Abstract
Surface of detonation nanodiamonds was functionalized for the covalent attachment of immunoglobulin, and simultaneously bovine serum albumin and Rabbit Anti-Mouse Antibody. The nanodiamond-IgGI125 and RAM-nanodiamond-BSAI125 complexes are stable in blood serum and the immobilized proteins retain their biological activity. It was shown that the RAM-nanodiamond-BSAI125 complex is able to bind to the target antigen immobilized on the Sepharose 6B matrix through antibody–antigen interaction. The idea can be extended to use nanodiamonds as carriers for delivery of bioactive substances (i.e., drugs) to various targets in vivo.
Keywords: Nanodiamonds, Ligand, Protein immobilization, Nanocarrier, Targeted delivery
Introduction
Recently, much attention has been drawn to the studies on developing systems for address delivery of diagnostic and therapeutic agents to biological targets. For these purposes, liposomes [1,2] and various polymers [3,4] are known examples. Other carriers such as nanoparticles of metals [5-7] and nanotubes [8] represent an attractive alternative. It is obvious that such systems must contain a ligand exhibiting high selectivity and affinity towards a biological target to which they carry the desired substance. For example, antibodies are often used as targeting agents [9].
Detonation nanodiamonds with a high colloidal stability in aqueous and non-aqueous media are a promising material for the targeted delivery of substances [10-12]. Earlier studies have shown that the nanodiamonds effectively adsorb various biomolecules [13-17], allow obtaining sterile hydrosols for all types of injections including intravenous ones [18] and show a high biocompatibility in vivo [19,20].
In this work, we investigated the possibility of conjugating nanodiamonds with iodine-125 labeled immunoglobulin (IgGI125) and bovine serum albumin (BSAI125), and Rabbit Anti-Mouse Antibody (RAM), as well as properties of the obtained complexes.
Experimental Details
A nanodiamond powder produced at Krasnoyarsk Research Centre with aggregate sizes varying in the range 30–125 nm was modified to impart a high colloidal stability in hydrosols. The modification method, physical and chemical properties of the nanodiamonds have been described elsewhere [10,11]. Nanodiamond hydrosols were prepared using DI water with concentration of the dispersed phase 15.0 g l−1. Milli-Q system (Millipore, USA) was used for producing DI water.
Mouse immunoglobulin G (IgG) (Medix Biochemical, Finland); bovine blood serum (Mikrogen, Russia); BSA (Amersham, England); Sepharose 6B (Pharmacia, Sweden); Rabbit AntiMouse Antibody (RAM) (Imtek Ltd., Russia); NaI125 (RITVERC, Russia); chloramine-T and sodium metabisulphite (Amersham, England) were used as acquired.
IgGI125 and BSAI125 were produced as follows. A chloramine-T solution (10 μl) at concentration of 2.5 mg ml−1 and NaI125 solution (40 μl) of total activity ~20 MBk were added to IgG (or BSA) solution (50 μl) at a protein concentration of 1 mg ml−1 in 0.5 M phosphate buffer (pH 7.0). The samples were stirred and incubated at room temperature for 2 min. Then, a sodium metabisulphite solution (100 μl) at concentration of 5 mg ml−1 was added to the samples. Unbound radioactive marker (I125) was removed by chromatography on a mini-column with Sepharose 6B (8 × 20 mm) at eluent flow rate of 1 ml/min, with 0.5 M phosphate buffer (pH 7.0). A fraction containing protein IgGI125 (or BSAI125) was collected at the column output, and its total radioactivity was evaluated using gamma-counter “NK-Narkotest” (VNIIMP, Russia).
Adsorption of IgGI125 on the nanodiamonds particles was performed in the following way. A radioactive protein solution was mixed with the nanodiamond hydrosol (protein-to-nanodiamond ratio—1:10 (w/w)). The suspension obtained was incubated during 1 h at room temperature and stirred continually throughout the incubation. Nanodiamonds with the adsorbed protein were collected by centrifugation at 16,000 g for 5 min at 20 °C. The supernatant was taken from sample and its residual radioactivity was measured to evaluate the amount of non-adsorbed protein. The precipitate was repeatedly washed with the PBS-buffer to remove the unbound protein, every time re-suspending the nanoparticles in a fresh buffer, collecting them by centrifugation under the above-mentioned conditions and taking a supernatant for radioactivity evaluation.
