Abstract
A new nanocomposite fluorescence probe with thioglycolic acid (TA) functional layers embedded inside the hydroxyapatite nanoribbon spherulites has been synthesized. The fluorescence intensity of the novel probe is about 1.5–3.3-fold increase compared with the probe containing no TA. When used to detect cadmium ion, the most of original assembly nanoribbon spherulites structure in the novel probe is found to have been damaged to new flake structures. The mechanism of determining cadmium ion in alcohol solution has been studied. The present systematic study provides significant information on the effect of assembly nanostructure on the metal-enhanced fluorescence phenomenon.
Keywords: Composite materials, Nanocomposite fluorescence probe, Cadmium ion, Hydroxyapatite
Introduction
Hydroxyapatite (Ca10(PO4)6(OH)2, HAP) is a calcium phosphate salt and being used as an artificial bone material due to its biocompatibility, bioactivity, osteoconductivity, nontoxicity, nonimmunogenicity, and noninflammatory behavior. [1]. Current preparation methods of HAP chiefly include conventional wet chemical [2], template synthesis [3], calcination of xenogenic bone [4], and hydrothermal conversion of calcium carbonate exoskeleton [5]. The properties of HAP differ with preparation route, reaction ingredients, etc. Among many properties, crystallographic changes directly affect the activity of HAP; thus, it is necessary to study its morphology and crystallography before practical application [6,7].
Many studies have recognized the ability of HAP to bind divalent heavy metal ions and have shown that synthetic HAP has a high removal capacity for Pb, Zn, Cu, Cd, Co, and Sb in aqueous solutions [8-20]. Mechanisms such as dissolution of HAP and precipitation of metal phosphates [10,11], surface complexation [11,12], ion exchange [13], substitution of Ca in HAP by metals during coprecipitation [21] have been proposed in order to describe the uptake of heavy metals from aqueous solutions by synthetic HAP. It is difficult to detect the formation of Cd complexes or modification of the structure of HAP by Cd due to the similar ionic radii of Ca (0.99 Å) and Cd (0.97 Å). Moreover, few studies about microanalysis in organic compounds by nanotechnology have been reported. Therefore, the corresponding research will be explored in present work.
The HAP nanoribbon spherulites, which had been obtained in our previous studies, have large surface, good bio-consistency and high physical/chemical activities [7]. Because of a lot of hydroxy groups in their surface, the HAP nanoribbon spherulites can be modified to prepare probe to detect heavy metals in organic compounds [22,23]. In this paper, we have prepared a novel HAP nanocomposite for developing a simple and rapid approach to increase the fluorescence sensitivity of Cd2+ in alcohol solutions.
Experimental Section
The Preparation of Hydroxyapatite Nanoribbon Spherulites
The HAP nanoribbon spherulites were prepared according to our previous study showed in reference [7]. The eggshell membrane was made by removing the outer shell of a fresh eggshell and washing with deionized water. Then it was fixed in a container to separate it into two horizontal compartments, into which 20 mL of 0.1 mol/L CaCl2 and 20 mL of 0.06 mol/L KH2PO4 solutions were added, respectively. The ethylenediamine was put on both sides of the membrane to modulate the pH value to 7.4. All reagents used were analytical purity class. Thereafter, the reaction container was kept at room temperature for about 10 h. The product was obtained by centrifugal separation, then washed with deionized water and dried in a desiccator.
Typical Preparation of Nanocomposite Fluorescence Probe
About 0.005 g of the above HAP product and 5.0 μL thioglycolic acid (TA) were put in a beaker with 5.0 mL alcohol solution. Then the system was vibrated ultrasonically for 2 min. After that, the reaction system was centrifugated at 1,500 rpm for 10 min, and the nanocomposite fluorescence probe settled to the bottom of the container.
The morphologies of the products were investigated through transmission electron microscopy (TEM, Hitachi-800) and scanning electron microscopy (SEM, Philips S-4800). The optical properties of the nanocomposite fluorescence probe in alcohol solution were studied through fluorescent spectroscopy (Perkin-Elmer LS-55) and Fourier transform infrared spectroscopy (FT-IR).
Results and Discussions
Morphologies of Products
A typical TEM image of the HAP products (Fig. 1a) shows they are spherulites with a diameter of about 2.5 μm [7]. The high magnification TEM image in Fig. 1b, d indicates that the spherulites consist of a large number of nanoribbons with large surface area. They contain a large number of hydroxy groups. So they can readily be combined with organic fluorescent compounds that contain carboxyl or hydroxy groups. The electron diffraction (ED) lattice of selected region suggests that the nanoribbons were single crystals (Fig. 1c).
