Abstract
Basic fibroblast growth factor (bFGF), a protein, plays a key role in wound healing and blood vessel regeneration. However, bFGF is easily degraded in biologic systems. Mesoporous silica nanoparticles (MSNs) with well-tailored porous structure have been used for hosting guest molecules for drug delivery. Here, we report an in situ route to load bFGF in MSNs for a prolonged release. The average diameter (d) of bFGF-loaded MSNs is 57 ± 8 nm produced by a water-in-oil microemulsion method. The in vitro releasing profile of bFGF from MSNs in phosphate buffer saline has been monitored for 20 days through a colorimetric enzyme linked immunosorbent assay. The loading efficiency of bFGF in MSNs is estimated at 72.5 ± 3%. In addition, the cytotoxicity test indicates that the MSNs are not toxic, even at a concentration of 50 μg/mL. It is expected that the in situ loading method makes the MSNs a new delivery system to deliver protein drugs, e.g. growth factors, to help blood vessel regeneration and potentiate greater angiogenesis.
Keywords: In situ loading method, Protein release, Nanoparticles
Introduction
Basic fibroblast growth factor (bFGF), an 18 kDa protein involved in angiogenesis, has been shown to stimulate endothelial cell proliferation and to promote the physical organization of endothelial cells into tube-like structures, indicative of new blood vessel growth [1]. Studies have demonstrated that bFGF has a high therapeutic potential for tissue regeneration. For instance, delivery of bFGF to damaged tissues stimulates regeneration of muscle, heart, cartilage, and nerves [2-4]. For skin wound healing, bFGF significantly accelerates soft tissue formation, re-epithelialization, and collagen maturation in human and animals [5-7]. However, direct injection of bFGF into a host leads to rapid diffusion and degradation of the protein [8]. Many attempts have been made to keep bFGF stable in vitro, or in vivo. For instance, Gospodarowicz and Cheng demonstrated that bFGF coupled with heparin was protected from heat and acid deactivation [9]. Furthermore, in order to avoid the over-dosage and degradation of bFGF, various lipid and biocompatible polymers have been used as carriers for the delivery of growth factors including, the encapsulation of heparin–sepharose-bound bFGF in alginate microspheres [10], the impregnation of collagen sponges with heparin–bFGF–fibrin mixtures [11], bFGF incorporation into hyaluronate gels [12], and gelatin hydrogels [2,12]. However, the release of bFGF from these polymer-based delivery systems was completed within only 3 days. Choi and Park reported that bFGF-loaded poly(d,l-lactide-coglycolide) (PLGA) particles (diameter, d = 200 nm) produced by chemical cross-linking showed a longer release period, around 2 weeks [13]. However, it was difficult to control the release rate and period due to the complicated releasing mechanism for the polymers. Moreover, the major challenge is to eliminate the use of high temperatures during the drug loading process to polymer nanoparticles. For instance, it takes at least 70 °C to entrap the drug in the nanoshell of a lipid polymer, which is not feasible to load temperature sensitive protein, such as bFGF [14]. Furthermore, polymer-based release carriers keep the therapeutic agents entrapped, adsorbed or chemically coupled onto the polymer matrix, which, unfortunately, has side effects on the loaded bFGF because of the crosslinking agents, temperature, and un-desired pH values during the polymer preparation [15].
Inorganic nanoparticles (diameter, 1 <d < 100 nm) have special properties (e.g. large surface to volume ratio and high atomic fractures), which make them suitable carriers in the blood stream [16]. Quite recently, mesoporous silica nanoparticles (MSNs) have attracted a lot of attention for their unique structure features, including large surface areas (800 m2 g−1), tunable pore sizes (2–10 nm in diameter), and well-defined surface properties [17]. In addition, MSNs have been approved by the Food and Drug Administration (FDA) as a new biocompatible material. Furthermore, MSNs show multifunctional surface modification, controlled releasing capability, and good thermal stability [18,19], which make MSNs an ideal nonviral carrier for gene, and/or drug delivery. For instance, Lin and Wang utilized the MSNs with honeycomb structure for delivering DNA and chemicals into plants [17]. In the case of the MSNs system, the drugs and imaging agents are encapsulated with covalently bound caps that physically block the drugs from leaching out. Molecules entrapped inside the pores are released by the introduction of uncapping triggers (chemicals that cleave the bonds attaching the caps to the MSNs). However, it takes extra processes to modify the MSNs with capping and uncapping agents. In addition, very few efforts have been made to load protein within silica nanoparticles at room temperature. The possible reason is that the protein is easily denatured during the chemical reactions that are normally required during the synthesis and loading of nanoparticles.
