Summary
To determine if short-term calorie restriction reverses vascular endothelial dysfunction in old mice, old (O, n=30) and young (Y, n=10) male B6D2F1 mice were fed ad libitum (AL) or calorie restricted (CR, ∼30%) for 8 weeks. Ex vivo carotid artery endothelium-dependent dilation (EDD) was impaired in OAL vs. YAL (74±5 vs. 95±2% of maximum dilation, P<0.05), whereas OCR and YCR did not differ (96±1 vs. 94±3%). Impaired EDD in OAL was mediated by reduced nitric oxide (NO) bioavailability associated with decreased endothelial NO synthase expression (aorta) (P<0.05), both of which were restored in OCR. Nitrotyrosine, a cellular marker of oxidant modification, was markedly elevated in OAL (P<0.05), whereas OCR was similar to Y. Aortic superoxide production was 150% greater in OAL vs. YAL (P<0.05), but normalized in OCR, and TEMPOL, a superoxide dismutase (SOD) mimetic that restored EDD in OAL (to 97±2%), had no effect in Y or OCR. OAL had increased expression and activity of the oxidant enzyme, NADPH oxidase, and its inhibition (apocynin) improved EDD, whereas NADPH oxidase in OCR was similar to Y. Manganese SOD activity and sirtuin1 expression were reduced in OAL (P<0.05), but restored to Y in OCR. Inflammatory cytokines were greater in OAL vs. YAL (P<0.05), but unaffected by CR. Carotid artery endothelium-independent dilation did not differ among groups. Short-term CR initiated in old age reverses age-associated vascular endothelial dysfunction by restoring NO bioavailability and reducing oxidation stress via reduced NADPH oxidase-mediated superoxide production and stimulation of anti-oxidant enzyme activity, and upregulates sirtuin1.
Introduction
The risk of cardiovascular diseases is markedly increased in older adults (Lakatta & Levy 2003) and this is linked to the development of vascular endothelial dysfunction, most commonly demonstrated as impaired endothelium-dependent dilation (EDD). The latter is mediated by a reduction in the bioavailability of the dilating molecule nitric oxide (NO) and is linked to the development of vascular oxidative stress (Celermajer et al. 1994; DeSouza et al. 2000; Taddei et al. 2001). Thus, therapeutic strategies that reduce vascular oxidative stress, increase NO bioavailability and reverse age-associated impairments in EDD have important clinical implications for the prevention of cardiovascular diseases in older adults.
Calorie restriction, defined as a reduction in energy intake without malnutrition, extends lifespan and is associated with enhanced physiological function in several species (Weindruch & Sohal 1997; Masoro 2005). Although little is known about its potential effects on vascular aging, recent observations indicate that life-long calorie restriction preserves EDD with aging in rats (Ungvari et al. 2008; Csiszar et al. 2009). This was associated with greater protein expression of endothelial NO synthase (eNOS) and evidence for less production of superoxide in large elastic arteries (Ungvari et al. 2008; Csiszar et al. 2009), suggesting the possibility of enhanced NO bioavailability and reduced vascular oxidative stress.
Recent findings indicate that shorter-term calorie restriction may produce some of the same effects on longevity and physiological function in rodents as life-long restriction of energy intake (Cao et al. 2001; Dhahbi et al. 2004; Goto 2006). However, it is unknown if short-term calorie restriction can improve or reverse vascular endothelial dysfunction associated with aging and, if so, the mechanisms by which this effect is mediated.
In the present study we used a recently established model of age-associated endothelial dysfunction in large arteries (Lesniewski et al. 2009) to test the hypothesis that short-term calorie restriction initiated late in life restores EDD by improving NO bioavailability as a result of reducing oxidative stress. We also determined the role of reduced arterial superoxide production, as well as the expression and activities of the oxidant enzyme nicotinamide adenine dinucleotide phosphate (NADPH)-oxidase and antioxidant enzymes in mediating these effects of calorie restriction. Arterial expression of the deacetylase sirituin 1 (SIRT1--silent mating type information regulation 2 homolog) was determined because it activates eNOS (Mattagajasingh et al. 2007) and is implicated in the physiological effects of calorie restriction (Cohen et al. 2004; Csiszar et al. 2009). Because life-long calorie restriction can have anti-inflammatory effects (Spaulding et al. 1997; Ungvari et al. 2008; Csiszar et al. 2009), we also assessed arterial expression of inflammatory proteins. Finally, vasodilation in response to a NO donor (i.e., endothelium-independent dilation) was assessed to determine if improvements in EDD with calorie restriction might be mediated by increases in vascular smooth muscle responsiveness to NO.
Results
Food intake, body and fat pad mass, and metabolic characteristics
Daily food intake during the 8-week experimental period was 10% less in old ad libitum (OAL) vs. young ad libitum (YAL) (4.4±0.1 vs. 4.9±0.1g, P<0.05), whereas all calorie restricted (CR) mice ate 4.1g, 3.7g and 3.2g, respectively, in weeks 1, 2 and 3-8.
