Abstract
In vitro, D2 dopamine receptors can exist in low and high affinity states for agonists, and increases of D2 receptors in high affinity state have been proposed to underlie dopamine receptor supersensitivity in vivo. Deletion of the gene for dopamine β-hydroxylase (DBH) causes mice to become hypersensitive to the effects of psychostimulants, and in vitro radioligand binding results suggest an increased percentage of D2 receptors in a high affinity state. To determine whether DBH knockout mice display an increase of high affinity state D2 receptors in vivo, we scanned DBH knockout and control mice with the agonist PET radioligand [11C]MNPA, which is thought to bind preferentially to the high affinity state of the D2 receptor. In addition, we performed in vitro binding experiments on striatal homogenates with [3H]methylspiperone to measure Bmax values and the percentages of high and low affinity states of the D2 receptor. We found that the in vivo striatal binding of [11C]MNPA was similar in DBH knockout mice and heterozygous controls and the in vitro Bmax values and percentages of D2 receptors in the high affinity state, were not significantly different between these two groups. In summary, our results suggest that DBH knockout mice have normal levels of D2 receptors in the high affinity state and that additional mechanisms contribute to their behavioral sensitivity to psychostimulants.
Keywords: [11C]MNPA, D2 dopamine receptor, high affinity state, dopamine β-hydroxylase, PET
Introduction
The D2 dopamine receptor exists in two affinity states for agonists in vitro: a low affinity state and a high affinity state that reflects an active form of the receptor that is competent for signaling (Sibley et al., 1982). An increase in the ability of agonists to promote the high affinity coupling state of the D2 receptor has been proposed to underlie behavioral D2 supersensitivity in several animal models such as unilaterally 6-OHDA-lesioned and amphetamine-sensitized rats (Seeman et al., 2002; Seeman et al., 2005), but until recently there has been no way to assess an increase in the high affinity state of D2 receptors in vivo. In theory, a full D2 receptor agonist radioligand should selectively label high affinity state receptors in vivo. Notably, one such compound, [11C]-(+)-PHNO, failed to detect an increase in high affinity state D2 receptors in rats with behavioral D2 hypersensitivity (McCormick et al., 2009).
A common polymorphism of the gene for dopamine β-hydroxylase (DBH), which converts dopamine into norepinephrine, has been linked to psychostimulant abuse and psychotic symptoms in humans (Weinshenker and Schroeder, 2007). DBH knockout mice have been studied as an animal model of this human polymorphism and of psychostimulant abuse. DBH knockout mice, which are deficient in norepinephrine, are hypersensitive to the behavioral effects of cocaine, amphetamine, and the D2 agonist quinpirole (Schank et al., 2006; Weinshenker et al., 2002). This hypersensitivity was surprising, since DBH knockout mice have reduced basal levels of dopamine as well as reduced dopamine release after amphetamine challenge. An explanation for this apparent paradox was apparently provided by in vitro receptor binding studies that reported a higher percentage of striatal D2 receptors in the high affinity state in DBH knockouts in comparison to heterozygote controls, which was hypothesized to represent a compensatory response to low extracellular DA levels in the knockouts (Schank et al., 2006; Seeman et al., 2005).
The purpose of this study was to test in vivo the hypothesis that DBH knockout mice have an increased percentage of D2 receptors in the high affinity state and to determine whether DBH knockout mice might provide a model for increases in high affinity state D2 receptors in vivo. For this purpose, we scanned DBH knockout mice with the D2 agonist radioligand [11C]MNPA. In addition, we performed in vitro binding experiments on striatal homogenates with the antagonist radioligand [3H]methylspiperone to measure Bmax values and the percentages of high and low affinity states of the D2 receptor.
Materials and methods
Radioligand preparation
[11C]MNPA was prepared as previously described (Steiger et al., 2009). The specific activity of [11C]MNPA at the time of injection was 82 ± 24 GBq/µmol (n = 8 syntheses). Chemical purity was >98%, radiochemical purity was >95%, and mean injected activity was 13 ± 6 MBq, which was accompanied by 0.17 ± 0.09 nmol of carrier.
Animals
Dopamine β-hydroxylase knockout mice and heterozygous controls (31 ± 5 g) were reared as previously described (Thomas et al., 1998; Weinshenker et al., 2002). We used heterozygous animals as controls as they are indistinguishable from wild-type mice, have normal levels of catecholamines and have been used as controls in prior studies (Thomas et al., 1998; Weinshenker et al., 2002).