Figure 1.
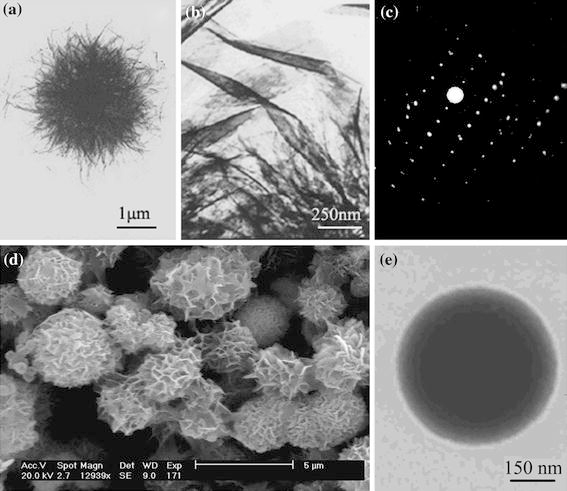
TEM images (a, b), ED pattern (c) and SEM image (d) of HAP nanoribbon spherulites and fluorescence probe (e)
The HAP nanoribbon spherulites were treated with TA. The TEM images of the final fluorescent probe material (Fig. 1e) indicate that they are spherical with a diameter of about 2.2 μm, which is less than that of HAP spherulites products (containing no TA). After treating with TA, the nanoribbons have almost disappeared in TEM images, the surface is smooth, and there is visible darkness in the center (Fig. 1e), which suggests that TA is embedded inside the spherulites instead of just adhering to the surface of spherulites. This may be the strong effects between TA and HAP nanoribbon spherulites. As the HAP nanoribbon spherulite is a porous structure composed of interlaced nanoribbons, one of these effects is likely to be hydrogen bonds between the –OH groups in HAP spherulite and the –COOH groups in TA. The other is the strong surface adsorbability of HAP spherulites to TA molecules due to its large surface area. These two effects combine TA and HAP nanoribbon spherulites tightly. When the probe was used to detect cadmium ion, the most of original spherulites structure of the probe is damaged (Fig. 2c). We also captured a process image that showed the spherulites have separated to new flakes. The mechanism was presumably the ion exchange between Cd2+ and Ca2+, the exposed Ca2+ on surface of probe will be Cd2+ replaced [17], which could be depicted by the following general equation:
Figure 2.
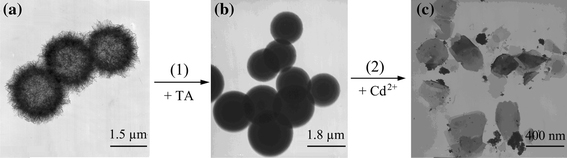
Formation and detection TEM images of HAP fluorescence probes
And the assembling bondings of nanoribbons were broken by the transmitting energy in the process of reaction, the probe with spherulites shape transformed to separation flakes. The more exact mechanism has not claimed and it will be conducted in our further exploitation.
Fluorescence Phenomenon of Probe in Alcohol Solution
Enhanced Fluorescence Intensity of Probe by Cadmium Ion
We have investigated the fluorescence phenomenon of probe in alcohol solution. When the excitation wavelength is 254 nm, the nanocomposite fluorescence probe in alcohol solution appears weak emission peak at 425 nm. When the fluorescence probe meets cadmium ions, the fluorescence intensity enhances obviously (shown in Fig. 3). Under the same concentration of nanocomposite fluorescence probe, the fluorescence intensity enhances with the increasing concentration of cadmium ions. The nanocomposite fluorescence probe shows fluorescence intensity 1.5–3.3-fold increase compared with the probe containing no TA.
Figure 3.
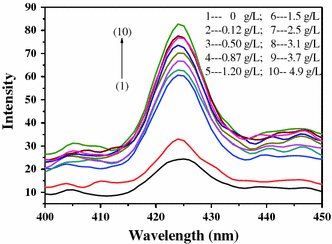
PL spectra of fluorescence probe under different concentrations of cadium ion (the concentration of TA is 1 mL/L; the concentration of HAP is 1 g/L)
Appropriate Concentrations of TA and HAP
Because the interactions among TA, HAP, and cadmium ions are complicated, we should select the appropriate concentrations of TA and HAP in order to get the appropriate fluorescence intensity. In all solutions, the solvent is alcohol.