For immobilization of IgGJ125, the surface of nanodiamonds was chemically activated with p-benzoquinone (vide infra)—a well-known method used for covalent coupling of enzymes to carriers [21,22]. For this, phosphate buffer, 20 mM, pH 8.0 and 100 mg of hydroquinone dissolved in aqueous ethanol were consequently added with stirring to 5 ml of 1.5 wt% nanodiamond hydrosol. The suspension was incubated for 2 h at room temperature, washed with DI water followed by thorough washing with 1 M NaCl and rinsing with DI water.
Immobilization of the radioactive protein was carried out in 100 mM Na-bicarbonate buffer (pH 8.0). For these purposes, IgGI125 solution was mixed with the modified nanodiamond hydrosol (protein-to-nanodiamond ratio—1:10 (w/w)). The suspension obtained was incubated at continual stirring for overnight at 4 °C. Nanodiamonds with the immobilized protein were collected by centrifugation at 16,000 g for 5 min at 20 °C. The supernatant was taken from sample, and the amount of non-immobilized protein was determined by its residual radioactivity. The precipitate of nanodiamonds was repeatedly washed with the PBS-buffer to remove the unbound proteins. Simultaneous covalent immobilization of the two proteins (RAM and BSAI125) on the nanodiamonds was performed in the same manner.
Evaluation of the stability of these complexes in blood serum included two stages. At first, nanodiamond precipitates containing the adsorbed or immobilized IgGI125 preliminarily washed with PBS-buffer were treated with serum as follows. The precipitates were re-suspended in serum, incubated for 5 min at 20 °C and then the particles were collected by centrifugation at 16,000 g for 5 min at 20 °C. Supernatants were taken from samples and their residual radioactivities were measured. The treatment was performed repeatedly, each time suspending the particles in a fresh serum, incubating and collecting them by centrifugation. The residual radioactivity was measured after each centrifugation. At the second stage, chromatography of the obtained supernatants was carried out on the column with Sepharose 6B (10 × 200 mm) equilibrated with 150 mM NaCl at eluent flow rate 1 ml/min and fractions of 0.5 ml were collected. Thereafter, we evaluated the radioactivity of all fractions obtained after chromatography. The same method was used to determine the stability of the RAM–nanodiamond–BSAI125 complex.
Sedimentation stability of the nanodiamond-IgGI125 and RAM-nanodiamond-BSAI125 complexes obtained after covalent immobilization of proteins was evaluated as follows. After immobilization of IgGI125 (or RAM and BSAI125), unbound proteins were removed by chromatography on the column with Sepharose 6B (10 × 200 mm) equilibrated with 150 mM NaCl at eluent flow rate 1 ml/min. Total fraction containing the nanodiamond–IgGI125 (or RAM–nanodiamond–BSAI125) complex was blended with blood serum. The mixture was placed in an ice bath and sonicated at 22 kHz (six times for 10 s, with 20 s intervals). After that, samples of 1 ml were collected and centrifuged at 16,000 g and 20 °C. The time of centrifugation was individually selected for each sample. The residual radioactivity of supernatants obtained by centrifugation was measured.
Specific interaction of the RAM–nanodiamond–BSAI125 complex with a target antigen was analyzed in the following way. A sample of blood serum containing the RAM–nanodiamond–BSAI125 complex was produced using the method described in the previous paragraph. Two portions of this sample (1 ml each) were loaded into two mini-columns packed with 1 ml of Sepharose 6B. A control column contained the adsorbent with the immobilized BSA, while an experimental column contained the adsorbent with the immobilized mouse IgG. Each column was washed with 5 ml of serum, and the residual radioactivity of the washed adsorbents was evaluated.
