Abstract
We previously reported that higher serum concentrations of C-reactive protein (CRP) are associated with shorter survival in men with castration-resistant prostate cancer (CRPC). To confirm this finding in an independent data set, we used 119 CRPC patients enrolled in 6 phase II clinical trials and examined the relationship of CRP, alkaline phosphatase, hemoglobin, age, ECOG PS, and prostate specific antigen (PSA) with survival. Median follow-up was 19.7 months (0.9–98.5 months) and 89% have died. After analyzing the form of the risk function using the generalized additive model method, univariate and multivariate Cox proportional hazard models were used to assess associations between baseline individual categorical and continuous variables. Quartiles of CRP were: 1: 0–1.0, 1.1–4.9, 5.0–17.0, and 17.1 to 311 mg/L. In a Cox multivariate model, log2(CRP) (HR 1.106 p=0.013) as well as hemoglobin and alkaline phosphatase were independently associated with survival, confirming that higher CRP is associated with shorter survival in CRPC. Since CRP is a marker of inflammation, this finding suggests that inflammation may play an important role in the natural history of advanced prostate cancer. CRP is a readily measurable biomarker that has the potential to improve prognostic models and should be validated in a prospective clinical trial.
Keywords: prostate cancer, c-reactive protein, prognosis, inflammation, survival
INTRODUCTION
The complex relationship between inflammation and cancer has been well-described since the late 1800’s [1]. The principal purpose of an acute inflammatory response is to create a protective tissue microenvironment that allows for recognition and attempted repair of cell damage, as well as the elimination of pathogens and permanently damaged cells. Persistent inflammation, however, may promote tumor formation [2,3].
The intricate molecular and cellular mechanisms responsible for the association between inflammation and cancer have recently become subjects of intense study. Chronic inflammation is thought to induce carcinogenesis through a variety of mechanisms, including irreversible cellular and DNA damage through the generation of free radicals, and the promotion of rapid cellular growth through DNA and cellular replication [4]. Finally, a microenvironment rich in angiogenesis-promoting growth factors is created with the intent of repairing inflamed tissue, but instead establishes the ideal conditions conducive to tumor growth [2,3,5,6].
Well-established epidemiological studies have demonstrated that inflammatory diseases increase the risk of developing cancer. For example, gastric infection with Helicobacter pylori [7], inflammatory bowel disease [8], and chronic hepatitis [9] have all been linked to malignancies of the affected organs. In fact, it has been estimated that infections and inflammatory responses may be linked to upwards of 15% of worldwide cancer deaths [10]. Specifically regarding prostate cancer, it has been hypothesized that chronic intraprostatic inflammation—such as that associated with chronic prostatitis—may contribute to its development [11]. Several retrospective case-control studies have reported a positive association between prostatitis and prostate cancer [12]. Further supporting the link between chronic inflammation and cancer is the evidence that treatment with non-steroidal anti-inflammatory drugs (NSAIDs) has been found to decrease the incidence not only of prostate cancer [13,14], but also of several other solid tumors [15–17], although not all studies have upheld this finding [18–21].
C-reactive protein, an acute-phase reactant first described circa 1930, is a sensitive marker of tissue damage and inflammation [22,23]. A growing body of literature has described a correlation between circulating C-reactive protein serum levels and poor prognosis in patients with various solid tumors. Elevated CRP has been associated with shorter survival in melanoma [24], colorectal cancer [25], non-Hodgkins lymphoma [26], esophageal carcinoma [27], cervical cancer [28], endometrial cancer [29], ovarian cancer [30] and renal cell carcinoma [31].
We previously reported that higher serum concentrations of C-reactive protein (CRP) are associated with shorter overall survival in patients with CRPC, and that it also predicts a lower probability of PSA response to docetaxel-based therapy [32]. In this study, we sought to confirm these findings in an independent data set.
MATERIAL AND METHODS
Patients
Patients with castrate-resistant prostate cancer (CRPC) from six institutional phase II clinical trials were included in this analysis. Detailed eligibility criteria and the treatment in each of these studies have previously been described [33–38]. Regimens tested included calcitriol + docetaxel, calcitriol + docetaxel + estramustine, calcitriol + carboplatin, imatinib and zoledronic acid, and abarelix. All patients had evidence of progression after standard androgen suppression therapy. 90.8% of patients had metastases and 15.5% had prior chemotherapy exposure. The median follow-up time was 19.7 months (range 0.9–98.5 months), and 89.1% of patients have died. Institutional Review Board approval was obtained for all studies and for biomarker analyses, and informed consent was obtained from all patients contributing samples.