PET studies
A total of 20 mice were imaged: 10 heterozygous and 10 knockout mice, with each group containing 3 females and 7 males. PET scans were performed on the Advanced Technology Laboratory Animal Scanner (Seidel et al., 2003). Images were acquired and data analyzed with a reference tissue model as previously described (Ichise et al., 2003; Seneca et al., 2008). The outcome measure was binding potential (BPND), which is the ratio at equilibrium of specific binding to nondisplaceable uptake. The cerebellum was used as the reference region.
In vitro radioligand binding
Membrane homogenates were prepared from dissected striata of DBH knockout and heterozygous mice. 3–4 striata from two mice were pooled per experiment (a total of 5 mice of each genotype) and binding was performed as previously described (Skinbjerg et al., 2009). In brief, for competition assays, membranes were incubated in binding buffer containing 0.2 mM sodium metabisulfite, 50 nM ketanserin, ~0.2 nM [3H]methylspiperone (85.5 Ci/mmol, Perkin Elmer Life and Analytical Science, MA, USA), and increasing concentrations of dopamine with and without the addition of 100 µM GTP. For saturation binding experiments, membranes were incubated with increasing concentrations (~0.02 nM to 2 nM) of [3H]methylspiperone. The total receptor density was measured as Bmax expressed as fmol/mg protein.
Results
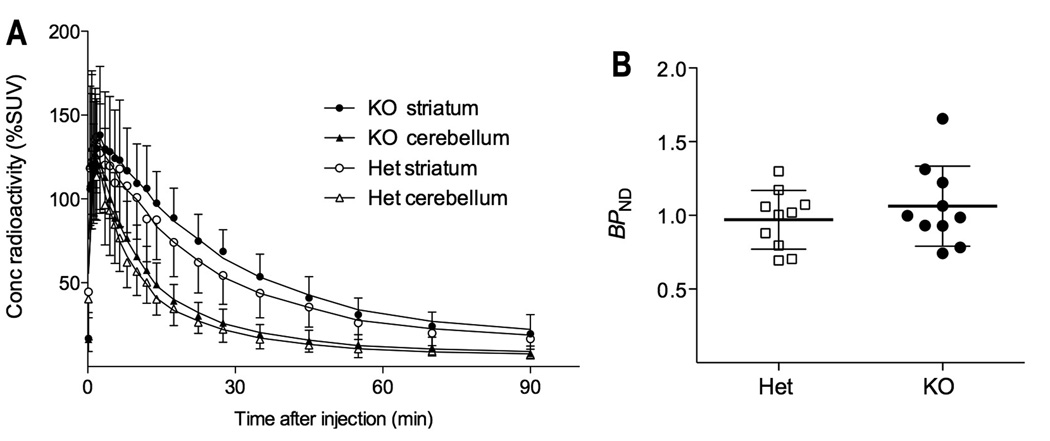
Following the intravenous injection of the agonist [11C]MNPA, the radioactivity was concentrated in the striatum and had a similar time course in DBH knockout and heterozygous mice (Fig. 1A). Using the cerebellum as a reference region, the ratio at equilibrium of specific binding to nondisplaceable uptake (BPND) was not significantly different (BPND= 0.97 ± 0.06 and 1.06 ± 0.09 for heterozygous and knockout mice repectively, P = 0.866, n = 10 of each genotype) in these two groups of mice (Fig. 1B).
Figure 1.
A) Time-activity curves for [11C]MNPA and B) binding potential (BPND) in DBH knockout and heterozygous control mice. BPND was insignificantly different between knockout (1.07 ± 0.3) and heterozygous controls (0.98 ± 0.2; mean ± SD, with 10 mice in each group).
To separately measure receptor density (Bmax) and radioligand affinity (KD), we performed in vitro binding studies with the antagonist [3H]methylspiperone. Both Bmax and KD values were not significantly different between DBH knockout and heterozygous mice. For knockout mice, the average Bmax = 164 ± 31 fmol/mg and the average KD = 0.39 ± 0.21 nM measured in striatal membranes from 5 animals. For heterozygous mice, the average Bmax = 148 ± 30 fmol/mg and the average KD = 0.33 ± 0.19 nM, measured in striatal membranes from 5 animals. Both the Bmax (P = 0.72) and KD (P = 0.82) values were not significantly different between groups.
