Abstract
Background and objective
Young mice do not develop measurable periodontal bone loss, unless heavily infected with human periodontal pathogens. However, mice with genetically altered immune system are unable to control their own oral flora and develop periodontitis early in life. Based on the potential of the indigenous oral microbiota to cause periodontitis, we hypothesized that normal mice may ultimately develop inflammatory periodontal bone loss, i.e., as a function of age. If confirmed, this could serve as an aging model of chronic periodontitis.
Materials and methods
Periodontal bone levels were measured as the distance from the cementoenamel junction (CEJ) to the alveolar bone crest (ABC), in young (8-10 weeks of age), old (≥ 18 months of age), and mice of intermediate ages. Differential expression of inflammatory mediators in the gingivae of young and old mice was determined by quantitative real-time PCR.
Results
In comparison to young mice, old mice displayed significantly (p < 0.05) increased periodontal bone loss, accompanied by elevated expression of proinflammatory cytokines (interleukin-1β, tumor necrosis factor-α, and interleukin-17A) and innate immune receptors involved in the induction or amplification of inflammation (Toll-like receptor 2, CD14, CD11b, CD18, complement C5a receptor, and triggering receptor expressed on myeloid cells-3).
Conclusion
Mice develop naturally-induced periodontal bone loss as a function of age. This aging model of periodontitis represents a genuinely chronic model to study mechanisms of periodontal tissue destruction.
Keywords: Animal model, Alveolar bone, Chronic periodontitis, Inflammation, Innate immunology
Introduction
Mice have been extensively used to study aspects of periodontal disease, including the inflammatory host response and induction of alveolar bone loss (reviewed in ref. 1). In this regard, mice constitute a relatively inexpensive and convenient model, there is a wealth of information on their immune system and, importantly, the availability of lines of transgenic mice with targeted gene deletions offers important mechanistic tools (1). These can be used to dissect proximal and downstream events in periodontal pathogenesis, such as mechanisms of microbial sensing and activation of specific proinflammatory pathways. In contrast, causal mechanistic relationships between suspected etiologic factors and periodontal breakdown cannot normally be addressed in human studies due to important ethical considerations (1).
Induction of experimental mouse periodontitis is typically achieved by oral gavage with human periodontal pathogens (typically with three or more doses of 109 bacteria), such as Porphyromonas gingivalis, Tannerella forsythia, and Aggregatibacter actinomycetemcomitans (2-5). Induction of measurable periodontal bone loss requires several weeks following oral infection (2), although the placement of pathogen-soaked ligatures around molar teeth accelerates this process and bone loss becomes evident within days (6). Several mouse strains have been used in periodontal studies, although BALB/c mice are considered the model of choice due to their increased susceptibility to infection-induced periodontal bone loss (2). Because sham infected mice in these models do not typically develop appreciable periodontal bone loss, this might give the impression that mice do not develop naturally occurring periodontitis, i.e., induced by their own oral flora. In this regard, although animal vendors may claim that experimental mice are pathogen-free, this does not necessarily mean that they are free of potential periodontal pathogens. In fact, the reason that sham infected mice do not develop obvious signs of periodontitis is likely due to their young age (usually used when 8-12 week old), consistent with the fact that periodontitis is normally associated with advanced age (7).
When their immune status is genetically altered, however, young mice do develop periodontitis even when they are not inoculated with human pathogens. Indeed, young mice with impaired mobilization of leukocytes to sites of infection, owing to combined P/E-selectin deficiency, display massive oral bacterial colonization and induction of gingival inflammation and alveolar bone loss (8). Similar bacteriological findings and periodontitis features are observed in young mice whose neutrophils display impaired bacterial killing (and hence unable to control their oral flora) due to genetically ablated lysosomal-associated membrane protein-2 (9). In both cases, induction of inflammation and periodontal bone loss is prevented by antibiotics, thus further confirming indigenous bacterial involvement in the disease (8, 9). These findings strongly suggest that the indigenous oral microbiota of mice has the potential to cause periodontitis. We thus hypothesized that mice develop inflammatory periodontal bone loss as a function of age. Our findings presented here have confirmed this hypothesis. The significance of this “aging model of periodontitis” is that it can be productively used to determine, under a genuinely chronic process, the role of suspected immune receptors or signaling molecules in destructive periodontal inflammation.
