Abstract
Polpyrrole (PPy)/Ag nanocomposites were successfully synthesized at the interface of water and ionic liquid by one-step UV-induced polymerization. Highly dispersed PPy/Ag nanoparticles were obtained by controlling the experimental conditions. The results of Fourier-transform infrared spectroscopy, X-ray diffraction, transmission electron microscopy and X-ray photoelectron spectroscopy revealed that the UV-induced interface polymerization leaded to the formation of PPy incorporating silver nanoparticles. It was also found that the electrical conductivity of PPy/Ag nanocomposite was about 100 times higher than that of pure PPy.
Keywords: Polypyrrole, Ag, Ionic liquid, Nanocomposites
Introduction
Nanocomposites of conducting polymers and metallic nanoparticles have attracted considerable attention in recent years because of their potential applications in various areas, such as electrocatalysis, chemical sensors and optoelectronic devices [1-4]. They have synergistic chemical and physical properties based on the constituent polymer and introduced metal. Extraordinary physicochemical properties of such nanocomposites can be attributed to high surface area and quantum size effect [5-8]. Various methods for the preparation of these composites have been described. In general, these following routes are used: (a) where the monomer or polymer acts as a reductant for the metal, yielding the nanocomposite in powder or thin film forms [9,10] or (b) preparation of the nanoparticles followed by either chemical polymerization around the particles or dispersion of the nanoparticles in a polymer matrix [11,12].
In parallel with the development of the above nanocomposites, the topic of “green” chemistry and chemical processes has been emphasized [13]. Ionic liquids, defined herein as salts that melt at or below 100°C, can provide unique properties, such as non-flammability, low volatility, high thermal and electrochemical stability, and ionic conductivity [14-17]. They are regarded as environmentally friendly solvents. In particular, the imidazolium ionic liquids associated with specific anions are known to self-organize in a way that is adaptable to the fabrication of nanostructures of metal and conducting polymers [18-22]. However, it is still appealing to study the relationship between the different ionic liquids and the structures of the nanomaterials fabricated in them.
In this paper, we presented a simple and environmentally friendly method to synthesize PPy/Ag nanocomposites at the interface of water and ionic liquid. During the formation process of PPy/Ag, ultraviolet light was introduced in order to improve the reaction rate and distribution level of Ag nanoparticles. Fourier-transform infrared (FTIR), X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS) and transmission electron microscopy (TEM) were used to characterize the PPy/Ag nanocomposites. It was observed that PPy/Ag nanocomposites possess enhanced higher conductivity since silver was a conductor.
Experimental Section
Polyvinylpyrrolidone (PVP), 1-butyl-3-methylimidazolium tetrafluoroborate (BmimBF4) and AgNO3 were purchased from Aldrich. Pyrrole was distilled twice under reduced pressure, stored under nitrogen and refrigerated in the dark. Other reagents were used as received without further treatment.
In a typical synthesis of PPy/Ag nanocomposites, 2 mmol of pyrrole was dissolved in 10 mL of BmimBF4. Then, AgNO3 was dissolved in aqueous solution with or without 0.1 M PVP. The two solutions were then carefully transferred to a beaker and an interface was generated between two layers. The solutions were placed under a UV lamp (500 W) set at 254 nm for 60 min at room temperature. After that, the UV-light was removed and the reaction was continued for 10 h. The precipitate was centrifuged and washed with distilled water and ethanol for several times, respectively. The final product was dried under vacuum at 40°C for 24 h.
The morphologies were measured by transmission electron microscopy (TEM, JEM-200CX). Fourier-transform infrared spectra (FTIR) of the samples were recorded on a Bruker VECTOR22 spectrometer using KBr pressed disks. XRD patterns were taken with a Shimadzu XD-3A instrument with a Cu Ka X-ray source. XPS was performed using a Kratos AXIS HSi spectrometer equipped with a monochromatized Al Kα X-ray source (1,486.6 eV photons). The conductivities were measured by using the standard four-probe method at room temperature.