Sample Handling and Assays
Blood samples were collected from 119 CRPC patients prior to initiation of therapy. All baseline samples were plasma, except for one serum sample. Plasma was separated by centrifugation at 3000 revolutions per minute (rpm), and samples were stored at −80°C. CRP concentration was measured using turbidimetric measurement of agglutinated anti-CRP antibody/CRP complexes (Roche Diagnostics, Indianapolis, IN).
Statistical Methods
The endpoint of interest was overall survival (OS), defined as the time from day 1 of the start of therapy to death from any cause. Baseline covariates included in the analyses were age, alkaline phosphatase, Eastern Cooperative Oncology Group (ECOG) performance status, hemoglobin, PSA, and CRP. Because the distribution of alkaline phosphatase, CRP and PSA were skewed, a logarithmic transformation (base 2) was applied. Generalized Additive Models [39] were used to visually assess functional relationships between the continuous covariates and the risk of death. The risk function of each covariate was analyzed using the GAM package available in the R (www.r-project.org) software. We were thus able to determine if a covariate would be best analyzed as a continuous or categorical predictor in subsequent analyses [40]. Multivariate analysis was performed using the stepwise Cox regression in order to assess the association between the above modified covariates and overall survival. Statistical significance was defined as a P values less than 0.05.
RESULTS
Patient Demographics
Baseline characteristics of the 119 patients are shown in Table 1. 90.8% had radiographically demonstrated metastases while the others had CRPC manifested by a rising PSA on androgen suppression therapy only. 57 of these patients were enrolled in clinical trials of docetaxel-based chemotherapy while the remainder received other investigational regimens. None of these patients received the current FDA-approved regimen of docetaxel and prednisone. Quartiles of CRP were: 1: 0–1.0, 1.1–4.9, 5.0–17.0, and 17.1 to 311 mg/L, somewhat lower than in our previous study.
Table 1.
Baseline Characteristics of Patients
| Treatment regimen | |
| calcitriol + docetaxel (n) | 34 |
| abarelix + orchiectomy (n) | 12 |
| abarelix + LHRH antagonist (n) | 18 |
| calcitriol + docetaxel + estrumustine (n) | 23 |
| carboplatin + calcitriol (n) | 17 |
| imatinib + zoledronic acid (n) | 15 |
| Total (n) | 119 |
| Median age (range), years | 71.9 (45.8–91.5) |
| ECOG performance status | |
| 0 | 34.5% |
| > 0 | 65.5% |
| Median PSA (range), ng/mL | 80.8 (0.8–2113) |
| Median hemoglobin (range), g/dL | 12.4 (8.4–16.7) |
| Median alkaline phosphatase (range), U/L | 113 (33–1304) |
| Median CRP (range), mg/L | 5.0 (1–311) |
| Metastasis (all sites) | 90.8% |
| Chemotherapy naive | 84.5% |
| Subjects still alive | 10.9% |
| Median follow-up time (range), months | 19.7 (0.9–98.5) |
Overall Survival
Based on the GAM analysis, the risk function for the log2 transformed baseline alkaline phosphatase was best analyzed categorically in two groups, above or below 238.9 U/L, whereas log2 hemoglobin displayed a decrease in risk. The risk of death increased as log2 (CRP) increased. Baseline age was categorized into three groups (50–60 years, 60–80 years and greater than 80 years of age). The risk function increased with the log2 (PSA) and then decreased for log2 (PSA) >8, thus the log2 (PSA) variable was categorized into two groups (log2 (PSA) ≤ 8 or >8).
