Abstract
Water-solublel-arginine-capped Fe3O4 nanoparticles were synthesized using a one-pot and green method. Nontoxic, renewable and inexpensive reagents including FeCl3,l-arginine, glycerol and water were chosen as raw materials. Fe3O4 nanoparticles show different dispersive states in acidic and alkaline solutions for the two distinct forms of surface bindingl-arginine. Powder X-ray diffraction and X-ray photoelectron spectroscopy were used to identify the structure of Fe3O4 nanocrystals. The products behave like superparamagnetism at room temperature with saturation magnetization of 49.9 emu g−1 and negligible remanence or coercivity. In the presence of 1-ethyl-3-(dimethylaminopropyl) carbodiimide hydrochloride, the anti-chloramphenicol monoclonal antibodies were connected to thel-arginine-capped magnetite nanoparticles. The as-prepared conjugates could be used in immunomagnetic assay.
(See supplementary material 1)
Keywords: Magnetite, Superparamagnetic, Solvothermal, Amino acid, Nanocrystals
Introduction
In the last decade, inherently safer nanomaterials and nanostructured devices were widely fabricated with the “green chemistry” principles [1-13]. It is important to design synthetic methodologies that possess the minimization or even total elimination toxicity to the environment and human health in green chemistry [1,14]. The nontoxic, renewable raw materials and environmentally benign solvents are generally considered in a green synthetic strategy [1]. As society and environment can benefit from the products, green chemistry can convey a responsible attitude to public toward the development of nanoscience and nanotechnology [14].
Magnetite (Fe3O4) nanoparticles have attracted intensive interests for a wide range of fields, including magnetic fluids, immobilization of proteins, peptides and enzymes, immunoassays, drug or gene delivery magnetic resonance imaging, data storage, environmental remediation [15-25]. The Fe3O4 nanoparticles perform best in most of biomedicinal applications when the size of the nanoparticles is around 10–20 nm. In this range, an individual nanoparticle becomes a single magnetic domain and shows superparamagnetic behavior above blocking temperature [26,27]. Large numbers of methods have been developed for the synthesis of high-quality Fe3O4 nanoparticles of various surface modifier based on the thermal decomposition of iron organometallic compounds in a high-boiling point organic solvent [28-37]. When those magnetite nanoparticles are applied in biomedical fields, surface post-treatments are usually needed.
In the present work, we described a facile and green approach toward synthesis and stabilization of Fe3O4 nanoparticles. Water and glycerol were used as environmentally benign solvents in the synthesis. Inartificial amino acidl-arginine was chosen as the nontoxic, renewable stabilizing agent.
Experimental Section
Materials
Chloramphenicol (CAP) and 1-ethyl-3-(dimethylaminopropyl) carbodiimide hydrochloride (EDC) were purchased from Sigma–Aldrich. o-Phenylenediamine (OPD) was purchased from Xinjingke Biotechnology. Hydrogen peroxide (30%) was supplied by Guangmang Chemical Co. The anti-CAP monoclonal antibody and HRP-CAP conjugates were produced by our lab. Other analytical grade chemicals were purchased from Shanghai Chemical Reagents Company. All of the chemicals were used as received without further purification.
Buffers and solutions used were listed below:
a. Phosphate-buffered saline (PBS): 138 mM NaCl, 1.5 mM KH2PO4, 8 mM Na2HPO4·H2O and 2.7 mM KCl, pH = 7.4.
b. Washing buffer (PBST): PBS containing 0.05 (v/v) Tween 20.
c. Citrate buffer: 19 mM citric acid, 33.5 mM Na2HPO4·H2O, pH = 5.0
d. Substrate solution: 5 mg OPD, 12.5 mL citrate buffer, 2.5 μL H2O2(30%).
e. Stopping solution: 2 N HCl.