Results and Discussion
Recently, conducting polymers with various nanostructures have been synthesized in the biphasic ionic liquid/water system [23]. Both water and ionic liquid are environmental benign solvents. In this work, PPy/Ag nanocomposites were successfully synthesized through the redox reaction of AgNO3 and pyrrole monomer at the water/ionic liquid interface with the aid of UV-light. TEM image of a typical PPy/Ag nanocomposite, with the size range of 80–140 nm prepared in the presence of PVP, was shown in Fig. 1a. In comparison with the morphology of the nanocomposite prepared in the absence of PVP (Fig. 1b), there were two major findings that can be easily observed: (1) there was no obvious change in the average particle size for the nanoparticles with and without PVP and (2) the degree of dispersion and the uniformity of the resultant composite nanoparticles prepared in the presence of PVP were effectively improved. Therefore, PVP as an anchor agent played an important role during the synthesis process. Shin et al. [24] synthesized silver nanoparticles stabilized with amphiphilic PVP and suggested that PVP promoted the nucleation of metallic silver. The amphiphilic PVP would be beneficial for the adherence between the polymer shell and the inorganic core. In the present work, when the mixture containing pyrrole and AgNO3 was irradiated by UV-light, the silver ions may first interact with PVP, and then are reduced to silver atoms. At the same time pyrrole radical cation was formed. Herein, PVP acted as a useful stabilizer to promote a strong interaction between the Ag nanoparticles and the PPy matrix. PVP might provide active sites on the silver particles so as to induce the growing polycationic PPy chains to complete the coating of PPy layers, resulting in the formation of PPy/Ag nanocomposites. In addition, the effect of the feed ratio of AgNO3 to pyrrole monomer (represented by [AgNO3]/[pyrrole]) on the resultant products was also studied. As shown in Fig. 2, PPy-coated Ag nanocomposite with a diameter of 80–130 nm was uniform in size and well dispersed. Moreover, the size of Ag increased gradually with the increase of [AgNO3]/[pyrrole].
Figure 1.
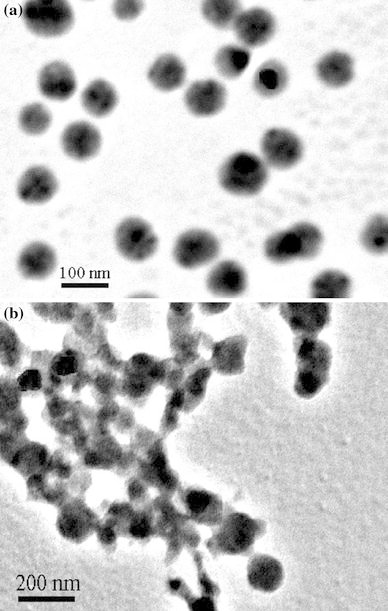
TEM images of PPy/Ag nanocomposites prepared at [AgNO3]/[pyrrole] of 0.8 with (a) and without (b) of PVP
Figure 2.
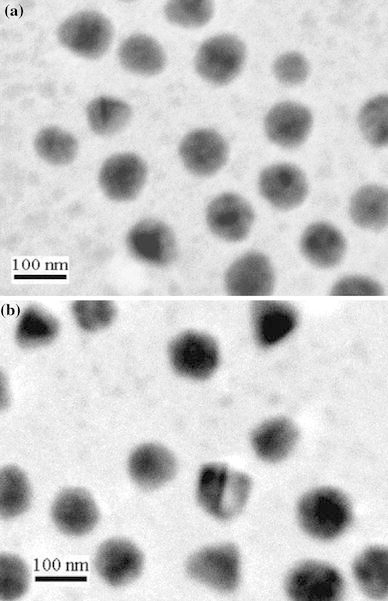
TEM images of PPy/Ag nanocomposites prepared at [AgNO3]/[pyrrole] of 0.2 (a) and 0.5 (b)
The conductivity of the PPy/Ag nanocomposite prepared at [AgNO3]/[pyrrole] of 0.8 was found to be 12.8 S/cm, whereas that of the pure PPy was found to be 0.14 S/cm [25]. There was an increase of about two orders of magnitude in conductivity upon incorporation of silver in the PPy matrix based on the present method. Because silver is a conductor, the higher conductivity of the nanocomposites should be ascribed to the combination of silver in the nanocomposites. As shown in Table 1, with the increase of feed ratios of AgNO3 to pyrrole monomer, the conductivity of the nanocomposite increased perhaps due to the increase of Ag content in the nanocomposite.
