Abstract
Apoptotic cell death is mediated by caspase activation. Autophagy involves the sequestration of cytoplasmic contents into autophagosomes for traffic to lysosomes for degradation. While autophagy is antiapoptotic, increased numbers of autophagosomes have been associated with forms of non-apoptotic cell death. Apoptosis and autophagy may be co-regulated in the same directions, as the anti-apoptotic Bcl-2 and Bcl-xL proteins negatively regulate autophagy by binding to Beclin 1 (mammalian Atg6), and pro-apoptotic BH3-only proteins may reverse this effect by displacing these interactions. Here we show that apoptosis can suppress autophagy. Apoptosis induced by the pro-apoptotic protein Bax reduced autophagy by enhancing caspase-mediated cleavage of Beclin 1 at D149. After cleavage, both N and C-terminal Beclin 1 fragments change their localisations and these fragments do not interact normally with Vps34, which is required for autophagy. The cleavage of Beclin 1 is a critical event whereby caspases inhibit autophagy, as a non-cleavable Beclin 1 mutant restored autophagy in cells overexpressing Bax.
Keywords: apoptosis, autophagy, cleavage, caspase
Macroautophagy, which we will call autophagy, is a bulk degradation system that mediates clearance of long-lived cytoplasmic proteins, including aggregate-prone proteins, certain pathogens (like mycobacteria and herpes viruses) and organelles (like mitochondria) 1-3, 4. In mammalian cells, autophagosome formation begins with a nucleation step, where membranes of unknown origins form phagophores, which then expand and fuse to form completed double-membrane vesicles called autophagosomes. Autophagosomes are formed at random sites in the cytoplasm, then traffic along microtubules in a dynein-dependent fashion towards the microtubule-organising centre where they are more likely to encounter lysosomes 5. After fusion with the lysosomes, the contents of the autophagosomes are degraded 6.
Autophagy is controlled by two ubiquitin-like conjugation processes. The first involves conjugation of Atg12 to Atg5. Atg5-Atg12 conjugates are localised to phagophores and dissociate after mature autophagosomes are formed 7. Atg5-Atg12 conjugation is regulated by the activity of the class III PI-3-kinase, Vps34 8. Vps34 activity is positively regulated by the mammalian orthologue of yeast Atg6, called Beclin 1 9. The second modification involves conjugation of microtubule-associated protein 1 light chain 3 (MAP-LC3/Atg8/LC3) to phosphatidylethanolamine (PE). LC3 (cytosolic) is cleaved at its C-terminus by Atg4 to form LC3-I. LC3-I is covalently conjugated to PE to form LC3-II. LC3-II (membrane associated) is specifically targeted to Atg5-Atg12-associated, expanded phagophores and remains associated with autophagosomes even after fusion with lysosomes, after which LC3-II can be delipidated and recycled. LC3 is the only known protein that specifically associates with autophagosomes and not with other vesicular structures. Thus, LC3-II levels correlate with autophagosome number, which can also be assessed by scoring LC3-positive vesicles 10.
Apoptosis, also called type 1 cell death, is a form of cell suicide mediated by caspase activation 11. While autophagy has been demonstrated as a cytoprotective and antiapoptotic process, increased numbers of autophagosomes have been associated with forms of non-apoptotic cell death, called type 2 cell death 11-14. Both apoptosis and autophagy can be regulated by Bcl-2 family proteins. Bcl-2 family proteins have been divided into subgroups based on the presence of their Bcl-2 homology (BH) domain(s) 15. The related anti-apoptotic proteins Bcl-2 and Bcl-xL contain four BH domains (BH1-BH4). Bad, Noxa, Bim, Bid, Puma, and Bnip3/Nix each contain a single BH3 domain, and are a group of pro-apoptotic proteins. Bax and Bak contain three BH domains (BH1-BH3), and either protein is required for mitochondrial outer membrane permeabilisation leading to cytochrome c release, which, in turn, results in the activation of a cascade of caspase enzymes that ultimately execute apoptosis 16,17. BH3-only proteins appear to kill cells by binding to the BH3 receptor, the groove formed by the BH1, BH2 and BH3 domains. This process may either activate Bax/Bak, or may induce death by displacing anti-apoptotic BH1-4 (Bcl-2/Bcl-xL) proteins which inhibit Bax/Bak when bound to them 16-18.