Synthesis ofl-Arginine-Capped Fe3O4 Nanoparticles
l-Arginine (3.0 g) and FeCl3(0.5 g) were added to a component solvent containing glycerol (10 mL) and water (10 mL). A transparent solution formed through sonication of this mixture. This solution was transferred into a Teflon-lined stainless steel autoclave with a capacity of 50 mL and maintained at 200°C for 6 h. Then, the autoclave was cooled to room temperature naturally. The product was washed with distilled water to remove residue of solvent and unboundl-arginine, finally dried by vacuum freeze-desiccation technology before characterization. During each step, the product was separated from the suspension by magnetic force.
Preparation of Magnetic Nanoparticles Conjugates
A solution was formed by mixing 250 μL Fe3O4 nanoparticles suspension and 1 mL phosphate-buffered saline (PBS). Then, 10 μL of anti-CAP monoclonal antibody and 1 mg of 1-ethyl-3-(dimethylaminopropyl) carbodiimide hydrochloride (EDC) were added. Afterward, the mixture was incubated overnight with light shaking at room temperature. Excess EDC and the supernatant were removed by magnetic separation, and the precipitate was washed three times with PBS. Antibody-labeled magnetic nanoparticles were redispersed in PBS (1 mL) and stored at 4°C for use.
Immunomagnetic Assay
The above store suspension (100 μL) was added to a tube and rinsed three times with washing buffer (PBST) in a magnetic field. Then, 100 μL conjugates of chloramphenicol and horseradish peroxidase (CAP-HRP) were injected. The incubation was performed for 2 h at room temperature with constant shaking. The sample was washed three times with PBST as earlier. Substrate solution (100 μL) was added, and the reaction was kept for 15 min. Finally, stopping solution (2 N HCl) was used to stop the reaction, and the absorbance was determined at 492 nm. A comparative experiment was performed just replaced magnetic nanoparticles conjugates with unlabeled magnetic nanoparticles.
Characterization
XRD patterns were recorded on the X-ray diffractometer (Bruker D8) with a graphite monochromator and Cu Kα radiation (λ = 1.5418 Å) in the range of 10–80° at room temperature. The morphology of the products was determined with transmission electron microscopy (JEM-100CXII) with an accelerating voltage of 80 kV. The nanocrystals dispersed in water were cast onto a carbon-coated copper grid. Magnetization measurements of the nanocomposites were performed with a Micromag 2900 at room temperature under ambient atmosphere. X-ray photoelectron spectra (XPS) were measured with X-Ray photoelectron spectroscopy XPS (ESCALAB 250). Enzyme immunoassay (ELISA) was performed with an automatic microplate reader KHB ST-360 from Shanghai Zhihua Medical Instrument Ltd.
Results and Discussion
Black products were prepared via a one-step solvothermal method.l-Arginine, an alkaline amino acid with guanidino group, was served as capped reagent in this reaction. The crystallinity and phase purity were determined by powder X-ray diffraction (XRD) as shown in supporting information. All diffraction peaks could be assigned to inverse spinel Fe3O4 phase (JCPDS card 19-0629). No other crystalline impurity was detected. The lattice constant calculated from this pattern was 8.389 Å, which is very close to the reported value. A representative TEM image ofl-arginine-capped Fe3O4 nanoparticles dispersed in acidic solution is shown in Fig. 1a, which indicates that Fe3O4 nanoparticles have an average diameter of 13 nm. The high-resolution transmission electron microscopy (HRTEM) image (Fig. 1b) suggests the crystalline nature of Fe3O4 nanoparticles with a clearly resolved lattice spacing of around 0.483 nm, corresponding to that of (111) of inverse spinel Fe3O4 crystal. All the spots of Fourier transformed pattern (Fig. 1c) obtained from the HRTEM image in Fig. 1b can be indexed as those peculiar to the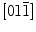 zone axis of face centered cubic Fe3O4.
zone axis of face centered cubic Fe3O4.