When the excitation wavelength is 254 nm and the concentration of cadmium ion is 1.5 g/L and TA is absent, the fluorescence intensity enhanced with the increasing concentration of HAP (shown in Fig. 4a). The alcohol solution of cadmium chloride is not photoluminescent, whereas as 1.2 g/L of HAP or 0.6 mL/L of TA is added, the weak emission peak at 425 nm appeared, and the TA system is stronger. When both 1.2 g/L of HAP and 0.6 mL/L of TA are added to the alcohol solution, the intensity is stronger than adding HAP or TA only (shown in Fig. 4b). When the concentration of cadmium ion is 1.5 g/L and TA is 1 mL/L, the fluorescence intensity reduced with the increasing concentration of HAP (shown in Fig. 4c). When the concentration of cadmium ion is 1.5 g/L and HAP is 1 g/L, the fluorescence intensity enhances with the increasing concentration of TA (shown in Fig. 4d) at first, but remains the same when the concentration of TA is more than 1 mL/L. On summarizing our observations, we chose the suitable concentration of TA is 1 mL/L and HAP is 1 g/L.
Figure 4.
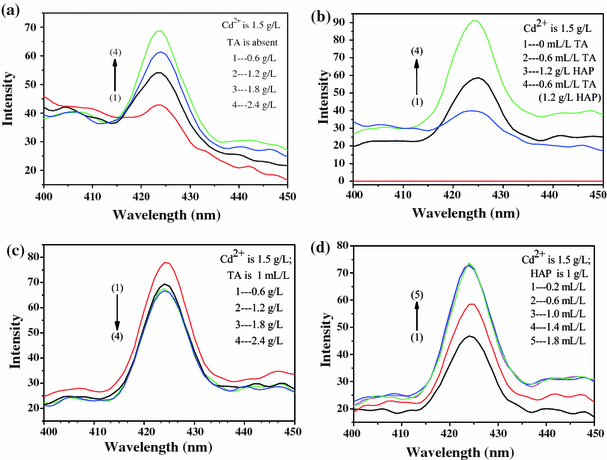
a PL spectra under different concentration of HAP; b PL spectrum under different conditions; c PL spectra under different concentrations of HAP; d PL spectra under different concentrations of TA
FT-IR Analysis of Probes
Figure 5a shows a widening in FT-IR absorbing peaks. The νP-O vibration absorbing peak of HAP nanoribbons spherulites in Fig. 5a appears almost at the identical wavelength with that of HAP bulk materials at 1,040 cm−1[3,7], but it has an obvious widening. This is because the νP-O vibration energy has changed by the small-size effect and interface-effect of nanomaterials. Figure 5b is a spectrum of the novel probe, in which, the absorption peak at 2,530 cm−1 belongs to the νS-H, and the widening of νP-O vibration is softened because of weaker interaction-effect between HAP and TA.
Figure 5.
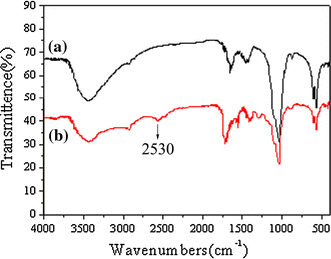
FT-IR spectra of HAP (a) and fluorescence probe (b)
Conclusions
A new kind of nanocomposite fluorescence probe that gives very strong fluorescence upon the addition of cadmium ion is explored. The mechanism of determining cadmium ion in alcohol solution (shown in Fig. 6) is proposed, which is similar to that in aqueous solutions [10-13]. The typical probes consist of HAP nanoribbon spherulite and proper amount of TA. When determining cadmium ion in alcohol, the fluorescence probe is found to have mostly separated to flake structures. Because of the enhancement of the emission intensity,the fluorescence probe can also be used to detect the heavy metal such as Cd2+, Zn2+, Cu2+, etc.
Figure 6.
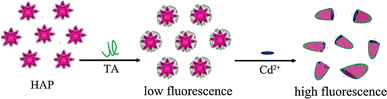
Schematic representation of the determination process of HAP nanocomposite fluorescence probe
Contributor Information
Jin-Ku Liu, Email: jkliu@ecust.edu.cn.
