Abstract
Advances in liquid chromatography-mass spectrometry (LC-MS) can be used to measure steroid hormone metabolites in vitro and in vivo. We find that LC-Electrospray Ionization (ESI)-MS using a LCQ ion trap mass spectrometer in the negative ion mode can be used to monitor the product profile that results from 5α–dihydrotestosterone(DHT)-17β-glucuronide, DHT-17β-sulfate, and tibolone-17β-sulfate reduction catalyzed by human members of the aldo-keto reductase (AKR) 1C subfamily and assign kinetic constants to these reactions. We also developed a stable-isotope dilution LC-electron capture atmospheric pressure chemical ionization (ECAPCI)-MS method for the quantitative analysis of estrone (E1) and its metabolites as pentafluorobenzyl (PFB) derivatives in human plasma in the attomole range. The limit of detection for E1-PFB was 740 attomole on column. Separations can be performed using normal-phase LC because ionization takes place in the gas phase rather than in solution. This permits efficient separation of the regioisomeric 2- and 4-methoxy-E1. The method was validated for the simultaneous analysis of plasma E2 and its metabolites: 2-methoxy-E2, 4-methoxy-E2, 16α-hydroxy-E2, estrone (E1), 2-methoxy-E1, 4-methoxy-EI, and 16α-hydroxy-E1 from 5 pg/mL to 2,000 pg/mL. Our LC-MS methods have sufficient sensitivity to detect steroid hormone levels in prostate and breast tumors and should aid their molecular diagnosis and treatment.
Keywords: steroid conjugates, catechol estrogens, electron capture atmospheric pressure chemical ionization, normal phase HPLC
1. Introduction
Radioimmunoassay or ELISA based methods were once considered state-of-the art methods for measuring steroid metabolites in biospecimens. These approaches now appear to be fraught with difficultly. First, they can only measure one analyte at a time, thus multiple assays are required to measure all the metabolites from a single steroid hormone. Second, steroid metabolites have highly related structures and in a biospecimen mixtures of stereoisomers, regioisomers or compounds that differ by only the substitution of a carbonyl group for an alcohol exist. It is thus not possible to control for interference in the immunoassay from both known and unknown structurally related steroids that may be present in the biological matrix. Third, these immunological approaches do not give any structural validation of the analyte. Fourth, in many instances antisera do not exist for all the steroid metabolites of interest to allow immunodetection in the first place. This is certainly true for the detection of conjugated steroids.
The reliability of radioimmunoassays for steroid hormones have also been questioned by position papers which have documented the large inter-laboratory variability that exists in measuring plasma testosterone [1–3] and the difficulty in measuring 17β-estradiol (E2) and its metabolites in plasma and urine [4–6]. In contrast, gas chromatography-mass spectrometry (GC-MS) coupled with stable isotope dilution methodology is sensitive, specific, and accurate and has been used for the quantitative analysis of steroid hormones in biological samples such as urine and plasma [7, 8]. Unfortunately this method requires extremely tedious extraction and derivatization procedures for each sample. However, when used in conjunction with electron capture negative chemical ionization and tandem MS, very low detection limits can be obtained for plasma E2 (0.0.63 pg/mL] [9]. A further drawback of the GC-MS methods is that, steroid conjugates cannot be analyzed directly. LC-MS can now circumvent many of these problems. We have developed negative ion LC-ESI/MS in order to analyze multiple steroid conjugates directly. We demonstrate the utility of the method by showing that it can be used to conduct product profiling of the enzymatic reduction of endogenous DHT-conjugates and conjugates derived from the hormone replacement therapeutic tibolone catalyzed by members of the aldo-keto reductase (AKR) 1C subfamily.
By contrast, LC-ESI/MS of underivatized estrogens are relatively insensitive in both positive and negative ionization modes so it cannot be used to determine the concentrations of estrogens and their metabolites which are present in the low pg/mL range [10]. To circumvent this problem, we have developed stable isotope dilution LC-ECAPCI/MS methodology, which can detect estrogen PFB derivatives in the attomol range on column [10]. This has made it possible to quantify multiple estrogen metabolites with high sensitivity in the same chromatographic run. We demonstrate the utility of this approach by analyzing E1 and E2, together with their corresponding 2- and 4-methoxy and 17α-hydroxy metabolites in plasma. These methods can be adapted to measure targeted steroid metabolomes within prostate and breast tumor biopsy samples.
2. Methods
2. 1. Materials
DHT, DHT-17β-glucuronide (DHTG), DHT-17β-Sulfate (DHTS), 3α-hydroxy-5α-androstane-17β-glucuronide (3α-Diol-17G), and 3β-hydroxy-5α-androstane-17-sulfate (3β-Diol-17-S) were obtained from Steraloids (Wilton, NH, U.S.A.). The latter compound was custom synthesized by Steraloids. E2, E1, 2-methoxy-E2, 4-methoxy-E2, 16α-hydroxy-E2, 2-methoxy-E1, 4-methoxy-E1, and 16α-hydroxy-E1 were obtained from Steraloids Inc. (Newport, RI). 16,16,17-[2H3]-E2, 2,4,17-[2H3]-16α-hydroxy-E2, and 2,4,16,16-[2H4]-E1 were obtained from C/D/N Isotopes (Pointe-Claire, Quebec, Canada). [2H3]-2-methoxy-E2, [2H3]-4-methoxy-E2, [2H3]-2-methoxy-E1, [2H3]-4-methoxy-E1, and 2,4,16-[2H3]-E2 were synthesized using standard procedures from [2H3]-methyl iodide (C/D/N Isotopes) and unlabeled E2 or E1. 2,4,16-[2H3]-16α-hydroxy-E1 was prepared by sodium borodeuteride/PdCl2 (Sigma-Aldrich, St. Louis, MO) reduction in CH3OD (Sigma-Aldrich) of 2,4-dibromo-17, 17-ethylenedioxy-1,3,5(10)-estratriene-3,16α-diol (prepared by standard procedures from estrone) followed by acid hydrolysis. Tibolone (Tib), Tib-17β-Sulfate (TibS), 3α- and 3β-hydroxy-Tib (3α- and 3β-OH-Tib), and 3α- and 3β-OH-Tib-17S were provided by N.V. Organon (Oss, Netherlands). Pentafluorobenzyl bromide (R-bromo-2,3,4,5,6-pentafluorotoluene) was obtained from Sigma-Aldrich Chemical Co. (Milwaukee,WI). Pyridine nucleotides were purchased from Roche Applied Science (Indianapolis, IN, USA). HPLC grade water, Optima grade acetonitrile, methanol, hexane, isopropanol, water, ethyl acetate, and potassium bromide were purchased from Fisher Scientific Co.(Fair Lawn, NJ). Ethanol was from Pharmco (Brookfield, CT). Ammonium acetate was obtained from J. T. Baker (Phillipsburg, NJ). Heparinized male human plasma was from Biological Specialty Corporation (Colmar, PA). All other reagents were purchased from Sigma-Aldrich and were of ACS (American Chemical Society) grade or better. Recombinant AKR1C enzymes were over-expressed and purified to homogeneity as previously described [11].
2.2. Identification of Steroid Conjugates Produced by AKRs using LC-MS
Products formed during the reduction of conjugated steroids catalyzed by AKR1C enzymes were prepared for LC-MS analyses as follows. Reaction mixtures contained 100 mM potassium phosphate buffer (pH 7.0), steroid (36 μM DHTG, 45 μM DHTS or TibS), 0.5 mM NADPH, and 4% methanol in 450 μl of total volume. The reaction was initiated by the addition of purified enzyme (buffer for no-enzyme control, 13–33 μg of AKR1C1–AKR1C4) and incubated for 0–90 min at 37 °C. Reaction mixtures were extracted twice with 1.5 mL water-saturated ethyl acetate. The pooled organic extracts were vacuum dried and the residues were re-dissolved in 200 μl 50% methanol.
2.3. LC Separation of Steroid Conjugates
Chromatography was performed using a Waters Alliance 2690 HPLC system (Waters Corporation, Milford, MA) coupled to the mass spectrometer. For DHTG and DHTS, a SunFire C8 column (4.6 × 150 mm, 3.5 μm Waters), total run time 30 min was employed. Solvent A was 5 mM aqueous ammonium acetate in water, and solvent B was 5 mM ammonium acetate in acetonitrile. The linear gradient used was as follows: 30% solvent B at 0 and 3 min, 50% solvent B at 13 min, 80% solvent B at 14 and 19 min, and 30% solvent B at 20 and 30 min with a flow rate of 0.3 mL/min. For TibS, a Jupiter C18 column (2.0 × 150 mm, 5 μm, Phenomenex, Torrance, CA) total run time 22 min was employed. Solvent A was 5 mM aqueous ammonium acetate, and solvent B was 5 mM ammonium acetate in acetonitrile. The linear gradient was as follows: 20% B at 0 and 2 min, 30% B at 8 and 12 min, and 20% B at 14 min with a flow rate of 0.5 mL/min. All separations were performed at ambient temperature.
2.4. MS of Steroid Conjugates
Analyses were conducted using an LCQ ion trap mass spectrometer (Thermo Fisher, San Jose, CA) equipped with an electrospray ionization source. The mass spectrometer was operated in the negative ion mode with a potential of 4.5 kV applied to the electrospray ionization needle. Operating conditions for DHTG and DHTS were as follows: heated capillary temperature 220 °C, capillary voltage − 4 V, tube lens offset 10 V, nitrogen was used for the sheath gas at 80 psi, and for the auxiliary at 10 (arbitrary units). Operating conditions for TibS were as follows: heated capillary temperature 230 °C, capillary voltage −23 V, tube lens offset −25 V, nitrogen was used for the sheath gas at 50 psi, and for the auxiliary gas at 30 (arbitrary units). Full scanning analyses were performed in the range of m/z 100 to m/z 600. Products of the reactions were identified based on their LC retention times and mass spectra relative to those observed with the authentic standards. The molecular ions monitored for the steroid conjugates were as follows: DHTG [M-H−; m/z = 465]; 3α- and 3β-Diol-17G [M-H−; m/z = 467]; DHTS [M-H−; m/z = 369]; 3α-and 3β-Diol-17S [M-H−; m/z = 371]; TibS [M-H−; m/z = 391.5]; 3α- and 3β-OH-TibS [M-H−; m/z = 393.5].
2.5. LC Separation of Estrogen PFB Derivates
Chromatographic separation of the estrogen PFB derivatives was conducted using a YMC Silica column (250 mm × 4.6 mm i.d., 5 μm particle size, 120 Å pore size, Waters) heated to 40 °C. Solvent A was hexane and solvent B was isopropanol/hexane (30:70, v/v). The LC conditions were as follows: 2 % solvent B at 0 and 2 min, 10 % solvent B at 5 min, 20 % solvent B at 12 min, 50 % solvent B at 22 min, and 24 min, 2 % solvent B at 28 min and 35 min with a flow rate of 0.9 mL/min. A post-column flow-rate of 0.5 mL/min was used to prevent the source from becoming contaminated with carbon deposits. Pre-column filters (2 μm; Alltech, Deerfield, IL) were used to protect the column from particulate matter.
2.6. ECAPCI of Estrogen PFB Derivates
A TSQ 7000 (Thermo Fisher) mass spectrometer was operated in the APCI negative ion mode under the following conditions: vaporizer temperature 500 °C, heated capillary temperature 200 °C, corona needle discharge current 15 μA, sheath gas pressure (nitrogen) 20 psi, auxiliary gas (nitrogen) 15 (arbitrary units). The parent and daughter resolution settings were of 0 V for the methoxy compounds and 10 V for all other analytes and internal standards. The [M-PFB]− ions of the analytes were filtered through the first quadrupole, and collision-induced dissociation (CID) was performed using argon as the collision gas at 3.0 mTorr in the second (rf-only) quadrupole. Product ions were then detected in the third quadrupole. Collision energies were optimized for each analyte, and ranged from 25 eV to 45 eV (Table 3). MRM transitions were performed as shown in Table 3.
Table 3.
LC-ECAPCI/MRM/MS Analysis of Estradiol and its Metabolites
| Estrogen | [M-PFB] parent ion (m/z) | Product ion (m/z) | Collision energy (eV) | Retention time (min) |
|---|---|---|---|---|
| estradiol (E2) | 271 | 183 | 43 | 17.4 |
| [2H3]-estradiol (E2-D3) | 274 | 185 | 43 | 17.4 |
| 2-methoxy-E2 (2-MeO-E2) | 301 | 286 | 28 | 17.7 |
| [2H3]-2-methoxy-E2 (2-MeO-E2-D3) | 304 | 286 | 28 | 17.7 |
| 4-methoxy-E2 (4-MeO-E2) | 301 | 286 | 28 | 18.5 |
| [2H3]-4-methoxy-E2 (4-MeO-E2-D3) | 304 | 286 | 28 | 18.5 |
| 16α-hydroxy-estradiol (16α-OH-E2) | 287 | 171 | 40 | 22.6 |
| [2H4]-16α-hydroxy-estradiol (16α-OH-E2-D4) | 291 | 173 | 40 | 22.7 |
| Estrone (E1) | 269 | 145 | 45 | 6.95 |
| [2H4]-estrone (E1-D4) | 273 | 147 | 45 | 6.96 |
| 4-methoxy-E1 (4-MeO-E1) | 299 | 284 | 28 | 7.39 |
| [2H3]-4-methoxy-E1 (4-MeO-E1-D3) | 302 | 284 | 28 | 7.40 |
| 2-methoxy-E1 (2-MeO-E1) | 299 | 284 | 28 | 8.37 |
| [2H3]-2-methoxy-E1 ([2-MeO-E1-D3) | 302 | 284 | 28 | 8.44 |
| 16α-hydroxy-estrone (16α-OH-E1) | 287 | 171 | 40 | 22.6 |
| [2H4]-16α-hydroxy-estrone (16α-OH-E1-D4) | 291 | 173 | 40 | 22.7 |
2.7. Sample Preparation for LC-ECAPCI/MS Analysis
Heparinized male human plasma was allowed to thaw. Plasma aliquots (1 mL) were transferred to 10 mL glass centrifuge tubes. An aliquot (10 μL) of analyte solution of the appropriate concentration was added to each sample using a dedicated microsyringe, followed by vortex mixing. This was followed by an aliquot (10 μL; 4 ng) of internal standard solution, which was added using another dedicated microsyringe. Samples were vortex mixed for 1 min, and 4 mL of ethyl acetate was added. This mixture was mechanically shaken for 1 h, followed by centrifugation at 4000 RPM for 5 min. The samples were then placed at −20 °C and the lower phase was allowed to freeze, after which the supernatant was transferred by decantation into a new 5 mL glass centrifuge tube and evaporated to dryness under nitrogen at 37 °C using an N-Evap Analytical Evaporator (Organomation, Berlin, MA). Methanol (200 μL) was added to the dried samples, followed by vortex mixing for 2 min. Water (800 μL) was then added, and the samples were vortex-mixed again. The reconstituted solution was then placed on a C18 solid phase extraction (SPE) cartridge (7mm/3mL Empore C18 Standard Density Extraction Disk Cartridges, 3M, St. Paul, MN), which had been pre-conditioned with 0.5 mL of methanol and 0.5 mL of water. This cartridge was placed inside a 15 mL plastic centrifuge tube (Fisher Scientific). All samples and washes were eluted through the SPE cartridge by centrifugation at 1800 RPM for 3 min. The cartridge was washed sequentially with 1 mL of water and 0.3 mL of methanol/water (20:80, v/v). The cartridges were then transferred to a new 15 mL plastic centrifuge tube and the analytes were eluted with 0.3 mL of methanol. The resulting eluate was transferred to a 1.8 mL plastic centrifuge tube (Fisher Scientific), and evaporated to dryness under nitrogen at 37 °C.
2.8. PFB Derivatization
After drying, samples were reconstituted in 100 μl of acetonitrile, and were treated with 100 μl of pentafluorobenzyl bromide in acetonitrile (1:19, v/v), followed by 100 μl of ethanolic potassium hydroxide (2:100, w/v). After vortex mixing, samples were allowed to react at room temperature for 15 min, evaporated to dryness under nitrogen, and reconstituted in 200 μl of isopropanol/hexane (2:98, v/v), 90 μl of which was injected on the LC.
2.9. Data Analysis
Calibration curves ranged from 5 pg/mL to 1,000 pg/mL For each curve, 8 different concentrations, distributed over the entire concentration range, were used. Peak height ratios between the analytes and their respective internal standard were calculated from each sample using Finnigan LCquan version 1.2 software. The data were fit to a linear least-squares regression curve with a weighting index of 1/x. A water blank, plasma blank, and plasma blank spiked with internal standard were also processed with each calibration curve.
2. 10. Accuracy and Precision
Five replicates of each quality control (QC) sample at each concentration level were processed and analyzed along with the 8 standard curve samples. The lower quality control sample (LQC), middle quality control sample (MQC), and high quality control sample (HQC) were analyzed on three separate days, while the lower limit of quantitation (LLOQ) samples were analyzed on 1 day only. Assay accuracy was assessed by comparing means of the measured analyte concentrations with the theoretical concentrations in the QC samples. Within-day precision was expressed as percent relative standard deviation (% RSD) and was obtained by calculating the percent ratio between the relative standard deviation of five replicates (n = 5) and their mean at each concentration within the same validation run. Inter-day precision, defined as percent relative standard deviation (% RSD) of three different validation runs (n = 3) was also assessed.
2. 11. Determination of steady state kinetic parameters
Initial rates of the NADPH dependent reduction of ketosteroids and their conjugates catalyzed by AKR1C were measured using a Hitachi F-2500 fluorescence spectrophotometer (Hitachi America, Ltd.; New York, NY) by monitoring the change in fluorescence emission of NADPH. Excitation and emission wavelengths were set at 340 nm and 450 nm, respectively. Changes in fluorescence units were converted to nanomoles of cofactor by using standard curves of fluorescence emission versus known NADPH concentrations. Data were analyzed by nonlinear least-squares fitting to the equation,
where υ is the initial velocity, [E] and [S] are the total molar concentrations of the enzyme and steroid substrate, respectively, kcat (s−1) is the turnover number, and Km (μM) is the apparent Michaelis-Menten constant for the steroid substrate.
3. Results
3. 1. Metabolism of Endogenous Steroid Conjugates by Aldo-Keto Reductase (AKR) 1C Subfamily Enzymes
The four aldo-keto reductase (AKR) 1C subfamily members found in humans (AKR1C1–AKR1C4) have been shown to catalyze the NADPH dependent reduction of 3-ketosteroids to yield 3α- or 3β-hydroxysteroids with different stereochemical preferences based on steroid substrate. For example, AKR1C2 is predominately a 3α-HSD with DHT; while AKR1C1 is predominately a 3β-HSD with the same substrate [12]. By contrast the same two enzymes convert tibolone (a hormone replacement therapeutic pro-drug) only to its 3β-hydroxysteroid (estrogenic metabolite) form [13]. Several other human AKRs, not involved in steroid metabolism, were shown to catalyze the reduction of glutathionyl conjugates of 4-hydroxy-2-nonenal [14]. These observations led us to ask whether human AKR1C1–1C4 isoforms were capable of metabolizing steroid conjugates e.g. glucuronides and sulfates. This required the development of LC-ESI/MS methods using negative ionization for product profiling so that kinetic constants could be assigned to the respective reactions with confidence.
The LC-ESI/MS method for detecting steroid conjugates was validated as follows: Linear standard curves were obtained in the range 0.5 μg/mL to 50 μg/mL in the reaction buffer for steroid conjugates (data not shown). Replicate determinations (n=3) of quality control samples were conducted at the low concentration (1.0 μg/mL), mid-range concentration (25 μg/mL), and high concentration (50 μg/mL) on three separate days. The intra- and inter-day accuracy of all quality control samples was within 100 ± 15 % of theoretical with a precision (coefficient of variation) better than 15 %.
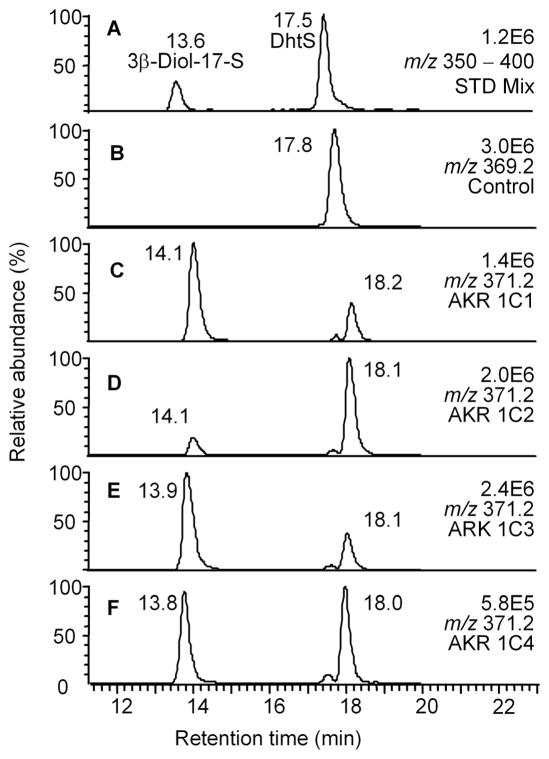
Using DHTG as substrate LC-ESI/MS product profiling showed that AKR1C1–AKR1C3 all converted this substrate to 3β-Diol-17G (RT =7.4 min); while AKR1C4 converted DHTG to 3α-Diol-17G (RT= 12.6 min), Figure 1. An authentic synthetic standard only existed for 3α-Diol-17G but the 3α-isomer was positively identified based on its molecular ion which is identical to that for the 3β-isomer and its retention time (see below). DHTS was next tested as substrate for the AKR1C1-AKR1C4 isoforms. LC-ESI-MS product profiling showed that the product profile for the sulfate conjugate was identical to that observed for free DHT. Thus AKR1C1 produced 3β-Diol-17S (RT 13.6 min); AKR1C2 produced 3α-Diol-17S (RT 18.1 min); and AKR1C3 and AKR1C4 produced mixtures of the 3α- and 3β-isomers, Figure 2. An authentic synthetic standard only existed for 3β-Diol-17S but the 3α-isomer was positively identified based on its molecular ion which is identical to that for the 3β-isomer. Moreover, the elution order in the ion-chromatogram for the glucuronide and sulfate conjugate products was the same: 3β-isomer > parent conjugate > 3α-isomer. The unexpected findings are that both glucuronide and sulfate conjugates could be reduced by AKR1C enzymes. Moreover, with DHTG and DHTS there was an inversion of stereochemical preference for AKR1C2 producing a 3β-isomer with the first substrate and a 3α-isomer with the second substrate.
Fig. 1.
LC-MS analysis of the reduction of DHTG catalyzed by human AKR1C isoforms. The ion chromatogram (m/z 450–500) of a mixture of authentic standards of DHTG and 3α-Diol-17-G; B through F, corresponding ion chromatograms of reaction samples containing no enzyme (B) and AKR1C1–4 (C–F). Samples were prepared as described in the experimental section. Reproduced with permission from the American Society of Biochemists and Molecular Biologists.
Fig. 2.
LC-MS analysis of the reduction DHTS catalyzed by human AKR1C isoforms. The ion chromatogram (m/z 350–400) of a mixture of authentic standards of DHTS and 3β-Diol-17-S; B through F, corresponding ion chromatograms of reaction samples containing no enzyme (B) and AKR1C1–4 (C–F). Samples were prepared as described in the experimental section. Reproduced with permission from the American Society of Biochemists and Molecular Biologists.
Assignment of catalytic efficiencies (kcat/Km) to these reactions for each enzyme isoform were then made based on fluorimetric assays which monitored the concurrent consumption of NADPH associated with the appearance of products followed by LC-ESI-MS, Table 1. It was also found that the kcat/Km values for the reduction of DHT and DHTG for AKR1C4 were unaffected by the presence of the glucuronide group but were depressed for the reduction of DHTG by AKR1C1, and AKR1C2. It was also found that the kcat/Km values for the reduction of DHT and DHTS for AKR1C1, AKR1C2 and AKR1C4 were unaffected by the presence of the sulfate group. This led to the conclusion that AKR1C enzymes can work equally well on conjugated as well as free DHT [15].
Table 1.
Kinetic Parameters for the Reduction of DHT, Tib and their ConjugatesCatalyzed by AKR1C Isoforms.
| AKR1C1 | AKR1C2 | AKR1C4 | ||
|---|---|---|---|---|
| DHT | kcat (Min−1) | 0.74 ± 0.03 | 1.98 ± 0.12 | 3.08 ± 0.15 |
| Km (μM) | 4.2 ± 0.5 | 2.9 ± 0.2 | < 0.2 | |
| kcat/Km (Min−1M−1) | 1.8 × 105 | 6.8 × 105 | > 1.5 × 107 | |
| DHTG | kcat (Min−1) | 0.53 ± 0.03 | 0.24 ± 0.03 | 3.28 ± 0.08 |
| Km (μM) | 16.1 ± 2.4 | 4.1 ± 1.2 | < 0.2 | |
| kcat/Km (Min−1M−1) | 3.3 × 104 | 5.9 × 104 | > 1.6 × 107 | |
| DHTS | kcat (Min−1) | 0.75 ± 0.05 | 1.0 ± 0.1 | 3.07 ± 0.07 |
| Km (μM) | 5.2 ± 0.8 | 4.2 ± 0.5 | < 0.2 | |
| kcat/Km (Min−1M−1) | 1.4 × 105 | 2.4 × 105 | > 1.5 × 107 | |
| Tib | kcat (Min−1) | 0.88 ± 0.06 | 12.7 ± 3.7 | 1.83 ± 0.06 |
| Km (μM) | 0.76 ± 0.13 | 0.87 ± 0.34 | 1.02 ± 0.07 | |
| kcat/Km (Min−1M−1) | 1.2 × 106 | 1.4 × 107 | 1.8 × 106 | |
| Ki (μM) | - | 0.7 ± 0.29 | - | |
| TibS | kcat (Min−1) | 1.9 ± 0.3 | 12.1 ± 2.7 | 1.20 ± 0.03 |
| Km (μM) | 2.5 ± 0.28 | 1.2 ± 0.5 | 0.5 ± 0.1 | |
| kcat/Km (Min−1M−1) | 7.6 × 105 | 1.0 × 107 | 2.4 × 106 | |
| Ki (μM) | - | 2.1 ± 0.6 | - |
Steady state parameters determined by fluorimetric assay. Values are given as means ± SD (n>2). Values for AKR1C3 catalyzed reactions were not determined due to low turnover.
3. 2. Metabolism of Conjugates of Synthetic Steroids by Aldo-Keto Reductase (AKR)1C Subfamily Enzymes
We extended our studies on the reduction of steroid conjugates to include therapeutically relevant steroids. Tibolone is a hormone replacement therapeutic and pro-drug [16, 17]. It has been previously shown that AKR1C isoforms catalyze the reduction of tibolone to its 3α- and 3β-hydroxymetabolites [13, 18]. These reactions are important since these are the estrogenic metabolites of the pro-drug and their tissue specific formation may contribute to the tissue selective estrogen properties of Tibolone. By contrast TibS, 3α-OH-TibS and 3β-OH-TibS are the major inactive metabolites of Tibolone [19].
The ability of AKR1C1–AKR1C4 isoforms to reduce TibS was therefore examined. Incubation with recombinant AKR1C1–AKR1C4 followed by product profiling by LC-ESI/MS in the negative ion mode showed that AKR1C1–AKR1C3 all reduced TibS to 3β-OH-TibS (RT =9.8 min). By contrast AKR1C4 reduced TibS to a mixture of the 3α- and 3β-hydroxy isomers (RT 7.0 and 9.9 mins, respectively) Figure 3. Identification of the products was unambiguous since authentic synthetic standards were available which showed identical RT and negative molecular ions. Assignment of catalytic efficiencies to these reactions was again performed by conducting fluorimetric assays which measured the consumption of NADPH, Table 1. These assays revealed that the reduction of TibS occurred with similar catalytic efficiencies to those reported for the reduction of tibolone by each AKR1C isoform [12].
Fig. 3.
LC-MS analysis of the reduction of TibS catalyzed by human AKR1C isoforms. A, The ion chromatogram (m/z 200–400) of a mixture of authentic standards of TibS, 3α-OH-TibS, and 3β-OH-TibS; B through F, corresponding ion chromatograms of reaction samples containing no enzyme (B) and AKR1C1–4 (C–F). Samples were prepared as described in the experimental section.
Reproduced with permission from the American Society of Biochemists and Molecular Biologists.
3.3. Analysis of Estrogens by LC-ECAPCI/MS
Increased risk to breast cancer is associated with life-time exposure to estrogens. Some estrogen metabolites are thought to be genotoxic e.g. 4-hydroxy-17β-estradiol (4-OHE2) while others are thought to be non-genotoxic and anti-proliferative e.g. 2-hydroxy-17β-estradiol (2-OHE2) [20–23]. The balance between these competing metabolic pathways may be an important determinant of breast cancer risk, yet the immunochemical methods available for quantifying endogenous E2 and its metabolites in plasma have proved to be controversial [4–6]. Stable isotope LC-MS-based methods can potentially resolve problems in estrogen quantification through the rigorous application of stable isotope dilution methodology. Unfortunately, underivatized estrogens are relatively insensitive to conventional LC-ESI/MS-based methods. Therefore, we developed LC-ECAPCI/MS methodology, coupled with the use of PFB derivatives in order to substantially improve the sensitivity of detection for plasma estrogens. In this method, the nitrogen sheath gas is bombarded with electrons from the coronal discharge to yield a nitrogen radical cation and a low energy thermal electron [10] (Figure 4). An E1 molecule modified to have a high collision cross section through formation of electron capturing PFB-ether derivative (Figure 5) will then undergo highly efficient dissociative electron capture. This results in the formation of an intense M-PFB negative ion through the loss of a PFB radical. Similar results have been obtained with other electron capturing groups such as p-nitrobenzyl (PNB, Figure 5) [24]. MRM analysis of the transition m/z 269 → 145 which monitors the fragmentation of the C and D rings upon CID provides the necessary rigor for unequivocal identification of E1 [25]. The limit of detection (LOD) is estimated as 740 attomoles and 140 attomoles for E1 and 2-methoxy-E1, respectively Table 2. Thus, ECAPCI/MS was found to increase sensitivity over traditional negative ion APCI by 25–50 fold.
Fig. 4.
Proposed mechanism for EC-APCI/MS. Reproduced with permission from Analytical chemistry
Fig. 5.
Estrone derivatives that provide increased LC-MS sensitivity compared with underivatized estrone.
Table 2.
Limits of Detection Using APCI and ECAPCI for Selected Estrogen Metabolites.
| Analyte | Negative APCIa LOD (fmol) | ECAPCIb LOD (amol) | Increasedc Sensitivity |
|---|---|---|---|
| estrone | 18.52 | 740 | 25-fold |
| 2-methoxyestrone | 8.33 | 170 | 50-fold |
underivatized analyte;
PFB derivative,
negative ion APCI of underivatized sample compared with ECAPCI of PFB-derivative.
3.4. Analysis of Plasma Estrogens by Stable Isotope Dilution LC-ECAPCI/MS
The specificity of the LC-ECAPCI/MS method was further increased by the use of stable isotope labeled analogs as internal standards for each analyte and the use of normal phase LC for the separation of isomeric estrogens. The stable isotope dilution LC-ECAPCI/MS method was validated for the simultaneous detection of E2 and seven of its plasma metabolites: 2-methoxy-E2, 4-methoxy-E2, 16α-hydroxy-E2, E1, 2-methoxy-E1, 4-methoxy-E1, and 16α-hydroxy-E1 (Table 3). Typical chromatograms for male plasma samples to which authentic estrogens had been were added are shown for E2 and its metabolites (Figure 6) and for E1 and its metabolites (Figure 7). Linear standard curves were obtained in the range 5 pg/mL to 2,000 pg/ml plasma for E2 and E1 (Figure 8) as well as the other six E2 metabolites (data not shown). Replicate determinations (n=5) of quality control samples were conducted one day at the LLOQ (5 pg/mL). They were conducted on three separate days for the LQC (10 pg/mL), MQC (50 pg/mL), and HQC (500 pg/mL) on three separate days. The intra- and inter-day accuracy of all quality control samples was within 100 ± 15 % of theoretical with a precision (coefficient of variation) better than 15 %. The method can also be further modified by the inclusion of a hydrolysis step prior to extraction, where the plasma is treated with β-glucuronidase/arylsulfatase step to release the free steroids.
Fig. 6.
Stable isotope dilution LC-ECAPCI/MRM/MS analysis of E2 and metabolites.
Fig. 7.
Stable isotope dilution LC-ECAPCI/MRM/MS analysis of E1 and metabolites.
Fig. 8.
Standard curves for E1 and E2 in the range of 5 pg/mL to 2000 pg/mL. Linear regression lies were obtained with r2 values of 0.998 or better.
4. Discussion
Hydroxysteroid dehydrogenases (HSDs) belong to two gene superfamilies (AKRs) and the short-chain dehydrogenase/reductases [26]. Historically many substrate specificity studies have been performed with these enzymes in which the basis of the assay is to monitor the formation or consumption of NAD(P)H linked to the oxidation or reduction of a hydroxy- or ketosteroid. Assay validation is usually performed in discontinuous assays which rely upon separating substrate from product by TLC and identifying unknowns by co-migration with authentic synthetic standards. Often the TLC systems do not have sufficient resolving power to separate all mixtures of stereoisomers of interest and product identify is inferred based on reactions with structurally related steroid substrates. However, bacterial [27–29] and mammalian HSDs [12,30] are also not always positional or stereo- specific increasing the number of products possible. The problem of product identity can be exacerbated if steroid conjugates are examined as substrates since conjugates do not migrate from the origin of normal-phase TLC plates.
We have used LC-MS methods to resolve the products of reactions catalyzed by steroid hormone transforming AKRs [15, 18]. We show that LC-ESI/MS in the negative ion mode provides sufficient resolving power to generate umambiguous product profiles for the reduction of steroid conjugates of DHT and the synthetic steroid tibolone catalyzed by AKR1C isoforms. These methods also highlight the danger of using one reaction to determine the stereochemical course of another. Thus AKR1C2 will produce the 3β-hydroxysteroid product from DHTG but the 3α-hydroxysteroid product from DHTS. Knowing the product profiles has enabled us to assign kinetic constants (Km, kcat and kcat/Km) to each of the reactions with confidence. For AKR1C4 catalytic efficiency for the reduction of DHT, DHTG and DHTS are unaltered by the presence of the conjugate group raising the possibility that conjugation reactions (Phase 2 reactions) could precede reduction reactions (Phase I reactions). In liver we proposed that the conjugation pathway occurs first for DHT since 17β-hydroxy-5α-androstane-3α-glucuronide would be the expected metabolite if reduction occurred first but this product is not detected in the plasma or urine. Instead the 3α-OH-DHTG is observed which would be consistent with reduction occurring after conjugation, i.e. the Phase 2 enzyme reaction occurs first [15].
For AKR1C2 and AKR1C4 we show that the catalytic efficiencies for tibolone and TibS are similar again raising the issue that reduction of the 3-ketosteroid could follow 17β-sulfation. If tibolone conjugation precedes reduction in any tissue, the estrogenic 3α- and 3β-hydroxy-tibolones would not form since they would be present as their 17β-sulfates. The difference in the balance between reduction of tibolone or sulfation of tibolone could contribute to the selective tissue estrogenic effects of the drug in post-menopausal women.
We have also exploited the power of stable-isotope dilution-LC-ECAPCI/MS to reliably quantify plasma E1 and E2 and their downstream metabolites. The use of an electron capturing derivative such as PFB increases sensitivity by approximately 50-fold compared with conventional LC-MS methodologies employing underivatized estrogens. This level of sensitivity is required to analyzed estrogen metabolites in the plasma of post-menopausal women where basal levels are in the low pg/mL range [25].
Stable isotope dilution LC-MS methods can provide the optimal specificity for estrogen analysis because internal standards with identical physicochemical properties to the relevant analytes are carried through the entire analysis procedure. This makes it possible to compensate for losses that occur through all steps. Equally important is the ability of the stable isotope analogs to act as carriers and prevent non-specific losses through binding to glassware or other surfaces when the corresponding endogenous analytes are present in only trace amounts. Furthermore, the endogenous analyte has to meet three strict criteria in order to satisfy the constraints of the analytical method – it must have the same parent ion, product ion as the authentic material, and the relative retention time to the internal standard should be identical from run to run. Ideally, [13C]-estrogen analogs should be used as internal standards but they have only recently become available and so it has been necessary to used deuterium labeled analogs, which elute slightly ahead of the protium forms under reversed phase conditions or slightly after the protium forms under normal-phase conditions. Therefore, differential suppression or enhancement of ionization could still affect the quality of the analytical data. Fortunately, [13C]-analogs of many estrogens have become available recently from Cambridge Isotope Laboratories (Andover, MA), so it should be possible to introduce another level of rigor by employing these standards in the future instead of the deuterium labeled versions.
Endogenous estrogens are not effectively ionized using conventional ESI or APCI methodology. Therefore, it is necessary to enhance their ionization characteristics by derivatization and three approaches have been described. The first approach uses conventional derivatization coupled with LC-ESI/MS. This approach was exemplified by studies of the Ziegler [31], Yamashita [32], and Spink [33] groups in which a dansyl (D), picolinoyl (P), or pyridyl-3-sulfonyl (PS) group is attached to the 3-hydroxy moiety of the estrogen (Figure 5). The second approach involves the preparation of pre-ionized (quaternized) derivatives, so that ionization is not required in the ESI source of the mass spectrometer. This approach was exemplified by studies of the Chen [34], Higashi [35], and Adamec [36] groups in which an N-methyl-2-pyridyl (MP), 1-(2,4-dinitro-5-fluorphenyl-4,4,-dimethylpiperazinyl (MPPZ), or a N-methyl-nicotinyl (NMN) group is attached to the 3-hydroxy moiety of the estrogen (Figure 5). The third approach, which we have pioneered, involves the preparation of an electron capturing PFB derivative of the estrogen (Figure 5) coupled with the use of ECAPCI/MS. The Higashi group [24] has also explored the utility of ECAPC/MS for estrogen analysis by using different electron capturing derivatives such as PNB (Figure 5).
The three derivatization strategies described are all capable of quantifying plasma estrogens in the low pg/mL range. Therefore, in the future, we and others will be able to rigorously establish the precise levels of individual estrogens that are present in the plasma of post-menopausal women. The ratio of 4-methoxy-estrogens to 2-methoxy-estrogens provides an indirect measurement of the catechol estrogens 4-hydroxy-E2/4-hydroxy-E1 and 2-hydroxy-E2/2-hydroxy-E1. This is potentially important since the 4-hydroxylated catechols are considered to be genotoxic estrogens while the 2-hydroxy catechols are metabolized very rapidly to 2-methoxy-estrogens that are considered to be anti-proliferative and protective against mammary carcinogenesis [20–23].
Hormone dependent malignancies also synthesize steroid hormones in the tumor to drive tumor growth [37,38] and this explains the effectiveness of aromatase inhibitors in treating breast cancer in post-menopausal women and the use of 5α-reductase inhibitors in treating prostate cancer [39–43]. Considerable effort has been made in diagnosing these tumors in terms of the presence of nuclear receptors, real-time-PCR to measure transcript levels of steroidogenic enzymes, and immunohistochemical approaches to measure both receptor and enzyme levels. A characteristic property less developed which is a pre-requisite to complete the molecular pathology of these tumors are robust methods to measure intra-tumoral levels of estrogens and androgens. The methods we have developed offer promise to measure steroid metabolomes within prostate and breast tumor biopsy samples and the information gleaned could be used to determine treatment paradigm.
The regulation of ligand occupancy of nuclear receptors is often governed by pairs of HSDs. For example regulation of the ER is governed by type 1 17β-HSD (which reduces E1 to E2) and by type 2/4 17β-HSD (which oxidizes E2 to E1) [44–47], while regulation of the AR is governed by AKR1C2 (which reduces 5α-DHT to 3α-diol) and by HSD17B6 (which oxidizes 3α-diol to DHT) [48, 49]. Thus a component of measuring intra-tumoral levels of steroid hormones is to distinguish between the intracrine formation of ketosteroids from hydroxysteroids. The LC/ECAPCI-MS method requires derivatization to an electron capturing group. This can be accomplished using the pentafuorobenzyl bromide or pentafluorobenzylcarboxymethoxime derivative, for hydroxysteroids and ketosteroids, respectively. The derivatization with pentafluorobenzylcarboxymethoxime was developed originally for EC negative chemical ionization gas-chromatography-MS of keto-steroids Alternatively, conventional derivatives such as N-hydroxy-oximes [50] or pre-ionized derivatives such as those formed by the Girard T reagent [51] could be employed to improve sensitivity of keto-steroid detection by ESI/MS or APCI/MS [52, 53]. The next phase of our work will be to implement such methods.
Acknowledgments
This work was supported by the following research grants: P30ES013587 and R01CA90744 from the National Institutes of Health and by a Prostate Cancer Foundation Challenge Grant (TMP), by R01CA091016 (IAB), and by a Pilot-Project Grant awarded from P30ES13587 (YJ).
Abbreviations
- 3α-Diol-17G
3α-hydroxy-5α-androstane-17β-glucuronide
- 3β-Diol-17G
3β-hydroxy-5α-androstane-17β-glucuronide
- 3α-Diol-17S
3α-hydroxy-5β-androstane-17β-sulfate
- 3β-Diol-17S
3β-hydroxy-5α-androstane-17β-sulfate
- dihydrotestosterone
DHT, 17α-hydroxy-5α-androstan-3-one
- DHTG
DHT-17β-glucuronide
- DHTS
DHT-17β-sulfate
- E1
estrone
- E2
17β-estradiol
- Tibolone
[7α,17α]-17-hydroxy-7-methyl-19-norpregn-5(10)-en-20-yn-3-one
- TibS
Tibolone-17β-sulfate
- 3α-OH-Tib
3α-hydroxy-tibolone
- 3β-OH-Tib
3β-hydroxy-tibolone
- 3α-OH-TibS
3α-hydroxy-tibolone-17β-sulfate
- and 3β-OH-TibS
3β-hydroxy-tibolone-17β-sulfate
- 2-methoxy-E1
2-methoxy-estrone
- 4-methoxy-E1
4-methoxy-estrone
- 2-methoxy-E2
2-methoxy-17β-estradiol
- 4-methoxy-E2
4-methoxy-17β-estradiol
- 2-OH-E1
2,3-dihydroxy-estrone
- 2-OH-E2
2,3-dihydroxy-17β-estradiol
- 4-OH-E1
3,4-dihydroxyestrone
- 4-OH-E2
3,4-dihydroxy-17β-estradiol
- AcN
acetonitrile
- AKR
aldo-keto reductase
- PFB
pentafluorobenzyl
- HQC
high quality control sample
- LLQC
lower limit of quantitation
- LQC
lower quality control sample
- MQC
middle quality control sample; and QC quality control sample.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Moal V, Mathieu E, Reynier P, Malthièry Y, Gallois Y. Low serum testosterone assayed by liquid chromatography-tandem mass spectrometry. Comparison with five immunoassay techniques. Clin Chim Acta. 2007;386(1–2):12–9. doi: 10.1016/j.cca.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 2.Thienpont LM, Van Uytfanghe K, Blincko S, Ramsay CS, Xie H, Doss RC, Keevil BG, Owen LJ, Rockwood AL, Kushnir MM, Chun KY, Chandler DW, Field HP, Sluss PM. State-of-the-art of serum testosterone measurement by isotope dilution-liquid chromatography-tandem mass spectrometry. Clin Chem. 2008;54(8):1290–7. doi: 10.1373/clinchem.2008.105841. [DOI] [PubMed] [Google Scholar]
- 3.Wang C, Catlin CH, Demers LM, Starcevic B, Swerdloff RS. Measurement of total serum testosterone in adult men: comparison of current laboratory methods versus liquid chromatography-tandem mass spectrometry. J Clin Endocrinol Metab. 2004;89(6):534–43. doi: 10.1210/jc.2003-031287. [DOI] [PubMed] [Google Scholar]
- 4.Giese R. Measurement of endogenous estrogens: analytical challenges and recent advances. J Chromatogr A. 2003;1000(1–2):401–12. doi: 10.1016/s0021-9673(03)00306-6. [DOI] [PubMed] [Google Scholar]
- 5.Stanczyk F, Lee JS, Santen RJ. Standardization of steroid hormone assays: why, how and when? Cancer Epidemiology, Biomarkers & Prevention. 2007;16(9):1713–9. doi: 10.1158/1055-9965.EPI-06-0765. [DOI] [PubMed] [Google Scholar]
- 6.Xu X, Roman JM, Issaq HJ, Keefre LK, Veenstra TD, Ziegler RG. Quantitative measurement of endogenous estrogens and estrogen metabolites in human serum by liquid chromatography-tandem mass spectrometry. Anal Chem. 2007;79(20):7813–21. doi: 10.1021/ac070494j. [DOI] [PubMed] [Google Scholar]
- 7.Adlercreutz H, Tikkanen MJ, Hunneman DH. Mass fragmentographic determination of eleven estrogens in the body fluids of pregnant and nonpregnant subjects. J Steroid Biochem. 1974;5(3):211–7. doi: 10.1016/0022-4731(74)90134-4. [DOI] [PubMed] [Google Scholar]
- 8.Adlercreutz H, Kiuru P, Rasku S, Wähälä K, Fotsis T. An isotope dilution gas chromatographic-mass spectrometric method for the simultaneous assay of estrogens and phytoestrogens in urine. J Steroid Biochem Mol Biol. 2004;92(5):399–411. doi: 10.1016/j.jsbmb.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 9.Zacharia LC, Dubey RK, Jackson EK. A gas chromatography/mass spectrometry assay to measure estradiol, catecholestradiols, and methoxyestradiols in plasma. Steroids. 2004;69(4):255–61. doi: 10.1016/j.steroids.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 10.Singh G, Gutierrez A, Xu K, Blair IA. Liquid chromatography/electron capture atmospheric pressure chemical ionization/mass spectrometry: analysis of pentafluorobenzyl derivatives of biomolecules and drugs in the attomole range. Anal Chem. 2000;72(14):3007–3013. doi: 10.1021/ac000374a. [DOI] [PubMed] [Google Scholar]
- 11.Burczynski ME, Harvey RG, Penning TM. Expression and characterization of four recombinant human dihydrodiol dehydrogenase isoforms: oxidation of trans-7,8-dihydroxy-7,8-dihydrobenzo[a]pyrene to the activated o-quinone metabolite benzo[a]pyrene-7,8-dione. Biochemistry. 1998;37(32):6781–6790. doi: 10.1021/bi972725u. [DOI] [PubMed] [Google Scholar]
- 12.Steckelbroeck S, Jin Y, Gopishetty S, Oyesanmi B, Penning TM. Human cytosolic 3α-hydroxysteroid dehydrogenases of the aldo-keto reductase superfamily display significant 3β-hydroxysteroid dehydrogenase activity: Implications for steroid hormone metabolism and action. J Biol Chem. 2003;279(11):10784–10795. doi: 10.1074/jbc.M313308200. [DOI] [PubMed] [Google Scholar]
- 13.Steckelbroeck S, Jin Y, Oyesanmi B, Kloosterboer HJ, Penning TM. Tibolone is metabolized by the 3α/3β-hydroxysteroid dehydrogenase activities of the four human isozymes of the aldo-keto reductase 1C subfamily: inversion of stereospecificity with a Δ5(10)-3-ketosteroid. Mol Pharmacol. 2004;66(6):1702–1711. doi: 10.1124/mol.104.004515. [DOI] [PubMed] [Google Scholar]
- 14.Ramana K, Dixit BL, Srivastava S, Balendiran GK, Sivastava SK, Bhatnagar A. Selective recognition of glutathiolated aldehydes by aldose reductase. Biochemistry. 2000;39(40):12172–80. doi: 10.1021/bi000796e. [DOI] [PubMed] [Google Scholar]
- 15.Jin Y, Duan L, Lee SH, Kloosterboer HJ, Blair IA, Penning TM. Human cytosolic hydroxysteroid dehydrogenases of the aldo-keto reductase superfamily catalyze reduction of conjugated steroids: implications for phase I and phase II steroid hormone metabolism. J Biol Chem. 2009;284(15):10013–22. doi: 10.1074/jbc.M809465200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Albertazzi P, di Micco R, Zanardi E. Tibolone: a review. Maturitas. 1998;30(3):295–305. doi: 10.1016/s0378-5122(98)00059-0. [DOI] [PubMed] [Google Scholar]
- 17.Moore R. Livial: a review of clinical studies. Br J Obstet Gynaecol. 1999;106(Suppl 19):1–21. [PubMed] [Google Scholar]
- 18.Stecklebroeck S, Oyesanmi B, Jin Y, Lee SH, Kloosterboer HJ, Penning TM. Tibolone metabolism in human liver is catalyzed by 3α/3β-hydroxysteroid dehydrogenase activities of the four isoforms of the aldo-keto reductase (AKR)1C subfamily. J Pharmacol Exp Therap. 2006;316(3):1300–1309. doi: 10.1124/jpet.105.091587. [DOI] [PubMed] [Google Scholar]
- 19.Vos RM, Krebbers SF, Verhoeven CH, Delbressine LP. The in vivo human metabolism of tibolone. Drug Metab Dispos. 2002;30(2):106–112. doi: 10.1124/dmd.30.2.106. [DOI] [PubMed] [Google Scholar]
- 20.Chen JQ, Brown TR, Yager JD. Mechanisms of hormone carcinogenesis: evolution of views, role of mitochondria. Adv Exp Med Biol. 2008;630:1–18. [PubMed] [Google Scholar]
- 21.Eliassen AH, Missmer SA, Tworoger SS, Hankinson SE. Circulating 2-hydroxy- and 16α-hydroxy estrone levels and risk of breast cancer among postmenopausal women. Cancer Epidemiology, Biomarkers & Prevention. 2008;17(8):2029–35. doi: 10.1158/1055-9965.EPI-08-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Russo J, Hasan Lareef M, Balogh G, Guo S, Russo IH. Estrogen and its metabolites are carcinogenic agents in human breast epithelial cells. J Steroid Biochem Mol Biol. 2003;87(1):1–25. doi: 10.1016/s0960-0760(03)00390-x. [DOI] [PubMed] [Google Scholar]
- 23.Santen R, Cavalieri E, Rogan E, Russo J, Guttenplan J, Ingle J, Yue W. Estrogen mediation of breast tumor formation involves estrogen receptor-dependent, as well as independent, genotoxic effects. Ann NY Acad Sci. 2009;1155:132–40. doi: 10.1111/j.1749-6632.2008.03685.x. [DOI] [PubMed] [Google Scholar]
- 24.Higashi T, Takayama N, Nishio T, Taniguchi E, Shimada K. Procedure for increasing the detection responses of estrogens in LC-MS based on introduction of a nitrobenzene moiety followed by electron capture atmospheric pressure chemical ionization. Anal Bioanal Chem. 2006;386(3):658–665. doi: 10.1007/s00216-006-0371-z. [DOI] [PubMed] [Google Scholar]
- 25.Singh G, Gutierrez A, Xu K, Blair IA. Liquid chromatography/electron capture atmospheric pressure chemical ionization/mass spectrometry: analysis of pentafluorobenzyl derivatives of biomolecules and drugs in the attomole range. Anal Chem. 2000;72(14):3007–13. doi: 10.1021/ac000374a. [DOI] [PubMed] [Google Scholar]
- 26.Penning TM. Molecular endocrinology of hydroxysteroid dehydrogenases. Endocrine Reviews. 1997;18(3):281–305. doi: 10.1210/edrv.18.3.0302. [DOI] [PubMed] [Google Scholar]
- 27.Abalain JH, Di Stefano S, Abalain-Colloc ML, Floch HH. Cloning, sequencing and expression of Pseudomonas testosteroni gene encoding 3α-hydroxysteroid dehydrogenase. J Steroid Biochem & Mol Biol. 1995;55(2):233–8. doi: 10.1016/0960-0760(95)00170-5. [DOI] [PubMed] [Google Scholar]
- 28.Ghosh D, Wawrzak Z, Weeks CM, Duax WL, Erman M. The refined three-dimensional structure of 3α,20β-hydroxysteroid dehydrogenase and possible roles of the residues conserved in short-chain dehydrogenases. Structure. 1994;15(10):629–40. doi: 10.1016/s0969-2126(00)00064-2. [DOI] [PubMed] [Google Scholar]
- 29.Strickler R, Covey DF, Tobiase B. Study of 3α,20 β-hydroxysteroid dehydrogenase with an enzyme-generated affinity alkylator: dual enzyme activity at a single active site. Biochemistry. 1980;19(22):4950–4. doi: 10.1021/bi00563a002. [DOI] [PubMed] [Google Scholar]