Materials and Methods
BALB/cByJ mice (8-10 weeks of age [young] or ≥ 18 months of age [old], as well as mice of intermediate ages) were obtained from the National Institute of Aging. All animal procedures were approved by the Institutional Animal Care and Use Committee, in compliance with established Federal and State policies. Assessment of periodontal bone loss in defleshed maxillae of euthanized mice was performed under a dissecting microscope (x40) fitted with a video image marker measurement system (VIA-170K; Fryer). The procedure involved measuring the distance from the cementoenamel junction (CEJ) to the alveolar bone crest (ABC) on 14 predetermined points on the buccal surfaces of the maxillary molars (2, 5). The CEJ-ABC distances were totaled for each mouse, which constituted the unit of analysis. Gingival tissue was excised from around the maxillary molars for use in quantitative real time PCR to assess mRNA expression of periodontal disease markers and other molecules of interest. Briefly, RNA was extracted using the PerfectPure RNA cell kit (5 Prime, Fisher) and quantified by spectrometry at 260 and 280 nm. The RNA was reverse-transcribed using the High-Capacity cDNA Archive kit (Applied Biosystems) and quantitative real-time PCR with cDNA was performed using the ABI 7500 Fast System, according to the manufacturer's protocol (Applied Biosystems). TaqMan probes, sense primers, and antisense primers for expression of genes shown in Figures 2 and 3, or a house-keeping gene (GAPDH) were purchased from Applied Biosystems. Data were evaluated by analysis of variance and the Tukey-Kramer Multiple Comparisons Test using the InStat program (GraphPad Software). Where appropriate (comparison of two groups only), two-tailed t tests were performed. P < 0.05 was taken as the level of significance. All experiments were performed at least twice for verification.
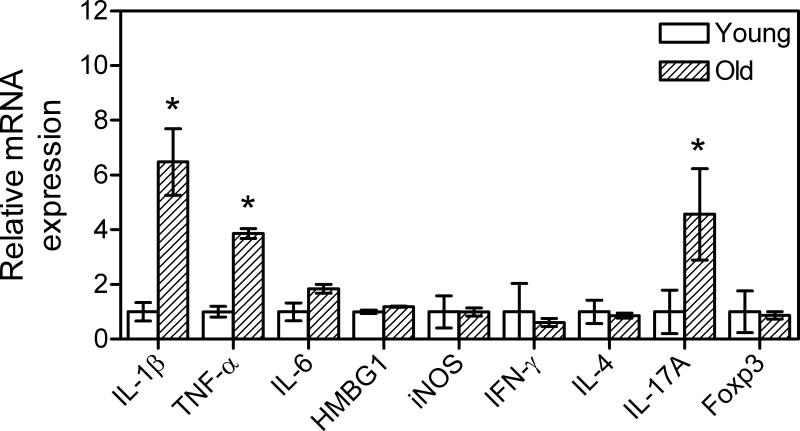
Fig. 2.
Relative expression of inflammatory mediators in the gingivae of young and old mice. Quantitative real-time PCR (qPCR) was used to determine gingival mRNA expression levels for the indicated molecules (normalized against GAPDH mRNA levels). The gingivae used were excised from young (8-10 weeks of age) and old (≥ 18 months of age) BALB/c mice. Results are shown as fold induction relative to young. Each data point represents the mean ± SD of 10 separate expression values, corresponding to qPCR analysis of total gingival RNA from individual mice. A minimum of two-fold difference was a requirement for further testing of statistical significance. Asterisks indicate statistically significant (p < 0.05) differences between old and young mice.
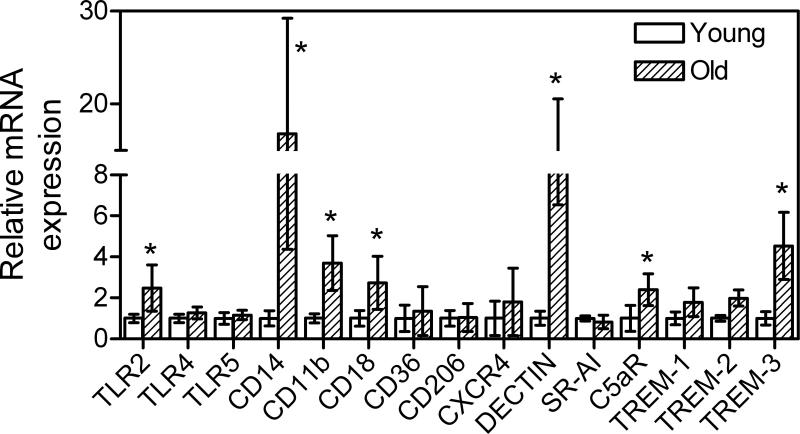
Fig. 3.