Figure 1.
a TEM image of the Fe3O4 nanocrystal. b HRTEM image of single Fe3O4 nanoparticle c FFT of HRTEM image in (b)
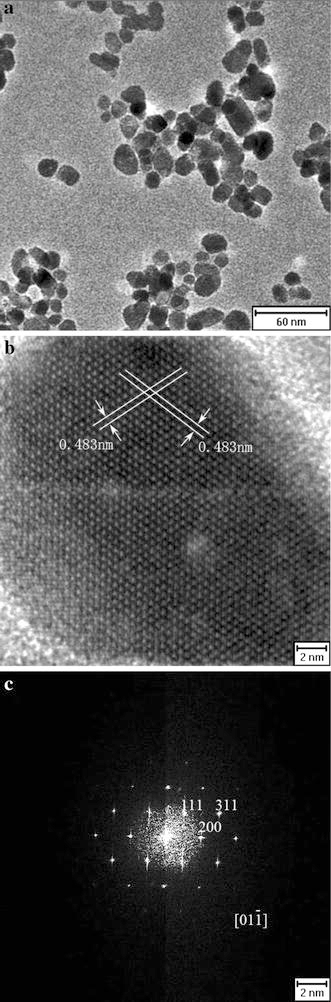
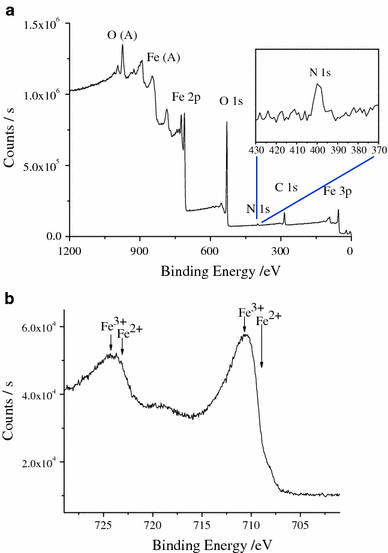
X-ray photoelectron spectroscopy (XPS) was used to further confirm the products. From spectra in Fig. 2a, the peaks of the C 1 s, O 2p, N 1 s, Fe 3p and Fe 2p indicate thel-arginine molecules are located on the surface of Fe3O4 nanoparticles. In Fig. 2b, Fe 2p3/2 and Fe 2p1/2 double peaks correspond to binding energies of 710.55 and 723.70 eV, respectively. The double peaks are broadened due to the appearance of Fe2+(2p3/2) and Fe2+(2p1/2), in agreement with the literature that the peaks broaden for Fe3O4 on the appearance of Fe2+(2p3/2) and Fe2+(2p1/2) [38,39]. This phenomenon confirms the product is Fe3O4 rather than γ-Fe2O3. As is shown in the magnetic hysteresis loop of l-arginine-capped Fe3O4 nanoparticles (Fig. 3), the nanocrystals behave with superparamagnetism at room temperature with saturation magnetization of 49.9 emu g−1 and negligible remanence or coercivity.
Figure 2.
a The XPS of thel-arginine-capped Fe3O4 nanoparticles. Evidence for the existence ofl-arginine coating can be found. b The details of the Fe 2p1/2 and Fe 2p3/2 peaks
Figure 3.
Magnetic hysteresis loop measured at room temperature for thel-arginine-capped Fe3O4 nanoparticles. The NPs show superparamagnetic properties at room temperature, and the Ms is about 49.9 emu g−1
The different dispersing state of Fe3O4 nanoparticles in acidic and alkaline solutions can be clearly observed by naked eye, as shown by the supporting information Fig. S2. Fe3O4 nanoparticles dispersed in an alkaline solution completely precipitated in a few minutes, while they are stable in an acidic solution for at least 1 month and could be moved by a magnet just like ferrofluid. When the suspensions were filtrated with 0.45 μm filtration membrane, we got colorless and transparent liquid as Fe3O4 nanoparticles could not pass filtration membrane in alkaline solution. On the other hand, black and homogeneous solution was collected in acidic solution.l-Arginine is an inartificial amino acid. The amino group and the acid group could exist in the form of ammonium ions and carboxylate ions, respectively, under certain conditions [40]. It has been reported that both amine and acid groups are able to attach onto iron oxide surface [17,25]. When the guanidino group of l-arginine attaches onto the surface of iron oxide, the nanoparticles are expected to have distinct states in solutions with different pH value. Although the isoelectric point (pI) of pure l-arginine is 10.76 [40], the isoelectric point is expected to change for the attachment of the guanidino group in l-arginine to Fe3O4 nanoparticles. The new isoelectric point will be the average of the pKa of the carboxylic acid group and the pKb of the amine group [40] and therefore, ca. 5.61. As illustrated in Scheme 1, in an acidic solution, the l-arginine molecules exist in the cationic form due to the formation of ammonium ions. These ammonium ions may prevent formation of hydrogen bonds between Fe3O4 nanoparticles. In an alkaline solution, surface-bound l-arginine molecules are negatively charged due to the formation of carboxylate ions which readily form hydrogen bonds with surface-bound amine groups of neighboring Fe3O4 nanoparticles. This phenomenon is similar to the case of lysine-capped gold nanoparticles [41].
