP–antibiotic mixture and to simultaneously investigate the penetration of particles into agar.
We explored the antibacterial activity of a mixture of gentamicin and colloidal-gold particles (average diameter, 15 nm) toward E. coli К12, by using the agar-well-diffusion method, enumeration of colony-forming units (CFUs), and turbidimetry. Gentamicin was chosen on the basis of the following reasons. First, as an aminoglycoside antibiotic, gentamicin is of unquestionable practical interest. Being a mixture of gentamicins C1, C2, and C1a, it is bacteriostatic to many gram-positive and gram-negative microorganisms, including E. coliProteusSalmonella, and penicillin-resistant Staphylococcus strains. The mechanism of gentamicin action is linked to disruption of ribosomal synthesis of protein, and microbial resistance to gentamicin develops fairly slowly. Gentamicin is a major agent used to treat severe purulent infection, especially that caused by a resistant gram-negative flora. As a broad-spectrum antibiotic, gentamicin is often prescribed for patients with mixed infection and also when the infecting agent has not been identified. Sometimes gentamicin is effective when other antibiotics display insufficient activity [50].
Second, gentamicin was chosen because, as found previously [45], a mixture of gentamicin and gold NPs has the most enhanced activity toward E. coli. It is this result, along with the need to study particle penetration into agar, that prompted this research.
Experimental Section
Preparation of Gold NPs
Gold NPs were prepared by the reduction of tetrachloroauric acid with sodium citrate [51]. A 242.5-mL portion of 0.01% aqueous tetrachloroauric acid (Aldrich, USA) was heated on an MR 3001 magnetic stirrer (Heidolph, Germany) in an Erlenmeyer flask fitted with a water-cooled reflux tube. This was followed by the addition of 7.5 mL of 1% aqueous sodium citrate (Fluka, Switzerland) to the flask. The mean particle diameter (16 nm) was controlled by spectrophotometric calibration [52].
Preparation of a Gentamicin–NP Mixture
We used an aqueous stock solution of gentamicin sulfate (Fluka, Switzerland; activity, 636 U mg−1; concentration, 4.5 mg mg−1). Immediately before being added to the culture medium or to the gel wells, the antibiotic solution was mixed 1:1 either with 2 mM K2CO3or with gold NPs in the same solution. In agar-well-diffusion experiments, we also made a series of twofold dilutions of the free-gentamicin solution and of the gentamicin–NP mixture.
The formation of Au–Gm complex can be easily monitored by UV–Vis spectra (S-300 spectrophotometer, Analytik Jena, Germany). The initial 16-nm gold colloid exhibits well-known plasmon resonance near 520 nm. Immediately after addition of Gm, we observed drastic change in the colloid color from wine red to purple blue. To follow such kinetics in detail, we decreased the concentration of Gm 20 times (up to 0.05 μg mL−1) as compared to the average concentration used in microbial assay (Table 1). The time-dependent UV–Vis spectra were recorded after mixing (1:1 v/v) Au colloid with Gm at timed interval 30 s (step, 1 s). A portion of spectra is shown in Fig. 1. The appearance of a new red-shifted peak near 650–670 nm is a typical signature of a fast NPs aggregation. Indeed, such a phenomenon has been described in numerous reports; for details, the readers are referred to review Ref. [53].
Table 1.
Antibacterial action of gentamicin and a gentamicin–NP mixture onE. coliK12
| Gentamicin concentration (mg mL−1) | Inhibition-zone diameter (mm) |
|
|---|---|---|
| Gentamicin | Gentamicin + NPs | |
| 0.563 |
9.9 ± 0.6 |
9.9 ± 0.6 |
| 1.13 |
11.6 ± 0.2 |
11.5 ± 0.2 |
| 2.25 | 12.3 ± 0.6 | 12.1 ± 0.55 |
Figure 1.
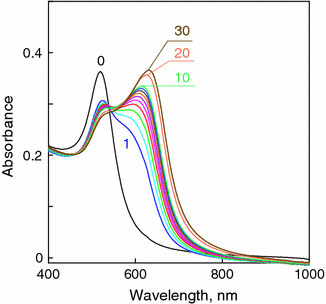
Time-dependent absorption spectra of NPs–Gm mixture (1:1 v/v). The final concentration of NPs and Gm are 0.15 mM and 0.05 μg mL−1, respectively. Thenumbersnear curves designate the time after mixing (seconds), thecurve 0is the initial spectrum without Gm
Bacterial Strain and Growth Conditions
E. coli К12 obtained from this institute’s collection was used for this study. The strain was grown in Luria–Bertani (LB) medium at 37 °C. All inoculation experiments used an overnight accumulation culture grown to stationary phase in advance. The initial culture absorbance A600 was 0.04. Bacterial growth was assessed by using the time-dependent absorbance curve. The cell concentration was estimated by the turbidity-spectra method [54].