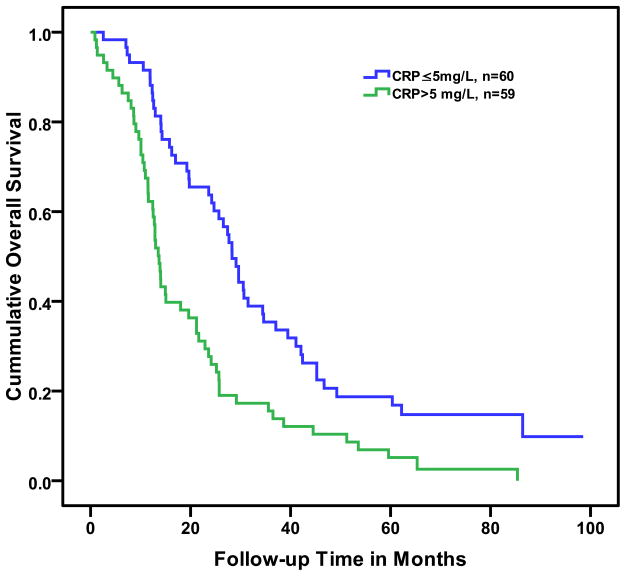
In the univariate Cox proportional hazard model, log2 (CRP) was a significant predictor of shorter overall survival. The impact of an elevated baseline CRP on overall survival is shown in Figure 1.
Figure 1.
Kaplan-Meier curve of below the median (≤5 mg/L) or above the median (>5 mg/L) C-reactive protein (CRP) on overall survival in men with castrate-resistant prostate cancer (log rank p < 0.0001).
Using multivariate analysis with the stepwise Cox regression model, we analyzed age, alkaline phosphatase, Eastern Cooperative Oncology Group (ECOG) performance status, hemoglobin, PSA, and CRP. In this multivariate analysis (Table 2), an elevated CRP remained a significant independent predictor of shorter survival (p value=0.013), showing a 10.6% increase in risk for every doubling of CRP. Baseline hemoglobin was inversely and significantly associated with overall survival (p=.006). Alkaline phosphatase was also significantly associated with overall survival (p value=0.014), showing a 2-fold increase in risk of death for a baseline level greater than 238.9 U/L. Age, ECOG performance status, and PSA were not significant predictors of survival in the multivariate model. A stratified analysis by disease extent (metastatic vs. PSA only) yielded the same results, but lacked power in the PSA only subgroup.
Table 2.
Independent Risk Factors for Death
| Prognostic Factor | HR (95% CI) | P |
|---|---|---|
| CRP (continuous, per each doubling of CRP) | 1.106 (1.022–1.197) | 0.013 |
| Alkaline phosphatase (categorical, above vs. below 238.9 U/L) | 1.944 (1.144–3.302) | 0.014 |
| Hemoglobin (continuous, per each g/dL) | 0.819 (0.711–0.943) | 0.006 |
DISCUSSION
In our independent data set of patients with CRPC, we confirmed our initial finding that elevated baseline C-reactive protein is associated with shorter overall survival. Recent evidence has suggested that elevated CRP is not only a marker of inflammation and cancer, but also plays a functional role in the proliferation of tumor cells. CRP has been found to inhibit apoptosis of myeloma cells, thereby directly regulating tumor cell growth and survival [41]. Moreover, HMG-CoA reductase inhibitors, commonly known as statins, decrease levels of circulating CRP [42]; a finding that, in addition to the primary lipid lowering properties, may be another mechanism by which statins may decrease the risk of prostate cancer and other solid tumors [43,44]. The possibility that CRP may contribute to the pathogenesis of cancer indicates that CRP (in addition to inflammation) may be a potential target for novel cancer treatments.
Our current study has some limitations. As in our previous study, a sample size of 119 patients is modest for a complete analysis of potential prognostic markers in men with CRPC. Additionally, although prognostic models that predict the overall survival probability for patients with CRPC are well established in the medical literature [45,46], we were not able to include all of these available predictors in this current study as these data were not uniformly collected in all patients. A larger sample size that includes data from a comprehensive set of prognostic factors would be needed to incorporate CRP into current prognostic models. Our effort was more modest, however, and sought to confirm our initial finding that CRP elevations are associated with shorter survival in an unrelated patient group. For this purpose, our patient sample proved adequate. With only 57 patients receiving docetaxel-based chemotherapy, our study did not have sufficient power to determine if CRP was associated with the probability of PSA decline in response to docetaxel-based chemotherapy in CRPC.
Similarly to all studies of CRP, we were unable to determine the specific causes of CRP elevation in these patients and unable to distinguish between increased CRP due to the presence of cancer from increased CRP related to other medical conditions. Although a formal determination of the cause of death in our patient subset was not performed, we would expect that the vast majority of these patients with CRPC die of prostate cancer rather than other co-morbid conditions [47].