Scheme 1.

Illustration of the assembly of dispersedl-arginine-capped Fe3O4 nanoparticles in water at different pH values. The inset is the structure ofl-arginine
To demonstrate potential biomedical applications ofl-arginine-capped Fe3O4 nanoparticles, magnetite nanoparticles were bioconjugated with anti-CAP monoclonal antibody to form the immunomagnetic beads (IMB) via the classical EDC activation [42,43]. Then, they are used in the immunological test. The result showed that the mixture containing anti-CAP monoclonal antibody-labeled magnetic nanoparticles had a deep yellow color (Fig. 4 right) after color development, and the absorbance was 2.113, while the comparative one had no obvious color change (Fig. 4 left) at the same time and the absorbance was 0.065. It was suggested that l-arginine-capped Fe3O4 nanoparticles were successfully attached to the anti-CAP monoclonal antibody.
Figure 4.

Photographs of color development of unlabeled (left) and antibody-labeled (right) magnetic nanoparticles
Conclusions
We have synthesizedl-arginine-capped superparamagnetic Fe3O4 nanoparticles via a simple and green method in water and glycerol component solvent. The synthesized Fe3O4 nanoparticles have an average diameter of 13 nm and the saturation magnetization reaches to 49.9 emu g−1 with negligible remanence or coercivity. With superparamagnetic properties and the active groups on the surface of the nanoparticles, their application for magnetic separation and concentration in immunoassays were further demonstrated. These products are expected to have more extensive applications in biomedical fields.
Supplementary Material
The online version of this article (doi:10.1007/s11671-009-9480-x) contains supplementary material, which is available to authorized users.
Acknowledgments
Financial support from the Program for New Century Excellent Talents in University (NCET-06-0586), the Key Project of Chinese Ministry of Education (No. 109098), and the National Basic Research Program of China (973 Program 2005CB623601, 2007CB936602) is gratefully acknowledged. Prof. Xi acknowledges the financial support from the National Natural Science Foundation of China (No. 20675048), the National High-Tech Research and Developmental Program of China (863 Program, No. 07AA10Z435 and 2007AA06A407).
References
- Anastas PT, Warner JC. Green Chemistry: Theory and Practice. Oxford university Press Inc, New York; 1998. [Google Scholar]
- Matlack AS. Introduction to Green Chemistry. Marcel Decker, New York; 2001. [Google Scholar]
- Raveendran P, Fu J, Wallen SL. J. 2003. p. 13940. COI number [1:CAS:528:DC%2BD3sXot12ms7k%3D] [DOI] [PubMed]