CFU Enumeration
A bacterial suspension was mixed 1:1 with either a free-gentamicin solution or a gentamicin–NP mixture and was incubated at 37 °C for 1 h. For each treatment, six 10-fold serial dilutions were made. A 200-μL volume of the resultant suspension was uniformly spread onto overnight-dried solid LB medium with a sterile spatula. After cultivation at 37 °C for 24 h, all the colonies grown were enumerated, and the mean values and maximal scatter in CFUs were determined.
Microbial Assay
Antibacterial activity was studied by the agar-well-diffusion method, wherein a bacterial suspension was added to sterile nutrient agar at 45 °C and the mixture was solidified on a Petri dish. A 20-mL volume of the medium was poured into a Petri dish (diameter, 90 mm) on a horizontally leveled surface. After the medium had solidified, 4-mm-diameter wells were made in the agar (at six wells per dish) that were equidistant from one another and from the dish edge. The wells received either 20 μL of the free-antibiotic solution or 20 μL of the antibiotic–NP mixture. The Petri dishes were incubated in a thermostat at 37 °C for 24 h. After incubation, the diameter of the zone of bacterial-growth inhibition was measured with an accuracy of ±0.1 mm. The mean inhibition-zone diameter and the maximal data scatter also were determined. All experiments were repeated thrice.
Determination of the Minimum Inhibitory and Maximum Tolerant Concentrations
In experiments to determine the minimum inhibitory concentration (MIC) and the maximum tolerant concentration (MTC, equivalent to the “no observed effect concentration”), culturing was done in microtitration-plate wells for 3 h. The initial culture absorbance A600was 0.04. The MIC was taken to be the gentamicin concentration at which the A600of the bacterial suspension after incubation was almost the same as the initial A600, and the MTC was numerically equal to the gentamicin concentration at which the parameters of culture growth were close to those for the control culture (without the antibiotic).
Atomic Absorption Spectroscopy
Ashing of samples was done with the addition of sulfuric acid at 600–630 °C. The ash was then dissolved in a mixture of concentrated hydrochloric and nitric acids. The solution was evaporated to dryness, a necessary amount of 0.5 N hydrochloric acid was added, and the sample thus prepared was analyzed for gold on an AAS-3 atomic absorption spectrometer (Carl Zeiss, Germany). The resonance line was 242.8 nm, and the spectral slit width was 0.35 nm. Under such conditions, the limit of detection is 0.02 μg mL−1and the linear working region is up to 20 μg mL−1.
Results and Discussion
Effect of the Antibiotic Concentration
Figure 2ais a photo of a Petri dish showing the zones of inhibition ofE. coligrowth upon addition of free-gentamicin and a gentamicin–NP mixture to the wells. The antibiotic concentration in the wells was decreased by twofold dilutions from 2.25 to 0.56 mg mL−1. It can be seen that the gentamicin–NP mixture retarded bacterial growth to a degree comparable to that demonstrated by the free antibiotic. When the free antibiotic and its mixture with NPs were diluted twofold, the diameter of the zone of culture-growth inhibition was reduced to the same extent in both cases. To obtain reliable statistical data, we ran five independent experiments, with three replicates per experiment. Figure 2band Table 1give averaged data indicating that the antibacterial action of gentamicin did not differ significantly from that of the gentamicin–NP mixture.
Figure 2.