The Bcl-2 family proteins also regulate autophagy, since Beclin 1 has a BH3 domain that binds to a hydrophibic groove in Bcl-2/Bcl-xL 19. The binding of Bcl-2/Bcl-xL to Beclin 1 impairs autophagy, as the complex containing Beclin 1 and Vps34 has less PI-3-kinase activity when associated with Bcl-2/Bcl-xL 20. The inhibitory effect of Bcl-2/Bcl-xL on autophagy can be suppressed if Beclin 1 is dissociated from these proteins by BH3-only proteins like Bad 21. Thus, apoptosis and autophagy may be co-regulated in the same directions. However, BH1-3 proteins, like Bax, have not been implicated in autophagy regulation.
Results
Bax inhibits autophagy
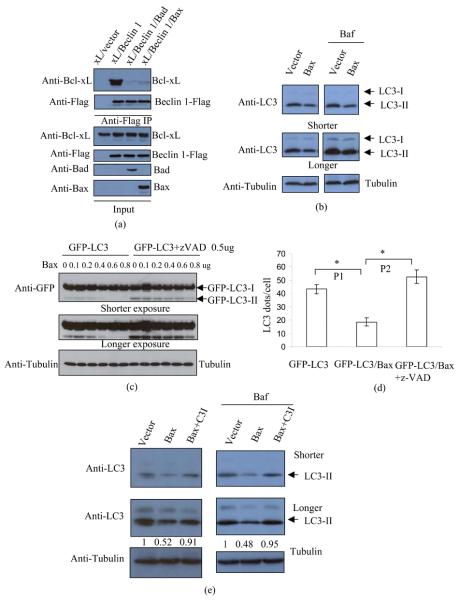
We initially tested whether Bax could disrupt the Beclin 1-Bcl-xL interaction, analogous to what was observed with BH3-only proteins, and confirmed that this was the case (Fig 1(a)). We then tested if Bax overexpression enhanced autophagy. In contrast to what was seen with Bad overrexpression 21, we found that Bax significantly reduced LC3-II levels (Fig 1(b)), and found that this effect was Bax-dose-dependent (Fig 1 (c), Fig S1 (a)-(b)). Bax also reduced LC3-II or GFP-LC3-II levels in cells treated with Bafilomycin A1 (Baf), which blocks LC3-II degradation, suggesting that Bax decreased autophagosome synthesis (Fig 1(b), Fig S1 (a)-(b)). We also tested whether Bax decreased autophagy in conditions where it was stimulated. Fig S2 shows that Bax still attenuated LC3-II synthesis in cells treated with the potent autophagy inducer, trehalose 22. The effects of Bax on autophagy appeared to be caspase-dependent, as they were largely reversed by the pan-caspase inhibitor, z-VAD-fmk. This effect was observed both by LC3-II immunoblotting (Fig 1(c)) and by scoring GFP-LC3 vesicle numbers (Fig 1(d)). High concentrations (100uM) of zVAD-fmk may inhibit cathepsins in cells 23 and calpain in vitro 24. The potential inhibitions of cathepsins and calpain are unlikely to have a major impact on our results, since we used a much lower concentration in the experiments (20uM). We confirmed that the decrease of LC3-II levels caused by Bax was dependent on caspase-3 activity, since a caspase-3 inhibitor reversed this effect (Fig 1(e)).
Fig 1. Bax reduces autophagosome formation.