- Raveendran P, Fu J, Wallen SL. Green Chem. 2006. p. 34. COI number [1:CAS:528:DC%2BD28XhsF2ntA%3D%3D] [DOI]
- Hu B, Wang SB, Wang K, Zhang M, Yu SH. J. 2008. p. 11169. COI number [1:CAS:528:DC%2BD1cXotVCltbo%3D] [DOI]
- Shankar SS, Rai A, Ankamwar B, Singh A, Ahmad A, Sastry M. Nat. 2004. p. 482. COI number [1:CAS:528:DC%2BD2cXlt1Ors74%3D]; Bibcode number [2004NatMa...3..482S] [DOI] [PubMed]
- Xie JP, Lee JY, Wang DIC, Ting YP. Small. 2007. p. 672. COI number [1:CAS:528:DC%2BD2sXktFGntLk%3D] [DOI] [PubMed]
- Wang Y, Maksimuk S, Shen R, Yang H. Green Chem. 2007. p. 1051. [DOI]
- Mukherjee P, Ahmad A, Mandal D, Senapati S, Sainkar SR, Khan MI, Parishcha R, Ajaykumar PV, Alam M, Kumar R, Sastry M. Nano Lett. 2001. p. 515. COI number [1:CAS:528:DC%2BD3MXmt1OksL8%3D]; Bibcode number [2001NanoL...1..515M] [DOI]
- Saito Y, Wang JJ, Smith DA, Batchelder DN. Langmuir. 2002. p. 2959. COI number [1:CAS:528:DC%2BD38XhvFymtLw%3D] [DOI]
- Mukherjee P, Ahmad A, Mandal D, Senapati S, Sainkar SR, Khan MI, Parishcha R, Ajaykumar PV, Alam M, Kumar R, Sastry M. Angew. 2001. p. 3585. COI number [1:CAS:528:DC%2BD3MXns1Wgsrw%3D] [DOI] [PubMed]
- Yang JH, Lu LH, Wang HS, Shi WD, Zhang HJ. Cryst. 2006. p. 2155. COI number [1:CAS:528:DC%2BD28XnsFemur8%3D] [DOI]
- Nadagouda MN, Varma RS. Green Chem. 2006. p. 516. COI number [1:CAS:528:DC%2BD28Xltlars70%3D] [DOI]
- Jennifer AD, Bettye LSM, James EH. Chem. 2007. p. 2228. [DOI] [PubMed]
- Neuberger T, Schöpf B, Hofmann H, Hofmann M, von Rechenberg BJ. J. 2005. p. 483. COI number [1:CAS:528:DC%2BD2MXktVagsbo%3D]; Bibcode number [2005JMMM..293..483N] [DOI]
- Liu JF, Zhao ZS, Jiang GB. Environ. 2008. p. 6949. COI number [1:CAS:528:DC%2BD1cXpvVSisLw%3D] [DOI] [PubMed]
- Wang LY, Bao J, Wang L, Zhang F, Li YD. Chem. 2006. p. 6341. COI number [1:CAS:528:DC%2BD28XptV2ntL4%3D] [DOI] [PubMed]
- Xu C, Xu K, Gu H, Zhong X, Guo Z, Zheng R, Zhang X, Xu B. J. 2004. p. 3392. COI number [1:CAS:528:DC%2BD2cXhsFyitrw%3D] [DOI] [PubMed]
- Jia X, Chen DR, Jiao XL, Zhai SM. Chem. 2009. p. 968. [DOI] [PubMed]
- Shi QX, Lu RW, Jin K, Zhang ZX, Zhao DF. Green Chem. 2006. p. 868. COI number [1:CAS:528:DC%2BD28XhtVCiu7rE] [DOI]
- Dyal A, Loos K, Noto M, Chang SW, Spagnoli C, Shafi KVPM, Ulman A, Cowman M, Gross RA. J. 2003. p. 1684. COI number [1:CAS:528:DC%2BD3sXmslaktQ%3D%3D] [DOI] [PubMed]
- Mirzabekov T, Kontos H, Farzan M, Marasco W, Sodroski J. Nat. 2000. p. 649. COI number [1:CAS:528:DC%2BD3cXktlSktbo%3D] [DOI] [PubMed]
- Won J, Kim M, Yi YW, Kim YH, Jung N, Kim TK. Science. 2005. p. 121. COI number [1:CAS:528:DC%2BD2MXlsF2htro%3D]; Bibcode number [2005Sci...309..121W] [DOI] [PubMed]
- Kotani M, Koike T, Yamaguchi K, Mizuno N. Green Chem. 2006. p. 735. COI number [1:CAS:528:DC%2BD28XnsFyitLs%3D] [DOI]