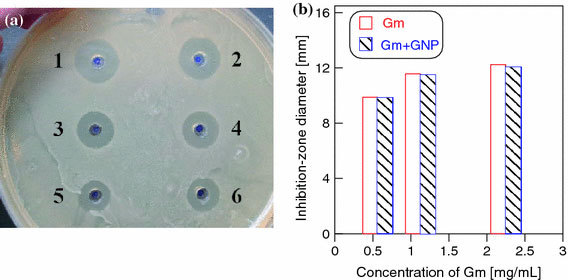
aZones of inhibition of the growth ofE. coliK12 on solid LB medium. Wells 1, 3, and 5 received gentamicin, whereas wells 2, 4, and 6 received gentamicin + gold NPs. The final antibiotic concentration in the wells was decreased by twofold dilutions and was 2.25 (wells 1 and 2), 1.13 (wells 3 and 4), and 0.563 mg mL−1(wells 5 and 6).bA diagram showing the averaged results from five independent experiments, with three replicates per experiment
Effect of the Residual Particles and Supernatant Liquids
We next answered the question whether the mixture NPs freed from unbound antibiotic in the solution showed antibacterial action. Because gold particles on their own did not have antibacterial activity (Fig. 3), our experiment allowed us to assess (to an extent) the degree of antibiotic binding to the particles and the possible enhancement of antibacterial activity through the agency of the particles. For this purpose, the gentamicin–NP mixture was centrifuged at 3000×g, and the sediment was stirred in the same volume of water and was applied to the wells.
Figure 3.
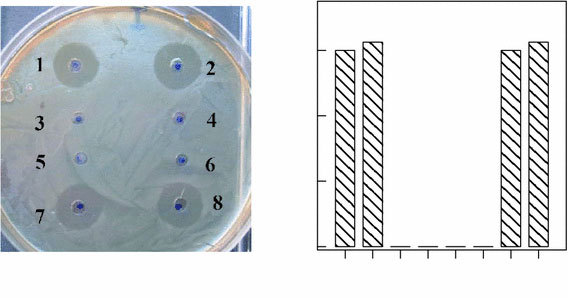
aAntibacterial effect of gentamicin (1), gentamicin + gold NPs (2), redissolved sediments (3, 4), the solvent (2 mM K2CO3) (5), a solution of gold NPs (6), and the supernatant liquids from both preparations (7, 8) on the growth ofE. coliK12.bA diagram showing the coincidence of the average inhibition-zone diameters for free gentamicin (1), its mixture with NPs (2), and the supernatant liquids from these preparation (7, 8), respectively
We found (Fig. 3, wells 3 and 4) that the sediment NPs did not cause the formation of a zone of culture-growth inhibition at all. Yet, the supernatant liquids resulting from centrifugation had the same degree of activity toward bacterial growth as did the initial gentamicin–NP mixture (Fig. 3, wells 7 and 8). We emphasize once again that in our control experiments, neither colloidal gold itself nor solvent (2 mM K2CO3) inhibited bacterial growth (Fig. 3, wells 5 and 6).
Effect of the NP Concentration
The absence of enhancement of the antibacterial action of the antibiotic–NP conjugates may have been due to the low concentration of particles themselves. Therefore, we examined the effect of the gold NP concentration on the antibacterial action of the conjugates. For this purpose, antibiotic solutions having the same concentration were mixed with equal volumes of 0.1, 0.5, and 1.0 mM gold solutions in 2 mM K2CO3before being added to the wells. Note that the gold concentration of as prepared 16-nm particles was about 0.3 mM. Accordingly, the mass/volume concentration is about 57 μg mL−1or, equivalently, the particle-number concentration is about 1.4 × 1012 mL−1. After the preparation of a concentrated stock solution, the above concentrations (0.1–1 mM) were obtained by corresponding dilutions. The results (Fig. 4) show that the antibacterial activity of the preparations decreased slightly with increasing particle concentration, but from a statistical analysis of the data, it follows that this effect is within the error and is not significant.
Figure 4.
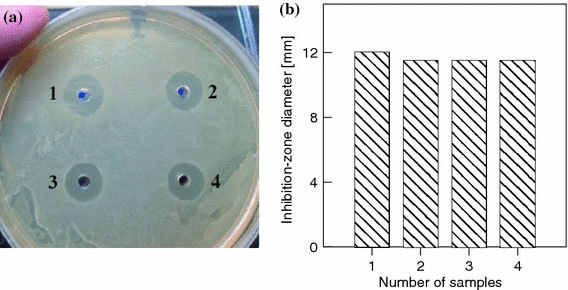
aZones of inhibition of the growth ofE. coliK12 upon application of gentamicin (1) and gentamicin–NP mixtures at particle concentrations of 0.1 (2), 0.5 (3), and 1.0 mM (4).bA diagram showing the averaged inhibition-zone diameters for samples 1–4
Diffusion of Free Gentamicin and Its Complexes with NPs into Agar
As said above, addition of the antibiotic to the NP sol led to aggregation, confirmed by changes in the colloid color and extinction spectrum and also by direct TEM images. Consequently, the absence of enhancement of the antibacterial action of the conjugates and particles sedimented from the antibiotic–NP mixtures could be explained by an inability of aggregated particles to penetrate into agar gel. To test this hypothesis, we poured 1.5% agar gel (in water) into 40-mm-diameter Petri dishes, made a well in the center of each dish, and applied an NP solution and a gentamicin–NP mixture to the wells. A day later, a red colloidal-gold halo was clearly seen around the well in the case of the NP solution, whereas a blue precipitate at the well bottom in the case of the mixture (Fig. 5).