Guang-Ming Li, Email: ligm@mail.tongji.edu.cn.
Acknowledgments
This work was supported by the State Key Laboratory of Pollution Control and Resource Reuse Foundation, China (NO.PCRRF09005).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Murugan R, Ramakrishna S. Cryst. 2005. p. 111. COI number [1:CAS:528:DC%2BD2cXnvVKltL0%3D] [DOI]
- López-Macipe A, Rodríguez-Clemente R, Hidalgo-López A, Arita I, García-Garduño MV, Rivera E, Castaño VM. J. 1998. p. 21. [DOI]
- Hu XJ, Liu JK, Qin XY, Huang J, Yi Y. NANO. 2009. p. 165. COI number [1:CAS:528:DC%2BD1MXhtFaqsLbE] [DOI]
- Kim SW, Seo DS, Lee JK. Appl. 2008. p. 388. COI number [1:CAS:528:DC%2BD1cXhtlWrsb7I]; Bibcode number [2008ApSS..255..388K] [DOI]
- Ripamonti U. J. 1991. p. 692. COI number [1:STN:280:DyaK3M3lvVektQ%3D%3D] [PubMed]
- Kay MI, Young RA, Posner AS. Nature. 1964. p. 1050. COI number [1:CAS:528:DyaF2MXjtlOmsg%3D%3D]; Bibcode number [1964Natur.204.1050K] [DOI] [PubMed]
- Liu JK, Wu QS, Ding YP. Eur. 2005. p. 4145. [DOI]
- Marchat D, B’Assollant D, Champion E. J. 2007. p. 453. COI number [1:CAS:528:DC%2BD2sXktlyitw%3D%3D] [DOI] [PubMed]
- Suzuki T, Hatsushika T, Michihiro M. J. 1982. p. 3605.
- Ma QY, Logan TJ, Traina SJ, Ryan JA. Environ. 1994. p. 1219. COI number [1:CAS:528:DyaK2cXktFKqur8%3D] [DOI] [PubMed]
- Xu Y, Schwartz FW, Traina SJ. Environ. 1994. p. 1472. COI number [1:CAS:528:DyaK2cXksFKmt70%3D] [DOI] [PubMed]
- Leyva AG, Marrero J, Smichowski P, Cicerone D. Environ. 2001. p. 3669. COI number [1:CAS:528:DC%2BD3MXlvVagt7k%3D] [DOI] [PubMed]
- Gómez del Río JA, Morando PJ, Cicerone DS. J. 2004. p. 169. [DOI] [PubMed]
- Peld M, Tonsuaadu K, Bender V. Environ. 2004. p. 5626. COI number [1:CAS:528:DC%2BD2cXnvVKltb8%3D] [DOI] [PubMed]
- Corami A, Mignardi S, Ferrini V. J. 2007. p. 164. COI number [1:CAS:528:DC%2BD2sXmvVeksbs%3D] [DOI] [PubMed]
- Smiciklas I, Dimovic S, Plecas I, Mitric M. Water Res. 2006. p. 2267. COI number [1:CAS:528:DC%2BD28XlvFagsrc%3D] [DOI] [PubMed]
- Yasukawa A, Yokoyama T, Kandori K, Ishikawa T. Colloids Surf. 2007. p. 203. COI number [1:CAS:528:DC%2BD2sXjsVSnu7w%3D] [DOI]
- Sheha RR. J. 2007. p. 18. COI number [1:CAS:528:DC%2BD2sXktFOmu7g%3D] [DOI] [PubMed]
- Corami A, Mignardi S, Ferrini V. J. 2008. p. 402. COI number [1:CAS:528:DC%2BD2sXhtlChurbM] [DOI] [PubMed]
- Choi S, Jeong Y. Fibers Polym. 2008. p. 267. COI number [1:CAS:528:DC%2BD1cXpslSjurg%3D] [DOI]
- Jeanjean J, Vincent U, Fedoroff M. J. 1994. p. 68. COI number [1:CAS:528:DyaK2cXhslansLw%3D]; Bibcode number [1994JSSCh.108...68J] [DOI]
- Krishanu R, Ramachandram B, Joseph RL. J. Phys. Chem. C. 2007. p. 7091. [DOI]
- Ray D, Bharadwa PKJ. Inorg. 2008. p. 2252. COI number [1:CAS:528:DC%2BD1cXjtVCqtbs%3D] [DOI] [PubMed]