(a) Bcl-xL (0.6ug), Bcl-xL (0.6ug)/Beclin 1 (0.6ug), Bcl-xL (0.6ug)/Beclin 1 (1.2ug)/Bad (0.6ug) and Bcl-xL (0.6ug)/Beclin 1 (1.8ug)/Bax (0.6ug) were transfected into HeLa cells. To achieve similar expression levels of Bcl-xL and Beclin 1, we used different amounts of Bcl-xL and Beclin 1 in each transfection. Empy vectors were used to keep the amount of DNA in each transfection constant. After 20 hours, cells were lysed and anti-Flag was used for immunoprecipitation. Immunoprecipitates were detected with anti-Bcl-xL and anti-Flag (Beclin 1), respectively. Total lysates were detected with anti-Bcl-xL, anti-Flag, anti-Bad and anti-Bax, respectively.
(b) HeLa cells were transfected with empty vector (lanes 1, 3), or Bax (lanes 2, 4). After 20 hours, one of the two sets (lanes 3-4) was treated with Bafilomycin A1 (Baf) for 4 hours. This treatment blocks LC3-II degradation and is saturating; thus, the changes in LC3-II reflect altered autophagosome synthesis 8. The cells were then lysed and subjected to SDS-PAGE and blotting with anti-LC3 (top), anti-tubulin (bottom) respectively. Note that lighter exposures were used in the Baf conditions to enable assessment of changes in LC3-II levels, since Baf dramatically increases LC3-II levels. Endogenous LC3-I levels appear very low in HeLa cells as LC3 antibody has stronger immunoreactivity to LC3-II than LC3 I 33.
(c) GFP-LC3 was co-transfected with increasing levels of Bax plasmid, as indicated in duplicate. (In all experiments of this type, the total amount of transfected DNA is kept constant by using empty vector.) After transfection, one set were treated with DMSO (lanes 1-6) and the other set were treated with z-VAD-fmk (20uM) (lanes 7-12). After 20 hours, cell lysates were subjected to western blot with anti-GFP (top and middle) and anti-tubulin (bottom).
(d) GFP-LC3/vector, or GFP-LC3/Bax were transfected into HeLa cells. The GFP-LC3/Bax transfections were treated with either DMSO or z-VAD-fmk (20uM), and GFP-LC3 transfection was also treated with DMSO following transfection. After 20 hours, cells were fixed and the numbers of GFP puncta were scored. Pictures were taken under fluorescent microcope. These transfections do not give rise to GFP-LC3 aggregates 5. *: P1<0.05; P2<0.01.
(e) Vector or Bax were transfected into HeLa cells. The Bax transfections were treated with either DMSO or caspase-3 inhibitor (Ac-DEVD-CHO) (20uM), and vector transfection was also treated with DMSO following transfection. In a parallel experiment, cells were treated with Bafilomycin A1 for the last 4 hours prior to harvesting. After 20 hours, cell lysates were subjected to western blots and probed with anti-LC3, anti-tubulin antibodies. LC3-II densitometry is indicated (versus tubulin).
Caspases cleave and inactivate Beclin 1
We tried to identify the mechanism that Bax reduces autophagy, and noted that Bax reduced Beclin 1 expression (Fig 2(a)). Since, pan-caspase inhibition stabilised Beclin 1 in the presence of Bax (Fig 2(a), Fig S3(a)-(b)) and also rescued the Bax-mediated inhibition of autophagy (Fig 1(c) and 1 (d), Fig S3(b)), we considered that Beclin 1 may be a caspase substrate and that this may be the mechanism for these Bax effects.
Fig 2. Beclin 1 is cleaved by caspase-3 at D149.
(a) Beclin 1-Flag was co-transfected with increasing levels of Bax plasmid as indicated. After transfection, one set (lanes 1-6) were treated with DMSO and the other set were treated with z-VAD-fmk (lanes 7-12). After 20 hours, cell lysates were subjected to western blot with anti-Flag (top), anti-Bax (middle) and anti-tubulin (bottom). Note that Beclin 1 band intensities (versus tubulin) for 0 Bax are 1 (lane 1) and 1.04 (lane 7).