- Ge JP, Hu YX, Biasini M, Dong CL, Guo JH, Beyermann WP, Yin YD. Chem. 2007. p. 7153. COI number [1:CAS:528:DC%2BD2sXhtVeltbnN] [DOI] [PubMed]
- Lu AH, Salabas EL, Schüth F. Angew. 2007. p. 1222. COI number [1:CAS:528:DC%2BD2sXitF2lurs%3D] [DOI] [PubMed]
- Varadan VK, Chen LF, Xie JN. Nanomedicine: Design and Applications of Magnetic Nanomaterials. Nanosensors and Nanosystems John Wiley & Sons, Ltd New York; 2008. [Google Scholar]
- Sun SH, Zeng H, Robinson DB, Raoux S, Rice PM, Wang SX, Li GX. J. 2004. p. 273. COI number [1:CAS:528:DC%2BD3sXps1yms7o%3D] [DOI] [PubMed]
- Sun SH, Zeng H. J. 2002. p. 8204. COI number [1:CAS:528:DC%2BD38Xks1arurY%3D] [DOI] [PubMed]
- Xu ZC, Shen CM, Hou YL, Gao HJ, Sun SH. Chem. 2009. p. 1778. COI number [1:CAS:528:DC%2BD1MXksVOjsrg%3D] [DOI]
- Jana NR, Chen YF, Peng XG. Chem. 2004. p. 3931. COI number [1:CAS:528:DC%2BD2cXnsVaitb8%3D] [DOI]
- Teng XW, Yang H. J. 2003. p. 14559. COI number [1:CAS:528:DC%2BD3sXos1Wjs78%3D] [DOI] [PubMed]
- Park J, An K, Hwang Y, Park JG, Noh HJ, Kim JY, Park JH, Hwang NM, Hyeon T. Nat. 2004. p. 891. COI number [1:CAS:528:DC%2BD2cXhtVehtrjM]; Bibcode number [2004NatMa...3..891P] [DOI] [PubMed]
- Peng S, Sun SH. Angew. 2007. p. 4155. COI number [1:CAS:528:DC%2BD2sXmtlOrsb0%3D] [DOI] [PubMed]
- Shevchenko EV, Talapin DV, Kotov NA, O′Brien S, Murray CB. Nature. 2006. p. 55. COI number [1:CAS:528:DC%2BD28Xht1aqsQ%3D%3D]; Bibcode number [2006Natur.439...55S] [DOI] [PubMed]
- Hu FQ, Wei L, Zhou Z, Ran YL, Li Z, Gao MY. Adv. 2006. p. 2553. COI number [1:CAS:528:DC%2BD28XhtFWqtLrO] [DOI]
- Hui C, Shen CM, Yang TZ, Bao LH, Tian JF, Ding H, Li C, Gao HJ. J. 2008. p. 11336. COI number [1:CAS:528:DC%2BD1cXot1eru70%3D] [DOI]
- Pamela CC, Richard AH, Denise RF. Lippincotts Illustrated Reviews: Biochemistry. Lippincott Williams & Wilkins, Philadelphia; 2008. [Google Scholar]
- Temesghen W, Sherwood PMA. Anal. 2002. p. 601. COI number [1:CAS:528:DC%2BD38XmtVKjs7s%3D] [DOI] [PubMed]
- Li Z, Chen H, Bao HB, Gao MY. Chem. 2004. p. 1391. COI number [1:CAS:528:DC%2BD2cXit1Sqt70%3D] [DOI]
- Selvakannan PR, Mandal S, Phadtare S, Pasricha R, Sastry M. Langmuir. 2003. p. 3545. COI number [1:CAS:528:DC%2BD3sXhslSlt7k%3D] [DOI] [PubMed]
- Shelver WL, Kamp LM, Church JL, Rubio FM. J. 2007. p. 3758. COI number [1:CAS:528:DC%2BD2sXksFSns7g%3D] [DOI] [PubMed]
- Hottenstein CS, Jourdan SW, Hayes MC, Rubio FM, Herzog DP, Lawruk TS. Environ. 1995. p. 2754. COI number [1:CAS:528:DyaK2MXot1yltb8%3D] [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The online version of this article (doi:10.1007/s11671-009-9480-x) contains supplementary material, which is available to authorized users.