Relative expression of innate immune receptors in the gingivae of young and old mice. Quantitative real-time PCR (qPCR) was used to determine gingival mRNA expression levels for the indicated receptors (normalized against GAPDH mRNA levels). The gingivae used were excised from young (8-10 weeks of age) and old (≥ 18 months of age) BALB/c mice. Results are shown as fold induction relative to young. Each data point represents the mean ± SD of 10 separate expression values, corresponding to qPCR analysis of total gingival RNA from individual mice. A minimum of two-fold difference was a requirement for further testing of statistical significance. Asterisks indicate statistically significant (p < 0.05) differences between old and young mice.
Results and Discussion
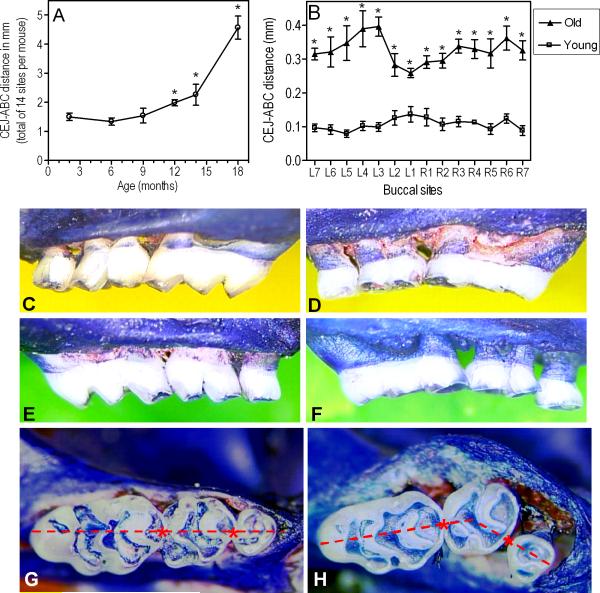
CEJ-ABC measurements in the maxillae of mice of various ages revealed an age-associated increase in periodontal bone loss, which reached statistical significance after 9 months of age (p < 0.05; Fig. 1A). The bone level differences between the two extreme age groups (8-10-week-old vs. ≥ 18-month-old) were significant at each buccal site examined (p < 0.05; Fig. 1B) and were clinically dramatic (Fig. 1 C-F), additionally involving molar tooth migration (Fig. 1 G,H). In fact, increased mobility of molar teeth or missing molars were seen in several old mice at the termination of the experiment.
Fig. 1.
Periodontal bone loss as a function of age in BALB/c mice. (A): Young (8-10 weeks of age), old (≥ 18 months of age), and mice of intermediate ages (6-, 9-, 12-, and 14-month-old) were used to determine their periodontal bone levels. The mm distance from the cementoenamel junction (CEJ) to the alveolar bone crest (ABC) was measured at 14 predetermined maxillary buccal sites and the readings were totaled for each mouse. The data are means ± SD (n = 5 mice). (B): Analytical data from the two extreme age groups (young vs. old). Each point corresponds to a measured site (L1-L7, left maxilla; R1-R7, right maxilla) and represents means ± SD (n = 5 mice). Asterisks denote significant (p < 0.05) differences in CEJ-ABC distances compared to young mice (the greater the CEJ-ABC distance, the greater the bone loss). The experiment was repeated with additional sets of 5 mice per group yielding consistent results. (C-F): Representative images from the maxillae of young (C, right; E, left) and old (D, right; F, left) mice. Extensive areas of resorbed alveolar bone are evident in the old mice. (G-H): Maxillary molar blocks seen from the occlusal surfaces of young (G) and old (H) mice. Note that all three molars in young mice are in line with each other (i.e., a straight line can connect their contact points, indicated by asterisks). Due to tooth mobility, this relationship did not apply to the molar blocks of old mice, many of which displayed overt migration of molars (especially of the second molar in buccal direction).