Figure 5.
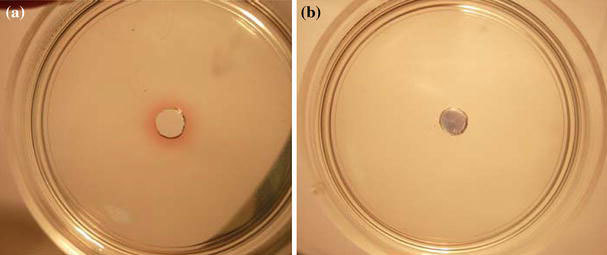
Petri dish with 1.5% agar gel at 24 h after application of an NP solution (a) and a gentamicin–NP mixture (b)
In order to independently estimate the content of gold in the diffusion zones, we used AAS. A ring-shaped piece of gel with an outside diameter of 15 mm and an inside diameter of 5 mm (the well diameter was 4 mm) was cut from the samples (Fig. 5) for AAS analysis of the gold content in the gel. The same procedure was used for the gels (Fig. 2) (for wells 2 and 6, which received the antibiotic–NP mixture and free NPs). Analysis showed that gold was totally absent in the agar gels around the mixture-containing wells but was present around the wells containing an NP solution (Table 2).
Table 2.
Analysis of gold content in the gel samples cut out around the wells at 24 h after the application of an NP solution and a gentamicin–NP mixture
| Sample | Fraction of the total Au mass in the sample (%) |
|---|---|
| Au in 1.5% agar gel |
9.3 × 10−4 |
| Gm + Au in 1.5% agar gel |
0 |
| Au in solid LB medium |
3.2 × 10−4 |
| Gm + Au in solid LB medium | 0 |
Experiments with Bacterial Suspensions
Our study with bacteria grown on a solid nutrient medium has shown the absence of NPs in the inhibition zone. It follows that the question of enhancement of or decrease in the antibacterial activity of gentamicin is meaningless in this context. Therefore, we decided to investigate the antibacterial activity of an antibiotic–NP mixture in liquid culture, in which NPs or aggregates have a chance of coming into contact with bacterial cells because of Brownian motion. The antibacterial activity of the preparations was assessed by the MIC and MTC of gentamicin and a gentamicin–NP mixture for E. coli K12. From spectroturbidimetric data [54], the initial cell density was 5 × 107 cells mL−1. Figure 6 shows that the absorbance of the control culture in an NP-containing medium did not differ within the limits of error from that in an NP-free medium. The main result of this experiment is that curves 3 and 4 for bacterial cells grown with free gentamicin and with a gentamicin–NP mixture do not differ from each other. Consequently, the antibacterial activity of the gentamicin–NP mixture does not exceed that of the native antibiotic not only on a solid nutrient medium, but also in a liquid medium. Quantitatively, this conclusion is shown in Table 3, which gives data on the MIC and MTC of the free antibiotic and its mixture with gold NPs.
Figure 6.
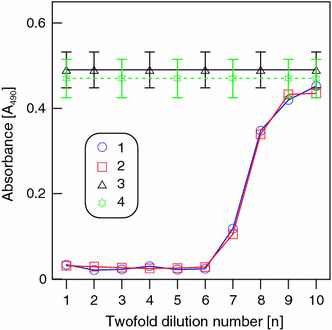
The absorbance (A490) ofE. coliK12 suspension after 3 h of incubation in LB nutrient medium versus the concentration of gentamicin (1) and a gentamicin–NP mixture (2). Thex-axis shows twofold dilutions of the preparations. Lines 3 and 4 show the average absorbance level in the control medium (3) and in a gentamicin-free medium containing 0.1 mM NPs (4)
Table 3.