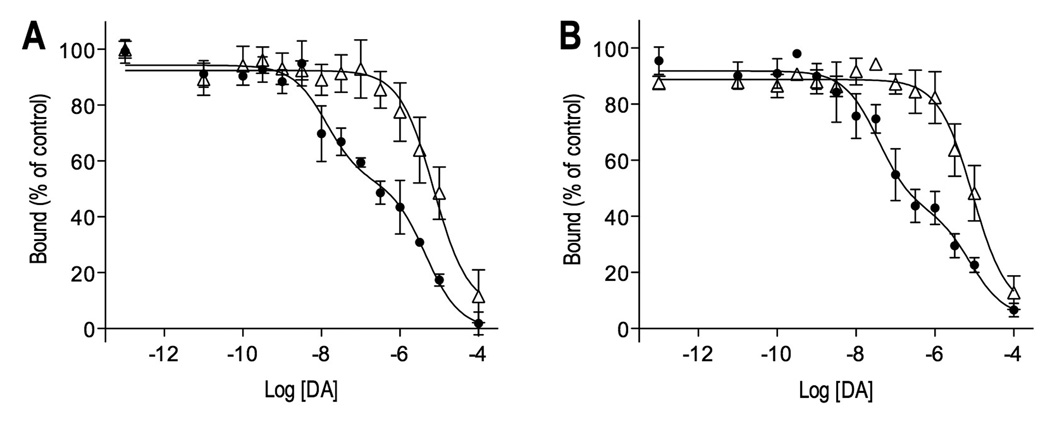
To determine the percentage of D2 receptors in the high affinity state, we performed competition binding studies with the antagonist [3H]methylspiperone and dopamine, in the absence and presence of GTP. In the absence of GTP, the percentage of high affinity receptors was similar in DBH knockout and heterozygous mice (56 ± 8% vs. 44 ± 6%, respectively, P = 0.23). In the presence of GTP, competition binding showed only low affinity binding for both knockout and heterozygous mice (Fig. 2 and table I).
Figure 2.
Competition binding experiments on striatal membrane homogenates with [3H]methylspiperone and dopamine in the absence (●) and presence (Δ) of guanine triphosphate (GTP). High affinity agonist binding was observed in the absence of GTP, but the proportion of high affinity binding was similar in heterozygous (A) and DBH knockout (B) mice. In the presence of GTP, one low-affinity binding site was observed. All experiments were performed in triplicate and repeated three times. KI values and proportion of high affinity agonist binding are shown in table I.
Table I.
Competition binding with [3H]methylspiperone and dopamine in the presence and absence GTP.
| Mouse strain | Ligand | KIlow (nM) |
KIhigh (nM) |
% High affinity |
|---|---|---|---|---|
| Heterozygote | DA | 2990 ± 420 | 15.7 ± 8.9 | 44 ± 6% |
| DA + GTP | 4030 ± 1400 | |||
| Knock out | DA | 4300 ± 670 | 17.0 ± 8.3 | 56 ± 8% |
| DA + GTP | 5250 ± 1900 | |||
Values are mean ± SD from three experiments performed in triplicate.
Discussion
The major finding of this study is that DBH knockout mice have the same density of D2 receptors in the high affinity state as heterozygous controls, based on both in vivo and in vitro measurements. The in vivo uptake of the agonist radioligand [11C]MNPA, which is a reflection of both receptor density and radioligand affinity, was similar in the two groups of animals. In addition, in vitro measurements using the antagonist radioligand [3H]methylspiperone showed that the density, affinity, and proportion of receptors in the high affinity state were not significantly different between these two groups.
Did endogenous dopamine block the in vivo radioligand binding of [11C]MNPA and artifactually produce these results? Notably, microdialysis studies reported that DBH knockout mice have significantly reduced basal levels of dopamine in striatum (~66% of control) (Schank et al., 2006). As PET radioligands are sensitive to in vivo competition of endogenous dopamine, reduced basal levels of dopamine would be expected to increase BPND in DBH knockout mice. However, the BPND of DBH knockout mice was not different from that of control animals. In addition, a number of recent publications suggested that only one affinity state of D2 receptor is detectable in vivo (Finnema et al., 2009; McCormick et al., 2009; McCormick et al., 2008). As suggested by Finnema and colleagues, the in vivo receptor is presumably in the high affinity state, because it is able to bind radioligand that is present at low (nanomolar to sub-nanomolar) concentrations.
Two potential confounding factors for PET imaging of small animals, such as mice, are partial volume effect (because of the small size of the target) and the radioligand occupying a significant percentage of receptors (because of the small number of receptors). As a result of limited spatial resolution of the PET camera, partial volume effects blunt the actual values of BPND. Ex vivo experiments in mice with the radioligand [3H]NPA, which pharmacologically is very similar to [11C]MNPA, reported BPND values of ~2.5 for striatum (Cumming et al., 2002), suggesting that our in vivo BPND values of ~1 were blunted by an expected 2.5 fold. These partial volume errors are assumed to be equal for heterozygous and knockout mice and would not have artifactually induced differences between the two groups. However, such partial volume errors would increase the magnitude of the difference necessary to be detected with in vivo imaging.
In addition to partial volume effects, the injected mass of radioligand in small animals may occupy a high percentage of receptors that violate the assumptions of tracer kinetic modeling. A rough estimate of receptor occupancy by [11C]MNPA can be made by dividing the maximum specific binding in striatum for [11C]MNPA (~2.8 nM) with reported Bmax values (~25 nM) of rat striatum (Malmberg et al., 1996). This estimation would result in ~11% occupancy (2.8/25) of striatal dopamine receptors, thus on the high side for accuracy of tracer kinetic modeling. In addition, agonists are thought to bind to a subset of D2 receptors in the high affinity state, which would yield a greater than 10% occupancy by [11C]MNPA. Nevertheless, the injected mass doses of radioligand were the same for knockout and controls and would not artifactually induce differences between the two groups. As mentioned for partial volume errors, the relative high mass dose will increase the magnitude of the differences necessary to be detected with in vivo imaging.
In addition to in vivo PET imaging, we also performed in vitro binding studies to measure the density and the percentage of D2 dopamine receptors in the high affinity state. In agreement with previous studies (Schank et al., 2006), we found no difference in the level of D2 receptor expression (Bmax) between DBH knockout and control mice. However, two prior studies reported that the percentage of D2 receptors in the high affinity state was increased in DBH knockout mice, although its statistical significance was not indicated (Schank et al., 2006; Seeman et al., 2005). In contrast, we did not find a statistically significant difference in the number of D2 receptors in the high affinity state between the DBH knockout and control mice. The reasons for these discrepant results are not clear, although they may be related to differences in methodology between the studies. In Schank et al. (2006) and Seeman et al. (2005), different approaches for detecting the high affinity state were used: [3H]raclopride binding in the presence and absence of GTP (which presumably caused endogenous dopamine to dissociate from the receptor leading to increased antagonist binding), as well as dopamine/[3H]raclopride and dopamine/[3H]domperidone competition assays. In contrast, we performed dopamine/[3H]methylspiperone competition assays coupled with computerized curve fitting to quantitate the high and low affinity states of the receptor in washed membrane preparations. Given this, we reasoned that an overall better approach would be to use an agonist radioligand, and to perform in vivo assessments, since the agonist is thought to bind preferentially to the high affinity state. Our current PET imaging results with [11C]MNPA indeed support the notion that there is no increase in the high affinity state of the D2 receptor in DBH knockout mice.
In summary, we found no significant differences of D2 receptors in the high affinity state between DBH knockout and control mice with either in vivo PET scanning or in vitro binding experiments. Our results do not support previous in vitro data and indicate that DBH knockout mice have normal densities of D2 dopamine receptors in high affinity state, suggesting that other mechanisms likely underlie their behavioral hypersensitivity to psychostimulants.
Acknowledgements
This research was supported by the Intramural Programs of NIMH (project #Z01-MH-002795-07) and of NINDS (NS002263-33). We thank Cheryl Morse for radiotracer production.
REFERENCES
- Cumming P, Wong DF, Gillings N, Hilton J, Scheffel U, Gjedde A. Specific binding of [11C]raclopride and N-[3H]propyl-norapomorphine to dopamine receptors in living mouse striatum: occupancy by endogenous dopamine and guanosine triphosphate-free G protein. J Cereb Blood Flow Metab. 2002;22:596–604. doi: 10.1097/00004647-200205000-00011. [DOI] [PubMed] [Google Scholar]
- Finnema SJ, Halldin C, Bang-Andersen B, Gulyas B, Bundgaard C, Wikstrom HV, Farde L. Dopamine D(2/3) receptor occupancy of apomorphine in the nonhuman primate brain-A comparative PET study with [11C]raclopride and [11C]MNPA. Synapse. 2009;63:378–389. doi: 10.1002/syn.20615. [DOI] [PubMed] [Google Scholar]
- Ichise M, Liow JS, Lu JQ, Takano A, Model K, Toyama H, Suhara T, Suzuki K, Innis RB, Carson RE. Linearized reference tissue parametric imaging methods: application to [11C]DASB positron emission tomography studies of the serotonin transporter in human brain. J Cereb Blood Flow Metab. 2003;23:1096–1112. doi: 10.1097/01.WCB.0000085441.37552.CA. [DOI] [PubMed] [Google Scholar]
- Malmberg A, Jerning E, Mohell N. Critical reevaluation of spiperone and benzamide binding to dopamine D2 receptors: evidence for identical binding sites. Eur J Pharmacol. 1996;303:123–128. doi: 10.1016/0014-2999(96)00080-5. [DOI] [PubMed] [Google Scholar]
- McCormick PN, Kapur S, Reckless G, Wilson AA. Ex vivo [11C]-(+)-PHNO binding is unchanged in animal models displaying increased high-affinity states of the D(2) receptor in vitro. Synapse. 2009;63:998–1009. doi: 10.1002/syn.20671. [DOI] [PubMed] [Google Scholar]
- McCormick PN, Kapur S, Seeman P, Wilson AA. Dopamine D2 receptor radiotracers [11C](+)-PHNO and [3H]raclopride are indistinguishably inhibited by D2 agonists and antagonists ex vivo. Nucl Med Biol. 2008;35:11–17. doi: 10.1016/j.nucmedbio.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Schank JR, Ventura R, Puglisi-Allegra S, Alcaro A, Cole CD, Liles LC, Seeman P, Weinshenker D. Dopamine beta-hydroxylase knockout mice have alterations in dopamine signaling and are hypersensitive to cocaine. Neuropsychopharmacology. 2006;31:2221–2230. doi: 10.1038/sj.npp.1301000. [DOI] [PubMed] [Google Scholar]
- Seeman P, Tallerico T, Ko F, Tenn C, Kapur S. Amphetamine-sensitized animals show a marked increase in dopamine D2 high receptors occupied by endogenous dopamine, even in the absence of acute challenges. Synapse. 2002;46:235–239. doi: 10.1002/syn.10139. [DOI] [PubMed] [Google Scholar]
- Seeman P, Weinshenker D, Quirion R, Srivastava LK, Bhardwaj SK, Grandy DK, Premont RT, Sotnikova TD, Boksa P, El-Ghundi M, O'Dowd BF, George SR, Perreault ML, Mannisto PT, Robinson S, Palmiter RD, Tallerico T. Dopamine supersensitivity correlates with D2High states, implying many paths to psychosis. Proc Natl Acad Sci U S A. 2005;102:3513–3518. doi: 10.1073/pnas.0409766102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidel J, Vaquero JJ, Green MV. Resolution uniformity and sensitivity of the NIH ATLAS small animal PET scanner: comparison to simulated LSO scanners without depth-of-interaction capability. IEEE Trans Nucl Sci. 2003;50:1347–1350. [Google Scholar]
- Seneca N, Zoghbi SS, Skinbjerg M, Liow JS, Hong J, Sibley DR, Pike VW, Halldin C, Innis RB. Occupancy of dopamine D(2/3) receptors in rat brain by endogenous dopamine measured with the agonist positron emission tomography radioligand [11C]MNPA. Synapse. 2008;62:756–763. doi: 10.1002/syn.20549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley DR, De Lean A, Creese I. Anterior pituitary dopamine receptors. Demonstration of interconvertible high and low affinity states of the D-2 dopamine receptor. J Biol Chem. 1982;257:6351–6361. [PubMed] [Google Scholar]
- Skinbjerg M, Namkung Y, Halldin C, Innis RB, Sibley DR. Pharmacological characterization of 2-methoxy-N-propylnorapomorphine's interactions with D2 and D3 dopamine receptors. Synapse. 2009;63:462–475. doi: 10.1002/syn.20626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiger C, Finnema SJ, Raus L, Schou M, Nakao R, Suzuki K, Pike VW, Wikstrom HV, Halldin C. A two-step one-pot radiosynthesis of the potent dopamine D2/D3 agonist PET radioligand [11C]MNPA. J Label Compd radiopharm. 2009;52:158–165. [Google Scholar]
- Thomas SA, Marck BT, Palmiter RD, Matsumoto AM. Restoration of norepinephrine and reversal of phenotypes in mice lacking dopamine beta-hydroxylase. J Neurochem. 1998;70:2468–2476. doi: 10.1046/j.1471-4159.1998.70062468.x. [DOI] [PubMed] [Google Scholar]
- Weinshenker D, Miller NS, Blizinsky K, Laughlin ML, Palmiter RD. Mice with chronic norepinephrine deficiency resemble amphetamine-sensitized animals. Proc Natl Acad Sci U S A. 2002;99:13873–13877. doi: 10.1073/pnas.212519999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinshenker D, Schroeder JP. There and back again: a tale of norepinephrine and drug addiction. Neuropsychopharmacology. 2007;32:1433–1451. doi: 10.1038/sj.npp.1301263. [DOI] [PubMed] [Google Scholar]