To date, several processes have been developed to produce MSNs. Zhao, et al. reported the generation of MSNs with 4.6–30 nm pores through triblock copolymer synthesis in 1998 [20]. Other methods include the sol–gel process [21], and the spray drying method [22]. Previous studies indicate that mesoporous silica can be synthesized in either the alkaline route, or the acid route. The acid route leads to a soft network because the hydrolysis is catalyzed easily compared to the condensation [23], while the alkaline route is favoured to both hydrolysis and condensation, and produces a condensed and compact structure. In this research, a weak acid-modified water-in-oil microemulsion is developed to encapsulate the bFGF within MSNs in situ. The in vitro releasing kinetics of bFGF from MSNs has been investigated through colorimetric enzyme linked immunosorbent assays (ELISAs). It is expected that this new delivery system can help tissue regeneration and wound healing by releasing the growth factors in a controlled and temporal manner.
Materials and Methods
Encapsulation of bFGF in MSNs
A weak acidic water-in-oil method followed by ammonium hydroxide treatment was developed to encapsulate bFGF in situ. Figure 1 illustrates the in situ loading of bFGF in MSNs through the micro-emulsion method. Unless otherwise stated, chemicals were obtained from Sigma–Aldrich. A volume of 0.3 mL acidic water (pH = 4) was mixed with oil phase, 8.5 mL cyclohexane, 2 mL non-ionic surfactants, triton X-100, and 2 mL co-surfactant, hexanol. The microemulsion was formed by stirring the mixture at 800 rpm until the solution became clear. About 200 μL tetraethoxysilane (TEOS) was added to the microemulsion, and the mixture was stirred for 1 h. The weak acid can promote the initial hydrolysis of TEOS. Following this, 28% ammonium hydroxide solution (NH4OH in H2O) was added to react with TEOS in microemulsion. Meanwhile, 10 μg bFGF was mixed in the solution. The hydrolysis and condensation reactions under the condition of pH = 9 were performed at room temperature for 24 h with continuous stirring. After the completion of the reaction, acetone was added to break the microemulsion, and recover the silica nanoparticles. The aqueous solution was collected to determine the concentration of free bFGF (Cf) by using ELISA. Nanoparticles were washed by ethanol–water (1:1) binary solution following reported procedure [24]. The total amount (yield) of freeze-dried bFGF-loaded MSNs was around 0.126 mg measured by a 0.001 mg balance.
Figure 1.
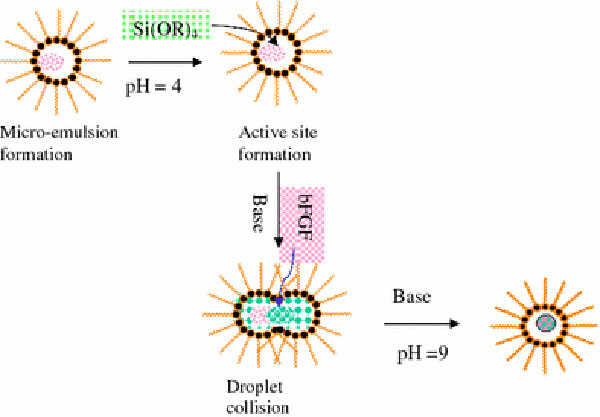
Illustration of the in situ loading of bFGF in MSNs through the microemulsion method
Nanoparticles Characterization
Mesoporous silica nanoparticles (MSNs) used for encapsulating bFGF were further investigated by Hitachi 3400-N scanning electron microscope (SEM) and transmission electron microscope (TEM-EM400) for measuring the particle size and size distribution. To prepare the TEM specimen, bFGF-loaded MSNs were re-dispersed in ethanol–water solution, and then one drop was added on the TEM Cu grid. Furthermore, Zeiss LSM 510 Duo Confocal microscope was carried to study on the uptake of MSNs in human umbilical vein endothelial cells (HUVEC).