Body weight did not differ among the groups at baseline: YAL 32.8±0.8 g, YCR 32.4±1.2 g, OAL 34.8±1.4 g, OCR 35.7±0.9 g. Body weight decreased over the feeding period similarly in the calorie restricted groups (YCR -9.6±1.5g, OCR -10.0±0.9), whereas there were no significant changes from baseline in the ad libitum fed groups (YAL +2.7±1.1g, P=0.14; OAL -3.1±0.8, P=0.21). At the end of the treatments, epididymal white adipose tissue mass was lower in the calorie restricted compared with the ad libitum fed animals at both ages (YCR 0.15±0.03 vs. YAL 0.71±0.22; OCR 0.11±0.02 vs. OAL 0.25±0.04 g, both P<0.05).
Blood glucose and plasma triglycerides were lower in both young and old calorie restricted animals compared with ad libitum fed controls of the same age, but there was no significant change in plasma insulin, free fatty acids or cholesterol associated with short-term calorie restriction (Table 1).
Table 1.
Metabolic characteristics
| Young ad libitum fed | Young calorie restricted | Old ad libitum fed | Old calorie restricted | |
|---|---|---|---|---|
| Glucose (mg/dL) | 137±4.4 | 55±3.3* | 123±4.0 | 74.5±5.4* |
| Insulin (ng/mL) | 0.65±0.18 | 0.45±0.03 | 0.80±0.11 | 0.64±0.23 |
| Free Fatty Acids (μM) | 613±76 | 653±42 | 746±132 | 749±76 |
| Triglycerides (mg/dL) | 149±30 | 20.0±8.3* | 133±17 | 19.2±2.8* |
| Cholesterol (mg/dL) | 96.2±9.8 | 83.3±16 | 93.0±18 | 90.2±7.8 |
Values are mean±SEM. P<0.05 ad libitum vs. calorie restricted within that age
Short-term calorie restriction restored EDD in old mice without influencing endothelium-independent dilation
Carotid artery pre-constriction to phenylephrine was not different in the ad libitum and calorie restricted groups (p=0.15). Peak carotid artery dilation in response to acetylcholine was impaired in OAL vs. YAL (74±5% vs. 95±2%, P<0.05), but was preserved in OCR (96±1%), not differing from YAL or YCR (94±3%) (left panel Figure 1). Neither vasodilatory sensitivity (IC50) to acetylcholine (YAL: 2.1E-8±9.8E-9, YCR: 1.8E-8±3.8E-9, OAL: 2.6E-8±1.43E-8, OCR: 2.6E-8±1.56E-8) nor endothelium-independent dilation to sodium nitroprusside (right panel Figure 1) differed among the groups (all P>0.5).
Figure 1. Endothelium-dependent and -independent dilation in young and old calorie restricted (YCR and OCR) and ad libitum fed (YAL and OAL) B6D2F1 mice.
Left: Dose-responses to the endothelium-dependent dilator acetylcholine (Ach) in the absence and presence of the endothelial nitric oxide synthase (eNOS) inhibitor N-G-nitro-L-arginine methyl ester (L-NAME) (YAL: n=5 YCR n=5, OAL: n=15 OCR: n=15). Right: Dose-responses to the endothelium-independent dilator sodium nitroprusside. Values are mean±SEM. * P<0.05 for main effect or interaction vs. other groups.
Short-term calorie restriction-related improvements in EDD in old mice were mediated by increases in NO bioavailability
NO inhibition with L-NAME (NG-nitro-L-arginine methyl ester) reduced peak carotid artery dilation to acetylcholine in all groups (P<0.005). However, the reduction in carotid artery dilation to acetylcholine after pretreatment with L-NAME compared to acetylcholine alone (NO-mediated dilation, left panel Figure 2) was smaller in OAL vs. YAL (P<0.05), and was associated with lower aortic eNOS protein expression (P<0.05, right panel Figure 2). In contrast, NO-mediated dilation and eNOS were preserved in OCR compared with YAL and YCR.
Figure 2. NO bioavailability and eNOS expression.
Left: Nitric oxide-dependent dilation was calculated as the maximal dilation of carotid arteries to acetylcholine (ACh) minus the maximal response to ACh in the presence of L-NAME (Max Dilationach -Max Dilationach+l-name) in calorie restricted and ad libitum fed young and old mice (YAL, YCR, OAL, OCR). Right: eNOS protein in aorta expressed relative to GAPDH and normalized to YAL mean value. Representative blot shown. Values are mean±SEM, * P<0.05.
Superoxide-associated oxidative stress is reduced in short-term calorie restricted old mice and contributes to preserved EDD
Nitrotyrosine staining (55kDa protein band), a cellular marker of oxidant modification of tyrosine residues of proteins, was 80% higher in aorta of OAL vs. YAL (P<0.05), whereas staining in OCR was markedly lower than OAL (P<0.005), somewhat lower than YAL and similar to YCR (left panel Figure 3).
Figure 3. Oxidative stress.