(b) Carboxyl terminal Flag-tagged Beclin 1 was transfected into HeLa cells in triplicate. The first set of transfected cells were treated with cycloheximide (CHX) for 5 h (lane 1); the second set of transfected cells were treated with TNF-α+ CHX for 5 hours (lane 2); the third set of transfected cells were treated with TNF-α +CHX and z-VAD-fmk for 5 hours (lane 3). The cell lysates were subjected to western blot with anti-Flag (10% gel).
(c) HeLa cells were treated DMSO, staurosporine (STS), and STS+z-VAD-fmk for 5 h, respectively. The cell lysates were subjected to blotting with anti-Beclin 1 (C-term) (top) and anti-tubulin (bottom). * shows 50kD band that may be a cleavage intermediate.
(d) Beclin 1, Beclin 1-D142E, Beclin 1-D149E or Beclin 1-D142 149E (C-terminal Flag tagged) were transfected into HeLa cells in duplicate. After 20 hours, one set of cells (lanes 5-8) were treated with TNF-α + CHX to induce caspases activation. As controls, another set of cells (lanes 1-4) were treated CHX only (which does not cause effective apoptosis by itself). Samples were subjected to 10% SDS-PAGE and blotting with anti-Flag.
(e) For caspase-3 in vitro cleavage assay, Beclin 1, Beclin 1-149E (C-terminal Flag tagged) were transfected into HeLa cells. After 20 hours, cells were lysed. The cell lysates were split into 2 parts. Caspase-3-containing bacterial lysate (lanes 3, 4) and control (lanes 1, 2) bacterial lysate were used to cleave Beclin 1 or Beclin 1-149E containing cell lysates. Samples were subjected to 12% SDS-PAGE and blotting with anti-Flag. Note that the extent of the Beclin 1 fragment migration differs in Fig 2(b)/Fig 2 (d) and Fig 2 (e) as different percentage gels were used.
(f) Alignment of Beclin 1 from different species, showing caspase consensus sequence.
The treatment of cells with both TNF-α and cycholoheximide (CHX) is an established method for caspase activation 25. Consistent with the Bax overexpression data, TNF+CHX reduced Beclin 1 levels by at least 50% and this effect was inhibited by caspase inhibition (Fig 2(b)). This is likely to be significant as a 50% reduction of Beclin 1 affects autophagic activity 26. The caspase-dependent reduction in full-length Beclin 1 levels was associated with the appearance of a fragment of this protein that was recognized by a tag on the C-terminus of exogenous Beclin 1 (Fig 2(b)). TNF-α +CHX treatment also caused a reduction in the levels of endogenous Beclin 1. We were unable to detect a Beclin 1 fragment using a Beclin 1 N-terminal antibody, suggesting rapid turnover of this fragment (Fig S4(a)). However, the caspase inhibitor, z-VAD-fmk abolished the ability of TNF+CHX to reduce Beclin 1 levels (Fig S4(b)). Autophagic degradation is unlikely to play a significant role in the Beclin 1 turnover under these conditions, since the potent chemical autophagy inducer trehalose failed to alter Beclin 1 level significantly in both Atg5 wild-type (autophagy-competent) and Atg5 null (autophagy-deficient) mouse embryonic fibroblasts (MEFs) (Fig S5). Consistent with these data, staurosporine, another apoptosis-inducing treatment, also resulted in Beclin 1 cleavage detected with an antibody directed to the C-terminus of endogenous Beclin 1 (Fig 2(c)). Interestingly, we observed an extra 50kD band in this assay, however this size band was not detected in the assay with Beclin 1-Flag. We cannot exclude the possibility that this 50kD band is an intermediate fragment.
To identify the caspase cleavage site, we screened the potential caspase consensus sequences within Beclin 1 and found that Asp149 (149D) was a preferred caspase cleavage site. To verify this caspase cleavage site, we mutated 149D to Glu (149E). Fig 2(d) shows, that, in contrast to wild type Beclin 1, Beclin 1-149E is no longer cleaved. Since caspase-3 is a major caspase acting in apoptotic execution pathways, we tested if it was able to cleave Beclin 1 in vitro. Fig 2(e) shows that caspase-3 is capable of cleaving wild-type Beclin 1 but not mutated Beclin 1-149E. These data revealed that Beclin 1 is a substrate of caspase-3 and that 149D is the major cleavage site. This caspase recognition sequence is well conserved in Beclin 1 orthologues in many species (Fig 2(f)). Notably, this site is not conserved in yeasts that are thought to lack caspases.