Together with our prior results, we now have two retrospective analyses of independent data sets that demonstrate that baseline elevated CRP is associated with shorter survival in CRPC. This readily measurable biomarker should now be examined prospectively in a larger study that incorporates a broad range of potential prognostic factors. If prospective evaluation further confirms our findings, the inclusion of CRP may enhance the performance of current prognostic models. Unlike many of the other prognostic markers, CRP levels, as well as the underlying inflammation, are potentially modifiable. Thus, a better understanding of how inflammation, and potentially CRP itself, affects cancer progression and treatment resistance may have the potential to point the way toward improved therapies and outcomes in advanced prostate cancer.
Acknowledgments
Supported in part by a generous donation from Robert and Diana Gerding. Biostatistics support was provided by the Biostatistics Shared Resource of the Knight Cancer Institute (NIH/NCI P30 CA069533).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–45. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 2.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–44. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 4.Hussain SP, Hofseth LJ, Harris CC. Radical causes of cancer. Nat Rev Cancer. 2003;3:276–85. doi: 10.1038/nrc1046. [DOI] [PubMed] [Google Scholar]
- 5.Ames BN, Gold LS, Willett WC. The causes and prevention of cancer. Proc Natl Acad Sci U S A. 1995;92:5258–65. doi: 10.1073/pnas.92.12.5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cerutti PA, Trump BF. Inflammation and oxidative stress in carcinogenesis. Cancer Cells. 1991;3:1–7. [PubMed] [Google Scholar]
- 7.Parsonnet J, Friedman GD, Vandersteen DP, et al. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127–31. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- 8.Rhodes JM, Campbell BJ. Inflammation and colorectal cancer: IBD-associated and sporadic cancer compared. Trends Mol Med. 2002;8:10–6. doi: 10.1016/s1471-4914(01)02194-3. [DOI] [PubMed] [Google Scholar]
- 9.Imperial JC. Natural history of chronic hepatitis B and C. J Gastroenterol Hepatol. 1999;14 (Suppl):S1–5. doi: 10.1046/j.1440-1746.1999.01903.x. [DOI] [PubMed] [Google Scholar]
- 10.Pisani P, Parkin DM, Munoz N, Ferlay J. Cancer and infection: estimates of the attributable fraction in 1990. Cancer Epidemiol Biomarkers Prev. 1997;6:387–400. [PubMed] [Google Scholar]
- 11.De Marzo AM, Marchi VL, Epstein JI, Nelson WG. Proliferative inflammatory atrophy of the prostate: implications for prostatic carcinogenesis. Am J Pathol. 1999;155:1985–92. doi: 10.1016/S0002-9440(10)65517-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dennis LK, Lynch CF, Torner JC. Epidemiologic association between prostatitis and prostate cancer. Urology. 2002;60:78–83. doi: 10.1016/s0090-4295(02)01637-0. [DOI] [PubMed] [Google Scholar]
- 13.Mahmud S, Franco E, Aprikian A. Prostate cancer and use of nonsteroidal anti-inflammatory drugs: systematic review and meta-analysis. Br J Cancer. 2004;90:93–9. doi: 10.1038/sj.bjc.6601416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nelson JE, Harris RE. Inverse association of prostate cancer and non-steroidal anti-inflammatory drugs (NSAIDs): results of a case-control study. Oncol Rep. 2000;7:169–70. doi: 10.3892/or.7.1.169. [DOI] [PubMed] [Google Scholar]
- 15.Corley DA, Kerlikowske K, Verma R, Buffler P. Protective association of aspirin/NSAIDs and esophageal cancer: a systematic review and meta-analysis. Gastroenterology. 2003;124:47–56. doi: 10.1053/gast.2003.50008. [DOI] [PubMed] [Google Scholar]
- 16.Dannenberg AJ, Subbaramaiah K. Targeting cyclooxygenase-2 in human neoplasia: rationale and promise. Cancer Cell. 2003;4:431–6. doi: 10.1016/s1535-6108(03)00310-6. [DOI] [PubMed] [Google Scholar]