Release Kinetic Study
0.037 mg bFGF-loaded MSNs (~30% of yield) were suspended in 20 mL phosphate buffer saline (PBS) at pH 7.4. The solution was divided into 20 microfuge tubes (1 mL each). The tubes were kept in an incubator at 37 °C. At predetermined intervals of time, the solution was centrifuged at 4500 rpm for 5 min to separate the released bFGF from the MSNs. The released sample was collected at 0.5, 1, 3, 6, and 12 h on the first day followed by sampling at every 24 h interval for first 120 h, i.e. 5 days, and then at every 48-h interval till the release was carried for 480 h (~20 days). The concentration of released bFGF as a function of the releasing time (t) was then quantitively determined through a colorimetric enzyme linked immunosorbent assay (ELISA) kit—Human bFGF ELISA Kit purchased from the RayBiotech, Inc., (Norcross, GA, USA). Briefly, standards and samples are pipetted into the wells, and bFGF presenting in a sample is bound to the wells by the immobilized antibody. Following several steps of washing and bio-conjugation, the amount of immobilized bFGF can be colorimetrically measured by plate reader at 450 nm. The limit of detection was 20 pg/mL for bFGF. All experiments were performed in triplicate. The releasing profile of bFGF refers to the relatively released concentration of (C*/C′) as a function oft. Here,C* represents the concentration of released bFGF in PBS at predetermined intervals of time.C′ is the total concentration of released bFGF from MSNs. In addition, the loading efficiency (R) of bFGF describes the capacity of the encapsulation method to load bFGF in MSNs. It can be estimated through two strategies as shown in Eq. 1.
| (1) |
whereRdenotes the loading efficiency (%);M′, the total amount (wt) of bFGF loaded in nanoparticles, which is converted fromC′ determined by ELISA kit;M0, the original amount (wt) of bFGF, that is, 10 μg. In Eq. 1,C0refers to the original concentration of bFGF in aqueous phase of microemulsion, i.e. 10 μg in 0.1 mL, andCfis the nonencapsulated free bFGF in the aqueous solution after breaking the microemulsion. In our study,Cfwas measured by the ELISA kit. The result indicated that two strategies gave similar values, i.e., the loading efficiency,R = 72.5 ± 3%.
Biocompatibility of MSNs
The MultiTox-Fluor Multiplex Cytotoxicity Assay (Promega) was used to investigate the biocompatibility or cytotoxicity of the pure MSNs. This assay simultaneously measures cell viability and cytotoxicity, which is independent on total cell number. Furthermore, human umbilical vein endothelial cells (HUVEC) from the American Type Culture Collection (ATCC) were cultured in CS-C medium without serum for endothelial cell lines (Sigma) supplemented with 10% FBS (Invitrogen) and endothelial cell attachment factor (E 9765) (Sigma) at 37 °C, 5% CO2. For the investigation of cytotoxicity of nanoparticles, 1,000 cells per well of 96-well dish were plated and allowed to adhere overnight. The medium was changed and replaced with fresh medium containing MSNs in next day. At specific time points, cytotoxicity and viability were measured as per manufacturer’s instructions using the MultiTox-Fluor Multiplex Cytotoxicity Assay. Six different concentrations of MSNs were used in the test, including 0.01, 0.1, 1, 5, 10, and 50 μg/mL. The control samples measured from day 1 to day 5 were the HUVEC cell culture media without adding MSNs. The cell viability was obtained by using the MultiTox-Fluor Multiplex Cytotoxicity Assay. According to the manufacturer’s instructions (http://www.promega.com/cnotes/cn015/CN015_11.pdf), the assay reagent is composed of two fluorogenic peptide substrates (GF-AFC, live-cell protease substrate and bis-AAF-R110, dead-cell protease substrate) to the assay buffer. This reagent was added to a 96-well plate. After at least 30 min of incubation at 37 °C, the resulting fluorescent signals for the living cell and dead cells were measured at an excitation of 400 nm and an emission of 505 nm, then at an excitation of 485 nm and an emission of 520 nm, respectively.