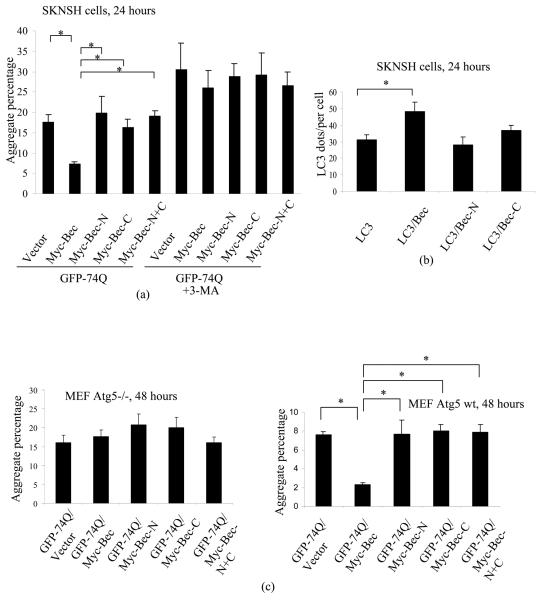
We tested if the N-terminal and C-terminal caspase cleavage products of Beclin 1 (Beclin 1 N and C) were still able to induce autophagy. We have established that autophagy regulates the clearance of mutant huntingtin (htt) fragments and other aggregate-prone proteins, and that the percentage of cells with aggregates of mutant htt correlates inversely with autophagic activity, all other things being equal 27,28. When Beclin 1 was co-transfected with a GFP-tagged exon 1 mutant htt fragment (GFP-74Q) in SK-N-SH human neuroblastoma cells, the aggregation of mutant htt was markedly reduced, compared with vector-cotransfected cells, as previously reported 29. However, neither Beclin 1 N or C by themselves, nor together, had significant effect on the clearance of mutant htt aggregation, suggesting that both Beclin 1-N and C have lost autophagic activity (Fig 3(a)). Likewise, full-length Beclin 1 overexpression consistently increased autophagosome numbers (Fig 3(b)), while its N-terminal and C-terminal caspase cleavage products had no significant effects. The effects of Beclin 1 on htt aggregation is autophagy dependent as it is lost in cells treated with the autophagy inhibitor, 3-methyl adenine (3MA) (Fig 3(a)) and in autophagy-deficient Atg5 null MEFs (Fig 3(c)). Consistently, Beclin 1, rather than Beclin 1-N or –C, reduced mutant httEx1 aggregation in Atg5 wild-type MEFs (Fig 3(c)). The expression levels of Myc-Beclin 1, Myc-Bec-N and Myc-Bec-C are shown in Fig S6.
Fig 3. Caspases-cleaved Beclin 1 fragments lose autophagic activity.
(a) GFP-tagged huntingtin exon 1 with 74 polyglutamines (GFP-74Q) was cotransfected with empty vector, Myc-Beclin 1 (Myc-Bec), Myc-Beclin 1 N-terminal fragment (Myc-Bec-N), Myc-Beclin 1 C-terminal fragment (Myc-Bec-C) and the combination of Myc-Bec-N and Myc-Bec-C (Myc-Bec-N+C) into HeLa cells in two sets. One set of experiments were treated with the autophagy blocker 3-methyl-adenine (3-MA) following transfection. After 24 hours, aggregates were scored under fluorescent microscope. *: P<0.05.
(b) GFP-LC3 was cotransfected with empty vector, Beclin 1, Beclin 1-N, Beclin 1-C into neuroblastma SK-N-SH cells. After 24 hours, GFP puncta were scored under fluorescent microscope. *: P<0.05.