- 17.Suh KS, Min SK. Flow cytometric DNA analysis of gastric cancer--correlation with histology and clinical outcome. J Korean Med Sci. 1993;8:348–54. doi: 10.3346/jkms.1993.8.5.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cook NR, Lee IM, Gaziano JM, et al. Low-dose aspirin in the primary prevention of cancer: the Women’s Health Study: a randomized controlled trial. JAMA. 2005;294:47–55. doi: 10.1001/jama.294.1.47. [DOI] [PubMed] [Google Scholar]
- 19.Daniels NA, Chen YH, Bent S. Antibiotic and anti-inflammatory use and the risk of prostate cancer. BMC Res Notes. 2009;2:57. doi: 10.1186/1756-0500-2-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eliassen AH, Chen WY, Spiegelman D, Willett WC, Hunter DJ, Hankinson SE. Use of aspirin, other nonsteroidal anti-inflammatory drugs, and acetaminophen and risk of breast cancer among premenopausal women in the Nurses’ Health Study II. Arch Intern Med. 2009;169:115–21. doi: 10.1001/archinternmed.2008.537. discussion 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Genkinger JM, De Vivo I, Stampfer MJ, Giovannucci E, Michaud DS. Nonsteroidal antiinflammatory drug use and risk of bladder cancer in the health professionals follow-up study. Int J Cancer. 2007;120:2221–5. doi: 10.1002/ijc.22546. [DOI] [PubMed] [Google Scholar]
- 22.Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003;111:1805–12. doi: 10.1172/JCI18921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tillett WS, Francis T. Serological Reactions in Pneumonia with a Non-Protein Somatic Fraction of Pneumococcus. J Exp Med. 1930;52:561–71. doi: 10.1084/jem.52.4.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tartour E, Dorval T, Mosseri V, et al. Serum interleukin 6 and C-reactive protein levels correlate with resistance to IL-2 therapy and poor survival in melanoma patients. Br J Cancer. 1994;69:911–3. doi: 10.1038/bjc.1994.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nozoe T, Matsumata T, Kitamura M, Sugimachi K. Significance of preoperative elevation of serum C-reactive protein as an indicator for prognosis in colorectal cancer. Am J Surg. 1998;176:335–8. doi: 10.1016/s0002-9610(98)00204-9. [DOI] [PubMed] [Google Scholar]
- 26.Legouffe E, Rodriguez C, Picot MC, et al. C-reactive protein serum level is a valuable and simple prognostic marker in non Hodgkin’s lymphoma. Leuk Lymphoma. 1998;31:351–7. doi: 10.3109/10428199809059228. [DOI] [PubMed] [Google Scholar]
- 27.Nozoe T, Saeki H, Sugimachi K. Significance of preoperative elevation of serum C-reactive protein as an indicator of prognosis in esophageal carcinoma. Am J Surg. 2001;182:197–201. doi: 10.1016/s0002-9610(01)00684-5. [DOI] [PubMed] [Google Scholar]
- 28.Polterauer S, Grimm C, Tempfer C, et al. C-reactive protein is a prognostic parameter in patients with cervical cancer. Gynecol Oncol. 2007;107:114–7. doi: 10.1016/j.ygyno.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 29.Schmid M, Schneitter A, Hinterberger S, Seeber J, Reinthaller A, Hefler L. Association of elevated C-reactive protein levels with an impaired prognosis in patients with surgically treated endometrial cancer. Obstet Gynecol. 2007;110:1231–6. doi: 10.1097/01.AOG.0000292085.50987.f2. [DOI] [PubMed] [Google Scholar]
- 30.Hefler LA, Concin N, Hofstetter G, et al. Serum C-reactive protein as independent prognostic variable in patients with ovarian cancer. Clin Cancer Res. 2008;14:710–4. doi: 10.1158/1078-0432.CCR-07-1044. [DOI] [PubMed] [Google Scholar]
- 31.Tatokoro M, Saito K, Iimura Y, Fujii Y, Kawakami S, Kihara K. Prognostic impact of postoperative C-reactive protein level in patients with metastatic renal cell carcinoma undergoing cytoreductive nephrectomy. J Urol. 2008;180:515–9. doi: 10.1016/j.juro.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 32.Beer TM, Lalani AS, Lee S, et al. C-reactive protein as a prognostic marker for men with androgen-independent prostate cancer: results from the ASCENT trial. Cancer. 2008;112:2377–83. doi: 10.1002/cncr.23461. [DOI] [PubMed] [Google Scholar]
- 33.Beer TM, Garzotto M, Katovic NM. High-dose calcitriol and carboplatin in metastatic androgen-independent prostate cancer. Am J Clin Oncol. 2004;27:535–41. doi: 10.1097/01.coc.0000136020.27904.9c. [DOI] [PubMed] [Google Scholar]
- 34.Tiffany NM, Ryan CW, Garzotto M, Wersinger EM, Beer TM. High dose pulse calcitriol, docetaxel and estramustine for androgen independent prostate cancer: a phase I/II study. J Urol. 2005;174:888–92. doi: 10.1097/01.ju.0000169261.42298.e6. [DOI] [PubMed] [Google Scholar]
- 35.Beer TM, Eilers KM, Garzotto M, Egorin MJ, Lowe BA, Henner WD. Weekly high-dose calcitriol and docetaxel in metastatic androgen-independent prostate cancer. J Clin Oncol. 2003;21:123–8. doi: 10.1200/jco.2003.05.117. [DOI] [PubMed] [Google Scholar]
- 36.Tiffany NM, Wersinger EM, Garzotto M, Beer TM. Imatinib mesylate and zoledronic acid in androgen-independent prostate cancer. Urology. 2004;63:934–9. doi: 10.1016/j.urology.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 37.Beer TM, Garzotto M, Eilers KM, Lemmon D, Wersinger EM. Targeting FSH in androgen-independent prostate cancer: abarelix for prostate cancer progressing after orchiectomy. Urology. 2004;63:342–7. doi: 10.1016/j.urology.2003.09.045. [DOI] [PubMed] [Google Scholar]
- 38.Beer TM, Garzotto M, Eilers KM, Lemmon D. Phase II study of abarelix depot for androgen independent prostate cancer progression during gonadotropin-releasing hormone agonist therapy. J Urol. 2003;169:1738–41. doi: 10.1097/01.ju.0000059584.47272.9d. [DOI] [PubMed] [Google Scholar]
- 39.Hastie T, Tibshirani R. Exploring the nature of covariate effects in the proportional hazards model. Biometrics. 1990;46:1005–16. [PubMed] [Google Scholar]
- 40.Garzotto M, Beer TM, Hudson RG, et al. Improved detection of prostate cancer using classification and regression tree analysis. J Clin Oncol. 2005;23:4322–9. doi: 10.1200/JCO.2005.11.136. [DOI] [PubMed] [Google Scholar]
- 41.Yang J, Wezeman M, Zhang X, et al. Human C-reactive protein binds activating Fcgamma receptors and protects myeloma tumor cells from apoptosis. Cancer Cell. 2007;12:252–65. doi: 10.1016/j.ccr.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 42.Albert MA, Danielson E, Rifai N, Ridker PM. Effect of statin therapy on C-reactive protein levels: the pravastatin inflammation/CRP evaluation (PRINCE): a randomized trial and cohort study. JAMA. 2001;286:64–70. doi: 10.1001/jama.286.1.64. [DOI] [PubMed] [Google Scholar]
- 43.Poynter JN, Gruber SB, Higgins PD, et al. Statins and the risk of colorectal cancer. N Engl J Med. 2005;352:2184–92. doi: 10.1056/NEJMoa043792. [DOI] [PubMed] [Google Scholar]
- 44.Shannon J, Tewoderos S, Garzotto M, et al. Statins and prostate cancer risk: a case-control study. Am J Epidemiol. 2005;162:318–25. doi: 10.1093/aje/kwi203. [DOI] [PubMed] [Google Scholar]
- 45.Halabi S, Small EJ, Kantoff PW, et al. Prognostic model for predicting survival in men with hormone-refractory metastatic prostate cancer. J Clin Oncol. 2003;21:1232–7. doi: 10.1200/JCO.2003.06.100. [DOI] [PubMed] [Google Scholar]
- 46.Armstrong AJ, Garrett-Mayer ES, Yang YC, de Wit R, Tannock IF, Eisenberger M. A contemporary prognostic nomogram for men with hormone-refractory metastatic prostate cancer: a TAX327 study analysis. Clin Cancer Res. 2007;13:6396–403. doi: 10.1158/1078-0432.CCR-07-1036. [DOI] [PubMed] [Google Scholar]
- 47.Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281:1591–7. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]