Table 1.
Conductivities of the products prepared at different ratios of AgNO3 and pyrrole monomer
| [AgNO3]/[pyrrole] | Conductivity (S/cm) |
|---|---|
| 1 | 13.5 |
| 0.8 | 12.8 |
| 0.5 | 9.6 |
| 0.2 | 3.2 |
| 0 | 0.14 |
Figure 3 showed the presence of a series of IR characteristic bands for the PPy/Ag nanocomposite and PPy. The peaks located at 1,540 and 1,454 cm−1 were related to the fundamental vibrations of the pyrrole rings in the protonated PPy [26]. Bands at 1,293 and 1,034 cm−1 were assigned to a combination C–H in-plane ring bending and the deformation of the five-membered ring that contains the C=C–N deformation [27]. The peak near 958 cm−1 may be attributed to the C–C out-of-plane ring deformation vibration. The peak at 1,163 cm−1 was related to C–N stretching wagging vibrations. In addition, the band at 1,655 cm−1 corresponded to the stretching vibration of the C=O group, which indicated that PVP was absorbed in the prepared nanocomposites.
Figure 3.
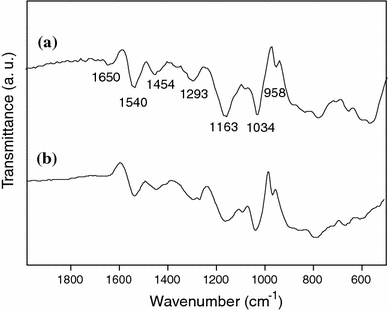
FTIR spectra of PPy/Ag nanocomposite prepared at [AgNO3]/[pyrrole] of 0.8 (a) and PPy (b)
XPS was further used to characterize the PPy/Ag nanocomposite. Figure 4 showed the XPS N 1s and Ag 3d core-level spectra of the nanocomposite. The N 1s spectrum was deconvoluted into three peak component with BEs at 399.5 and 401.2 eV, attributed to the –NH– and –N+– species [28]. Lower binding energy component (397.5 eV) attributed to uncharged deprotonated imine (=N–) nitrogen atoms or imine defects responsible for interruption of effective conjugation of PPy, was absent in the core-level N1s XPS spectrum of PPy/Ag nanocomposites. Ratio of the areas of the nitrogen peaks at 401.2 and 399.5 eV was 0.26 for the nanocomposites, which correlated well with the doped state of the nanostructured PPy [29]. The inclusion of zero valent Ag species in the nanocomposites was confirmed by the presence of major doublets at 367.9 and 373.9 eV attributed to Ag 3d5/2 and Ag 3d3/2, respectively [30]. The XPS results thus indicated that chemically doped PPy and elemental Ag were produced simultaneously in the present interface system.
Figure 4.
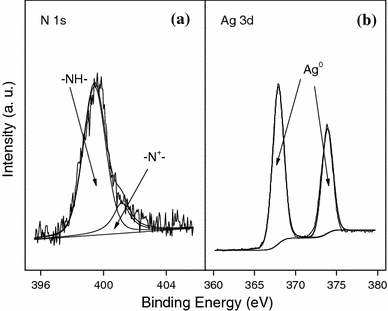
XPS N 1s (a) and Ag 3d (b) core-level spectra of PPy/Ag nanocomposite prepared at [AgNO3]/[pyrrole] of 0.8
The powder XRD pattern also confirmed the incorporation of Ag in the nanocomposites. As shown in Fig. 5a, 5b, the broad band appearing at 2θ value of 25° was ascribed to the amorphous PPy. Above 30°, four additional diffraction peaks at 38, 44, 65 and 77° that represented Bragg’s reflections from (111), (200), (220) and (311) planes of Ag were observed in Fig. 4a, which further indicated the existence of Ag nanoparticles in the nanocomposites.