(c) GFP-74Q was cotransfected with empty vector, Myc-Beclin 1 (Myc-Bec), Myc-Beclin 1 N-terminal fragment (Myc-Bec-N), Myc-Beclin 1 C-terminal fragment (Myc-Bec-C) and the combination of Myc-Bec-N and Myc-Bec-C (Myc-Bec-N+C) into Atg5−/− MEFs (mouse embryonic fibroblasts). After 48 hours, cells were fixed and aggregation was evaluated
GFP-74Q was cotransfected with empty vector, myc-Beclin 1 (myc-Bec), myc-Beclin 1 N-terminal fragment (myc-Bec-N), myc-Beclin 1 C-terminal fragment (myc-Bec-C), or the combination of myc-Bec-N and myc-Bec-C (myc-Bec-N+Bec-C) into Atg5 wild-type MEFs. After 48 hours, cells were fixed and aggregation was evaluated. *: P<0.05.
Non-caspase-cleavable Beclin 1 rescues autophagy in bax-expressing cells
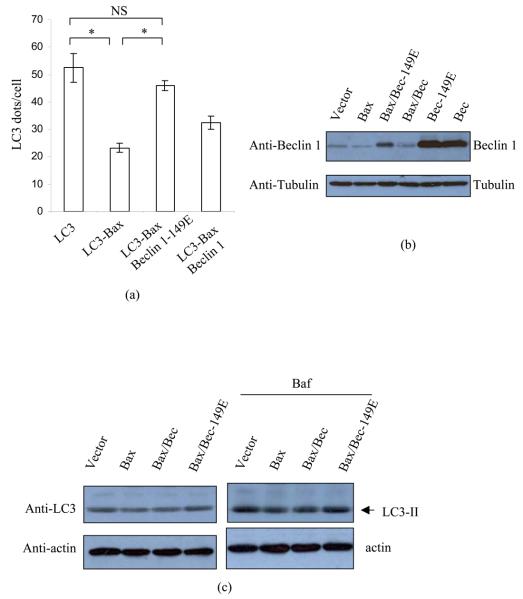
We tested if Beclin 1 cleavage was the major determinant of the inhibition of autophagy by caspases and confirmed that this was the case, since the Bax reduced autophagosome numbers in vector-transfected cells, but the autophagosome numbers were restored in Beclin 1-149E-transfected cells. The autophagosome numbers were partially restored by cleavable wild-type Beclin 1 (Fig 4(a)). We confirmed that the expression of Beclin 1-149E in the presence of Bax was similar to those of wild-type Beclin 1 in otherwise cells not exposed to Bax overexpression (Fig 4(b)). We then confirmed that Bax also reduced autophagosome numbers in starvation conditions, which normally increase the numbers of LC3 vesicles per cell (Fig S7(a)). Fig S7(b) shows that Beclin 1-149E exerted a protective effect against the reduction of autophagosomes induced by Bax in starvation conditions, in a way that was analogous to what we observed in full media. Likewise, Beclin 1 D149E restored LC3-II levels in Bax-transfected cells in both Baf or non-Baf treated cells in full media (Fig 4(c)).
Fig 4. Non-cleavable Beclin 1 restores Bax-reduced autophagy.
(a) GFP-LC3, GFP-LC3/Bax, GFP-LC3/Bax/Beclin 1-149E, GFP-LC3/Bax/Beclin 1 were transfected into HeLa cells. After 20 hours, cells were fixed and the numbers of GFP puncta per cell were scored. *: P<0.05.
(b) HeLa cells were transfected with empty vector, Bax, Bax/Beclin 1-149E and Bax/Beclin 1 respectively. After 20 hours, cell lysates were subjected to blotting, and probed with anti-Beclin 1 (N-) and tubulin antibodies.
(c) HeLa cells were transfected with vector, Bax, Bax/Beclin 1 and Bax/Beclin 1-149E respectively, in duplicate. After 20 hours, one set was treated with DMSO and the other set was treated Baf for 4 hours. Cell lysates were subjected to blotting with anti-LC3 and tubulin respectively.