The Fig. 1 findings suggest a high degree of naturally-induced periodontitis in old mice in sharp contrast to their young counterparts. This conclusion is consistent with additional data that the gingivae of old mice displayed significantly elevated expression of interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α) (p < 0.05; Fig. 2), which are major mediators of destructive bone resorption in periodontitis (10). Other inflammatory mediators, such as IL-6, the high-mobility group box-1 protein (HMBG1), and the inducible nitric oxide synthase (iNOS), were not differentially expressed in the gingivae from young and old mice (Fig. 2). We have also examined expression of interferon-γ (IFN-γ), IL-4, IL-17A, and forkhead box P (Foxp3), as signature molecules of the Th1, Th2, Th17, and Treg subsets of T lymphocytes, respectively. Interestingly, only IL-17A was differentially expressed, reaching significantly higher levels in the gingivae of old mice (p < 0.05 vs. young; Fig. 2). Although the precise role of T lymphocytes in periodontitis is still unclear, the emergence of Th17 as a specialized osteoclastogenic T cell subset suggests that it may play an important role in this chronic inflammatory disease (reviewed in ref. 11).
We moreover found that six out of fifteen investigated innate immune receptors were differentially expressed in the gingivae of young and old mice (Fig. 3). Specifically, the Toll-like receptor 2 (TLR2) and its functionally associated co-receptors CD14, CD11b, and CD18 (12) were expressed at significantly higher levels in the gingivae of old mice (p < 0.05 vs. young; Fig. 3). Also upregulated in old age were the β-glucan receptor Dectin-1, the complement receptor for the C5a anaphylatoxin (C5aR; CD88), and one of the members of the family of triggering receptors expressed on myeloid cells (TREM), specifically the TREM-3 (p < 0.05; Fig. 3). The increased expression of C5aR and TREM-3 in old age could contribute to heightened periodontal inflammation, since these receptors participate in the amplification of the host inflammatory response (13, 14). Although TLR2, CD14, and the CD11b/CD18 heterodimer (also known as complement receptor-3; CR3) can also contribute to inflammation by cooperatively inducing the production of proinflammatory cytokines such as TNF-α (12), the same receptors are components of the TLR2/CR3 inside-out signaling pathway (15). This pathway is exploited by P. gingivalis and Mycobacterium tuberculosis (and possibly other, as yet unidentified, pathogens) for evading immune elimination (reviewed in refs. 16, 17). However, whether this pathway is also exploited by mouse periodontal bacteria and promotes their chronic persistence and virulence is currently uncertain.
The molecules investigated and shown in Figs. 2 and 3 were also examined for possible differential expression in the spleens of young and old mice. However, no significant differences were found (not shown). Thus, the age-associated differential expression of certain inflammatory cytokines (IL-1β, TNF-α, and IL-17A) or innate immune receptors (TLR2, CD14, CD11b, CD18, C5aR, and TREM-3) in the gingivae possibly reflects specific microenvironmental influence rather than global age-dependent changes.
The fact that the age-associated increase in CEJ-ABC distances is accompanied by elevated expression of periodontal disease markers, certain clinical signs (increased tooth mobility and missing teeth), as well as observations of vertical bone loss (Fig. 1H), which cannot be explained by compensatory eruption in response to occlusal attrition, strongly supports real periodontal breakdown. In summary, our findings indicate that the periodontal tissues of aged mice show clear signs of increased inflammation and elevated alveolar bone loss compared to young mice. This aging model of periodontitis represents a genuinely chronic model to study mechanisms of periodontal tissue destruction. For example, the parallel aging of wild-type mice and mice with defined knock-out mutations will allow the identification of specific recognition and signaling pathways that protect against or exacerbate chronic inflammatory periodontitis. This knowledge can in turn be exploited for the development of novel therapeutic approaches in chronic periodontitis.
Acknowledgements
This study was supported by U.S. Public Health Service Grants DE015254 and DE018292 to G.H.
References
- 1.Graves DT, Fine D, Teng Y-TA, Van Dyke TE, Hajishengallis G. The use of rodent models to investigate host-bacteria interactions related to periodontal diseases. J Clin Periodontol. 2008;35:89–105. doi: 10.1111/j.1600-051X.2007.01172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker PJ, Dixon M, Roopenian DC. Genetic control of susceptibility to Porphyromonas gingivalis-induced alveolar bone loss in mice. Infect Immun. 2000;68:5864–5868. doi: 10.1128/iai.68.10.5864-5868.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garlet GP, Cardoso CR, Silva TA, et al. Cytokine pattern determines the progression of experimental periodontal disease induced by Actinobacillus actinomycetemcomitans through the modulation of MMPs, RANKL, and their physiological inhibitors. Oral Microbiol Immunol. 2006;21:12–20. doi: 10.1111/j.1399-302X.2005.00245.x. [DOI] [PubMed] [Google Scholar]
- 4.Sharma A, Inagaki S, Honma K, Sfintescu C, Baker PJ, Evans RT. Tannerella forsythia-induced alveolar bone loss in mice involves leucine-rich-repeat BspA protein. J Dent Res. 2005;84:462–467. doi: 10.1177/154405910508400512. [DOI] [PubMed] [Google Scholar]
- 5.Hajishengallis G, Shakhatreh M-AK, Wang M, Liang S. Complement receptor 3 blockade promotes IL-12-mediated clearance of Porphyromonas gingivalis and negates its virulence in vivo. J Immunol. 2007;179:2359–2367. doi: 10.4049/jimmunol.179.4.2359. [DOI] [PubMed] [Google Scholar]
- 6.Li CH, Amar S. Morphometric, histomorphometric, and microcomputed tomographic analysis of periodontal inflammatory lesions in a murine model. J Periodontol. 2007;78:1120–1128. doi: 10.1902/jop.2007.060320. [DOI] [PubMed] [Google Scholar]
- 7.van der Velden U. The onset age of periodontal destruction. J Clin Periodontol. 1991;18:380–383. doi: 10.1111/j.1600-051x.1991.tb02304.x. [DOI] [PubMed] [Google Scholar]
- 8.Niederman R, Westernoff T, Lee C, et al. Infection-mediated early-onset periodontal disease in P/E-selectin-deficient mice. J Clin Periodontol. 2001;28:569–575. doi: 10.1034/j.1600-051x.2001.028006569.x. [DOI] [PubMed] [Google Scholar]
- 9.Beertsen W, Willenborg M, Everts V, et al. Impaired phagosomal maturation in neutrophils leads to periodontitis in lysosomal-associated membrane protein-2 knockout mice. J Immunol. 2008;180:475–482. doi: 10.4049/jimmunol.180.1.475. [DOI] [PubMed] [Google Scholar]
- 10.Graves D. Cytokines that promote periodontal tissue destruction. J Periodontol. 2008;79:1585–1591. doi: 10.1902/jop.2008.080183. [DOI] [PubMed] [Google Scholar]
- 11.Gaffen SL, Hajishengallis G. A new inflammatory cytokine on the block: re-thinking periodontal disease and the Th1/Th2 paradigm in the context of Th17 cells and IL-17. J Dent Res. 2008;87:817–828. doi: 10.1177/154405910808700908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hajishengallis G, Tapping RI, Harokopakis E, et al. Differential interactions of fimbriae and lipopolysaccharide from Porphyromonas gingivalis with the Toll-like receptor 2-centred pattern recognition apparatus. Cell Microbiol. 2006;8:1557–1570. doi: 10.1111/j.1462-5822.2006.00730.x. [DOI] [PubMed] [Google Scholar]
- 13.Klesney-Tait J, Turnbull IR, Colonna M. The TREM receptor family and signal integration. Nat Immunol. 2006;7:1266–1273. doi: 10.1038/ni1411. [DOI] [PubMed] [Google Scholar]
- 14.Guo RF, Ward PA. Role of C5a in inflammatory responses. Annu Rev Immunol. 2005;23:821–852. doi: 10.1146/annurev.immunol.23.021704.115835. [DOI] [PubMed] [Google Scholar]
- 15.Harokopakis E, Hajishengallis G. Integrin activation by bacterial fimbriae through a pathway involving CD14, Toll-like receptor 2, and phosphatidylinositol-3-kinase. Eur J Immunol. 2005;35:1201–1210. doi: 10.1002/eji.200425883. [DOI] [PubMed] [Google Scholar]
- 16.Hajishengallis G, Harokopakis E. Porphyromonas gingivalis interactions with complement receptor 3 (CR3): innate immunity or immune evasion? Front Biosci. 2007;12:4547–4557. doi: 10.2741/2409. [DOI] [PubMed] [Google Scholar]
- 17.Hajishengallis G. Porphyromonas gingivalis-host interactions: Open war or intelligent guerilla tactics? Microbes Infect. 2009 doi: 10.1016/j.micinf.2009.03.009. doi:10.1016/j.micinf.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]