The MICs and MTCs of gentamicin and a gentamicin–NP mixture added to growingE. coliK12 cells
| Sample | MIC (μg mL−1) | MTC (μg mL−1) |
|---|---|---|
| Gm |
7.4 |
0.9 |
| Gm + Au | 7.4 | 0.9 |
Comparison of the Bactericidal Effects of Gentamicin and a Gentamicin–NP Mixture
In the final set of experiments, we compared the bacterial effects of the original antibiotic and a gentamicin–NP mixture. For this purpose, the cells were plated on gentamicin-free solid LB medium from the 10−6dilution of cultures incubated for 3 h with different preparations. For incubation, we used free gentamicin, a gentamicin–NP mixture, and colloidal NPs (control). The antibiotic concentrations were lowered by twofold dilutions from 240 to 3.7 μg mL−1. The CFU data for the minimal and maximal values are given in Table 4.
Table 4.
The results of CFU counts after culturing on solid LB medium
| Sample | CFU (cells mL−1) |
|
|---|---|---|
| 240 μg of the antibiotic per milliliter | 3.7 μg of the antibiotic per milliliter | |
| Control |
4.8 ± 0.4 × 109 |
4.6 ± 0.5 × 109 |
| NPs |
3.6 ± 0.9 × 109 |
2.8 ± 0.6 × 109 |
| Gm |
0 |
1.8 ± 0.5 × 109 |
| Gm + NPs | 0 | 0.96 ± 0.5 × 109 |
Table 4shows that gentamicin at 240 μg mL−1was bactericidal to 50 × 106bacterial cells mL−1both in a free state and in complex with NPs. The NPs decreased the CFU value, as compared with the control, but these differences were not significant. At a gentamicin concentration of 3.7 μg mL−1, the difference between the CFU values for free gentamicin and for the mixture was almost twofold, with the addition of NPs decreasing, not increasing, the bactericidal action of the antibiotic. However, because the CFU method is usually in error by an order of magnitude, this difference between the CFU values for gentamicin and for its mixture with NPs is not significant.
Conclusions
By using several methods, we have studied the effect of 16-nm gold NPs on the antibacterial activity of gentamicin. Within the limits of experimental error, no differences have been found between the antibacterial activity of gentamicin and that of a gentamicin–gold NP mixture at various gentamicin and particle concentrations. Sedimented gold NPs from the conjugates had no antibacterial activity, whereas the supernatant liquids from gentamicin–NP mixtures and free gentamicin demonstrated the same activity. Electron microscopy and the changes in the extinction spectra showed the presence of NP aggregates, which, on evidence derived by AAS, could not penetrate into gel. This explains the absence of growth inhibition upon addition of NP sediment to the wells. Furthermore, the same degree of activity of free gentamicin and the mixtures indicates that the amount of antibiotic that could bind to the particles is small. By the CFU method, we have found that the bactericidal action of a gentamicin–NP mixture does not differ from that of free gentamicin within the limits of error. Finally, the parameters of growth inhibition in a liquid bacterial culture (MIC and MTC) also were the same for gentamicin and for the gentamicin–NP mixture. In all our experiments, therefore, we have found no significant differences in antibacterial activity between the free antibiotic and the mixture either on a solid or in a liquid nutrient medium. Comparison of these data with the findings in the literature [37,40,41], showing enhancement of antibacterial activity in the presence of NPs, suggests that two conditions at minimum are necessary (but insufficient) for such effects to be observed. First, antibiotic–NP conjugates should be stabilized, and their spectrum and color should correspond to those of single-particle nonaggregated colloids. Second, the amount of the antibiotic covering the particle surface should be large enough to ensure an increase in the local antibiotic concentration at the site of bacterium–particle contact. Thus, although gold NPs themselves do not have any antimicrobial activity, they may act as drug curriers. In other words, because of the presence of gold NPs, the surface area increases and hence it carries a lot of drug on its surface. Obviously, when the amount of drug in proximity of a bacterium is more, the antibacterial property may be enhanced. For other possible explanations, the readers are referred to Ref. [40]. In our opinion, the mechanism(s) of possible enhancement of the antibacterial activity of conjugates is still an open question and needs further study.
Acknowledgments
This study was partially supported by grants from the Russian Foundation for Basic Research (Nos. 07-04-00301a, 07-04-00302a, 07-02-01434-a, 08-02-00399, and 09-02-00496-a), CRDF BRHE Annex (Y4-B-06-01), the Ministry of Science and Education of the Russian Federation by a Program on the Development of High School Potential (No. 2.2.1.1/2950), and from the Presidium of RAS Program “The Basic Sciences—to Medicine.” We thank Mr. D.N. Tychinin (IBPPM RAS) for help in preparation of the manuscript.
References
- Daniel MC, Astruc D. Chem. 2004. p. 293. COI number [1:CAS:528:DC%2BD3sXpvFGlur0%3D] [DOI] [PubMed]
- Dykman LA, Bogatyrev VA. Russ. 2007. p. 181. COI number [1:CAS:528:DC%2BD2sXlt1Oms74%3D] [DOI]
- L.A. Dykman, V.A. Bogatyrev, S.Y. Shchyogolev, N.G. Khlebtsov, Gold Nanoparticles: Synthesis, Properties, Biomedical Applications (Izdatel’stvo ‘‘Nauka’’, Moscow, 2008) (in Russian)
- Cheng MM, Cuda G, Bunimovich YL, Gaspari M, Heath JR, Hill HD, Mirkin CA, Nijdam AJ, Terracciano R, Thundat T, Ferrari M. Curr. 2006. p. 11. COI number [1:CAS:528:DC%2BD28XhtlGnt7g%3D] [DOI] [PubMed]
- Liao H, Nehl CL, Hafner JH. Nanomedicine. 2006. p. 201. COI number [1:CAS:528:DC%2BD28XpsFKisLw%3D] [DOI] [PubMed]
- Hu M, Chen J, Li Z-Y, Au L, Hartland GV, Li X, Marqueze M, Xia Y. Chem. 2006. p. 1084. COI number [1:CAS:528:DC%2BD28XhtFSgtL%2FK] [DOI] [PubMed]
- Shim S-Y, Lim D-K, Nam J-M. Nanomedicine. 2008. p. 215. COI number [1:CAS:528:DC%2BD1cXktFyjt7s%3D] [DOI] [PubMed]
- Glomm WR. J. 2005. p. 389. COI number [1:CAS:528:DC%2BD2MXktFKlt70%3D] [DOI]
- Kreibig U, Vollmer M. Optical Properties of Metal Clusters. Springer-Verlag, Berlin; 1995. [Google Scholar]
- Stewart ME, Anderton CR, Thompson LB, Maria J, Gray SK, Rogers JA, Nuzzo RG. Chem. 2008. p. 494. COI number [1:CAS:528:DC%2BD1cXhtFOnu74%3D] [DOI] [PubMed]
- Rosi NL, Mirkin CA. Chem. 2005. p. 1547. COI number [1:CAS:528:DC%2BD2MXisVymsLk%3D] [DOI] [PubMed]
- Liu X, Dai Q, Austin L, Coutts J, Knowles G, Zou J, Chen H, Huo Q. J. 2008. p. 2780. COI number [1:CAS:528:DC%2BD1cXhsF2rtbc%3D] [DOI] [PubMed]
- Baptista P, Pereira E, Eaton P, Doria G, Miranda A, Gomes I, Quaresma P, Franco R. Anal. 2008. p. 943. COI number [1:CAS:528:DC%2BD1cXlvFKnsr0%3D] [DOI] [PubMed]
- Gupta S, Huda S, Kilpatrick PK, Velev OD. Anal. 2007. p. 3810. COI number [1:CAS:528:DC%2BD2sXktVyhurc%3D] [DOI] [PubMed]
- Dykman LA, Sumaroka MV, Staroverov SA, Zaitseva IS, Bogatyrev VA. Biol. 2004. p. 75. COI number [1:CAS:528:DC%2BD2cXovVGhug%3D%3D] [DOI] [PubMed]
- Luo PG, Stutzenberger FJ. Adv. 2008. p. 145. COI number [1:CAS:528:DC%2BD1cXpsVKru7g%3D] [DOI] [PubMed]
- El-Sayed IH, Huang X, El-Sayed MA. Nano Lett. 2005. p. 829. COI number [1:CAS:528:DC%2BD2MXjt1SmtLg%3D]; Bibcode number [2005NanoL...5..829E] [DOI] [PubMed]
- Aaron J, de la Rosa E, Travis K, Harrison N, Burt J, José-Yakamán M, Sokolov K. Opt. Express. 2008. p. 2153. COI number [1:CAS:528:DC%2BD1cXhvFansbk%3D]; Bibcode number [2008OExpr..16.2153A] [DOI] [PubMed]
- Loo C, Hirsch L, Lee M, Chang E, West J, Halas N, Drezek R. Opt. 2005. p. 1012. COI number [1:CAS:528:DC%2BD2MXktlCjsLo%3D]; Bibcode number [2005OptL...30.1012L] [DOI] [PubMed]
- Park J, Estrada A, Sharp K, Sang K, Schwartz JA, Smith DK, Coleman C, Payne JD, Korgel BA, Dunn AK, Tunnell JW. Opt. Express. 2008. p. 1590. COI number [1:CAS:528:DC%2BD1cXhvFansbY%3D]; Bibcode number [2008OExpr..16.1590P] [DOI] [PubMed]
- Zharov V, Galanzha E, Shashkov E, Khlebtsov N, Tuchin V. Opt. 2006. p. 3623. Bibcode number [2006OptL...31.3623Z] [DOI] [PubMed]
- Mallidi S, Larson T, Aaron J, Sokolov K, Emelianov S. Opt. Express. 2007. p. 6583. COI number [1:CAS:528:DC%2BD2sXntlGlsbY%3D]; Bibcode number [2007OExpr..15.6583M] [DOI] [PubMed]
- Pissuwan D, Valenzuela SM, Cortie MB. Trends Biotechnol. 2006. p. 62. COI number [1:CAS:528:DC%2BD28XhtV2lsr8%3D] [DOI] [PubMed]
- Khlebtsov BN, Zharov VP, Melnikov AG, Tuchin VV, Khlebtsov NG. Nanotechnology. 2006. p. 5167. COI number [1:CAS:528:DC%2BD28Xht1OjtLbK]; Bibcode number [2006Nanot..17.5167K] [DOI]
- Donnelly JJ, Wahren B, Liu MA. J. 2005. p. 633. COI number [1:CAS:528:DC%2BD2MXlvFSjs7w%3D] [DOI] [PubMed]
- Paciotti GF, Kingston DGI, Tamarkin L. Drug Dev. 2006. p. 47. COI number [1:CAS:528:DC%2BD28XltF2msLc%3D] [DOI]
- Xu ZP, Zeng QH, Lu GQ, Yu AB. Chem. 2006. p. 1027. COI number [1:CAS:528:DC%2BD2MXht1aqur3P] [DOI]
- Bergen JM, von Recum HA, Goodman TT, Massey AP, Pun SH. Macromol. 2006. p. 506. COI number [1:CAS:528:DC%2BD28XnslChtbc%3D] [DOI] [PubMed]
- Han G, Ghosh P, Rotello VM. Nanomedicine. 2007. p. 113. COI number [1:CAS:528:DC%2BD2sXhvF2itrY%3D] [DOI] [PubMed]
- Paciotti GF, Myer L, Weinreich D, Goia D, Pavel N, McLaughlin RE, Tamarkin L. Drug Deliv. 2004. p. 169. COI number [1:CAS:528:DC%2BD2cXks1aiurc%3D] [DOI] [PubMed]
- Chen Y-H, Tsai C-Y, Huang P-Y, Chang M-Y, Cheng P-C, Chou C-H, Chen D-H, Wang C-R, Shiau A-L, Wu C-L. Mol. 2007. p. 713. COI number [1:CAS:528:DC%2BD2sXpt1Gitrs%3D] [DOI] [PubMed]
- Li J, Wang X, Wang C, Chen B, Dai Y, Zhang R, Song M, Lv G, Fu D. ChemMedChem. 2007. p. 374. COI number [1:CAS:528:DC%2BD2sXmtVWqu7w%3D] [DOI] [PubMed]
- Patra CR, Bhattacharya R, Wang E, Katarya A, Lau JS, Dutta S, Muders M, Wang S, Buhrow SA, Safgren SL, Yaszemski MJ, Reid JM, Ames MM, Mukherjee P, Mukhopadhyay D. Cancer Res. 2008. p. 1970. COI number [1:CAS:528:DC%2BD1cXjtFOhtrg%3D] [DOI] [PubMed]
- Joshi HM, Bhumkar DR, Joshi K, Pokharkar V, Sastry M. Langmuir. 2006. p. 300. COI number [1:CAS:528:DC%2BD2MXht1Kkt7zF] [DOI] [PubMed]
- Nie Z, Liu KJ, Zhong C-J, Wang L-F, Yang Y, Tian Q, Liu Y. Free Radical Biol. 2007. p. 1243. COI number [1:CAS:528:DC%2BD2sXhtVOis7nE] [DOI] [PubMed]
- Pissuwan D, Valenzuela SM, Miller CM, Cortie MB. Nano Lett. 2007. p. 3808. COI number [1:CAS:528:DC%2BD2sXhtlCks77O]; Bibcode number [2007NanoL...7.3808P] [DOI] [PubMed]
- Huang W-C, Tsai P-J, Chen Y-C. Nanomedicine. 2007. p. 777. COI number [1:CAS:528:DC%2BD1cXitlWgu7Y%3D] [DOI] [PubMed]
- Zharov VP, Mercer KE, Galitovskaya EN, Smeltzery MS. Biophys. 2006. p. 619. COI number [1:CAS:528:DC%2BD28XktlKrsg%3D%3D]; Bibcode number [2006BpJ....90..619Z] [DOI] [PMC free article] [PubMed]
- D.N. Williams, S.H. Ehrman, T.R.P. Holoman, J. Nanobiotechnol. 4, Art. No. 3 (2006). doi:10.1186/1477-3155-4-3.