To exclude the possibility that Beclin 1-149E acts as a caspase-3 inhibitor, we assayed caspase-3 activity in Bax-ovexpressing cells in the presence or absence of wild-type on Beclin 1-149E. As expected, caspase-3 activity was enhanced by Bax overexpression. However, this effect was not affected in cells overexpressing either wild-type Beclin 1 and Bax or Beclin 1-149E and Bax, compared to cells overexpressing Bax alone (Fig S8(a)). These data were confirmed by the cleavage assay of poly(ADP-ribose) polymerase (PARP), a classical caspase-3 substrate. Similar levels of the 85-kD PARP caspase fragment were detected in cells with Bax alone, Bax and Beclin 1 or Bax and Beclin 1-149E (Fig S8(b)).
Previously, we showed that Beclin 1 regulates Atg5-Atg12 conjugation, a step that precedes LC3-II formation 8. Fig S9 confirms that the levels of the endogenous Atg5-Atg12 conjugate are decreased in the Beclin 1-knockdown cells. Consistent with these findings, Bax decreased Atg5-Atg12 conjugation in vector-trasfected cells, while Beclin 1-149E partially restored the levels of the conjugate (Fig S10(a-b)).
Mechanism of the loss-of-function of Beclin 1 cleavage fragments
Beclin 1 is a predominantly cytoplasmic protein, enriched in mitochondria and ER 20,30. These localisations are important for its function(s). We tested if the localisations of caspase-cleaved fragments of Beclin 1 are different from full-length Beclin 1. We generated Beclin 1-GFP (Bec-GFP), N-terminal Beclin 1-GFP (1-149aa, Bec-N-GFP), and C-terminal Beclin 1-GFP (150-450aa, Bec-C-GFP). Bec-GFP was predominantly localised in the cytoplasm, as previously reported. However Bec-N-GFP was mainly localised in the nucleus, and Bec-C-GFP was localised in both cytoplasm and nucleus ((Fig 5(a-b)). Similar effects were observed with these constructs when tagged with Myc, rather than with GFP (Fig 5(c)). Since the interaction between Beclin 1 and Vps34 is essential for autophagy, we tested whether the cleaved Beclin 1 fragments still retained the ability to bind Vps34. Fig 5(d) and Fig S11 show that Beclin 1 N-terminal bound only weakly to Vps34, while this interaction is almost lost for Beclin 1 C-terminal. These data, along with the mislocalisation of cleaved Beclin 1, can explain why the cleavage results in a loss-of-function.
Fig 5. Mechanism of the loss-of-function of cleaved Beclin 1 fragments.
(a)-(b) Beclin 1-GFP (Bec-GFP), N-terminal caspase-cleaved fragment of Beclin 1 (Beclin 1-N)-GFP (Bec-N-GFP), C-terminal caspase-cleaved fragment of Beclin 1 (Beclin 1-C)-GFP (Bec-C-GFP) were transfected into HeLa cells respectively. After 18 hours, cells were fixed. The cells were later viewed under fluorescence microscope (a) and confocal microscope (b).
(c) Myc-Beclin 1 (Myc-Bec), Myc-Beclin 1-N (Myc-Bec-N), Myc-Beclin-C (Myc-Bec-C) were transfected into HeLa cells. After 20 hours, cells were fixed and stained with anti-Myc (9E10). The cells were viewed under confocal microscope.
(d) Vps34/vector, Vsps34/Beclin 1-Flag (Bec-Flag), Vps34/Beclin 1-N-Flag (Bec-N-Flag), Vps34/Beclin 1-C-Flag (Bec-C-Flag) were transfected into HeLa cells respectively. After 20 hours, cells were harvested and lysed. The cell lysates were subjected to anti-Flag antibody immunoprecipitation. The immunoprecipitates were used for SDS-PAGE and western blotting with anti-Vps34 antibody (top panel) and anti-Flag antibody (middle panel) respectively. The total lysates were detected with anti-Vps34 (bottom). