Abstract
Background
Glucocorticoid use is a major risk factor for osteoporosis. Overall rates of glucocorticoid use and bone health preventive measures in gastroenterology and hepatology populations are unknown.
Aims
We aimed to determine rates of glucocorticoid use and bone health preventive measures, to evaluate an education-based quality improvement initiative on bone health, and to assess improvement in health care practices of providers in regards to bone health recommendations.
Methods
A cross-sectional survey was offered to all patients visiting a tertiary care gastroenterology and hepatology clinic. A bone health education intervention was performed, followed by a repeat cross-sectional survey. Pearson’s chi square test statistic was used to evaluate interval improvement in bone health recommendations with the intervention. Predictive multiple logistic regression modeling was used to determine factors that influenced bone health recommendations by providers.
Results
A total of 552 patients and 725 patients completed the pre and post-intervention questionnaires respectively. The prevalence of glucocorticoid use was 12.9%. Bone health recommendations to patients on glucocorticoids did not improve with the intervention (63.0% vs.55.4%, p=0.42). The strongest predictor of bone health recommendations was autoimmune hepatitis (OR 6.60 95% CI 3.13, 13.90), followed by inflammatory bowel disease (OR 6.06 95%CI 3.92, 9.38), liver disease (OR 3.70 95% CI 2.45, 5.59), current smoking (OR 3.31 95% CI 2.32, 4.73) and history of osteoporosis/osteopenia (OR 2.72 95% CI 1.83, 4.03).
Conclusions
In spite of risk factors for osteoporosis in patients with digestive diseases, health care practices by providers in regards to bone health recommendations warrant further improvement.
Keywords: Osteoporosis; glucocorticoid use; quality improvement; digestive diseases; knowledge, attitudes and practices (KAP)
Introduction
It is estimated that over 200 million people worldwide have osteoporosis. The prevalence of osteoporosis is continuing to increase as the population ages. The major complication of osteoporosis is an increase in fragility fractures leading to morbidity, mortality, and decreased quality of life [1]. Patients with glucocorticoid-induced osteoporosis represent only a fraction of the osteoporotic population. However, the impact of the disease remains substantial in terms of complications. For example, patients who receive several courses of high-dose glucocorticoids (daily dose ≥15 mg and cumulative exposure >1 gm) have a substantially increased risk of fracture [2]. Therefore, it is important to intervene with preventive measures.
Despite data on the effectiveness of many pharmacologic therapies for osteoporosis prevention and treatment, only approximately 5–62% of patients in the United States and Europe on glucocorticoid therapy receive preventive therapies [3]. Gastroenterologists in particular have relatively few (<20%) of their at-risk patients on therapies to prevent osteoporosis [3,4]. Many gastrointestinal and hepatic disorders require treatment with corticosteroids, placing these patients at risk for osteoporosis and the complication of fracture. For example, it is estimated that patients with inflammatory bowel disease have a 40% greater risk of fracture than the general population, and this risk increases with age [5].
In 2003, the American College of Gastroenterology (ACG) and the American Gastroenterological Association (AGA) published guidelines on osteoporosis prevention and management in gastrointestinal and hepatic disorders [5–7]. These guidelines recommend lifestyle modifications and medical therapy, including supplemental calcium, vitamin D and bisphosphonates, to prevent or treat bone loss in patients who are on chronic glucocorticoid therapy. Kornbluth demonstrated that implementation of these guidelines led to the detection of osteopenia and osteoporosis and an initiation of specific therapies in a majority of gastroenterology clinic patients who met the guidelines’ criteria for dual energy x-ray absorptiometry (DEXA) screening [8]. The most frequent indication for implementing these guidelines was glucocorticoid use. Therefore, we aimed to determine the prevalence of glucocorticoid use in a tertiary care gastroenterology and hepatology practice. We then aimed to assess improvement in health care practices of providers in regards to bone health recommendations with a knowledge-based intervention. Finally, we aimed to evaluate factors that influenced bone health recommendations by health care providers.
Methods
Over a two month period in 2003, baseline data on osteoporosis risk factors, corticosteroid use, DEXA scan evaluation and treatment of osteoporosis was collected from consecutive patients visiting the tertiary care gastroenterology and hepatology clinic at University of North Carolina Hospitals (UNCH) in Chapel Hill, NC. The knowledge, attitudes and practices of health care providers were assessed via questionnaires filled out by patients. Data such as age, current corticosteroid and other medication use, smoking, alcohol intake, menopausal status, history of fragility fracture, family history of osteoporosis, personal history of osteoporosis and many other demographic characteristics were obtained. A provider and patient targeted intervention on osteoporosis risk factors, assessment and treatment was performed over a three month time period at a tertiary-care gastroenterology and hepatology clinic beginning in November of 2007. The provider intervention consisted of posted clinical reminders with a summary of the AGA guidelines on osteoporosis prevention and management. The patient intervention consisted of posters and educational materials placed in each examination room highlighting the importance of bone health. The providers underwent a voluntary web-based osteoporosis knowledge assessment evaluation prior to the intervention and after cessation of the intervention. This evaluation consisted of a web-based quiz based on a previously validated Osteoporosis Knowledge Assessment Tool and the AGA Guidelines on Osteoporosis [5,9]. The assessment tool was developed specifically for the purposes of this study. Following the three month intervention, the clinical reminders and the patient-centered posters and educational materials were removed. A questionnaire was then administered to all consecutive patients visiting the gastroenterology and hepatology clinic at UNCH. The survey was identical to the previous questionnaire distributed in 2003, with additional questions added about recognition of the intervention itself. The study protocol was approved by the Institutional Review Board at the University of North Carolina – Chapel Hill.
Statistical Analysis
Bivariate analyses were performed evaluating the characteristics of the population. The pre and post intervention characteristics were compared via student’s t-test for continuous variables and Pearson’s chi square test statistic for categorical variables. Improvement in physician osteoporosis knowledge post-intervention was assessed via paired t-test. Bone health recommendation by the tertiary care physician was the main outcome of interest. Recommendation of any bone health medication including calcium, vitamin D, bisphosphonate, calcitonin or hormone replacement therapy, lifestyle modification such as weight bearing exercise, smoking cessation or recommendation of a DEXA scan by the tertiary care gastroenterology or hepatology physician was considered to be a bone heath recommendation (binary outcome any/none). Improvement in bone health recommendations was assessed via Pearson’s chi square test statistic. Finally, a predictive logistic regression model was used to identify factors influencing bone health recommendations by health care providers. The fit of the predictive model was evaluated via a Hosmer-Lemeshow goodness of fit test.
For all analyses, p-values were two-sided, and a p-value of 0.05 or less was considered statistically significant. All statistical analyses were performed using Stata version 9.0 (Texas Station, TX).
Results
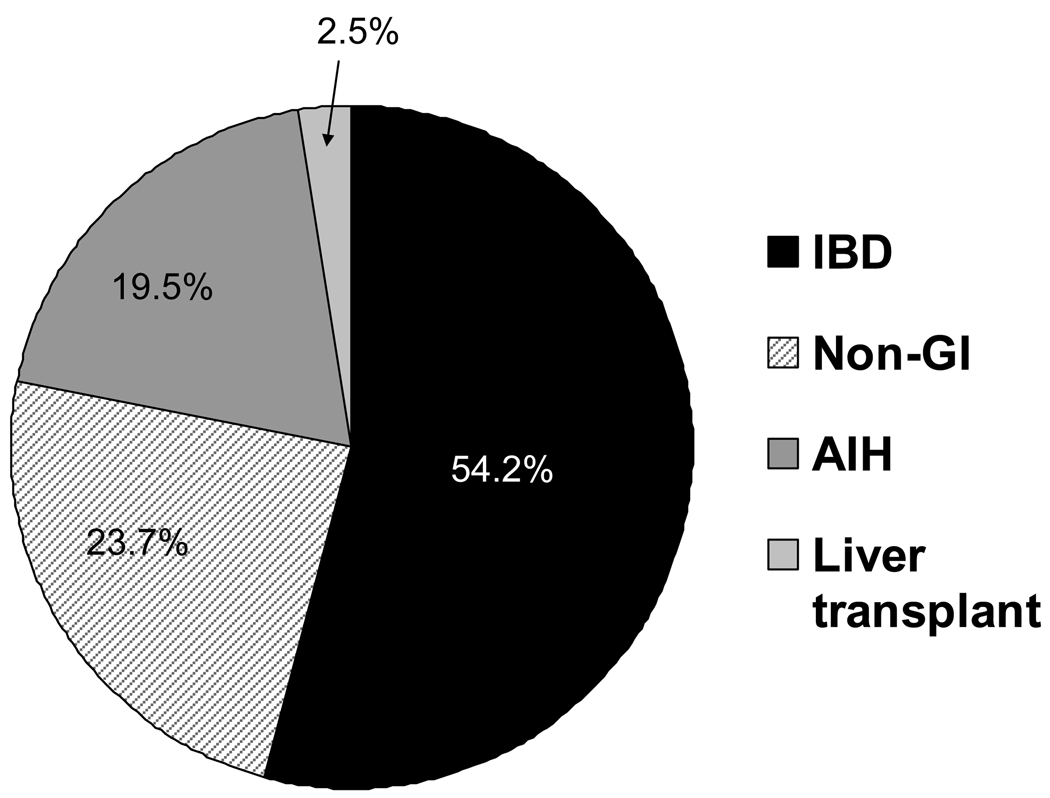
A total of 552 patients completed the baseline questionnaire (of 700 distributed surveys), and 725 patients completed the post-intervention questionnaire (of 1000 distributed surveys). Reasons for refusal included unwillingness to participate, Spanish speaking only, and lack of time. The overall characteristics of the population were similar pre and post intervention (Table 1). A higher percentage of the post-intervention population included patients returning for follow-up appointments, there were fewer smokers and a higher percentage had medication coverage in this group. Of note, there were similar rates of corticosteroid use and a similar percentage of the population had been previously diagnosed by a physician with osteoporosis. The overall prevalence of current corticosteroid use in the entire study population was 12.9%. The majority of these patients were taking corticosteroids for inflammatory bowel disease (IBD), followed by non-gastroenterology indications (including diagnoses such as asthma and arthritis), autoimmune hepatitis and liver transplant (Figure 1).
Table 1.
Pre and post-intervention characteristics of the tertiary-care gastroenterology and hepatology population
| Characteristic | Pre (n=552) | Post (n=725) | p value* |
|---|---|---|---|
| Mean age (SD), yr | 45.7 (15.3) | 47.5 (14.8) | 0.04 |
| Female gender (%) | 60.4 | 60.4 | 0.10 |
| Caucasians (%) | 78.6 | 76.5 | 0.47 |
| Return visits (%) | 68.1 | 75.4 | 0.01 |
| Have a primary care provider (%) |
89.3 | 88.9 | 0.84 |
| Diagnosis | |||
| IBD Diagnosis (%) | 25.5 | 21.0 | 0.08 |
| Liver disease/ transplant (%) |
27.1 | 29.6 | 0.39 |
| AIH diagnosis (%) | 3.9 | 5.1 | 0.37 |
| Other diagnosis (%) | 43.4 | 44.3 | 0.76 |
| Mean BMI (SD), kg/m2 | 26.7(6.5) | 27.5(6.7) | 0.05 |
| History of osteoporosis/penia (%) |
18.8 | 19.7 | 0.68 |
| Family history of osteoporosis/penia (%) |
26.6 | 32.2 | 0.05 |
| History of low/no impact fracture (%) |
10.8 | 8.7 | 0.23 |
| Post-menopausal (%) | 52.3 | 59.3 | 0.07 |
| Current smoking (%) | 39.6 | 21.2 | <0.01 |
| Current alcohol use (%) | 1.5 | 3.4 | 0.05 |
| Income | |||
| % < $25,000/yr | 34.9 | 33.1 | 0.55 |
| % $25–50,000/yr | 26.9 | 22.6 | 0.11 |
| % $51–75,000/yr | 14.4 | 15.3 | 0.69 |
| % $ >$75,000/yr | 23.8 | 29.0 | 0.06 |
| Education (% ≤ HS) | 30.7 | 26.6 | 0.14 |
| Medication coverage (%) | 82.7 | 89.1 | <0.01 |
| Insurance (%) | 94.0 | 93.0 | 0.47 |
| Current steroid use | 14.8 | 11.7 | 0.14 |
p-values obtained by Pearson’s chi square test for categorical variables and student’s t-test for continuous variables
Figure 1.
Reasons for corticosteroid use among current users of corticosteroids visiting a tertiary-care gastroenterology and hepatology clinic
When the population was limited only to those currently taking corticosteroids, there were a total of 61 corticosteroid users in the pre-intervention group and 77 in the post-intervention group. The rates of physician recommendation of specific bone health medications, lifestyle modifications and screening with DEXA were examined in the group of patients on corticosteroids who were established patients (return visits). Overall, health care practices by providers in regards to bone health recommendations were poor in both the pre and post intervention groups. There was no improvement in bone health recommendations with the intervention (63.0% pre-intervention versus 55.4% post-intervention for any bone health recommendation (p=0.42). Rates of previous DEXA scan and current bone health medication use in patients on corticosteroids were evaluated pre and post intervention (Table 2). Less than 1/2 of patients on corticosteroids were taking calcium, and less than 1/3 of these patients were taking concomitant vitamin D. Physician knowledge of osteoporosis prevention, screening and treatment (as measured by the provider osteoporosis knowledge assessment tool developed for this study), was similar pre and post intervention, with a mean quiz score of 79.3(8.4) pre intervention and 79.6(10.1) post intervention. There was no improvement with the intervention when individual physician scores were compared via paired t-test.
Table 2.
Health care practices of providers in regard to bone health recommendations and rates of bone health screening or treatment among corticosteroid users, pre and post intervention^
| n | Pre- intervention |
n | Post- intervention |
P value* |
|
|---|---|---|---|---|---|
|
Tertiary-care provider recommendations: |
n=46 | n=65 | |||
| Lifestyle Modifications | |||||
| Weight-bearing exercise | 5 | 11.1 | 4 | 16.7 | 0.42 |
| Smoking cessation** | 3 | 21.4 | 11 | 78.6 | 0.63 |
| Bone Mineral Density testing | 19 | 42.2 | 18 | 28.1 | 0.13 |
| Medications | |||||
| Calcium | 16 | 34.8 | 25 | 39.1 | 0.65 |
| Vitamin D | 7 | 15.2 | 15 | 23.1 | 0.31 |
| Bisphosphonate | 3 | 6.5 | 3 | 4.6 | 0.66 |
| Any bone health med | 19 | 41.3 | 27 | 41.5 | 0.98 |
| Any bone health recommendation# | 29 | 63.0 | 36 | 55.4 | 0.42 |
| Screening or medication use: | n=61 | n=77 | |||
| Had BMD testing in past | 35 | 59.3 | 41 | 55.4 | 0.65 |
| Medications | |||||
| Calcium | 21 | 34.4 | 36 | 46.8 | 0.14 |
| Vitamin D | 10 | 16.4 | 22 | 28.6 | 0.09 |
| Bisphosphonate | 6 | 9.8 | 11 | 14.3 | 0.43 |
| Any bone health med | 28 | 45.9 | 41 | 53.3 | 0.39 |
Bone health recommendation only assessed among corticosteroid users presenting for a return visit, screening or medication use assessed in all corticosteroid users
p-values obtained by Pearson’s Chi Square test (or Fisher’s Exact test when categories had few observations).
Among past or current smokers
Including lifestyle modifications, BMD testing or bone health medications
A predictive logistic regression model was fit to the data from the entire sample (n=1279). This model demonstrated that tertiary care physicians were most likely to recommend bone health medications, lifestyle changes or DEXA scan to patients if they had autoimmune hepatitis, inflammatory bowel disease, were current smokers, had liver disease or had a history of osteoporosis or osteopenia (Table 3). Notably, previous fracture, advanced age, steroid use and reduced BMI were not associated with tertiary physician bone health recommendations in this model. The model was evaluated with a Hosmer-Lemeshow goodness of fit test (p=0.20).
Table 3.
Predictive model of patient characteristics associated with bone health recommendation by tertiary care provider
| Characteristic | Odds Ratio for bone health recommendation |
95% Confidence Interval |
|---|---|---|
| History of osteoporosis or osteopenia |
2.72 | (1.83, 4.03) |
| Current smoking | 3.31 | (2.32, 4.73) |
| Inflammatory bowel disease | 6.06 | (3.92, 9.38) |
| Autoimmune hepatitis | 6.59 | (3.13, 13.90) |
| Liver disease* | 3.70 | (2.45, 5.59) |
All liver disease diagnoses other than autoimmune hepatitis, including liver transplant
Discussion
The study did not demonstrate a significant improvement in health care practices by providers in regards to bone health recommendations in patients on corticosteroids. This lack of improvement may be related to the knowledge-based intervention itself. The intervention was designed to be unobtrusive, but because of this, it may have failed to make an impact. Our providers’ knowledge of osteoporosis risk factors, screening and treatment was relatively good at baseline, therefore it is understandable that targeting knowledge improvement did not have a large impact. While high risk diagnoses such as inflammatory bowel disease and autoimmune hepatitis were predictors of bone health recommendations by tertiary care provider in our model, other important risk factors such as previous fragility fracture, reduced BMI, age and current glucocorticoid use were not predictive of bone health recommendation. Further improvement in recognition of these and other osteoporosis risk factors by gastroenterologists and hepatologists is necessary in order to improve the management of glucocorticoid induced osteoporosis in patients with digestive diseases.
The pharmacologic therapies most often recommended for the initial prevention and treatment of glucocorticoid-induced osteoporosis, regardless of the indication for the glucocorticoid, are calcium, vitamin D and bisphosphonates [5,6,10–13]. Our study found that overall rates of use of any of these bone health medications by patients on glucocorticoids were low (~40%). The data on efficacy of these medications for prevention and treatment of glucocorticoid-induced osteoporosis are primarily from patients with rheumatic diseases on relatively low average doses of prednisone. Recommended calcium doses are usually 1500 mg/day. However, calcium alone is not adequate to prevent or treat glucocorticoid-induced osteoporosis [3]. Vitamin D is also indicated, with increasing data that at least 800 IU is the optimum dosing of this medication [14]. A meta-analysis of vitamin D in glucocorticoid treated patients demonstrated a beneficial effect in bone mineral density and a reduction in vertebral fracture risk (RR 0.56, 95% CI 0.34–0.92) [15]. Bisphosphonates may actually be superior to vitamin D in glucocorticoid-induced osteoporosis [15]. One study by Devogelaer et al randomized 163 rheumatology patients on low-dose glucocorticoid therapy to calcium, vitamin D and placebo or alendronate. At one year, patients in the alendronate arm had an increase of 3.7% in the lumbar spine bone mineral density compared to a decrease of 1% in the placebo group [12]. This trial argues that combination therapy with calcium, vitamin D and bisphosphonates may be the most effective. Fewer studies have evaluated bisphosphonate use specifically in the gastroenterology patient population. Abitbol et al recently published a randomized controlled trial of an intravenous bisphosphonate for the prevention of steroid-induced bone loss in patients with inflammatory bowel disease. Patients receiving the bisphosphonate in addition to calcium and vitamin D had no change in their lumbar bone mineral density, compared to a reduction in lumbar bone mineral density in the control group treated only with calcium and vitamin D [16]. Bisphosphonates may represent an important tool in glucocorticoid-induced osteoporosis prevention in a gastroenterology patient population.
Efforts to improve the quality of care for glucocorticoid-induced osteoporosis have largely occurred in the primary care or rheumatology settings. The studies focusing on education of physicians alone have largely been unsuccessful. A randomized-controlled trial by Curtis et al used an internet based educational “toolbox” to educate physicians on glucocorticoid-induced osteoporosis. This intervention did not improve the quality of osteoporosis care delivered [17]. Another study randomized rheumatologists to an intervention consisting of education, discussion and audit of practice patterns. This intervention also did not improve the quality of care of glucocorticoid-induced osteoporosis [18]. One intervention consisting of education materials, guidelines and presentations to physicians and pharmacies in Australia did improve the use of calcium supplementation and pharmacologic therapies in hospitalized patients on glucocorticoid therapy (31% to 57%) [19]. However, an adjacent region was used as a control and there was neither patient nor provider level randomization. Based on these data, targeting barriers other than lack of knowledge could be the most beneficial in improving quality of care for glucocorticoid-induced osteoporosis prevention and management.
A recent study evaluated the effectiveness of the AGA guidelines on screening and treatment of patients at risk for osteoporosis. The AGA guidelines were implemented in 100 consecutive patients with inflammatory bowel disease who met criteria. The most frequent indication for osteoporosis evaluation was corticosteroid use (92%). Of the patients evaluated, 44% had osteopenia and 12% had osteoporosis. Specific therapies such as calcium, vitamin D and bisphosphonates were instigated in 69% of patients [8]. Additionally, Kane et al surveyed clinicians prior to and post distribution of the ACG guidelines on osteoporosis. A small percentage of providers read these guidelines, but among those that did, DEXA scans were ordered substantially more frequently and providers were more comfortable with management of osteoporosis [20]. Another study of awareness and implementation of osteoporosis guidelines in patients with inflammatory bowel disease by gastroenterologists demonstrated that most surveyed physicians did not use the guidelines on metabolic bone disease. Barriers to care that were cited included the assumption that another physician should manage the problem, lack of time, lack of knowledge and cost [21].
Data clearly show that reduction in bone mineral density is common in gastroenterology patients on glucocorticoid therapy and that provider adherence to recommended guidelines is low, although improvement is possible. In order to prevent long-term complications of osteoporosis in our patient population, we need to improve both recognition and management of glucocorticoid-induced osteoporosis. Curtis et al reported that gastroenterologists were less likely than internists to prescribe bisphosphonates to patients in whom these medications would be appropriate (OR 0.49 (95% CI 0.28–0.86) [22]. Therefore, our study is important in that it highlights the lack of bone health preventive measures initiated by tertiary care gastroenterologists and hepatologists for patients on glucocorticoid therapy.
One of the strengths of our study is that it is a large sample of consecutive patients visiting a tertiary care center. Not only was medication use recorded, but other lifestyle factors such as alcohol use, smoking status and weight-bearing exercise were collected. We were able to determine the indication for glucocorticoid use in this population, and were able to ascertain whether the patient recalled physician recommendations for the prevention, evaluation and management of glucocorticoid induced osteoporosis.
There are limitations to this study. Repeated cross-sectional surveys, as used in this study, test associations between variables before and after an intervention. However, this design may not have isolated the effects of the intervention. This study represents one population of patients at a tertiary care center and is likely not generalizable. However, the high rate of corticosteroid use we saw in our patient population was striking, and even if this rate is somewhat less in the general practice, it remains an important issue. The initial survey was performed in 2003, with a follow-up survey in 2007 after an intevention. Despite low levels of faculty turnover during this time period, there were likely differences in some of the physicians caring for patients at the UNCH clinic during these time periods. Individual differences in practice patterns may therefore have influenced our results. In our evaluation, we found differences by diagnosis in bone health recommendation by tertiary provider. For example, hepatologists may be better at bone health preventive care than general gastroenterologists as rates of bone health recommendation were higher in patients with hepatology diagnoses. We were unable to specifically differentiate by provider type in our model. All of the clinical characteristics, medication history and recall of tertiary physician recommendations were self-reported by the patients. Certainly, it is possible that recommendations were made that the patient did not remember. However, the patient must remember these recommendations in order to implement them, and therefore our outcome remains important. There are many patients who may have had osteoporosis, but were unaware of the complication since they had not previously been evaluated. This could introduce a bias into the results if it was differential. Our study also addressed only current corticosteroid use, not previous use. While we would be missing a percentage of the population that used these medications prior to the study, these data would likely be less reliable in terms of length of therapy, dose and concurrent medication use.
In conclusion, overall rates of corticosteroid use in a tertiary care gastroenterology and hepatology clinic are relatively high. Therefore, there is a population of patients at-risk for osteoporosis for whom the current model of practice is not successful. This and other quality improvement initiatives have been unsuccessful in improving bone health recommendations. Education and reminders of guidelines alone are not likely to impact practice patterns and improve prevention and recognition of glucocorticoid-induced osteoporosis in a gastroenterology and hepatology clinic. Consideration should be given in the future for further quality improvement initiatives, likely utilizing a systems-based approach, to improve implementation of bone health strategies by gastroenterologists and hepatologists.
Acknowledgments
The research was supported, in part, by grants from the NIH (T32 DK007634, P30 DK034987)
Footnotes
Financial Disclosures:
The authors have no financial disclosures.
Writing Assistance:
None
References
- 1.Reginster JY, Burlet N. Osteoporosis: a still increasing prevalence. Bone. 2006;38:S4–S9. doi: 10.1016/j.bone.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 2.De Vries F, Bracke M, Leufkens HG, et al. Fracture risk with intermittent high-dose oral glucocorticoid therapy. Arthritis Rheum. 2007;56:208–214. doi: 10.1002/art.22294. [DOI] [PubMed] [Google Scholar]
- 3.Curtis JR, Saag KG. Prevention and treatment of glucocorticoid-induced osteoporosis. Curr Osteoporos Rep. 2007;5:14–21. doi: 10.1007/BF02938618. [DOI] [PubMed] [Google Scholar]
- 4.Mudano A, Allison J, Hill J, Rothermel T, Saag K. Variations in glucocorticoid induced osteoporosis prevention in a managed care cohort. J Rheumatol. 2001;28:1298–1305. [PubMed] [Google Scholar]
- 5.American Gastroenterological Association medical position statement: guidelines on osteoporosis in gastrointestinal diseases. Gastroenterology. 2003;124:791–794. doi: 10.1053/gast.2003.50107. [DOI] [PubMed] [Google Scholar]
- 6.American Gastroenterological Association medical position statement: osteoporosis in hepatic disorders. Gastroenterology. 2003;125:937–940. doi: 10.1016/s0016-5085(03)01060-6. [DOI] [PubMed] [Google Scholar]
- 7.Bernstein CN, SK Guidelines for osteoporosis and inflammatory bowel disease. A guide to the diagnosis and management for the gastroenterologist (monograph) The American College of Gastroenterology. 2003 [Google Scholar]
- 8.Kornbluth A, Hayes M, Feldman S, et al. Do guidelines matter? Implementation of the ACG and AGA osteoporosis screening guidelines in inflammatory bowel disease (IBD) patients who meet the guidelines' criteria. Am J Gastroenterol. 2006;101:1546–1550. doi: 10.1111/j.1572-0241.2006.00571.x. [DOI] [PubMed] [Google Scholar]
- 9.Winzenberg TM, Oldenburg B, Frendin S, Jones G. The design of a valid and reliable questionnaire to measure osteoporosis knowledge in women: the Osteoporosis Knowledge Assessment Tool (OKAT) BMC Musculoskelet Disord. 2003;4:17. doi: 10.1186/1471-2474-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis: 2001 update. American College of Rheumatology Ad Hoc Committee on Glucocorticoid-Induced Osteoporosis. Arthritis Rheum. 2001;44:1496–1503. doi: 10.1002/1529-0131(200107)44:7<1496::AID-ART271>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 11.Adler RA, Hochberg MC. Suggested guidelines for evaluation and treatment of glucocorticoid-induced osteoporosis for the Department of Veterans Affairs. Arch Intern Med. 2003;163:2619–2624. doi: 10.1001/archinte.163.21.2619. [DOI] [PubMed] [Google Scholar]
- 12.Devogelaer JP, Goemaere S, Boonen S, et al. Evidence-based guidelines for the prevention and treatment of glucocorticoid-induced osteoporosis: a consensus document of the Belgian Bone Club. Osteoporos Int. 2006;17:8–19. doi: 10.1007/s00198-005-2032-z. [DOI] [PubMed] [Google Scholar]
- 13.Geusens PP, de Nijs RN, Lems WF, et al. Prevention of glucocorticoid osteoporosis: a consensus document of the Dutch Society for Rheumatology. Ann Rheum Dis. 2004;63:324–325. doi: 10.1136/ard.2003.008060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang BM, Eslick GD, Nowson C, Smith C, Bensoussan A. Use of calcium or calcium in combination with vitamin D supplementation to prevent fractures and bone loss in people aged 50 years and older: a meta-analysis. Lancet. 2007;370:657–666. doi: 10.1016/S0140-6736(07)61342-7. [DOI] [PubMed] [Google Scholar]
- 15.de Nijs RN, Jacobs JW, Algra A, Lems WF, Bijlsma JW. Prevention and treatment of glucocorticoid-induced osteoporosis with active vitamin D3 analogues: a review with meta-analysis of randomized controlled trials including organ transplantation studies. Osteoporos Int. 2004;15:589–602. doi: 10.1007/s00198-004-1614-5. [DOI] [PubMed] [Google Scholar]
- 16.Abitbol V, Briot K, Roux C, et al. A double-blind placebo-controlled study of intravenous clodronate for prevention of steroid-induced bone loss in inflammatory bowel disease. Clin Gastroenterol Hepatol. 2007;5:1184–1189. doi: 10.1016/j.cgh.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 17.Curtis JR, Westfall AO, Allison J, et al. Challenges in improving the quality of osteoporosis care for long-term glucocorticoid users: a prospective randomized trial. Arch Intern Med. 2007;167:591–596. doi: 10.1001/archinte.167.6.591. [DOI] [PubMed] [Google Scholar]
- 18.McDonough RP, Doucette WR, Kumbera P, Klepser DG. An evaluation of managing and educating patients on the risk of glucocorticoid-induced osteoporosis. Value Health. 2005;8:24–31. doi: 10.1111/j.1524-4733.2005.04007.x. [DOI] [PubMed] [Google Scholar]
- 19.Naunton M, Peterson GM, Jones G, Griffin GM, Bleasel MD. Multifaceted educational program increases prescribing of preventive medication for corticosteroid induced osteoporosis. J Rheumatol. 2004;31:550–556. [PubMed] [Google Scholar]
- 20.Kane S, Reddy D. Guidelines do help change behavior in the management of osteoporosis by gastroenterologists. Am J Gastroenterol. 2006;101:1841–1844. doi: 10.1111/j.1572-0241.2006.00662.x. [DOI] [PubMed] [Google Scholar]
- 21.Wagnon JH, Leiman DA, Ayers GD, Schwartz DA. Survey of gastroenterologists' awareness and implementation of AGA guidelines on osteoporosis in inflammatory bowel disease patients: are the guidelines being used and what are the barriers to their use? Inflamm Bowel Dis. 2009;15:1082–1089. doi: 10.1002/ibd.20857. [DOI] [PubMed] [Google Scholar]
- 22.Curtis JR, Westfall AO, Allison JJ, et al. Longitudinal patterns in the prevention of osteoporosis in glucocorticoid-treated patients. Arthritis Rheum. 2005;52:2485–2494. doi: 10.1002/art.21194. [DOI] [PubMed] [Google Scholar]