Figure 5.
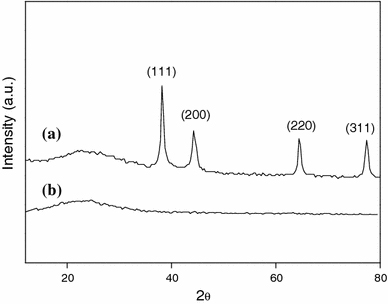
XRD patterns of PPy/Ag nanocomposite prepared at [AgNO3]/[pyrrole] of 0.8 (a) and PPy (b)
Conclusions
A simple one-step strategy for synthesizing PPy/Ag nanocomposites in the presence of PVP has been demonstrated. The oxidation of pyrrole and the formation of silver were initiated by UV irradiation, and then occurred simultaneously at the interface of water and ionic liquid. The formation of the nanocomposites was confirmed by TEM, XPS, FTIR and XRD analysis. Our method showed the merits of easier preparation and more environmental harmony. The nanocomposites may have potential application in many fields of biological separation, enzyme immobilization and biosensors.
Misc
An erratum to this article can be found athttp://dx.doi.org/10.1007/s11671-010-9759-y
Contributor Information
Liang Li, Email: msell08@163.com.
Xiaoming Yang, Email: yangxiaoming@suda.edu.cn.
Acknowledgments
The work is supported by Educational Bureau of Hubei Province (Q20091508), Scientific Research Key Project of Ministry of Education of China (209081), National Natural Science Foundation of China (20904044) and State Key Laboratory of Coordination Chemistry (Nanjing University).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Gallon BJ, Kojima RW, Kaner RB, Diaconescu PL. Angew. 2007. p. 7389. [DOI] [PubMed]
- Mangeney C, Bousalem S, Connan C, Vaulay MJ, Bernard S, Chehimi MM. Langmuir. 2006. p. 10163. COI number [1:CAS:528:DC%2BD28XhtFSqs7nO] [DOI] [PubMed]
- Yang W, Liu J, Zheng R, Liu Z, Dai Y, Chen G, Ringer S, Braet F. Nanos. 2008. p. 468. COI number [1:CAS:528:DC%2BD1cXhsVyhtrjO]; Bibcode number [2008NRL.....3..468Y] [DOI] [PMC free article] [PubMed]
- Kishore PS, Viswanathan B, Varadarajan TK. Nano. 2008. p. 14. COI number [1:CAS:528:DC%2BD1cXlslejsLo%3D] [DOI]
- Li X, Gao Y, Gong J, Zhang L, Qu L. J. Phys. Chem. C. 2009. p. 69. COI number [1:CAS:528:DC%2BD1cXhsVOnsLjO] [DOI]
- Tseng RJ, Huang JX, Ouyang J, Kaner RB, Yang Y. Nano Lett. 2005. p. 1077. COI number [1:CAS:528:DC%2BD2MXktVajt7g%3D]; Bibcode number [2005NanoL...5.1077T] [DOI] [PubMed]
- Wessling B, Thun M, Arribas-Sanchez C, Gleeson S, Posdorfer J, Rischka M, Zeysing B. Nano. 2007. p. 455. COI number [1:CAS:528:DC%2BD2sXhtl2rtrnJ] [DOI]
- Guo S, Dong S, Wang E. Small. 2009. p. 1869. COI number [1:CAS:528:DC%2BD1MXps1yrsr4%3D] [DOI] [PubMed]
- Xu J, Hu J, Quan B, Wei Z. Macromol. 2009. p. 936. COI number [1:CAS:528:DC%2BD1MXms1eltrs%3D] [DOI] [PubMed]
- Selvan ST, Spatz JP, Klok HA, Moller M. Adv. 1998. p. 132. COI number [1:CAS:528:DyaK1cXnvF2jtQ%3D%3D] [DOI]
- Xu P, Han X, Wang C, Zhou D, Lv Z, Wen A, Wang X, Zhang B. J. Phys. Chem. B. 2008. p. 10443. COI number [1:CAS:528:DC%2BD1cXptlCjsb0%3D] [DOI] [PubMed]
- Feng X, Mao C, Yang G, Hou W, Zhu J. Langmuir. 2006. p. 4384. COI number [1:CAS:528:DC%2BD28Xis1WltrY%3D] [DOI] [PubMed]
- Anastas PT, Warner JC. Warner, green chemistry: theory and practice. Oxford University Press, New York; 1998. [Google Scholar]
- Welton T. Chem. 1999. p. 2071. COI number [1:CAS:528:DyaK1MXkt1artrw%3D] [DOI] [PubMed]
- Wasserscheid P, Welton T. Welton, ionic liquids in synthesis. Wiley-VCH, Weinheim; 2003. [Google Scholar]
- Davis JH, Fox PA. Chem. 2003. p. 1209. [DOI] [PubMed]
- Salerno M, Patra N, Cingolani R. Nano. 2009. p. 865. COI number [1:CAS:528:DC%2BD1MXoslKisLc%3D] [DOI] [PMC free article] [PubMed]
- Li L, Huang Y, Yan G, Liu F, Huang Z, Ma Z. Mater. 2009. p. 8. COI number [1:CAS:528:DC%2BD1cXhtlartLbN] [DOI]
- Dinda E, Si S, Kotal A, Mandal TK. Chem. 2008. pp. 5528–5537. COI number [1:CAS:528:DC%2BD1cXoslantrs%3D] [DOI] [PubMed]
- Kubisa P. J. 2005. p. 4675. COI number [1:CAS:528:DC%2BD2MXhtVygsLjM]; Bibcode number [2005JPoSA..43.4675K] [DOI]
- Pringle JM, Ngamna O, Lynam C, Wallace GG, Forsyth M, MacFarlane DR. Macromolecules. 2007. p. 2702. COI number [1:CAS:528:DC%2BD2sXktFegu7w%3D]; Bibcode number [2007MaMol..40.2702P] [DOI]
- Wang Y, Yang H. J. 2005. p. 5316. COI number [1:CAS:528:DC%2BD2MXis1Ohtrs%3D] [DOI] [PubMed]
- Huang J, Kaner RB. J. 2004. p. 851. COI number [1:CAS:528:DC%2BD3sXhtVWisb%2FM] [DOI] [PubMed]
- Shin HS, Yang HJ, Kim SB. J. 2004. p. 89. COI number [1:CAS:528:DC%2BD2cXjsFOgs78%3D] [DOI] [PubMed]
- Yan F, Xue G, Zhou M. J. 2000. p. 135. COI number [1:CAS:528:DC%2BD3cXjtlOhtLc%3D] [DOI]
- Kumar DS, Nakamura K, Nishiyama S, Ishii S, Noguchi H, Kachiwagi K, Yoshida Y. J. 2003. p. 2705. COI number [1:CAS:528:DC%2BD3sXhvV2ktbY%3D]; Bibcode number [2003JAP....93.2705K] [DOI]
- Kostic R, Eakovic D, Stepanyan SA, Davidova IE, Gribov LA. J. 1995. p. 3104. COI number [1:CAS:528:DyaK2MXjvFKltLw%3D]; Bibcode number [1995JChPh.102.3104K] [DOI]
- Huang SW, Neoh KG, Kang ET, Han HS, Tan KL. J. 1998. p. 1743. COI number [1:CAS:528:DyaK1cXkvVehtLg%3D] [DOI]
- Menon VP, Lei J, Martin CR. Chem. 1996. p. 2382. COI number [1:CAS:528:DyaK28XltV2ks74%3D] [DOI]
- Moulder JF, Stickle WF, Sobol PE, Bomben KD. X-ray photoelectron spectroscopy. Perkin–Elmer, Eden Prairie; 1992. [Google Scholar]