Results and Discussion
Basic FGF encapsulated in MSNs in situ has been developed by a water-in-oil microemulsion. The volume of water droplets in oil phase can be controlled by adjusting the ratio of water and cyclohexane. Moreover, the boundary between the water droplets and the oil phase is tailored by adjusting the concentrations of surfactant, Triton X-100, and co-surfactant, hexanol [18]. In this acidic water-in-oil microemulsion, the hydrolysis of TEOS was promoted under the acidic condition at the initial stage, which leads to the formation of fuzzy active seeds to eventually build the porous structure at nano-scale. The addition of ammonium hydroxide then reacted with the active sites of TEOS in the microemulsion to perform the hydrolysis and condensation reactions. Basic FGF is a protein with a length of 155 amino acids and an isoelectric point of 9.6, which makes it stable in a weak basic solution. Therefore, NH4OH as a base catalyst for the hydrolysis and condensation reactions could trap the dissolved bFGF in the negative charge of nanoparticles with the form of Si–O− on the surface while still retaining protein integrity. Figure 2a shows the SEM micrograph of the bFGF-loaded MSNs that are spherical. The average particle size (d) of the SiO2 NPs was 57 ± 8 nm, which is smaller than most of polymer nano-containers. It is also noted that there was a lightly broad size distribution. In addition, the high resolution TEM indicates the porous structure clearly. The pore size of silica NP is around 7–10 nm in diameter as shown in Fig. 2b.
Figure 2.
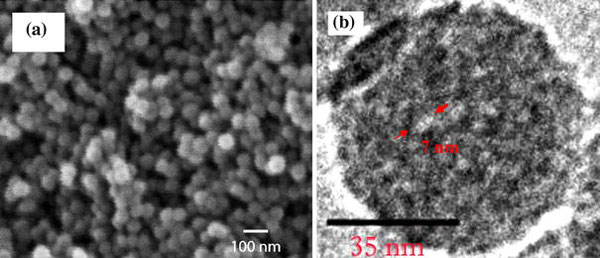
aSEM micrograph of bFGF-loaded MSNs.bHigh resolution TEM micrograph: the pore on the MSN is around 7–10 nm in diameter as labeled byarrows
The structure, shape, and chemicals of MSNs do not change in vitro and/or in vivo because of their chemical and biological stability. The releasing kinetics of encapsulated bFGF is, therefore, mainly controlled by diffusion mechanisms [25]. While, polymers acted as nanocarriers to deliver proteins they suffer from the swelling and/or degradation, which possibly leads to a complicated and nonsatisfied releasing profile.
Using an ELISA measurement, the releasing profile of bFGF from SiO2 NPs was investigated as shown in Fig. 3, which plots the correlation of the relative releasing concentration (C*/C′) of bFGF from MSNs as a function of time (t). The releasing profile can be divided into two regions with increasing t. First a fast releasing curve appeared when t increased to the first 12 h. A mild releasing rate was then observed followed the first stage. Furthermore, 50% of encapsulated bFGF was released in 8 days, and the maximum concentration of released bFGF could be detected at t = 20 days. The effect of particle size on the releasing rate has been reported [24]. In most cases, smaller nanoparticles offer prolonged releasing kinetics. Other possible factors related to the releasing rate include the pore size and the properties of payload, and so on.
Figure 3.
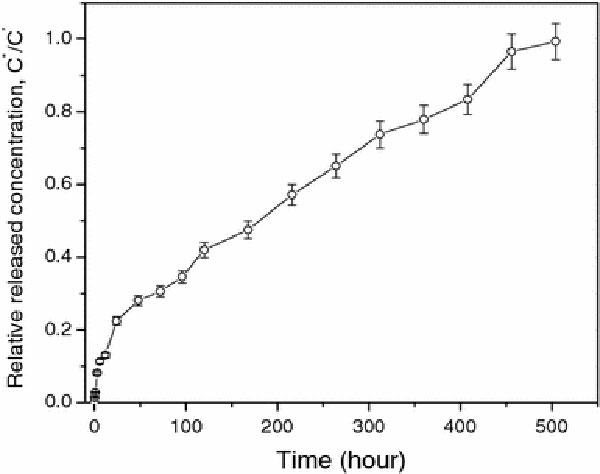
Releasing profile of bFGF from MSNs as a function oft
The result as shown in Fig. 3 indicates that this delivery system based on MSNs can achieve prolonged release of FGF-2 for at least 3 weeks in order to initiate angiogenesis within a cell delivery matrix when the particles is around 40 nm. In addition, avoiding the “initial burst”, in which a large percentage of the drug is released within the first 24–48 h, is a challenge to most delivery vehicles [19]. As seen in our studies, the “initial burst” is also hard to avoid completely for the silica NPs encapsulating bFGF. The reason is possibly related to the high solubility of bFGF in buffer, and the tunable nano-channels in MSNs as showed in Fig. 2b. Our efforts are continuously focusing on how to control the initial burst and increase the loading efficiency.
On the other hand, the biocompatibility of pure MSNs was studied for 5 days by using the MultiTox-Fluor Multiplex Cytotoxicity Assay. Confluent HUVEC cells were used in this assay as they are endothelial cells that undergo angiogenesis, a potential therapeutic target for the bFGF-releasing MSNs technology. This assay simultaneously measures cell viability and cytotoxicity, thereby enabling the quantitative assessment of population growth while controlling for differences in total cell number. The MultiTox-Fluor Assay simultaneously measures two protease activities; one is a marker of cell viability, and the other is a marker of cytotoxicity. The live-cell protease activity is restricted to intact viable cells and is measured using a fluorogenic, cell-permeant, peptide substrate (glycyl-phenylalanyl-aminofluorocoumarin; GF-AFC). The viability (% of control) as a function of the concentration of MSNs during the period from day 1 to day 5 is shown in Fig. 4. The cell viability (% of control) are obtained by normalizing the value of the control sample (no MSNs) in viable assay. The error bar is the calculated standard deviation. When the concentration of pure MSNs in the assay was increased from 0.01 to 50 μg/mL, the cell viability (%) increased up to 150% as compared to the control sample, and the number of viable cells shows a subtle fluctuation with the increasing time. Even the highest concentration, 50 μg/mL, may inhibit proliferation initially, but this effect only lasts for 1 day, as the cells appeared to recover. Recent reports on nanoparticle-induced lung epithelial cell proliferation indicated that MSNs are able to induce the activation of protein kinases [26]. Meanwhile, our study indicated that there is no obvious increase of dead cell (% of control) in the cytotoxicity assay during the test period. The results indicate that MSNs are not toxic to HUVEC cells, but rather promotes the cell proliferation. The internalization of pure MSNs (50 μg/mL) was further examined. MSNs were added in cell culture Petri dishes. After 30 min incubation of HUVEC with MSNs, the cells were fixed at the bottom of Petri dish. Normally, organic dye, fluorescein isothiocyanate (FITC), is used in cell stain solution. Figure 5 indicates FITC was absorbed on the MSNs. It may be caused by the porous structure and large surface area of silica nanoparticles. MSNs display green dots in HUEVC cell under confocal microscopy. It demonstrates that the MSNs can access the cytoplasm via an endocytic mechanism [27], but only stay outside of the nuclei of the cells. The morphology of HUVEC cells did not vary after internalization of MSNs, indicating that the MSNs are not only a biocompatible delivery system for growth factors/proteins, but also an ideal biomaterial for the application in bio-imaging.
Figure 4.
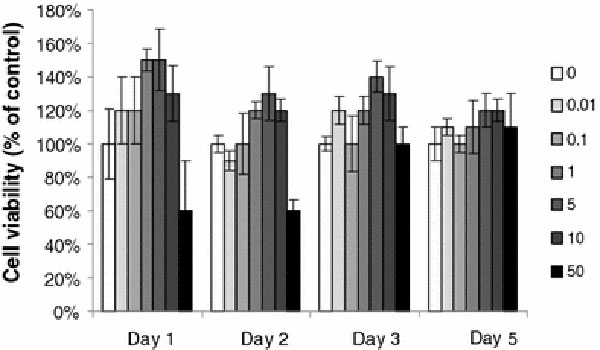
Cytotoxicity of MSNs on HUVEC was determined with MultiTox-Fluor Multiplex Cytotoxicity Assay kit (Promega). Cells without MSNs treatment were used as controls
Figure 5.
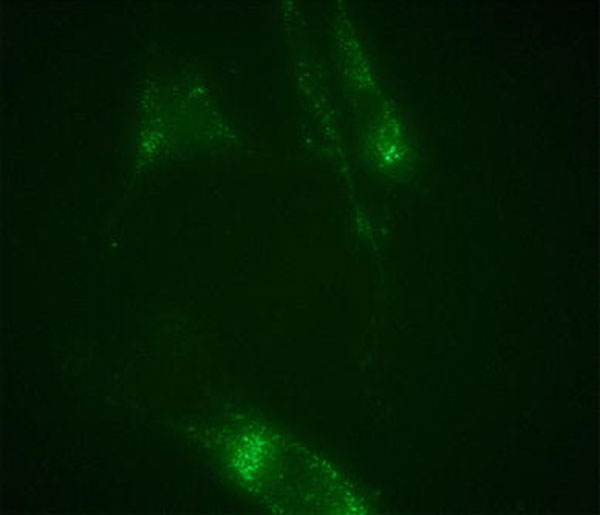
Confocal microscope image of the uptake of MSNs in cell (FITC from cell stain solution are absorbed on the surface of MSN due to their porous structure and large surface area)
Conclusions
This study develops a route for in situ loading of bFGF within MSNs at room temperature through a developed water-in-oil microemulstion method. MSN as a new bFGF vehicle system prolongs the release of bFGF for 20 days. The results of cytotoxicity assay show that MSNs are not toxic, even when administered at high concentrations (50 μg/mL). Interestingly, the viability assay shows that MSNs could promote the cell proliferation. This new delivery system may help blood vessel regeneration and potentiate greater angiogenesis. In addition, this research indicates that the porous silica nanoparticles could be potential carriers for immobilizing dyes as a biomarker.
Acknowledgments
This work was supported by the Discovery Grant (to Dr. Zhang, # 346202) of the Natural Sciences and Engineering Research Council of Canada (NSERC), and the financial support from the University of Western Ontario (UWO).
References
- Ornitz DM, Itoh N. Genome Biol. 2001. pp. 1–12. [DOI] [PMC free article] [PubMed]
- Ohta M, Suzuki Y, Chou H, Ishikawa N, Suzuki S, Tanihara M, Mizushima Y, Dezawa M. J. Biomed. Mater. Res. A. 2004. pp. 661–668. COI number [1:CAS:528:DC%2BD2cXhtFSis77M] [DOI] [PubMed]
- Tabata Y, Yamada K, Miyamoto S, Nagata I, Kikuchi H, Aoyama I, Tamura M, Ikada Y. Biomaterials. 1998. pp. 807–815. COI number [1:CAS:528:DyaK1cXjvVant7s%3D] [DOI] [PubMed]
- Ishii I, Mizuta H, Sei A, Hirose J, Kudo S, Hiraki Y. J. Bone Joint Surg. Br. 2007. pp. 246–255. [DOI] [PubMed]
- Ono I, Gunji H, Zhang JZ, Maruyama K, Kaneko F. Burns. 1995. pp. 352–355. COI number [1:STN:280:DyaK28%2FltlyhtA%3D%3D] [DOI] [PubMed]
- Nicole SG, Isik F, Heimbach DM, Gordon D. J. Surg. Res. 1994. pp. 226–234. [DOI] [PubMed]
- Chen WY, Rogers AA, Lydon MJ. J. Invest. Dermatol. 1992. pp. 559–564. COI number [1:STN:280:DyaK3s%2Fls1Kmsg%3D%3D] [DOI] [PubMed]
- Nakamura T, Ebihara I, Nagaoka I, Tomino Y, Nagao S, Takahashi H, Koide H. J. Am. Soc. Nephrol. 1993. pp. 1378–1386. COI number [1:CAS:528:DyaK3sXitlWlsro%3D] [DOI] [PubMed]
- Gospodarowicz D, Cheng J. J. Cell Physiol. 1986. pp. 475–484. COI number [1:CAS:528:DyaL28XlvVelt78%3D] [DOI] [PubMed]
- Edelman ER, Mathiowitz E, Langer R, Klagsbrun M. Biomaterials. 1991. pp. 619–626. COI number [1:CAS:528:DyaK3MXms1eht7k%3D] [DOI] [PubMed]
- Deblois C, Cote MF, Doilon CL. Biomaterials. 1994. pp. 665–672. COI number [1:CAS:528:DyaK2cXls1eqtbg%3D] [DOI] [PubMed]
- Liu LS, Thompson Y, Poser JW, Spiro RC (1998) In: Proceedings of the 25th International Symposium on Controlled Release Bioactive Materials 25:996–997.
- Choi SH, Park TG. Int. J. Pharm. 2006. pp. 223–228. COI number [1:CAS:528:DC%2BD28XhvF2lsr0%3D] [DOI] [PubMed]
- Muller RH, Jacobs C. Int. J. Pharm. 2002. pp. 151–161. COI number [1:CAS:528:DC%2BD38Xislyrsbk%3D] [DOI] [PubMed]
- Hile DD, Amirpour ML, Akgerman A, Pishko MV. J. Control Release. 2000. pp. 177–185. COI number [1:CAS:528:DC%2BD3cXhvF2lu7k%3D] [DOI] [PubMed]
- Zhang J, Lan CQ, Post M, Simard B, Deslandes Y, Hsieh TH. Cancer Genomics Proteomics. 2006. pp. 147–151. COI number [1:CAS:528:DC%2BD28XnslCmur4%3D] [PubMed]
- Torney F, Trewyn BG, Lin VSY, Wang K. Nat. Nanotechnol. 2007. pp. 295–300. COI number [1:CAS:528:DC%2BD2sXns1Ogtbc%3D]; Bibcode number [2007NatNa...2..295T] [DOI] [PubMed]
- Slowing II, Vivero-Escoto JL, Wu CW, Lin VSY. Adv. Drug Deliv. Rev. 2008. pp. 1278–1288. COI number [1:CAS:528:DC%2BD1cXosVOnsrg%3D] [DOI] [PubMed]
- Bharali DJ, Klejbor I, Stachowiak EK, Dutta P, Roy I, Kaur N, Bergey EJ, Prasad PN, Stachowiak MK. Proc. Natl. Acad. Sci. USA. 2005. pp. 11539–11544. COI number [1:CAS:528:DC%2BD2MXoslKiu7o%3D]; Bibcode number [2005PNAS..10211539B] [DOI] [PMC free article] [PubMed]
- Zhao DY, Feng JL, Huo QS, Melosh N, Fredrickson GH, Chmelka BF, Stucky GD. Science. 1998. pp. 548–549. COI number [1:CAS:528:DyaK1cXotVOitQ%3D%3D]; Bibcode number [1998Sci...279..548Z] [DOI] [PubMed]
- Nandiyanto ABD, Kim SG, Iskandar F, Okuyama K. Microporous Mesoporous Mater. 2009. pp. 447–453. COI number [1:CAS:528:DC%2BD1MXisFKisLo%3D] [DOI]
- Nandiyanto ABD, Iskandar F, Okuyama K. Chem. Lett. 2008. pp. 1040–1043. COI number [1:CAS:528:DC%2BD1cXht1ynsL3J] [DOI]
- Sanchez C, Ribot F. New J. Chem. 1994. pp. 1007–1010. COI number [1:CAS:528:DyaK2cXntVKhurY%3D]
- Bagwe RP, Yang C, Hilliard LR, Tan W. Langmuir. 2004. pp. 8336–8342. COI number [1:CAS:528:DC%2BD2cXmt1Sntr8%3D] [DOI] [PubMed]
- Tanaka H, Matsumura M, Veliky A. Biotechnol. Bioeng. 1984. pp. 53–58. COI number [1:CAS:528:DyaL2cXosVahsw%3D%3D] [DOI] [PubMed]
- Sydlik U, Bierhals K, Soufi M, Abel J, Schins R, Unfried K. Am. J. Physiol. Lung Cell Mol. Physiol. 2006. pp. 725–733. [DOI] [PubMed]
- Trewyn BG, Nieweg JA, Zhao Y, Lin VSY. Chem. Eng. J. 2008. pp. 23–29. COI number [1:CAS:528:DC%2BD1cXitFamur8%3D] [DOI]


