Abstract
Mechanisms of implicit spatial and temporal orienting were investigated by using a moving auditory stimulus. Expectations were set up implicitly, using the information inherent in the movement of a sound, directing attention to a specific moment in time with respect to a specific location. There were four conditions of expectation: temporal and spatial expectation; temporal expectation only; spatial expectation only; and no expectation. Event-related brain potentials were recorded while participants performed a go/no-go task, set up by anticipation of the reappearance of a target tone through a white noise band. Results showed that (1) temporal expectations alone speeded reaction time and increased response accuracy; and (2) implicit temporal expectations alone independently enhanced target detection at early processing stages, prior to motor response. This was reflected at stages of perceptual analysis, indexed by P1 and N1 components, as well as in task-related stages indexed by N2; and (3) spatial expectations had an effect at later response-related processing stages but only in combination with temporal expectations, indexed by the P3 component. Thus, the results, in addition to indicating a primary role for temporal orienting in audition, suggest that multiple mechanisms of attention interact in different phases of auditory target detection. Our results are consistent with the view from vision research that spatial and temporal attentional control is based on the activity of partly overlapping, and partly functionally specialized neural networks.
INTRODUCTION
The ability to identify an object is greatly improved by being able to anticipate its occurrence (Posner, 1980). Expectancy for an object can be directed explicitly, such as by cueing the observer to a particular location in space (Posner, Snyder, & Davidson, 1980), or implicitly, by the inherent trajectory of an object in time and space (Doherty, Rao, Mesulam, & Nobre, 2005). Most models of selective attention have focused on how visual attention is explicitly directed in space, and the space-based accounts of visual attention have widely been used to model auditory attention. This has resulted in a major focus of auditory research on locating sounds in space. However, in contrast to the visual domain, spatial information is processed indirectly and rather imprecisely in audition (Moore, 1997; Middlebrooks & Green, 1991). Temporal processing, on the other hand, is a central part of auditory cognition. Spoken speech and melody in music derive coherence and meaning from sound elements as they unfold in time. Thus, the auditory modality necessitates a high degree of temporal acuity. Despite the importance of temporal processing, the timing mechanisms of audition are not well understood (Mauk & Buonomano, 2004; Ivry, 1996).
In the visual domain, temporal predictability contributes to enhanced neural processing in the perceptual analysis of a stimulus. Doherty et al. (2005) demonstrated that implicit expectations formed by temporal regularities in the movement of an object in space interacted with spatial attention only at early, perceptual processing stages. At late response-specific processing stages, temporal and spatial expectations separately influenced target detection. Thus, in the visual domain, temporal expectations appear to selectively activate brain areas implicated in motor preparation and selection, but not in sensory processing per se. In the current study, we hypothesized that implicit temporal expectations would influence early perceptual processing because temporal processing is crucial in audition, and interact with spatial expectations only in later, task-related stages. To test effects of temporal and spatial expectation on auditory target detection, we used an auditory analogue of the naturalistic paradigm used by Doherty et al. (2005). Expectations were set up implicitly using information inherent in the movement of a sinusoidal sound. Auditory attention was directed to a specific moment in time, with respect to a specific location, through anticipation of target reappearance behind an occluding noise. As motion perception is based on the integration of spatial and temporal information, the task is well suited for exploring interactions between these neural mechanisms.
Event-related brain potentials (ERPs), which provide high temporal resolution, were recorded to evaluate the processing stages that implicit temporal and spatial expectations had on early perceptual and later response-related processes in auditory target detection. P1 and N1 are modality-specific components that reflect early perceptual processes associated with obligatory onset detection of an acoustic event (Näätänen & Picton, 1987). Auditory P1 (peaking around 50 msec from onset detection) and N1 (peaking at about 100 msec) components have different neural substrates but are both generated within auditory cortices (Liégeois-Chauvel, Musolino, Badier, Marquis, & Chauvel, 1994; Näätänen & Picton, 1987). Thus, P1 and N1 evoked by sound reflect the earlier stages of stimulus analysis involving auditory cortex. The later task-specific components (N2 and P3) are thought to be largely non-modality-specific, having a broader distribution of subcortical and cortical generators that give rise to the scalp-recorded potential (Oken, 1997; Picton, 1992). Unlike P1 and N1, which are obligatory in nature, N2 and P3 are elicited only when attention is directed toward the sounds. They reflect attention-based processes in target detection that integrate information from earlier systems (Picton, 1992). Thus, the latency and amplitude of these components are modulated not only by stimulus probability (bottom–up processes) but also by expectation and task difficulty (top–down processes) in target detection.
In the current study, the P1, N1, N2, and P3 components were dependent measures used to assess early perceptual and later task-related processing of implicit temporal and spatial orienting in auditory target detection. We predicted that temporal factors alone could modulate early, modality-specific ERP components (e.g., P1 or N1) because of the high reliance on temporal information for identifying meaningful auditory events. In contrast, we predicted that effects of spatial and temporal attention would interact at later processing stages, associated with task-related processes that are considered non-modality-specific (indexed by N2 and P3). The notion is that stimulus-driven factors (implicit orienting) interact with task requirements (auditory target detection) engaging a broader neural network, consistent with previous studies showing partly overlapping neural generators for visual spatial and temporal attention (Coull & Nobre, 1998).
METHODS
Participants
Twelve healthy right-handed adults (6 women) between the ages of 21 and 44 years (M = 29 years; SD = 6.8 years), with no reported neurological or hearing-related problems, were paid to participate in the study. All participants passed a hearing screening (20 dB HL at 500, 100020 dB HL at 500, 2000, and 4000 Hz). All procedures were approved by the Internal Review Board and Committee for Clinical Investigations of the Albert Einstein College of Medicine where the study was conducted. After the experimental protocol was explained, participants gave informed consent. Data from one participant were excluded from analysis due to excessive artifact in the EEG recording. Data from the remaining 11 participants were included in this report.
Stimuli and Procedure
Stimuli consisted of 12 pure tones (880 Hz) and 1 complex tone (f0 880 Hz; harmonics: 1760, 2640, and 3520 Hz), all with a tone duration of 100 msec (5 msec rise/fall times), and a white noise stimulus (tone duration 200 msec, 5 msec rise/fall times). The sounds were generated and presented via insert earphones using Tucker Davis Technologies (TDT; Alachua, FL, USA) software (RPvds and ActiveX Controls) and hardware (RX8 Multi I/0 Processor, Stereo Power Amp, RBOX-Button Box). Sounds (pure tones, white noise, and complex tones) were lateralized (McEvoy, Picton, & Champagne, 1991) in steps of 0.1 msec interaural time difference (ITD) (delay times were 0.7, 0.6, 0.5, 0.4, 0.3, 0.2, 0.1, and 0 msec) to create the perception of 14 different spatial positions outside the head (Figure 1). Sounds were presented in serial order by delay time, so that the sounds were perceived as traveling in a trajectory from the right to the left ear across the top of the head (or vice versa). In all conditions, the noise stimulus was presented successively after the final sinusoidal sound (no silence between them), followed by the interstimulus interval (ISI) and presentation of the final sinusoidal tone. This was done to give the effect that the noise stimulus was occluding “tones” in its virtual space so the next actual tone (the target or nontarget) could be anticipated to emerge on the other side of the noise barrier. A go/no-go paradigm was used, in which the final tone that reappeared after the occluding white noise was either a target (complex, p = .5) or a non-target (pure, p = .5), randomly presented. Thus, there were 14 tones comprising one trial (including the noise stimulus and final tone), each requiring a go or no-go response according to whether the final sound was a target. A 1500-msec period of silence separated each trial. Sound level was calibrated to 82 dB ppe SPL using a Brüel & Kjær sound level meter (model 2200) with an artificial ear.
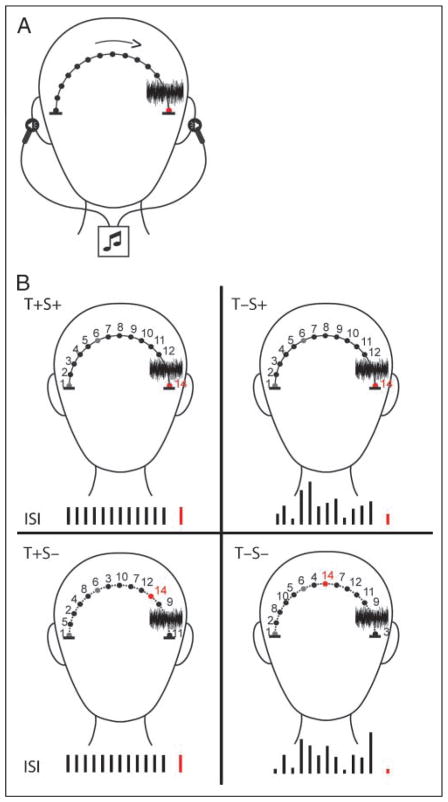
Figure 1.
Schematic of stimulus paradigm. (A) Experimental set-up. Tones were presented by insert earphones, using interaural time difference (ITD) cues to simulate perception of a moving stimulus from the left-to-right (or right-to-left) side of the head. Blackened circles depict the perceived tone locations. A white noise stimulus (noise spectrogram) occurred prior to the final tone (indicated by the red circle). The final tone was either a target or nontarget stimulus. (B) Four conditions of expectation are depicted. Numbers illustrate the position and order of the tones across the head. Black bars denote the length of the ISI. The red bar denotes the final tone of the trial (go/no-go). Temporal expectations (T+) were created by holding the timing of the stimulus constant. Spatial expectations (S+) were created by presenting the tones in a spatially constant motion along the trajectory (solid line). The absence of expectation was created by presenting tones with a random ISI along the trajectory (T−), random spatial distribution along the trajectory (S−), or both (T−S−). The first and sixth positions (gray circles) and position of the noise stimulus were fixed so that direction of motion could still be perceived even with irregular movement in time and space (dashed line).
Figure 1 displays an illustration of the four experimental conditions in which implicit expectations were set up by “where” (spatial) and “when” (temporal) a sound would reappear after an occluding noise. In all four conditions (temporal plus spatial expectation [T+S+]; temporal expectation only [T+S−]; spatial expectation only [T−S+]; neither temporal or spatial expectation [T−S−]), the motion of the trajectory from one side of the head to the other was maintained by keeping the spatial position of the first, sixth, and noise stimuli fixed. This was done so that, in each condition, the sound followed a trajectory and the movement along it was either regular or irregular in time and space. In the conditions that spatial expectation was present (T+S+, T−S+), spatial positions moved serially from one side of the head to the other in 0.1-msec ITD steps. This builds perception of the sound moving spatially on a constant trajectory. In the conditions that temporal expectation was present (T+S+, T+S−), the sound moved isochronously with a constant ISI of 200 msec. This builds anticipation of when the target will reappear from behind the noise. In the conditions in which spatial expectation was absent (T+S−, T−S−), sounds were randomized by position (except for the fixed positions). This caused perception of a sound skipping randomly across the head, making it difficult to anticipate the spatial position of target reappearance. In the conditions in which temporal expectation was absent (T−S+, T−S−), sounds were presented with a randomized ISI (0–550 msec for pure tones, 0–900 msec for the white noise stimulus). This was perceived as a randomly fluttering stimulus that made it difficult to anticipate the moment in time of target reappearance. The overall length of each trial was also varied so the listener could not use the occluding noise barrier as a temporal cue for behavioral response in temporal absent conditions. Additionally, the four conditions were randomly presented within each block of trials to ensure that the noise barrier was not a reliable temporal cue on its own in any condition. The perceived motion of the sound in a constant spatial trajectory of positions outside the head from left-to-right and right-to-left was confirmed in a pilot study with four subjects, none of whom participated in the main experiment.
Participants were seated in a comfortable chair in a sound-attenuated room. They were instructed to listen to the sounds and use the information inherent in the trajectory of the moving sound to press a response key when the target stimulus was detected. Participants maintained fixation on a cross at the center of a video monitor, to minimize eye and head movements. Seven blocks of 56 trials were presented, in which the four conditions were randomly presented with 14 trials of each condition in each block. In half of the trials, the sound moved from left to right, and in the other half the sound moved from right to left. The total session time, including electrode placement and breaks, was approximately 2 hr.
Data Analysis
Behavioral Measures
For each participant, reaction time (RT), hit rate (HR), and false alarm rate (FAR) were calculated separately in each condition. A response was considered correct if it occurred between 200 and 900 msec from stimulus onset. Behavioral criterion for inclusion in the study was a task accuracy of more than 61%. All participants met this criterion (Table 1).
Table 1.
Behavioral Results for All Conditions (Mean Reaction Time in msec [RT], Hit Rate [HR], and d′, with Standard Deviation in Parentheses)
| Condition | RT | HR | d′ |
|---|---|---|---|
| T+S+ | 354 (72) | 0.99 (1.4) | 4.31 (0.4) |
| T+S− | 344 (79) | 0.99 (1.0) | 4.23 (0.5) |
| T−S+ | 376 (92) | 0.99 (1.3) | 4.02 (0.5) |
| T−S− | 369 (88) | 0.99 (1.7) | 4.12 (0.6) |
As RT allows conclusions to be made only about the speed of motor processing, we additionally calculated d′ to derive a measure of sensitivity for identifying the target (McMillan & Creelman, 1991). The measure d′ is derived from the signal detection theory (Green & Sweets, 1966), measuring the separation between two classes of stimuli on a hypothetical inner perceptual dimension upon which the subject’s decision is based. In the present experiment, we used a “yes–no” model for independent observations to obtain d′. “Hits” were correct button presses to the complex tone and “misses” were the absence of a button press to the complex tones. “Correct rejections” were correct no-go responses to the pure tones, and “false alarms” were button presses to the pure tones. As there were many instances in which subjects had perfect performance (i.e., 1 or 0 for hits or false alarms), which can result in statistically infinite d′, we adjusted the scores slightly to avoid infinite d′ by adding 0.5 to all the cells and dividing the resulting scores by the number of trials (n + 1) related to the proportion (Macmillan & Creelman, 2005). The result was a number for each subject, in each condition, on a scale from 0 to 4.65, in which 4.65 indicated the highest sensitivity for detecting the target.
Effects of temporal and spatial expectations on RT and sensitivity were calculated using repeated measures analysis of variance (ANOVA) with factors of temporal expectation (present or absent) and spatial expectation (present or absent).
Electroencephalogram Recording and Data Analysis
EEG was recorded continuously with an electrode cap having 32 scalp locations (modified 10–20 International System) Fpz, Fz, Cz, Pz, Oz, Fp1, Fp2, F7, F8, F3, F4, FC5, FC6, FC1, FC2, T7, T8, C3, C4, CP5, CP6, CP1, CP2, P7, P8, P3, P4, O1, O2, plus electrodes placed on the right (RM) and left (LM) mastoids. Horizontal electrooculogram (EOG) was recorded using F7 and F8 electrode sites, and vertical EOG was recorded using a bipolar montage between FP2 and an external electrode placed beneath the right eye. The reference electrode was attached to the tip of the nose. The EEG and EOG were digitized (Neuroscan Synamps amplifier, Compumedics Corp., El Paso, TX) at a sampling rate of 500 Hz (band-pass 0.05–100 Hz), and then off-line filtered between 1 and 30 Hz. Target and nontarget stimuli (go and no-go stimuli) were time-locked to the EEG, and then analyzed in epochs of 600 msec (which includes a 100-msec prestimulus period), separately in each condition (T+S+, T+S−, T−S+, T−S−). The mean amplitude in the prestimulus period served as the baseline for the amplitude measurements. Epochs with an electrical change from baseline exceeding ±75 μV were excluded from further averaging. This procedure resulted in removal of the majority of trials contaminated by eye movement and other artifacts. On average, 10% of the epochs were artifact-rejected for target trials and 10% for nontarget trials.
Table 2 summarizes the peak latency of the components (and time window) used to calculate, separately, the mean amplitude of the P1, N1, N2, and P3 components for each individual. Peak latency was derived from the grand-mean waveforms at the electrodes with the greatest signal-to-noise ratio. To test for the presence of the components, one-sample t tests were used to determine if the mean amplitude of the ERP components, as measured in their respective latency ranges, was significantly greater than zero (in either polarity). Repeated measures ANOVA were conducted to examine effects of expectation (T+S+, T+S−, T−S+, T−S−), response requirements (go, no-go trials), and scalp distribution (P1 component: Fz, Cz, F3, F4, FC1, FC2, C3, C4; N1 component: Fz, Cz, F3, F4, FC1, FC2, C3, C4; N2 component: Fz, Cz, Pz, F3, F4, FC1, FC2, C3, C4, CP1, CP2, P3, P4; P3 component: Cz, Pz, C3, C4, CP1, CP2, P3, P4) on the mean latency and amplitude of each component. Because of possible violations of compound symmetry and sphericity, the Greenhouse–Geisser (G–G) adjusted univariate test was used and p values were reported. Significance was tested with a two-tailed criterion and a 95% confidence interval.
Table 2.
Peak Latency of the ERP Components (msec) with Time Interval (msec) Used to Measure Mean Amplitude in Parentheses
| P1 (Fz) | N1 (Fz) | N2 (Fz) | P3 (Pz) |
|---|---|---|---|
| 44 (29–59) | 116 (101–131) | 188 (173–203) | 322 (292–352) |
Electrode of maximal signal-to-noise ratio used to obtain peak measurements is shown in parentheses for each component.
Correlations between mean RT and mean ERP amplitude (collapsed across electrodes used in the analysis) were calculated for each condition using the Pearson product–moment correlation coefficient.
RESULTS
Behavioral Results
Table 1 provides a summary of the behavioral results. Target responses were best when subjects could use temporal expectations to implicitly anticipate the timing of target appearance. Temporal expectation speeded RT [F(1, 10) = 15.4, p = .003], whereas spatial expectation provided no such enhancement on its own [F(1, 10) = 4.2, p = .68] and did not interact with temporal expectation [F(1, 10) = 0.069, p = .798]. Temporal expectation also led to greater sensitivity (d′) for identifying the complex tone [F(1, 10) = 5.9, p = .04], and there was no benefit to performance from anticipating spatial direction [F(1, 10) = 0.004, p = .95], and no interaction between factors [F(1, 10) = 1.06, p = .33]. Thus, only temporal expectations influenced target performance.
ERP Results
Early Auditory ERP Components: P1 and N1
P1 component
Table 3 presents the mean amplitudes of the ERPs elicited in the P1 latency range. P1 was not statistically present, insofar as the mean amplitude of the waveforms was not significantly different from zero. This may be due to the low amplitude of the P1 component, as is generally observed in adults. However, as a component peak in the normal P1 latency range in adults (~50 msec from stimulus onset) was observed in the waveforms (Figure 3, Fz electrode), we calculated effects of expectation and response requirements on the P1 amplitude. We recognize therefore that further statistical results for the P1 component should be interpreted with caution.
Table 3.
Mean Amplitudes (μV) of P1, N1, and N2, P3 Components (Standard Deviation in Parentheses) for Go and No-go Trials
| P1 (Fz) | N1 (Fz) | N2 (Fz) | N2 (Cz) | P3 (Pz) | |
|---|---|---|---|---|---|
| Go Trials | |||||
| T+S+ | −0.69 (2.85) | −4.96* (3.19) | −3.41* (3.52) | −3.86* (3.65) | 6.81* (3.17) |
| T+S− | −0.62 (1.76) | −5.63* (3.05) | −4.09* (3.00) | −4.01* (3.93) | 5.68* (3.40) |
| T−S+ | 0.34 (2.03) | −5.39* (2.36) | −2.73* (2.53) | −3.53* (3.00) | 4.59* (3.30) |
| T−S− | −0.07 (1.25) | −3.72* (3.14) | −3.19* (3.22) | −3.69** (3.94) | 5.22* (3.28) |
| No-go Trials | |||||
| T+S+ | −1.21 (2.43) | −5.08* (2.86) | −4.64* (2.6) | −2.74* (2.68) | 1.52** (1.85) |
| T+S− | −0.71 (2.02) | −4.34* (1.88) | −4.12* (1.81) | −1.86* (1.87) | 0.96 (2.26) |
| T−S+ | 0.13 (1.00) | −3.72* (1.33) | −2.7* (2.28) | −1.68** (2.04) | 0.23 (1.78) |
| T−S− | 0.81 (1.53) | −2.62* (1.96) | −1.88** (2.31) | −0.99 (2.13) | 0.90 (1.65) |
Electrode used to obtain peak latency for statistical measurement is shown in parentheses.
Asterisks show results of one-sample t tests: when the mean amplitude of the ERP components was significantly greater than zero (in the positive or negative direction).
p < .01.
p < .05.
Figure 3.
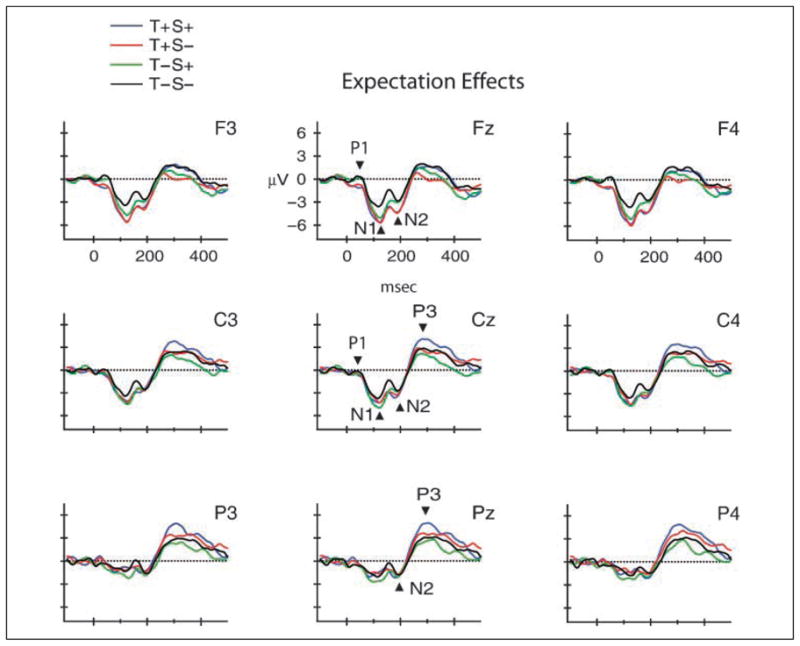
Temporal and spatial expectation effects. Grand-mean waveforms (collapsed across response requirements) are displayed to show effects of expectation on early and late auditory target detection (T+S+, blue line, T+S−, red line, T−S+, green line, T−S−, black line). Midline and lateral electrodes are displayed as in Figure 2. N1, N2, and P3 components are labeled at the midline electrodes, with arrows pointing to the component peaks. N1 amplitude was enhanced at fronto-central scalp sites when the target could be anticipated by implicit spatial or temporal cues. N2 effect of temporal expectation is best seen at frontal electrodes. Synergistic effect of temporal and spatial expectation is best seen at parietal electrodes (P3).
ANOVA revealed an effect only of temporal expectations on P1 amplitude at frontal electrode sites [Temporal expectation × Electrode interaction: F(7, 70) = 3.7, ε = .29, p = .04; Tukey HSD post hoc calculations showed significant effects at Fz, F3, F4, FC2; which can be seen in Figure 3, Fz electrode]. However, this effect was a smaller P1 amplitude for temporal expectations at frontal sites. There were no significant main effects of temporal expectation, spatial expectation, task requirements, or electrode [F(1, 10) = 2.99, p = .11; F(1, 10) = 2.04, p = .18; F(1, 10) < 1, p = .40; F(7, 70) < 1, p = .89, respectively], and no other significant interactions with any of the factors [Temporal × Spatial: p = .95; Temporal × Task: p = .43; Spatial × Task: p = .10; Spatial × Electrode: p = .09; Task × Electrode: p = .06; the remaining three- and four-way interactions were in the range p > .30–.79].
P1 amplitude was positively correlated with RT (r = .32, p = .03). Less positive (decreased) P1 amplitudes were associated with faster response times (shorter RTs).
N1 component
The N1 component was significantly elicited in all conditions (Table 3, FFigure 3), with a fronto-central scalp distribution [main effect of electrode: (7, 70) = 4.08, p = .02; Tukey HSD revealed the largest amplitude at Fz, F4, FC1, FC2]. There was a main effect of temporal expectation on N1 amplitude [F(1, 10) = 11.58, p = .007]. N1 amplitude was larger in conditions with temporal expectations (T+S+, T+S−) over frontal electrode sites [Temporal expectation × Electrode interaction: F(7, 70) = 5.76, ε = 0.23, p = .02; Tukey test shows effects at Fz, F3, F4 electrodes]. There was also a main effect of task requirements [F(1, 10) = 11.98, p = .06], with N1 amplitude larger for go than no-go trials. There was no main effect of spatial expectation (p > .11), and no interactions (Temporal × Spatial: p > .34; Temporal × Task: p > .34; Spatial × Task: p > .75; Spatial × Electrode: p > .07; Task × Electrode: p > .46; the remaining three- and four-way interactions were in the range p > .21–.91). An additional post hoc ANOVA was conducted to test the effects of expectation by electrode site (due to near significance of the Spatial × Electrode factor), which revealed that N1 amplitude was larger at frontal electrode sites for conditions with expectations (T+S+, T+S−, T−S+) compared to no expectations (T−S−) [Expectation × Electrode interaction: F(21, 210) = 4.76, p < .00001]. This effect can be seen in Figure 3 (Fz electrode).
N1 amplitude was positively correlated with RT (r = .444, p = .003). Faster response times (decreased RT) were associated with more negative amplitude (decreased amplitude).
Later ERP Components N2 and P3
N2 component
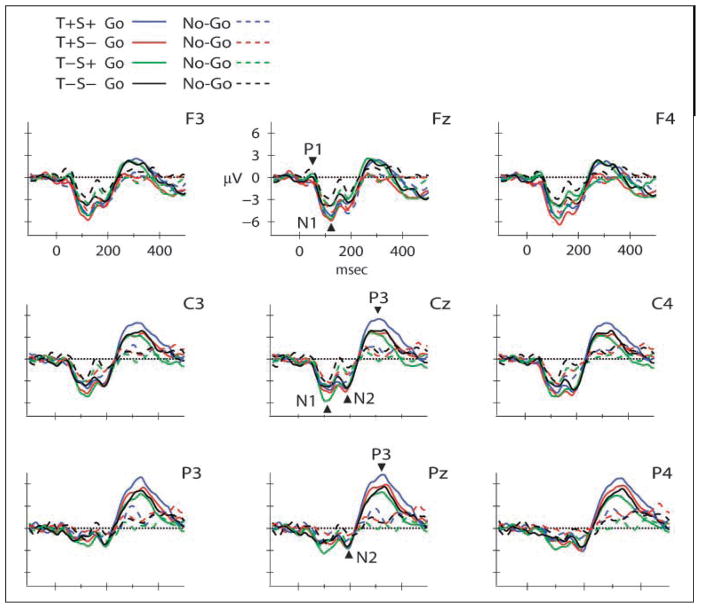
Table 3 presents the mean amplitudes for the N2 component at frontal (Fz) and central (Cz) electrode sites. The overall distribution of the N2 was fronto-central [main effect of electrode: F(12, 120) = 8.44, ε = .13, p < .005]. Tukey HSD calculations showed that it was largest at Fz, Cz, F3, F4, FC1, FC2, C3, C4, CP1, CP2. N2 amplitude was more negative when temporal expectations could be formed [T+S+, T+S− conditions; main effect of temporal expectation: F(1, 10) = 5.46, p = .04] (Figure 3, Fz electrode). N2 amplitude was also influenced by response requirements. N2 was significantly more negative for go trials over centro-parietal scalp sites [Response requirements × Electrode interaction: F(12, 120) = 7.54, ε = .25, p < .001] (Figure 2, Cz electrode). The effect of temporal expectation was fronto-central, whereas the effect of response requirements was centro-parietal (Tukey HSD tests show effects at Cz, Pz, FC1, FC2, C3, C4, CP1, CP2, P3, P4). Spatial expectation did not exert influence on N2 amplitude [F(1, 10) < 1, p > .48]. There was no main effect of response requirements [F(1, 10) = 3.86, p > .08], and no other interactions (Temporal × Spatial: p > .88; Temporal × Task: p > .40; Spatial × Task: p > .17; Temporal × Electrode: p > .06; Spatial × Electrode: p > .14; the remaining three- and four-way interactions were in the range p > .07–.99). The Temporal × Electrode interaction showing a frontal effect was nearly significant (p > .06). When collapsed across response requirements, this interaction was significant in a post hoc ANOVA with factors of expectation and electrode [F(36, 360) = 2.8744, p < .001], with post hoc tests showing larger amplitude for temporal expectations (T+S+, T+S−) at frontal electrode sites (Fz, F3, F4, FC1, FC2). Effects of response requirements observed at centro-parietal sites were not influenced by temporal expectations (Figure 2, Cz electrode), whereas effects of temporal expectation at fronto-central sites were not influenced by response requirements (Figure 3, Fz electrode).
Figure 2.
Response requirements effects. Grand-mean waveforms are displayed at the midline (Fz, Cz, Pz), and lateral, frontal (F3, F4), central (C3, C4), and parietal (P3, P4) electrodes by condition (T+S+, blue; T+S−, red; T−S+, green; and T−S−, black), separated by response requirements (go solid lines/no-go dashed lines). Positive polarity is upward. N1, N2, and P3 are labeled at the midline, with arrows pointing toward the peak of the component. Effects of the go response are reflected in N1, N2, and P3 components.
N2 amplitude positively correlated with RT (r = .63, p < .001). This indicates that when subjects responded faster (shorter RT), N2 amplitudes were larger (more negative).
P3 component
Table 3 shows the mean P3 amplitude for each condition, and results of a one-sample t test which shows that the P3 was significantly elicited for go trials (Figure 2, Pz electrode). This is also seen in a main effect of response requirements on P3 amplitude [F(1, 10) = 15.02, p = .0003]. P3 amplitude was larger for go than no-go trials. There was also a main effect of electrode [F(7, 70) = 7.6, ε = .30, p = .003], showing an overall parietal distribution of P3 amplitude, with post hoc analyses showing it was largest at parietal sites (Pz, P3, P4). The interaction between response requirements and electrode [F(7, 70) = 5.53, ε = .22, p = .02] showed that the go P3 amplitude was more positive than no-go trials at parietal electrodes (Tukey HSD post hoc revealed maximum amplitudes at Pz, P3, and P4 electrodes). This is consistent with P3 component being elicited for target trials only, and suggests that P3 mainly reflects response requirements in target detection.
Temporal expectations modulated P3 amplitude [main effect of temporal expectation: F(1, 10) = 5.3, p = .04; post hoc analyses showed that P3 amplitude was larger for T+S+, T+S− conditions], whereas spatial expectations did not [no main effect of spatial expectation: F(1, 10) = 0.05, p = .83]. However, there was an interaction between temporal and spatial expectations [F(1, 10) = 7.23, p = .02]. Tukey HSD analysis showed a significant difference between T+S+ and T−S+ conditions. The amplitude of P3 elicited when both temporal and spatial expectations could be formed (T+S+) was significantly larger than when only spatial expectations could be formed (T−S+). P3 amplitudes also did not significantly differ when time was the only cue that could be used to anticipate the target (T+S+ vs. T+S−). There were no other interactions (Temporal × Task: p > .25; Spatial × Task: p > .34; Temporal × Electrode: p > .10; Spatial × Electrode: p > .70; the remaining three- and four-way interactions were in the range p > .20–.74). As with the N2 component, P3 task effects did not interact with expectation effects.
P3 amplitude negatively correlated with RT (r = −.402, p < .01). The correlation is negative because faster response times (RT decreases) were associated with larger P3 amplitude (increases in a positive direction). In general, all component amplitudes were larger (in their respective negative or positive direction) with faster response times.
DISCUSSION
Spatial and temporal expectations were set up by the trajectory of a moving sound to investigate the role of implicit expectations in auditory target processing. Behavioral and ERP results were consistent with theories that substantiate a primary role of temporal orienting in audition. Implicitly directed temporal auditory attention modulated the amplitude of ERP components independently from spatially directed attention at earlier, sensory processing stages (indexed by P1 and N1), and at target detection stages prior to motor response (indexed by N2). In contrast, at later processing stages associated with response requirements (indexed by P3), implicit temporal orienting interacted with spatial orienting. These results indicate that there are seemingly distinct mechanisms of spatial and temporal attentional control that can act independently at several processing stages prior to motor response (P1, N1, N2), but can also integrate information at stages involved in decision-making (P3). This result is consistent with theories of visual attention, suggesting that cortical brain areas involved in temporal and spatial orienting are task directed, involving multiple levels of analysis that include both functionally specialized and overlapping neural networks (Nobre, 2004; Corbetta & Shulman, 2002; Coull & Nobre, 1998).
Behavioral Results
Only temporal expectations enhanced auditory target detection. RT was shorter, and sensitivity (d′) for detecting the target was greater, when the timing of the moving sound could be anticipated. These behavioral findings are consistent with previous studies showing RT benefits in auditory temporal cueing paradigms that used endogenous cues to explicitly direct attention to a location in space (e.g., left vs. right) or temporal instant (Lange & Röder, 2006; Niemi & Näätänen, 1981; Jones, 1976; Snodgrass, 1969). They are also consistent with previous studies that used simple detection tasks and found null effects for spatial orienting (Spence & Driver, 1994; Buchtel & Butter, 1988), as we found no impact of implicitly derived spatial expectations on the speed or accuracy of target detection with this easy target detection task. However, other studies have found improvements in auditory target performance for endogenous spatial orienting when perceptually more challenging discrimination tasks were used (e.g., Spence & Driver, 1996; Mondor & Zatorre, 1995). In addition to the difference in ease of ability to detect the target, a possible reason why no effect on behavioral performance for spatial orienting was found in our studies compared to others is that we directed spatial attention by implicit expectation. Expectations were formed by regularities in the stimulus characteristics. In most studies that found spatial effects on target performance, spatial orienting was explicitly directed by pretarget cueing. Thus, effects of spatial orienting on behavioral performance may depend on both the ease of discriminability and on the manner in which auditory attention is directed in time or space.
Effects on Early Auditory Processing P1/N1
The ability to implicitly anticipate the target, by stimulus-driven regularities, modulated early auditory processing reflected in P1 and N1 components. At the earliest cortical level (P1), only temporal expectations modulated the P1 amplitude, and only at frontal electrode sites. This scalp topography is consistent with generators of the P1 component within primary auditory cortices (Yvert, Fischer, Bertrand, & Pernier, 2005; Liégeois-Chauvel et al., 1994). Interestingly, temporal expectations negatively modulated P1 amplitude. P1 was smaller when temporal expectations could be formed. The negative (lower amplitude) displacement of the P1 cannot be solely attributed to overlap with N1 (e.g., riding on the more negative N1 response that follows) because P1 evoked by spatial expectations was not similarly displaced, even when the adjacent N1 was similarly large (Figure 3, Fz, the T−S+ condition). Thus, implicitly directed temporal expectations appear to have early perceptual effects on target detection prior to motor response.
Conversely, in the visual modality, spatial expectations modulated P1 amplitude but temporal expectations did not (Doherty et al., 2005). Finding temporal effects on P1 in audition and spatial effects in vision is consistent with a fundamental difference between the two sensory systems. However, Doherty et al. also found that spatial and temporal expectations interacted to enhance the amplitude of P1, suggesting that the timing of visuospatial information can influence perceptual analysis. A similar interaction with timing was not found at this early level for audiospatial information, suggesting that temporal characteristics play a more dominant role in early perceptual phases of auditory information processing. Nonetheless, the specific circumstances that temporal expectations strongly or weakly modulate P1 amplitude are yet to be fully defined for the auditory modality.
As with P1, N1 was modulated only by temporal expectations, in fronto-central scalp locations, consistent with generators within auditory cortices (Näätänen & Picton, 1987). N1 was larger when temporal expectations could be formed. This result is consistent with Lange and Röder (2006) and Lange, Rösler, and Röder (2003), who investigated endogenously cued temporal attention in the auditory modality and found a larger N1 amplitude to stimuli at the attended point in time, over frontal scalp sites, and concluded that temporal orienting can improve early perceptual processing of auditory stimuli. In recent studies based on implicit orienting, Lange (2009) and Doherty et al. (2005) found attenuated N1 amplitude. Lange concluded the attenuation of N1 to be specific to implicit temporal expectations. However, it remains to be determined whether the modulation of N1 is due to the type of cueing or to other stimulus factors that interact with top–down control.
Unexpectedly, N1 amplitude was also modulated by response requirements: larger for go trials. Although N1 is not generally known to be modulated by response variables, its amplitude is enhanced by attention (Hillyard & Picton, 1987). Thus, the main effect of response requirements may reflect top–down control on early perceptual processes for a moving auditory stimulus. Alternatively, this enhancement may simply reflect a difference in stimulus characteristics that could occur by using complex tones for the target versus the pure tones that set up the expectation. However, it is not clear that N1 amplitude solely reflects spectral differences of tones (Tiitinen, Sivonen, Alku, Virtanen, & Näätänen, 1999) and a spectrally varied noise stimulus immediately preceded the target. Moreover, effects of temporal expectation were found, which are reflected in both trial types, go and no-go responses. Thus, effects on N1 cannot be solely attributed to the difference in stimulus characteristics. Moreover, Doherty et al. (2005) also reported an interaction of response requirements with temporal expectation, but which was seen in a diminished visual-evoked N1 amplitude.
In the current study, there were no spatial attention effects on N1, whereas in the visual modality, Doherty et al. found a main effect of both spatial and temporal attention and with no interaction. Thus, timing of stimuli has an early influence in vision and audition, but spatial orienting plays a lesser role in the earlier stages of audition. The absence of spatial effects on N1 may also be attributed to the type of paradigm used, the type of cue, or the nature of the task. Previous studies using endogenous cueing paradigms, for example, have shown influence of spatial and temporal expectations on N1 (Lange, Krämer, & Röder, 2006; Doherty et al., 2005; Lange et al., 2003; Eimer, 1998; Mangun, 1995; Woldorff & Hillyard, 1991; Giard, Perrin, Pernier, & Peronnet, 1988). Discrepancies in N1 findings among studies likely point toward the highly adaptive nature of the sensory systems, interacting with attention networks, to use the available information needed to perform a specific task.
Late Effects on Task-related Processing N2/P3
Non-modality-specific target effects, those involving more widespread neural generators, associated with attention-based processing, were reflected in elicitation of the N2 and P3 components (Nieuwenhuis, Yeung, van den Wildenberg, & Ridderinkhof, 2003; Squires, Donchin, Squires, & Grossberg, 1977). The neural substrate of the target-related P3 component that gives rise to the scalp-recorded potential is thought to include both cortical (e.g., inferior parietal lobe, medial temporal lobe, and association areas of cortex) and subcortical (e.g., hippocampus) structures (Oken, 1997; Picton, 1992). N2 and P3 enhancement for go trials is well documented (P3: Comercho & Polich, 1999; Picton, 1992; N2: Patel & Azzam, 2005; Schröger, 2005; Nieuwenhuis et al., 2003; Hoffman, 1990; Sams, Alho, & Näätänen, 1983; Näätänen, Gaillard, & Mäntysalo, 1978). In the current study, N2 and P3 amplitudes were both enhanced by response requirements (on go trials) over central and parietal scalp locations (consistent with the typical scalp topography of each component), which would be the expected target N2 and P3 effects (Figure 4).
Figure 4.
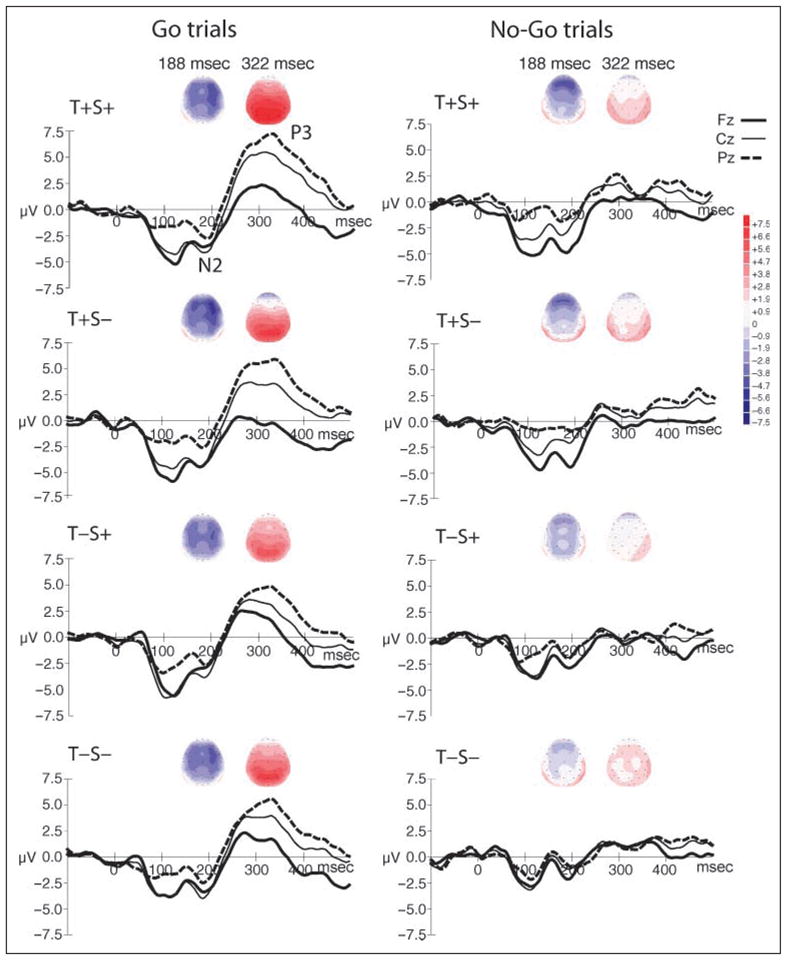
Spline-interpolated isovoltage maps of the grand-mean waveforms are shown separately for go and no-go trials, centered on the peak latency of the N2 and P3 components (188 and 322 msec, respectively) in each condition (T+S+, T+S−, T−S+, T−S−). Corresponding ERP graphs are displayed below the voltage maps, showing ERP waveforms from Fz (thick black solid line), Cz (thin black solid line), and Pz (thick black dashed line) electrodes. The y-axis shows the amplitude of the waveforms (+7.5 to −7.5 μV), which corresponds to the scale for the voltage maps in intensity of color (darker color for higher amplitude; red is positive polarity and blue is negative polarity). The N2 and P3 components are labeled in the top, left graph. The stronger frontal scalp distribution of N2 evoked when temporal expectation could be formed (T+S+, T+S−), which is observed in both go and no-go trials (left and right columns, blue color). The P3 component is clearly tied to response requirements, being elicited only in the go trials, with a strong focus at parietal electrodes (left column, red color).
Of interest in the current study were effects of expectation on the later, task-related processing stages. In this regard, N2 amplitude was modulated by temporal expectations, over more frontal scalp locations, with no influence of spatial expectations. Additionally, response requirements did not interact with expectation. That is, effects of response requirements were observed at centro-parietal sites and did not interact with temporal expectations, whereas effects of temporal expectation, observed at frontal sites, were not influenced by response requirements. Independent effects of response requirements and temporal expectations in the N2 range suggests that there are subcomponents of the N2 response, with different underlying neural generators, reflecting different aspects of target detection related to anticipation and to response requirements.
These results thus indicate that auditory target detection involves more than one attentional network interacting at different stages of the process. One involved in tracking (or searching for) the moving stimulus (i.e., the stimulus-driven characteristics directing attention to a time or place), and another in detecting and responding to the target stimulus. Frontal N2 may represent stimulus-driven effects, those in which the regularity of timing or spatial movement of the sound automatically engages the ventral fronto-parietal areas used in search tasks (Corbetta & Shulman, 2002), whereas N2 reflecting response requirements may involve a network including parietal structures more typically associated with target detection (Soltani & Knight, 2000).
Alternatively, the frontal N2 may be similar to that associated with the go/no-go aspect of the task. N2 elicitation has been associated with top–down inhibition of a response to no-go trials in lateral prefrontal cortex (Jonides, Smith, Marshuetz, Koeppe, & Reuter-Lorenz, 1998), operating prior to the motor response (Falkenstein, Hoormann, & Hohnsbein, 1999). In previous studies, using explicitly cued paradigms, N2 enhancement has been associated with inhibiting a response, being larger in amplitude for no-go versus go trials (Eimer, 1993; Kok, 1986). However, recently, it has also been shown that N2 may rather reflect conflict-monitoring by anterior cingulate cortex, in that the go/no-go task represents a conflict of responses (Nieuwenhuis et al., 2003). Nieuwenhuis et al. (2003) found that N2 was enhanced by infrequent stimuli in the go/no-go paradigm, regardless of their status. The current paradigm used only implicit cueing and the N2 no-go enhancement was not observed. Rather, we found larger N2 amplitude more frontally distributed for temporal expectations and centro-parietally distributed for go trials. There was a trend for a multiple interaction that would have indicated a larger frontal N2 amplitude for no-go response by temporal expectations, but this interaction did not reach significance after corrections for sphericity were applied. It is possible that the no-go inhibitory effect or reflection of conflict monitoring is more strongly observed in paradigms using explicit cues, not implicitly cued ones. The “conflict” may be derived prior to the actual target decision, possibly reflecting a conflict with the readiness state rather than the target itself.
Further, fronto-central distribution of N2 can also indicate generators within auditory cortex (Woods, 1990), whereas centro-parietal distribution indicates non-modality-specific generators. The frontally distributed temporal expectation effect on N2 may reflect processing related to the timing of auditory movement or to activity related to decision-making involving anterior cingulate cortex.
Late task-related processing, indicated by the P3 component, was largest over parietal electrodes in conditions with combined ability to anticipate spatial and temporal expectations (T+S+). This interaction was dominated by temporal expectations, as P3 amplitude was also larger in conditions with only temporal expectations (T+S−), compared to when participants could only use spatial expectations (T−S+) for target detection. Lange et al. (2003, 2006) also found P3 amplitude larger for temporal expectations, but not for spatial expectations, in a paradigm using explicit cueing, and suggested therefore that there are separate updating processes for spatial and temporal representations. In contrast, we found an interaction between temporal and spatial expectations at later stages reflected in P3 amplitude, which may be due to sound anticipation based on movement perception that encourages integration of spatial and temporal expectations in target detection. That is, in contrast to explicit cueing paradigms, it is possible that additional stimulus-driven expectations about stimulus location may be integrated to strengthen the effect of temporal stimulus expectations on processes that could ultimately be reflected in P3 amplitude. Our results are consistent with the notion that P3 is a sign of the integration of information from multiple brain areas to conscious target processing (Picton, 1992). Thus, for a moving stimulus, spatial and temporal expectations are used in combination to enhance target response. This would allow event processing based on multiple pieces of information, such as processes of evaluation completion (Verleger, 1988; Kutas, McCarthy, & Donchin, 1977), context updating (Donchin & Coles, 1988), or updating control processes (Metcalfe, 1994). These processes associated with the P3 component, involving generators within parietal cortex, are likely based on a more ubiquitous part of an attention control network (Coull & Nobre, 1998).
Conclusions
Results confirm a primary role of temporal orienting in audition. Temporal predictability alone modulated early perceptual stages (indexed by P1, N1) and, somewhat surprisingly, at early target detection stages of stimulus analysis (indexed by N2), whereas spatial predictability had no effect on target detection until later processes associated with responding to the target (P3). Only this latest stage (the target response phase) reflected synergistic effects of implicit spatial and temporal orienting. This is in contrast to the visual system, in which implicit expectations formed by temporal regularities in the movement of a visual object in space had influence at both early, perceptual (P1) and late, response-related processing stages of target detection (P3; Doherty et al., 2005). Thus, temporal predictability seems to play a greater role in early perceptual analysis in vision, than spatial orienting plays in modulating perceptual analysis in audition. However, in both modalities, temporal regularities can enhance target detection in early and late stimulus processing phases. Thus, implicit attention may be driven by the regularities extracted from the movement of the stimulus, which interacts with top–down processes and enhances target detection. This would engage both ventral and dorsal fronto-parietal areas involved in the search and detection task (Corbetta & Shulman, 2002), and could give rise to different components of N2, one with a frontal scalp distribution and one with a maximum over parietal scalp sites. Thus, the current results indicate that following a moving auditory stimulus engages multiple attentional networks, which is in line with the different phases of the search and detection process. In the early stages of stimulus analysis, implicit temporal expectations that arise from bottom–up, stimulus characteristics influence perceptual analysis and target detection, whereas in the later stages, spatial characteristics, specifically related to where the target would emerge, synergistically enhance the response phase of the task.
Acknowledgments
This research was supported by the Deutsche Forschungsge-meinschaft graduate program “Function of Attention in Cognition” (J. R.) and by the National Institutes of Health (DC004263, E. S.).
Footnotes
UNCITED REFERENCE
Kushnerenko, Čeponienė, Fellman, Huotilainen, & Winkler, 2001
References
- Buchtel HA, Butter CM. Spatial attentional shifts: Implications for the role of polysensory mechanisms. Neuropsychologia. 1988;26:499–509. doi: 10.1016/0028-3932(88)90107-8. [DOI] [PubMed] [Google Scholar]
- Comercho MD, Polich J. P3a and P3b from typical auditory and visual stimuli. Clinical Neurophysiology. 1999;110:24–30. doi: 10.1016/s0168-5597(98)00033-1. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Coull JT, Nobre AC. Where and when to pay attention: The neural systems for directing attention to spatial locations and to time intervals as revealed by both PET and fMRI. Journal of Neuroscience. 1998;18:7426–7435. doi: 10.1523/JNEUROSCI.18-18-07426.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty JR, Rao A, Mesulam MM, Nobre AC. Synergistic effect of combined temporal and spatial expectations on visual attention. Journal of Neuroscience. 2005;25:8259–8266. doi: 10.1523/JNEUROSCI.1821-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donchin E, Coles MGH. Precommentary: Is the P300 component a manifestation of context updating? Behavioral and Brain Sciences. 1988;11:355–441. [Google Scholar]
- Eimer M. Spatial cueing, sensory gating and selective response preparation: An ERP study on visuospatial orienting. Electroencephalography and Clinical Neurophysiology. 1993;88:408–420. doi: 10.1016/0168-5597(93)90017-j. [DOI] [PubMed] [Google Scholar]
- Eimer M. Mechanisms of visuospatial attention: Evidence from event-related brain potentials. Visual Cognition. 1998;5:257–286. [Google Scholar]
- Falkenstein M, Hoormann J, Hohnsbein J. ERP components in go/nogo tasks and their relation to inhibition. Acta Psychologica. 1999;10:267–291. doi: 10.1016/s0001-6918(99)00008-6. [DOI] [PubMed] [Google Scholar]
- Giard MH, Perrin F, Pernier J, Peronnet F. Several attention-related wave forms in auditory areas: A topographic study. Electroencephalography and Clinical Neurophysiology. 1988;69:371–384. doi: 10.1016/0013-4694(88)90008-9. [DOI] [PubMed] [Google Scholar]
- Green DM, Sweets JA. Signal detection theory and psychophysics. New York: Wiley; 1966. [Google Scholar]
- Hillyard SA, Picton TW. Electrophysiology of cognition. In: Plum F, editor. Handbook of physiology section 1: The nervous system. Volume V. Higher functions of the brain, part 2. Bethesda, MD: American Physiological Society; 1987. pp. 519–584. [Google Scholar]
- Hoffman JE. Event-related potentials and automatic and controlled processes. In: Rohrbaugh JW, Parasuraman R Jr, Johnson R, editors. Event related brain potentials. New York: Oxford University Press; 1990. pp. 145–157. [Google Scholar]
- Ivry RB. The representation of temporal information in perception and motor control. Current Opinion in Neurobiology. 1996;6:851–857. doi: 10.1016/s0959-4388(96)80037-7. [DOI] [PubMed] [Google Scholar]
- Jones MR. Time, our lost dimension: Toward a new theory of perception, attention, and memory. Psychological Review. 1976;83:323–355. [PubMed] [Google Scholar]
- Jonides J, Smith EE, Marshuetz C, Koeppe RA, Reuter-Lorenz PA. Inhibition in verbal working memory revealed by brain activation. Proceedings of the National Academy of Sciences, USA. 1998;95:8410–8413. doi: 10.1073/pnas.95.14.8410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok A. Effects of degradation of visual stimuli on components of the event-related potential (ERP) in go/nogo reaction tasks. Biological Psychology. 1986;23:21–38. doi: 10.1016/0301-0511(86)90087-6. [DOI] [PubMed] [Google Scholar]
- Kushnerenko E, Čeponienė R, Fellman V, Huotilainen M, Winkler I. Event-related potential correlates of sound duration: Similar pattern from birth to adulthood. NeuroReport. 2001;12:3777–3781. doi: 10.1097/00001756-200112040-00035. [DOI] [PubMed] [Google Scholar]
- Kutas M, McCarthy G, Donchin E. Augmenting mental chronometry: The P300 as a measure of stimulus evaluation time. Science. 1977;197:792–795. doi: 10.1126/science.887923. [DOI] [PubMed] [Google Scholar]
- Lange K. Brain correlates of early auditory processing are attenuated by expectations for time and pitch. Brain and Cognition. 2009;69:127–137. doi: 10.1016/j.bandc.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Lange K, Krämer UM, Röder B. Attending points in time and space. Experimental Brain Research. 2006;173:130–140. doi: 10.1007/s00221-006-0372-3. [DOI] [PubMed] [Google Scholar]
- Lange K, Röder B. Orienting attention to points in time improves stimulus processing both within and across modalities. Journal of Cognitive Neuroscience. 2006;18:715–729. doi: 10.1162/jocn.2006.18.5.715. [DOI] [PubMed] [Google Scholar]
- Lange K, Rösler F, Röder B. Early processing stages are modulated when auditory stimuli are presented at an attended moment in time: An event-related potential study. Psychophysiology. 2003;40:806–817. doi: 10.1111/1469-8986.00081. [DOI] [PubMed] [Google Scholar]
- Liégeois-Chauvel C, Musolino A, Badier JM, Marquis P, Chauvel P. Evoked potentials recorded from the auditory cortex in man: Evaluation and topography of the middle latency components. Electroencephalography and Clinical Neurophysiology. 1994;92:204–214. doi: 10.1016/0168-5597(94)90064-7. [DOI] [PubMed] [Google Scholar]
- Macmillan NA, Creelman CD. Detection theory: A user’s guide. 2. Mahwah, NJ: Erlbaum; 2005. [Google Scholar]
- Mangun GR. Neural mechanisms of visual selective attention. Psychophysiology. 1995;32:4–18. doi: 10.1111/j.1469-8986.1995.tb03400.x. [DOI] [PubMed] [Google Scholar]
- Mauk MD, Buonomano DV. The neural basis of temporal processing. Annual Review of Neuroscience. 2004;27:307–340. doi: 10.1146/annurev.neuro.27.070203.144247. [DOI] [PubMed] [Google Scholar]
- McEvoy LK, Picton TW, Champagne SC. Effects of stimulus parameters on human evoked potentials to shift in the lateralization of a noise. Audiology. 1991;30:286–302. doi: 10.3109/00206099109072892. [DOI] [PubMed] [Google Scholar]
- McMillan NA, Creelman CD. Detection theory: A user’s guide. Cambridge, UK: Cambridge University Press; 1991. [Google Scholar]
- Middlebrooks JC, Green DM. Sound localization by human listeners. Annual Review of Psychology. 1991;42:135–159. doi: 10.1146/annurev.ps.42.020191.001031. [DOI] [PubMed] [Google Scholar]
- Mondor TA, Zatorre RJ. Shifting and focusing auditory spatial attention. Journal of Experimental Psychology: Human Perception and Performance. 1995;21:387–409. doi: 10.1037//0096-1523.21.2.387. [DOI] [PubMed] [Google Scholar]
- Moore BCJ. An introduction to the psychology of hearing. 4. San Diego, CA: Academic Press; 1997. [Google Scholar]
- Näätänen R, Gaillard AWK, Mäntysalo S. Early selective attention effect on evoked potential reinterpreted. Acta Psychologica. 1978;42:313–329. doi: 10.1016/0001-6918(78)90006-9. [DOI] [PubMed] [Google Scholar]
- Näätänen R, Picton T. The N1 wave of the human electric and magnetic response to sound: A review and an analysis of the component structure. Psychophysiology. 1987;24:375–425. doi: 10.1111/j.1469-8986.1987.tb00311.x. [DOI] [PubMed] [Google Scholar]
- Niemi P, Näätänen R. Foreperiod and simple reaction time. Psychological Bulletin. 1981;89:133–162. [Google Scholar]
- Nieuwenhuis S, Yeung N, van den Wildenberg W, Ridderinkhof KR. Electrophysiological correlates of anterior cingulate function in a go/no-go task: Effects of response conflict and trial type frequency. Cognitive, Affective & Behavioral Neuroscience. 2003;3:17–26. doi: 10.3758/cabn.3.1.17. [DOI] [PubMed] [Google Scholar]
- Nobre AC. Probing the flexibility of attentional orienting in the human brain. In: Posner MI, editor. Cognitive neurosciences of attention. New York: The Guilford Press; 2004. pp. 157–179. [Google Scholar]
- Oken BS. Endogenous evoked potentials. In: Chiapa KH, editor. Evoked potentials in clinical medicine. 3. Philadelphia: Lippincott-Raven; 1997. pp. 529–563. [Google Scholar]
- Patel SA, Azzam PN. Characterization of N200 and P300: Selected studies of the event-related potential. International Journal of Medical Sciences. 2005;2:147–154. doi: 10.7150/ijms.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picton TW. The P300 wave of the human event-related potential. Journal of Clinical Neurophysiology. 1992;9:456–479. doi: 10.1097/00004691-199210000-00002. [DOI] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. Quarterly Journal of Empirical Psychology. 1980;32:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Posner MI, Snyder CR, Davidson BJ. Attention and the detection of signals. Journal of Experimental Psychology. 1980;109:160–174. [PubMed] [Google Scholar]
- Sams M, Alho K, Näätänen R. Sequential effects on the ERP in discriminating two stimuli. Biological Psychology. 1983;17:41–58. doi: 10.1016/0301-0511(83)90065-0. [DOI] [PubMed] [Google Scholar]
- Schröger E. The mismatch negativity as a tool to study auditory processing. Acta Acustica. 2005;91:490–501. [Google Scholar]
- Snodgrass JG. Foreperiod effects in simple reaction time: Anticipation or expectancy? Journal of Experimental Psychology. 1969;79:1–19. doi: 10.1037/h0026893. [DOI] [PubMed] [Google Scholar]
- Soltani M, Knight RT. Neural origins of the P300. Critical Reviews in Neurobiology. 2000;14:199–224. [PubMed] [Google Scholar]
- Spence CJ, Driver J. Covert spatial orienting in audition: Exogenous and endogenous mechanisms. Journal Experimental Psychology: Human Perception and Performance. 1994;20:555–574. [Google Scholar]
- Spence CJ, Driver J. Audiovisual links in endogenous covert spatial attention. Journal Experimental Psychology: Human Perception and Performance. 1996;22:1005–1030. doi: 10.1037//0096-1523.22.4.1005. [DOI] [PubMed] [Google Scholar]
- Squires NK, Donchin E, Squires KC, Grossberg S. Bisensory stimulation: Inferring decision-related processes from P300 component. Journal Experimental Psychology: Human Perception and Performance. 1977;3:299–315. doi: 10.1037//0096-1523.3.2.299. [DOI] [PubMed] [Google Scholar]
- Tiitinen H, Sivonen P, Alku P, Virtanen J, Näätänen R. Electromagnetic recordings reveal latency differences in speech and tone processing in humans. Cognitive Brain Research. 1999;8:355–363. doi: 10.1016/s0926-6410(99)00028-2. [DOI] [PubMed] [Google Scholar]
- Verleger R. Event-related potentials and cognition: A critique of the context updating hypothesis and an alternative interpretation of P3. Behavioral and Brain Sciences. 1988;11:343–427. [Google Scholar]
- Woldorff MG, Hillyard SA. Modulation of early auditory processing during selective listening to rapidly presented tones. Electroencephalography and Clinical Neurophysiology. 1991;79:170–191. doi: 10.1016/0013-4694(91)90136-r. [DOI] [PubMed] [Google Scholar]
- Woods DL. The physiological basis of selective attention: Implications of event-related potential studies. In: Rohrbaugh JW, Parasuraman R Jr, Johnson R, editors. Event-related brain potentials. Basic issues and applications. New York: Oxford University Press; 1990. pp. 178–209. [Google Scholar]
- Yvert B, Fischer C, Bertrand O, Pernier J. Localization of human supratemporal auditory areas from intracerebral auditory evoked potentials using distributed source models. Neuroimage. 2005;28:140–153. doi: 10.1016/j.neuroimage.2005.05.056. [DOI] [PubMed] [Google Scholar]