Results and Discussion
Nanodiamond–IgGI125 Complex Produced by Adsorption of Protein
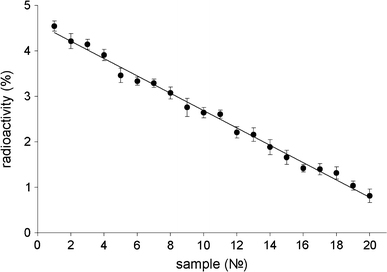
The results evidenced that IgGI125 is effectively adsorbed by nanodiamonds under the chosen conditions. After incubation with protein and removal of the particles by centrifugation, not more than 25–28% of the initial radioactivity of protein preparations was registered in supernatants. Multiple washing of the nanoparticles with adsorbed IgGI125 in PBS-buffer did not lead to desorption of protein. After the first two washings, supernatants display about 0.5–1% of the initial radioactivity. At the following washings, the level of radioactivity registered in supernatants did not exceed 0.1%. At the same time, it has been found that the obtained nanodiamond–IgGI125 complexes were not stable in blood serum. Repeated washings of precipitates with serum resulted in almost total desorption of IgGI125 (Fig. 1).
Figure 1.
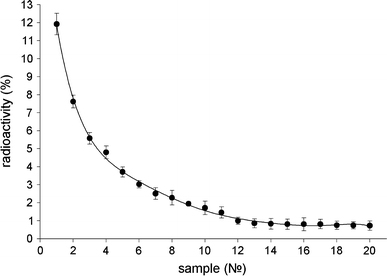
Radioactivity of supernatants after multiple washings with serum of a precipitate containing IgGI125 adsorbed on nanodiamonds (percentage of radioactivity of the initial protein IgGII125 sample)
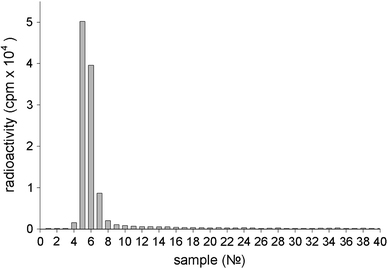
Protein desorption was also confirmed by analyzing the radioactivity of fractions obtained by the chromatography of supernatants. Results show (Fig. 2) that supernatants contained a significant amount of free IgGI125 (main peak of radioactivity—fractions 10–28) and a small amount of nanodiamonds with adsorbed proteins, which could not be removed by centrifugation (minor peak of radioactivity—fractions 5–7).
Figure 2.
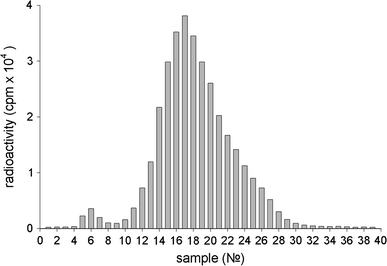
Typical distribution of radioactivity registered after Sepharose 6B chromatography of supernatants. The supernatants were obtained by washings with serum of the precipitate containing IgGI125 adsorbed on nanodiamonds
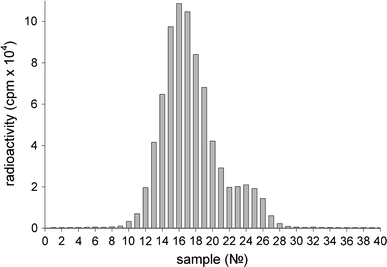
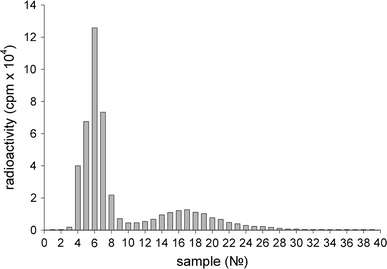
It should be noted that the main radioactive peak observed after chromatography of the supernatant (Fig. 2) completely corresponds to the radioactive peak observed after chromatography of the initial protein IgGI125 sample (Fig. 3). At chromatography of a serum sample with re-suspended nanoparticles after 20 washings (Fig. 1), one can observe a radioactivity distribution (Fig. 4) that is different to that at the chromatography of supernatants (Fig. 2).
Figure 3.
Distribution of radioactivity registered after Sepharose 6B chromatography of the initial protein IgGI125 sample
Figure 4.
Distribution of radioactivity registered after Sepharose 6B chromatography of a serum sample containing IgGI125 on nanodiamonds. The sample was obtained after 20 washings
In this case, the first radioactive peak (fractions 4–8) accounts for about 75% of the total radioactivity, which indicates the presence of the nanodiamond–IgGI125 complex. The second peak (fractions 12–28) amounting to about 25% of the total radioactivity bears evidence of a significant amount of desorbed (free) protein presented within the sample. Upon combining the fractions 4–8 (the first peak) and subsequent chromatography, the distribution of radioactivity was similar to that shown in Fig. 4.
In general, the experimental data obtained in this study show that it is impossible to get nanodiamond-IgGI125 complexes stable in blood serum by nonspecific adsorbing the protein molecules on the nanoparticles.
Nanodiamond–IgGI125 Complex Produced by Covalent Immobilization of Protein
The surface of detonation nanodiamond particles is covered with variety of functional groups, which originate from synthesis and purification processes. Among them, a hydroxyl group (–OH) is one of the most abundant [23]. This group was involved in a procedure for covalent coupling of IgG onto the nanodiamonds by treating the nanoparticles with benzoquinone, which is well known to provide chemical activation of hydroxyl containing surfaces [21]. The thus activated nanoparticles readily react with an amine group of the protein molecules. The reaction sequence of the benzoquinone activation and biochemical coupling is presented on Fig. 5.
Figure 5.
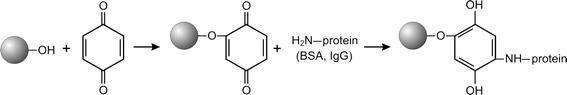
Schematic representation of protein immobilization on the benzoquinone activated nanodiamonds
Experiments showed the efficiency of covalent immobilization of IgGI125 on the surface of modified nanodiamonds. After incubation with protein and removal of the particles by centrifugation, not more than 25–30% of initial radioactivity of protein preparations was registered in supernatants. Like in protein adsorption (see above), serial washing of the immobilized IgGI125 with the PBS-buffer did not lead to desorption of the protein. The radioactivity level registered in the washings after removal of the particles was the same as in the previously described experiment—about 0.5–0.8% after the first two washings and less than 0.1% after the following washings. After the nanoparticles with immobilized protein were repeatedly washed with blood serum a significant level of radioactivity was registered in supernatants as well (Fig. 6).
Figure 6.
Radioactivity of supernatants after serial washings of the precipitate containing IgGI125 immobilized on nanodiamonds with serum (percentage of radioactivity of the initial protein IgGI125 sample)
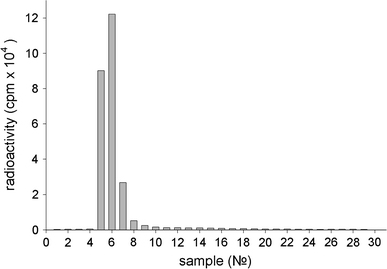
It would seem that these data indicate the instability of the nanodiamond-IgGI125 complex in serum. However, the radioactivity of fractions after chromatography of supernatants showed a radically different result. As shown in Fig. 7, only a single peak (fractions 4–8) is observed, which accounts for almost all radioactivity (~98%) and corresponds to the peak of the radioactive nanodiamond-IgGI125 complex.
Figure 7.
Typical distribution of radioactivity registered after Sepharose 6B chromatography of supernatants. The supernatants were obtained by washing the precipitate containing IgGI125 immobilized on nanodiamonds with serum
At chromatography of a serum sample with nanoparticles washed 20 times with serum (Fig. 5), the observed radioactivity distribution (Fig. 8) was similar to that at the chromatography of supernatants.
Figure 8.
Distribution of radioactivity registered after Sepharose 6B chromatography of a serum sample containing IgGI125 immobilized on nanodiamonds. The sample was obtained after 20 washings
These results allow a conclusion that the nanodiamond–IgGI125 complex obtained via the covalent attachment of protein is not just stable, but displays a high resistance to sedimentation in blood serum. This conclusion is confirmed with the tests on sedimentation of the complex in serum at centrifugation in a wide time interval. About 50% of the initial radioactivity was recorded in supernatants even after centrifuging a serum sample containing the nanodiamond–IgGI125 complex at 16,000 g for 1 h (Fig. 9).
Figure 9.
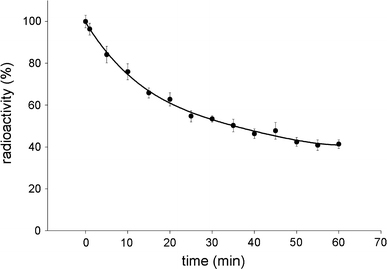
Residual radioactivity of supernatants versus time of centrifugation at 16,000 g for serum samples containing the nanodiamond–IgGI125 complex
RAM–Nanodiamond–BSAI125 Complex Obtained by Simultaneous Covalent Immobilization of Two Types of Proteins
The design of drug delivery systems on the base of particulate carriers must take into account the fact that after being immobilized on the carrier surface, ligand molecules should preserve their ability to bind to the target receptor molecule. It is known that immobilization by means of chemical attachment may result in a reduction in specific activity of a ligand (e.g., protein) and sometimes in its total deactivation [24]. We have investigated the possibility to design a model delivery system on the base of nanodiamonds. For this purpose, two types of proteins were simultaneously covalently immobilized on the nanoparticles—RAM, specifically interacting with a mouse IgG and serving as a ligand, and BSAI125 serving as a radioactive marker (Fig. 10). The results of the experiment showed that the RAM–nanodiamond–BSAI125 complex exhibits sustainability and high colloidal stability in blood serum as well as the nanodiamond–IgGI125.
Figure 10.
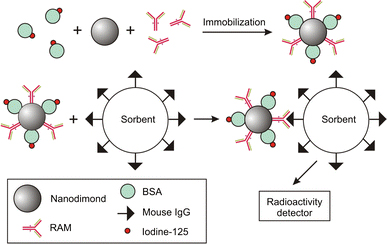
Schematic of experiment to illustrate the ability of the RAM–nanodiamond–BSAI125 complex to bind to the target antigen
Chromatography of serum samples containing the RAM–nanodiamond–BSAI125 complex using mini-columns Sepharose 6B with immobilized BSA (control) and mouse IgG (experimental) revealed that the complex is able to specifically bind to the target antigen. Once chromatography was performed and columns were washed, the radioactivity registered in the sorbent with immobilized mouse IgG significantly (more than nine times) exceeded that of the sorbent with immobilized BSA (Fig. 11).
Figure 11.
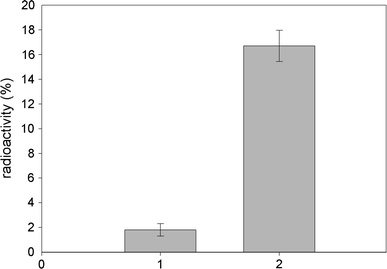
Radioactivity registered after chromatography of serum samples containing the RAM–nanodiamond–BSAI125 complex: 1—sorbent with immobilized BSA, 2—sorbent with immobilized mouse IgG (percentage of radioactivity of the initial samples used for chromatography)
The experimental data obtained in this study show that the ligand molecules (RAM) preserve their specific activity after covalent immobilization on the nanodiamonds and are able to attach to the target receptor molecules (mouse IgG). This at least was demonstrated by experiments in vitro.
Conclusion
Thus, this study shows the possibility of applying detonation nanodiamonds with a high colloidal stability in hydrosols for obtaining supramolecular nanodiamnd-IgGI125 and RAM–nanodiamond–BSAI125 complexes. The experiments showed that it is impossible to receive nanodiamond–IgGI125 complexes possessing stability in blood serum by non-specific absorption of the protein molecules on nanoparticles. However, in vitro experiments proved that complexes obtained by covalent immobilization of IgGI125 or simultaneous immobilization of RAM and BSAI125 demonstrate sustainability and high colloidal stability in blood serum. It was further demonstrated that a complex obtained by simultaneous covalent immobilization of RAM and BSAI125 on the nanoparticles is able to specifically bind to the target antigen (mouse IgG). The obtained results allow one to consider the possibility of applying nanodiamonds as carriers for address deliveries of bioactive substances (i.e., drugs) to various biological targets in vivo.
Acknowledgments
This work was supported by the Program #27 for Basic Research of the Presidium of RAS (project 3.6.3).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Harrington KL, Lewanski CR, Stewart SW. Clin. 2000. p. 16. COI number [1:STN:280:DC%2BD3c3htVGmuw%3D%3D] [DOI] [PubMed]
- Nobuto H, Sugita H, Kubo T, Shimose S, Yasunga Y, Murakami T, Ochi M. Int. J. Cancer. 2004. p. 627. COI number [1:CAS:528:DC%2BD2cXislCis7g%3D] [DOI] [PubMed]
- Ulbrich K, Subr V. Ad. 2004. p. 1023. COI number [1:CAS:528:DC%2BD2cXivVGitb4%3D] [DOI] [PubMed]
- Geng Y, Dalhaimer P, Cai S, Tsai R, Tewari M, Minko T, Discher DE. Nat. 2007. p. 249. COI number [1:CAS:528:DC%2BD1cXktVGgsbs%3D]; Bibcode number [2007NatNa...2..249G] [DOI] [PMC free article] [PubMed]
- Agasti SS, Chompoosor A, You C-C, Ghosh P, Kim CK, Rotello VM. J. 2009. p. 5728. COI number [1:CAS:528:DC%2BD1MXktFelsrk%3D] [DOI] [PMC free article] [PubMed]
- Kwangjae C, Wang X, Chen Z, Nie S, Shin DM. Clin. 2008. p. 1310. [DOI] [PubMed]
- Piao Y, Kim J, Na HB, Kim D, Baek JS, Ko MK, Lee JH, Shokouhimehr M, Hyeon T. Nat. 2008. p. 242. COI number [1:CAS:528:DC%2BD1cXisVSrs7s%3D]; Bibcode number [2008NatMa...7..242P] [DOI] [PubMed]
- Bianco A. Expert Opin. 2004. p. 57. COI number [1:CAS:528:DC%2BD2cXhtVOqtrrE] [DOI] [PubMed]
- Kirpotin DB, Drummond DC, Shao Y, Shalaby, Hong K, Nielsen UB, Marks JD, Benz CC, Park JW. Cancer Res. 2006. p. 6732. COI number [1:CAS:528:DC%2BD28XmsVamu7Y%3D] [DOI] [PubMed]
- Bondar VS, Puzyr AP. Phys. Solid State. 2004. p. 698.
- Puzyr AP, Bondar VS, Bukayemsky AA, Selyutin GE, Kargin VF. NATO Sci. 2005. p. 261. COI number [1:CAS:528:DC%2BD2MXps1Cit7k%3D]
- Ho D, editor. Nanodiamonds: applications in biology and nanoscale medicine. Springer, New York; 2009. [Google Scholar]
- Bondar VS, Pozdnyakova IO, Puzyr AP. Phys. Solid State. 2004. p. 737.
- Huang LC, Chang HC. Langmuir. 2004. p. 5879. COI number [1:CAS:528:DC%2BD2cXksV2rsbg%3D] [DOI] [PubMed]
- Puzyr AP, Baron AV, Purtov KV, Bortnikov EV, Skobelev NN, Mogilnaya OA, Bondar VS. Diamond Relat. 2007. p. 2124. COI number [1:CAS:528:DC%2BD2sXhtlGmt7fL] [DOI]
- Purtov KV, Burakova LP, Puzyr AP, Bondar VS. Nanotechnology. 2008. p. 1. [DOI] [PubMed]
- Wu VWK. Chem. 2006. p. 1380. COI number [1:CAS:528:DC%2BD2sXjsFOg] [DOI]
- Puzyr AP, Bortnikov EV, Skobelev HH, Tyan AG, Selimhanova ZU, Manaschev GG, Bondar VS. Siberian Medical Rev. 2005. p. 20.
- Puzyr AP, Bondar VS, Selimhanova ZU, Tyan AG, Bortnikov EV, Inzhevatkin EV. Siberian Medical Rev. 2004. p. 19.
- Schrand A, Hens SAC, Shenderova OA. Crit. 2009. p. 18. COI number [1:CAS:528:DC%2BD1MXmsFWrurs%3D] [DOI]
- Brandt J, Andersson LO. J. Porath Biochim. Biophys. Acta. 1975. p. 196. COI number [1:CAS:528:DyaE2MXktVejsL0%3D] [DOI] [PubMed]
- Mateescu M-A, Agostinelli E, Weltrowska G, Weltrowski M, Mondovi B. Biol. Metal. 1990. p. 98. COI number [1:CAS:528:DyaK3MXhsF2rsbc%3D] [DOI]
- Krueger A. J. 2008. p. 1485. COI number [1:CAS:528:DC%2BD1cXjvVensLk%3D]; Bibcode number [2008ipid.book.....K] [DOI]
- Simons BL, King MC, Cyr T, Hefford MA, Kaplan H. Protein Sci. 2002. p. 1558. COI number [1:CAS:528:DC%2BD38XktFWgs7k%3D] [DOI] [PMC free article] [PubMed]