Left: Nitrotyrosine (NT) abundance in aorta of young and old calorie restricted (YCR, OCR) and ad libitum fed (YAL, OAL) mice assessed by western blot analysis. Data are expressed in relation to GAPDH and normalized to YAL mean value. *P<0.05 YAL vs. OAL and #P<0.05 CR vs. AL groups. Below: Representative blot shown. Middle: Mean electron paramagnetic resonance (EPR) signal from aortic rings (3 mm) showing superoxide production. *P<0.05 YAL vs. OAL and #P<0.05 CR vs. AL groups. Right: Maximal dilation of carotid arteries to acetylcholine (ACh) and to ACh + TEMPOL in the absence or presence of the endothelial nitric oxide synthase (eNOS) inhibitor N-G-nitro-L-arginine methyl ester (L-NAME) in YAL, YCR, OAL and OCR mice. *P<0.05 vs. ACh + TEMPOL and #P<0.05 vs. Ach within groups. Values are mean±SEM.
Aortic superoxide production, measured directly by electron paramagnetic resonance, was 150% greater in OAL vs. YAL (P<0.05). In contrast, superoxide production in OCR was similar to YAL and not significantly different than YCR (middle panel Figure 3).
TEMPOL (4-hydroxy-2,2,6,6-tetramethylpiperidine 1-oxyl), a superoxide dismutase (SOD) mimetic, restored carotid artery dilation to acetylcholine in OAL to levels observed in YAL, but had no effect in OCR or the young groups (right panel Figure 3). eNOS inhibition with L-NAME abolished the selective TEMPOL-mediated restoration of the peak vasodilatory response to acetylcholine in OAL such that there were no longer differences among the groups (right panel, Figure 3).
Together these observations are consistent with the idea that short-term calorie restriction reduced superoxide bioactivity, which, in turn, increased NO bioavailability and improved EDD.
Down-regulation of NADPH oxidase contributes to reduced superoxide associated improvements in EDD in short-term calorie restricted old mice
NADPH oxidase activity and p67phox subunit expression were greater in aorta of OAL vs. YAL (P<0.05), whereas OCR demonstrated levels similar to YAL and YCR (left panel Figure 4).
Figure 4. NADPH oxidase.

Left: Enzyme activity and protein expression of NADPH oxidase (p67phox subunit) in aorta of ad libitum fed (AL) and calorie restricted (CR) young (Y) and old (O) mice (normalized to AL GAPDH controls). * P<0.05 vs. YAL group. Representative western blot shown below. Middle and Right: Endothelium-dependent dilation in the presence and absence of the NADPH-oxidase inhibitor apocynin in OAL and OCR (middle) and in YAL and YCR (right). P<0.05 for OAL vs. apocynin groups. Values are mean±SEM.
Apocynin, a NADPH oxidase inhibitor, restored carotid artery dilation to acetylcholine in OAL to that observed in YAL, but had no effect in OCR or the young groups (right panel Figure 4). There were no differences in dilation among the groups in the presence of apocynin.
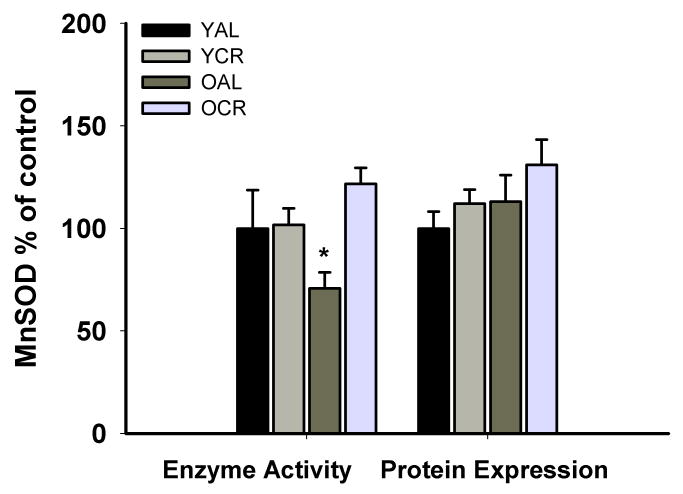
Short-term calorie restriction increased manganese SOD activity in old mice
Protein expression of manganese SOD (MnSOD) in aorta did not differ among the groups (Figure 5). However, aortic MnSOD activity was lower in OAL vs. YAL (P<0.05), whereas the activity in OCR was preserved at the levels of YAL and YCR (Figure 5). Catalase protein expression and activity were not significantly different among the groups (all P>0.05, data not shown).
Figure 5. Manganese superoxide dismutase.
Enzyme activity and protein expression of manganese superoxide dimustase (MnSOD) in aorta from young and old ad libitum fed (YAL and OAL) and calorie restricted mice (YCR and OCR). Values are mean±SEM, * P<0.05.
Short-term calorie restriction increases SIRT1 expression in aorta of old mice, but has no effect on inflammatory cytokines
Protein expression of the deacetylase SIRT1 was lower in aorta of OAL vs. YAL, whereas OCR demonstrated levels not significantly different from YAL and YCR (Figure 6). SIRT1 protein expression was positively related to peak carotid artery dilation in response to acetylcholine (r=0.63, P<0.001).
Figure 6. SIRT1.

Protein expression of SIRT1 in aorta of young and old calorie restricted (YCR and OCR) and ad libitum fed mice (YAL and OAL) (normalized to YAL GAPDH controls). Representative blot shown below. Values are mean±SEM.* P<0.05.
Expression of the inflammatory cytokines interleukin-1β, interleukin-6, interferon γ and tumor necrosis factor α was greater in OAL vs. YAL and in OCR vs. YCR (all P<0.05, Table 2). However, short-term calorie restriction had no significant effect on these inflammatory proteins in either young or old mice.
Table 2.
Inflammatory protein expression in aorta
| Young ad libitum fed | Young calorie restricted | Old ad libitum fed | Old calorie restricted | |
|---|---|---|---|---|
| IL1-β | 2.0 ± 0.4 | 1.2± 0.2 | 5.6± 1.4* | 9.5 ± 2.3 |
| IL-6 | 1.0± 0.2 | 1.2 ± 0.3 | 3.1 ± 0.6* | 5.0 ± 1.2 |
| INF-γ | 2.7±0.6 | 2.5±0.7 | 6.0±1.0* | 8.9±2.2 |
| TNF-α | 0.4±0.1 | 0.5±0.1 | 1.2±0.1* | 1.2±0.3 |
Values are mean±SEM and are expressed as pg/10μg total protein. P<0.05 vs. young ad libitum fed.
Discussion
EDD, NO bioavailability and eNOS
Life-long restriction of energy intake preserves EDD in skeletal muscle arterioles and aorta of F344 rats (Ungvari et al. 2008; Csiszar et al. 2009). Here we extend these findings by showing that short-term calorie restriction initiated late in life completely reverses the impairment in carotid artery EDD observed in old B6D2F1 mice. Endothelium-independent dilation to sodium nitroprusside was unchanged with energy intake restriction, indicating that the improvements in EDD were independent of changes in vascular smooth muscle sensitivity to NO.
Rather our findings show that the improvements in EDD were mediated by increases in NO bioavailability. This is supported by the fact that inhibition of NO production with L-NAME produced a suppression of acetylcholine-induced dilation in energy restricted old mice that was similar to that of young mice, such that EDD was not different in the ad libitum and calorie restricted groups in the absence of NO.
It is possible that increased expression of eNOS protein contributed to the increase in NO bioavailability associated with short-term calorie restriction by increasing NO production. We found that eNOS in the aorta of the energy intake restricted old mice was ∼30% greater than in old ad libitum fed mice, was similar to young mice, and correlated with peak dilation in response to acetylcholine in all animals. This is consistent with previous observations that eNOS is greater in large arteries of old rats subjected to life-long calorie restriction than in ad libitum fed old animals.
Oxidative stress
Independent of these increases in eNOS, our data demonstrate that the primary mechanism by which short-term energy intake restriction restores NO bioavailability and EDD in old mice is by reducing oxidative stress. Several lines of evidence support this idea.
Nitrotyrosine, a marker of cellular oxidative stress (Reiter et al. 2000), increases with aging vascular endothelial cells of humans and is inversely related to EDD (Donato et al. 2007). In the present study, nitrotyrosine was markedly lower in aorta of the old calorie restricted compared with old ad libitum fed mice, and was similar to levels observed in young mice. This extends to aging arteries previous observations of reduced oxidative modifications in other tissues in response to short- or long-term energy intake restriction (Gredilla et al. 2001; Bevilacqua et al. 2005).
Aortic superoxide production, measured directly with electron paramagnetic resonance, was markedly elevated in old mice that were ad libitum fed, but old mice who underwent short-term calorie restriction demonstrated much lower levels, not different than young mice. This finding is consistent with recent results using a fluorescent dye and a chemiluminescence method that suggest reduced superoxide production in arteries of life-long calorically-restricted old rats compared with ad libitum fed controls (Ungvari et al. 2008; Csiszar et al. 2009).
Finally, we found that a SOD mimetic restored EDD in ad libitum fed, but not calorie restricted old mice, indicating an absence of superoxide-mediated suppression of EDD in the latter group. This observation directly links the reduction in superoxide to improved vascular endothelial function, in agreement with recent findings in life-long calorically restricted rats (Csiszar et al. 2009). We extended these findings further by showing that inhibition of NO production with L-NAME abolished the selective improvement in the EDD response to TEMPOL in the ad libitum fed old mice.
Considered together, our data provide evidence that short-term energy intake restriction restores carotid artery EDD in old mice by reducing superoxide-induced oxidative stress, which, in turn, increases NO bioavailability.
NADPH oxidase
Our results also provide insight into the mechanisms by which short-term calorie restriction reduced vascular oxidative stress in old mice. NADPH oxidase is a major source of superoxide production in arteries (Zalba et al. 2000) that is increased with aging in humans (Donato et al. 2007) and, as confirmed here, in ad libitum fed rodents (Csiszar et al. 2007; Donato et al. 2007; Lesniewski et al. 2009). In the present study, we found that both protein expression and activity of this enzyme were lower in aorta of old energy intake restricted mice and similar to that of young mice. We then connected these changes directly to function by showing that inhibition of NADPH oxidase with apocynin selectively restored EDD in old ad libitum fed mice, indicating that the preserved EDD in the old calorie restricted mice was mediated primarily by reduced NADPH oxidase -mediated superoxide production.
Antioxidant enzymes
The expression of two important antioxidant enzymes, MnSOD and catalase, was not different in aorta of our groups. However, MnSOD activity was reduced in the old ad libitum fed animals and this was increased to levels of young mice in the calorie restricted old animals. That TEMPOL, a SOD mimetic, restored EDD in ad libitum fed, but not calorie restricted old mice supports the possibility that the increase in bioactivity of this endogenous antioxidant may have contributed to more effective scavenging of superoxide and reduced oxidative stress in arteries of the old energy restricted mice.
The mechanism by which calorie restriction restored MnSOD in our old mice is unclear, but nitrosylation of MnSOD reduces its catalytic activity (Yamakura et al. 1998; Guo et al. 2003). Thus, reduced nitrosylation of MnSOD could explain the increase in activity observed in the calorie restricted old animals. Because MnSOD deficiency is associated with oxidative stress and endothelial dysfunction with aging in mice (Brown et al. 2007), the increase in MnSOD activity could have contributed to the improvement in EDD with calorie restriction in our old mice.
SIRT-1
SIRT1 is a histone deacetyalse that increases in response to calorie restriction in a variety of tissues (Cohen et al. 2004). Deacetylation by SIRT1 activates eNOS and increases NO production and EDD (Mattagajasingh et al. 2007).
Protein expression of SIRT-1 is increased in coronary artery endothelial cells incubated with serum from calorie restricted rats (Csiszar et al. 2009). In the present study, SIRT1 expression in aorta of old ad libitum fed mice was ∼40% lower than in young controls, and short-term energy intake restriction restored the expression to young levels. Moreover, peak EDD was positively related to expression of SIRT-1. These observations are in agreement with previous work in young mice demonstrating increases in aortic expression of SIRT1 after one year of calorie restriction and that manipulation of SIRT1 expression was consistently related to NO-mediated EDD (Zhang et al. 2008).
Inflammatory cytokines
Life-long calorie restriction may suppress vascular inflammation with aging (Ungvari et al. 2008; Csiszar et al. 2009), and short-term calorie restriction inhibits signaling of the inflammatory nuclear transcription factor nuclear factor κB in the kidney of old rats (Jung et al. 2009). In the present study, protein expression of several pro-inflammatory cytokines was elevated in aorta of old compared with young ad libitum fed mice; however, calorie restriction did not affect expression in either age group. These observations suggest that short-term restriction of energy intake may not exert anti-inflammatory actions in arteries of old or young mice and, thus, may not contribute to the improved vascular endothelial function in old animals.
Circulating metabolic factors
In the present study, circulating glucose and triglyceride concentrations were lower in calorically restricted young and old animals, as reported previously in response to both short-term and life-long energy intake restriction (Lane et al. 2000; Mahoney et al. 2006). It is possible that these changes contributed to improvements in EDD in the old calorie restricted mice. However, the reductions in blood glucose and triglycerides were similar in young calorie restricted animals without any improvement in EDD.
Short-term vs. lifelong calorie restriction
Short-term calorie restriction initiated in old animals and life-long restriction of energy intake may represent different physiological states/stressors/stimuli. For example, whereas restriction of energy intake is a feature that is common to both interventions, weight loss occurs only during short-term calorie restriction initiated in old and young animals. We recently demonstrated that in middle-aged and older overweight and obese adult humans, energy intake restriction-based weight loss improves EDD by increasing NO bioavailability (Pierce et al. 2008). However, the old mice in the present study were of normal weight at baseline, and the body weight at the end of the 8-week feeding period was similar to that observed in old mice maintained on life-long calorie restriction (Turturro et al. 1999). Thus, the arterial adaptations to short-term calorie restriction in old animals may be the result of energy intake restriction, weight loss or both.
Conclusions
In conclusion, the results of the present study demonstrate that short-term calorie restriction initiated late in life restores vascular endothelial function in old mice. This is mediated by increased NO bioavailability as a result of reductions in superoxide-dependent oxidative stress and, perhaps, increased eNOS protein. Short-term restriction of energy intake in old mice may reduce oxidative stress by down-regulating NADPH oxidase and increasing MnSOD activity. Finally, short-term calorie restriction in old mice induces an increase in arterial expression of SIRT1, but has no obvious effect on arterial inflammatory cytokines.
Experimental Procedures
Ethical Approval
All animal procedures conformed to the Guide to the Care and Use of Laboratory Animals (NIH publication no. 85-23, revised 1996) and were approved by the UCB Animal Care and Use Committee.
Animals and calorie restriction
Younger (Y: 5-8 months) and older (O: 28-30 months) male B6D2F1 mice (n=40) obtained from the rodent colony of the National Institute on Aging were fed ad libitum (NIH-31 diet) for an acclimation period of two weeks. The young and old mice were then divided into two subgroups: one continued on the ad libitum regimen (YAL and OAL) and the other restricted to 3.2 g (NIH-31 fortified diet) for 6 weeks (YCR and OCR) after a 2-week progressive reduction in food intake (4.1g week 1 and 3.7g week 2). The mice were fed between 8.00 and 9.00 a.m. each day. All mice were housed in an animal care facility at the University of Colorado at Boulder on a 12:12 light:dark cycle and had continuous access to water. Age at sacrifice was 7-10 and 30-32 months (mean age: 7.4±0.6 and 8.2±0.7 months for YAL and YCR and 30.4±0.7 and 31.0±0.3 months for OAL and OCR respectively).
Vasodilatory responses
EDD and endothelium-independent dilation were determined in vitro in isolated carotid arteries as recently described in detail (Lesniewski et al. 2009). Mice were anesthetized using isoflurane and euthanized by exsanguination via cardiac puncture. The carotid arteries were carefully excised, cannulated onto glass micropipettes and secured with nylon (11-0) suture in myograph chambers (DMT Inc.) containing buffered physiological saline solution. The arteries were pressurized to 50 mmHg at 37° C and were allowed to equilibrate for 1 hour. After submaximal preconstriction with phenylepherine (2 μM), increases in luminal diameter in response to acetylcholine (ACh:1×10-9 to 1×10-4 mol/L) with and without co-administration of the NO synthase inhibitor NG-nitro-L-arginine methyl ester (L-NAME, 0.1mM/L, 30-minute incubation) or the superoxide dismutase (SOD) mimetic, 4-hydroxy-2,2,6,6-tetramethylpiperidine 1-oxyl (TEMPOL, 1mmol/L, 30 min incubation), were determined. EDD also was determined in the presence of the NADPH oxidase inhibitor apocynin (1mmol/L, 30 min incubation). Endothelium-independent dilation was determined by vasodilation in response to sodium nitroprusside (SNP: 1×10-10 to 1×10-4 mol/L).
Arterial protein expression and enzyme activities
Aortas were used as a surrogate large elastic artery to provide sufficient tissue for analysis of protein expression by western blot and enzyme activity as described previously (Cernadas et al. 1998; Blackwell et al. 2004; Ungvari et al. 2008; Lesniewski et al. 2009). Aortas were excised, cleared of surrounding tissues and frozen in liquid nitrogen before storage at -80°C. For assay the tissue was pulverized over liquid nitrogen and homogenized in ice-cold RIPA lysis buffer containing protease and phosphatase inhibitors (Protease Inhibitor Cocktail Tablet (Roche) and 0.01% phoshatase inhibitor cocktail (Sigma)). Fifteen μg of protein was loaded on 12% polyacrylamide gels, separated by electrophoresis and transferred onto nitrocellulose membranes for western blot analysis. Antibodies for western analysis included anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH, Cell Signaling, Danvers, MA), anti-p67phox, anti-eNOS (BD Biosciences, San Jose, CA), anti-nitrotyrosine, anti-SIRT1, anti-catalase (Abcam, Cambridge, MA), anti-manganese superoxide dismutase (MnSOD) (Stressgen, Ann Arbor, MI). Enzyme activity of MnSOD was determined in aortic lysates (1 μg protein) using a superoxide dismutase (SOD) activity assay kit in the presence of 1 mmol/L potassium cyanide to block copper-zinc SOD (CuZnSOD) activities. Enzyme activity for catalase was measured using a kit (Cayman Chemical Ann Arbor, MI). NADPH oxidase activity (10 μg total protein) was measured using a Amplex red xanthine/xanthine oxidase assay kit (Invitrogen, Carlsbad, CA) according to manufacturer instructions with NADPH (200 μmol/L/reaction) as the reaction substrate. Pro-inflammatory cytokines interleukin-1 beta, interleukin-6, interferon gamma and tumor necrosis factors alpha were measured using a multiplex SearchLight Chemiluminescent Array Kit (Thermo Fisher Scientific Inc.) according to manufacturer's instructions.
Metabolic Factors
At the end of the experimental period blood was collected through cardiac puncture and plasma was stored in -80C. Blood glucose was measured immediately using a glucose meter (One Touch Ultra, LifeScan, Inc., Milpitas CA). Plasma insulin was measured using an ELISA kit (Alpco, Salem, NH). Blood lipids (Free Fatty acids, Triglycerides and Cholesterol) were assessed using kits obtained from Wako Diagnostics, Richmond VA.
Superoxide production
Production of superoxide was measured by electron paramagnetic resonance (EPR) spectrometry using the spin probe 1-hydroxy-3-methoxycarbonyl-2,2,5,5-tetramethylpyrrolidine (CMH, Alexis Biochemicals). Stock solutions of CMH were prepared in ice-cold deoxygenated Krebs-HEPES buffer (mmol/L: NaCl, 99.01, KCl 4.69, CaCl2 2.50, MgSO4 1.20, K2HPO4 1.03, NaHCO3 25.0, glucose 11.10, Na-HEPES 20.00; pH 7.4) containing 0.1 mmol/L diethylenetriaminepenta-acetic acid (DTPA), 5 μmol/L sodium diethyldithiocarbamate (DETC) and pretreated with Chelex (Sigma) to minimize auto-oxidation of the spin probe. 3-mm aortic rings were washed once in PSS and again in modified Krebs-HEPES buffer. Rings were then incubated for 60 min at 37°C in 200 μL Krebs-HEPES buffer containing 0.5 mmol/L CMH and analyzed immediately on an MS300 X-band EPR spectrometer (Magnettech, Berlin, Germany). Instrument settings were: microwave frequency 9.43 Ghz, centerfield 3350 G, sweep 80 G, modulation amplitude 3 G, microwave power 10 mW, and receiver gain 50.
Statistics
Data are presented as mean±SEM. For the in vitro vasodilatory dose responses, group differences were determined by repeated measures ANOVA. For variables in which a significant interaction was found, comparisons between groups at particular doses were made using independent t-tests. For maximum dilation, protein expression and enzyme activities, comparisons between groups were made using ANOVA. Bivariate correlation analysis was performed to examine relations of interest. Significance was determined using P< 0.05.
Acknowledgments
We would like to thank Keri Nelson, Mark Blimline and Weston Blakeslee for technical assistance. The work was supported by the National Institutes of Health (AG006537, AG029337, AG013038, AG000279, AG029337, AG033196) and the Swedish Research Council.
Footnotes
Author Contributions: Catarina Rippe, Lisa A. Lesniewski, Melanie L. Connell, Anthony J. Donato, Thomas LaRocca and Douglas R. Seals contributed to the conception and design, analysis and interpretation of data and provided final approval of the paper.
References
- Bevilacqua L, Ramsey JJ, Hagopian K, Weindruch R, Harper ME. Long-term caloric restriction increases UCP3 content but decreases proton leak and reactive oxygen species production in rat skeletal muscle mitochondria. Am J Physiol Endocrinol Metab. 2005;289:E429–438. doi: 10.1152/ajpendo.00435.2004. [DOI] [PubMed] [Google Scholar]
- Blackwell KA, Sorenson JP, Richardson DM, Smith LA, Suda O, Nath K, Katusic ZS. Mechanisms of aging-induced impairment of endothelium-dependent relaxation: role of tetrahydrobiopterin. Am J Physiol Heart Circ Physiol. 2004;287:H2448–2453. doi: 10.1152/ajpheart.00248.2004. [DOI] [PubMed] [Google Scholar]
- Brown KA, Didion SP, Andresen JJ, Faraci FM. Effect of aging, MnSOD deficiency, and genetic background on endothelial function: evidence for MnSOD haploinsufficiency. Arterioscler Thromb Vasc Biol. 2007;27:1941–1946. doi: 10.1161/ATVBAHA.107.146852. [DOI] [PubMed] [Google Scholar]
- Cao SX, Dhahbi JM, Mote PL, Spindler SR. Genomic profiling of short- and long-term caloric restriction effects in the liver of aging mice. Proc Natl Acad Sci U S A. 2001;98:10630–10635. doi: 10.1073/pnas.191313598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol. 1994;24:471–476. doi: 10.1016/0735-1097(94)90305-0. [DOI] [PubMed] [Google Scholar]
- Cernadas MR, Sanchez de Miguel L, Garcia-Duran M, Gonzalez-Fernandez F, Millas I, Monton M, Rodrigo J, Rico L, Fernandez P, de Frutos T, Rodriguez-Feo JA, Guerra J, Caramelo C, Casado S, Lopez F. Expression of constitutive and inducible nitric oxide synthases in the vascular wall of young and aging rats. Circ Res. 1998;83:279–286. doi: 10.1161/01.res.83.3.279. [DOI] [PubMed] [Google Scholar]
- Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, Kessler B, Howitz KT, Gorospe M, de Cabo R, Sinclair DA. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science. 2004;305:390–392. doi: 10.1126/science.1099196. [DOI] [PubMed] [Google Scholar]
- Csiszar A, Labinskyy N, Jimenez R, Pinto JT, Ballabh P, Losonczy G, Pearson KJ, de Cabo R, Ungvari Z. Anti-oxidative and anti-inflammatory vasoprotective effects of caloric restriction in aging: role of circulating factors and SIRT1. Mech Ageing Dev. 2009;130:518–527. doi: 10.1016/j.mad.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiszar A, Labinskyy N, Smith K, Rivera A, Orosz Z, Ungvari Z. Vasculoprotective effects of anti-tumor necrosis factor-alpha treatment in aging. Am J Pathol. 2007;170:388–398. doi: 10.2353/ajpath.2007.060708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation. 2000;102:1351–1357. doi: 10.1161/01.cir.102.12.1351. [DOI] [PubMed] [Google Scholar]
- Dhahbi JM, Kim HJ, Mote PL, Beaver RJ, Spindler SR. Temporal linkage between the phenotypic and genomic responses to caloric restriction. Proc Natl Acad Sci U S A. 2004;101:5524–5529. doi: 10.1073/pnas.0305300101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE, Seals DR. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circ Res. 2007;100:1659–1666. doi: 10.1161/01.RES.0000269183.13937.e8. [DOI] [PubMed] [Google Scholar]
- Goto S. Health span extension by later-life caloric or dietary restriction: a view based on rodent studies. Biogerontology. 2006;7:135–138. doi: 10.1007/s10522-006-9011-4. [DOI] [PubMed] [Google Scholar]
- Gredilla R, Barja G, Lopez-Torres M. Effect of short-term caloric restriction on H2O2 production and oxidative DNA damage in rat liver mitochondria and location of the free radical source. J Bioenerg Biomembr. 2001;33:279–287. doi: 10.1023/a:1010603206190. [DOI] [PubMed] [Google Scholar]