- 30.Penning TM, Burczynski ME, Jez JM, Hung CF, Lin HK, Ma H, Moore M, Palackal N, Ratnam K. Human 3α-hydroxysteroid dehydrogenase isoforms (AKR1C1–AKR1C4) of the aldo keto reductase superfamily: functional plasticity and tissue distribution reveals roles in the inactivation and formation of male and female sex hormones. Biochem J. 2000;351(Pt 1):67–77. doi: 10.1042/0264-6021:3510067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu X, Veenstra TD, Fox SD, Roman JM, Issaq HJ, Falk R, Saavedra JE, Keefer LK, Ziegler RG. Measuring fifteen endogenous estrogens simultaneously in human urine by high-performance liquid chromatography-mass spectrometry. Anal Chem. 2005;77(20):6646–6654. doi: 10.1021/ac050697c. [DOI] [PubMed] [Google Scholar]
- 32.Yamashita K, Okuyama M, Watanabe Y, Honma S, Kobayashi S, Numazawa M. Highly sensitive determination of estrone and estradiol in human serum by liquid chromatography-electrospray ionization tandem mass spectrometry. Steroids. 2007;72(11–12):819–827. doi: 10.1016/j.steroids.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 33.Xu L, Spink DC. Analysis of steroidal estrogens as pyridine-3-sulfonyl derivatives by liquid chromatography electrospray tandem mass spectrometry. Anal Biochem. 2008;375(1):105–114. doi: 10.1016/j.ab.2007.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin YH, Chen CY, Wang GS. Analysis of steroid estrogens in water using liquid chromatography/tandem mass spectrometry with chemical derivatizations. Rapid Commun Mass Spectrom. 2007;21(13):1973–1983. doi: 10.1002/rcm.3050. [DOI] [PubMed] [Google Scholar]
- 35.Nishio T, Higashi T, Funaishi A, Tanaka J, Shimada K. Development and application of electrospray-active derivatization reagents for hydroxysteroids. J Pharm Biomed Anal. 2007;44(3):786–795. doi: 10.1016/j.jpba.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 36.Yang WC, Regnier FE, Sliva D, Adamec J. Stable isotope-coded quaternization for comparative quantification of estrogen metabolites by high-performance liquid chromatography-electrospray ionization mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;870(2):233–40. doi: 10.1016/j.jchromb.2008.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Labrie F, Belanger A, Simard J. Intracrinology. Autonomy and freedom of peripheral tissues. Annals Endocrinology. 1995;56(1):23–29. [PubMed] [Google Scholar]
- 38.Labrie F, Luu-The V, Lin SX, Simard J, Labrie C, El-Alfy M, Pelletier G, Belanger A. Intracrinology: role of the family of 17β-hydroxysteroid dehydrogenases in human physiology and disease. J Mol Endocrinol. 2000;25(1):1–16. doi: 10.1677/jme.0.0250001. [DOI] [PubMed] [Google Scholar]
- 39.Brodie A, Liu Q, Liu Y, Long B. Aromatase inhibitors and their antitumor effects in model systems. Endocrine-Related Cancer. 1999;6(2):205–210. doi: 10.1677/erc.0.0060205. [DOI] [PubMed] [Google Scholar]
- 40.Brodie A, Garrett WM, Hendrickson JR, Tsai-Morris CH, Marcotte PA, Robinson CH. Inactivation of aromatase in vitro by 4-hydroxy-4-androstene-3,17-dione and 4-acetoxy-4-androstene-3,17-dione and sustained effects. Steroids. 1981;38(6):693–702. doi: 10.1016/0039-128x(81)90087-8. [DOI] [PubMed] [Google Scholar]
- 41.Bull HG, Garcia-Calvo M, Andersson S, Baginsky WE, Chan HK, Ellsworth DE, Miller RR, Stearns RA, Bakshi R, Rasmusson GH, Tolmna RL, Myers RW, Kozarich JW, Harris GS. Mechanism-based inhibition of human steroid 5α-reductase by finasteride: enzyme-catalyzed formation of NADP+-dihydrofinasteride, a potent bisubstrate analog. J Amer Chem Soc. 1996;118(10):2359–2365. [Google Scholar]
- 42.Gormley GJ. 5α-Reductase inhibitors in prostate cancer. Endocrine-Related Cancer. 1996;3(1):57–67. [Google Scholar]
- 43.Miller WR. Aromatase Inhibitors. Endocrine-Related Cancer. 1996;3(1):65–79. [Google Scholar]
- 44.Adamski J, Normand T, Leenders F, Monte D, Begue A, Stehelin D, Jungblut PW, de Launoit Y. Molecular cloning of a novel widely expressed human 80 kDa 17β-hydroxysteroid dehydrogenase IV. Biochem J. 1995;311(Pt 2):437–43. doi: 10.1042/bj3110437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Andersson S. 17β-hydroxysteroid dehydrogenase: isozymes and mutations. J Endocrinol. 1995;146(2):197–200. doi: 10.1677/joe.0.1460197. [DOI] [PubMed] [Google Scholar]
- 46.Andersson S, Geissler WM, Patel S, Wu L. The molecular biology of androgenic 17β-hydroxysteroid dehydrogenases. J Steroid Biochem Mol Biol. 1995;53(1–6):37–9. doi: 10.1016/0960-0760(95)00039-3. [DOI] [PubMed] [Google Scholar]
- 47.Labrie F, Luu-The V, Lin SX, Labrie C, Simard J, Breton R, Belanger A. The key role of 17β-hydroxysteroid dehydrogenases in sex steroid biology. Steroids. 1997;62(1):148–158. doi: 10.1016/s0039-128x(96)00174-2. [DOI] [PubMed] [Google Scholar]
- 48.Bauman DR, Steckelbroeck S, Williams MV, Peehl DM, Penning TM. Identification of the major oxidative 3α-hydroxysteroid dehydrogenase in human prostate that converts 5α-androstane-3α,17β-diol to 5α-dihydrotestosterone: A potential therapeutic target for androgen dependent disease. Mol Endocrinol. 2006;20(2):444–458. doi: 10.1210/me.2005-0287. [DOI] [PubMed] [Google Scholar]
- 49.Penning TM, Bauman D, Jin Y, Rizner TL. Identification of the molecular switch that regulates access of 5α-DHT to the androgen receptor. Mol Cell Endocrinol. 2007;265–266:77–82. doi: 10.1016/j.mce.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee SH, Blair IA. Characterization of 4-oxo-2-nonenal as a novel product of lipid peroxidation. Chem Res Toxicol. 2000;13:698–702. doi: 10.1021/tx000101a. [DOI] [PubMed] [Google Scholar]
- 51.Griffiths WJ, Liu S, Alvelius G, Sjovall J. Derivatization for the characterisation of neutral oxosteroids by electrospray and matrix-assisted laser desorption/ionisation tandem mass spectrometry: the Girard P derivative. Rapid Commun Mass Spectrom. 2003;17(9):924–935. doi: 10.1002/rcm.1002. [DOI] [PubMed] [Google Scholar]
- 52.Higashi T, Takayama N, Kyutoku M, Shimada K, Koh E, Namiki M. Liquid chromatography-mass spectrometric assay of androstenediol in prostatic tissue: influence of androgen deprivation therapy on its level. Steroids. 2006;71(11–12):1007–13. doi: 10.1016/j.steroids.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 53.Higashi T, Yamauchi A, Shimada K, Koh E, Mizokami A, Namiki M. Determination of prostatic androgens in 10 mg of tissue using liquid chromatography-tandem mass spectrometry with charged derivatization. Anal Bioanal Chem. 2005;382(4):1035–43. doi: 10.1007/s00216-005-3233-1. [DOI] [PubMed] [Google Scholar]