- Gu H, Ho PL, Tong E, Wang L, Xu B. Nano Lett. 2003. p. 1261. COI number [1:CAS:528:DC%2BD3sXmsFOitr0%3D]; Bibcode number [2003NanoL...3.1261G] [DOI]
- Rosemary MJ, MacLaren I, Pradeep T. Langmuir. 2006. p. 10125. COI number [1:CAS:528:DC%2BD28XhtVyrsb7I] [DOI] [PubMed]
- Shrivastava S, Bera T, Roy A, Singh G, Ramachandrarao P, Dash D. Nanotechnology. 2007. p. 225103. Bibcode number [2007Nanot..18v5103S] [DOI] [PubMed]
- Shahverdi AR, Fakhimi A, Shahverdi HR, Minaian S. Nanomedicine. 2007. p. 168. COI number [1:CAS:528:DC%2BD2sXnsVOrurw%3D] [DOI] [PubMed]
- Zhang Y, Peng H, Huang W, Zhou Y, Yan D. J. 2008. p. 371. COI number [1:CAS:528:DC%2BD1cXpvVKht7o%3D] [DOI] [PubMed]
- Grace AN, Pandian K. Colloids Surf. 2007. p. 63. COI number [1:CAS:528:DC%2BD2sXitFekuro%3D] [DOI]
- Grace AN, Pandian K. J. 2007. p. 96. [DOI]
- Saha B, Bhattacharya J, Mukherjee A, Ghosh AK, Santra CR, Dasgupta AK, Karmakar P. Nanoscale Res. 2007. p. 614. COI number [1:CAS:528:DC%2BD1cXlslejsL8%3D]; Bibcode number [2007NRL.....2..614S] [DOI]
- Selvaraj V, Alagar M. Int. 2007. p. 275. COI number [1:CAS:528:DC%2BD2sXlsFags7o%3D] [DOI] [PubMed]
- Khlebtsov NG, Dykman LA, Krasnov YM, Melnikov AG. Colloid J. 2000. p. 765. COI number [1:CAS:528:DC%2BD3MXhs1Srsw%3D%3D] [DOI]
- V.G. Maidannik, I.V. Maidannik, Spravochnik sovremennykh lekarstvennykh sredstv (Handbook of Modern Medicines) (Izdatel’stvo ‘‘AST’’, Moscow, 2005) (in Russian)
- Frens G. Nat. 1973. p. 20. COI number [1:CAS:528:DyaE3sXns1ansg%3D%3D]; Bibcode number [1973NPhS..241...20F]
- Khlebtsov NG. Anal. 2008. p. 6620. COI number [1:CAS:528:DC%2BD1cXoslKmtbk%3D] [DOI] [PubMed]
- N.G. Khlebtsov, A.G. Melnikov, L.A. Dykman, V.A. Bogatyrev, in Photopolarimetry in Remote Sensing, ed. by G. Videen, Y.S. Yatskiv, M.I. Mishchenko, NATO Science Series, II. Mathematics, Physics, and Chemistry, vol. 161 (Kluwer, Dordrecht, 2004), pp. 265–308.
- Shchyogolev SY, Khlebtsov NG, Schwartsburd BI. Proc. SPIE. 1993. p. 67. Bibcode number [1993SPIE.1981...67S] [DOI]