- Guo W, Adachi T, Matsui R, Xu S, Jiang B, Zou MH, Kirber M, Lieberthal W, Cohen RA. Quantitative assessment of tyrosine nitration of manganese superoxide dismutase in angiotensin II-infused rat kidney. Am J Physiol Heart Circ Physiol. 2003;285:H1396–1403. doi: 10.1152/ajpheart.00096.2003. [DOI] [PubMed] [Google Scholar]
- Jung KJ, Lee EK, Kim JY, Zou Y, Sung B, Heo HS, Kim MK, Lee J, Kim ND, Yu BP, Chung HY. Effect of short term calorie restriction on pro-inflammatory NF-kB and AP-1 in aged rat kidney. Inflamm Res. 2009;58:143–150. doi: 10.1007/s00011-008-7227-2. [DOI] [PubMed] [Google Scholar]
- Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation. 2003;107:139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- Lane MA, Tilmont EM, De Angelis H, Handy A, Ingram DK, Kemnitz JW, Roth GS. Short-term calorie restriction improves disease-related markers in older male rhesus monkeys (Macaca mulatta) Mech Ageing Dev. 2000;112:185–196. doi: 10.1016/s0047-6374(99)00087-1. [DOI] [PubMed] [Google Scholar]
- Lesniewski LA, Connell ML, Durrant JR, Folian BJ, Anderson MC, Donato AJ, Seals DR. B6D2F1 Mice Are a Suitable Model of Oxidative Stress – Mediated Impaired Endothelium-Dependent Dilation With Aging. J Gerontol A Biol Sci Med Sci. 2009;64:9–20. doi: 10.1093/gerona/gln049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney LB, Denny CA, Seyfried TN. Caloric restriction in C57BL/6J mice mimics therapeutic fasting in humans. Lipids Health Dis. 2006;5:13. doi: 10.1186/1476-511X-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masoro EJ. Overview of caloric restriction and ageing. Mech Ageing Dev. 2005;126:913–922. doi: 10.1016/j.mad.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Mattagajasingh I, Kim CS, Naqvi A, Yamamori T, Hoffman TA, Jung SB, DeRicco J, Kasuno K, Irani K. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 2007;104:14855–14860. doi: 10.1073/pnas.0704329104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce GL, Beske SD, Lawson BR, Southall KL, Benay FJ, Donato AJ, Seals DR. Weight loss alone improves conduit and resistance artery endothelial function in young and older overweight/obese adults. Hypertension. 2008;52:72–79. doi: 10.1161/HYPERTENSIONAHA.108.111427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter CD, Teng RJ, Beckman JS. Superoxide reacts with nitric oxide to nitrate tyrosine at physiological pH via peroxynitrite. J Biol Chem. 2000;275:32460–32466. doi: 10.1074/jbc.M910433199. [DOI] [PubMed] [Google Scholar]
- Spaulding CC, Walford RL, Effros RB. Calorie restriction inhibits the age-related dysregulation of the cytokines TNF-alpha and IL-6 in C3B10RF1 mice. Mech Ageing Dev. 1997;93:87–94. doi: 10.1016/s0047-6374(96)01824-6. [DOI] [PubMed] [Google Scholar]
- Taddei S, Virdis A, Ghiadoni L, Salvetti G, Bernini G, Magagna A, Salvetti A. Age-related reduction of NO availability and oxidative stress in humans. Hypertension. 2001;38:274–279. doi: 10.1161/01.hyp.38.2.274. [DOI] [PubMed] [Google Scholar]
- Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD, Hart RW. Growth curves and survival characteristics of the animals used in the Biomarkers of Aging Program. J Gerontol A Biol Sci Med Sci. 1999;54:B492–501. doi: 10.1093/gerona/54.11.b492. [DOI] [PubMed] [Google Scholar]
- Ungvari Z, Parrado-Fernandez C, Csiszar A, de Cabo R. Mechanisms underlying caloric restriction and lifespan regulation: implications for vascular aging. Circ Res. 2008;102:519–528. doi: 10.1161/CIRCRESAHA.107.168369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weindruch R, Sohal RS. Seminars in medicine of the Beth Israel Deaconess Medical Center. Caloric intake and aging. N Engl J Med. 1997;337:986–994. doi: 10.1056/NEJM199710023371407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakura F, Taka H, Fujimura T, Murayama K. Inactivation of human manganese-superoxide dismutase by peroxynitrite is caused by exclusive nitration of tyrosine 34 to 3-nitrotyrosine. J Biol Chem. 1998;273:14085–14089. doi: 10.1074/jbc.273.23.14085. [DOI] [PubMed] [Google Scholar]
- Zalba G, Beaumont FJ, San Jose G, Fortuno A, Fortuno MA, Etayo JC, Diez J. Vascular NADH/NADPH oxidase is involved in enhanced superoxide production in spontaneously hypertensive rats. Hypertension. 2000;35:1055–1061. doi: 10.1161/01.hyp.35.5.1055. [DOI] [PubMed] [Google Scholar]
- Zhang QJ, Wang Z, Chen HZ, Zhou S, Zheng W, Liu G, Wei YS, Cai H, Liu DP, Liang CC. Endothelium-specific overexpression of class III deacetylase SIRT1 decreases atherosclerosis in apolipoprotein E-deficient mice. Cardiovasc Res. 2008;80:191–199. doi: 10.1093/cvr/cvn224. [DOI] [PMC free article] [PubMed] [Google Scholar]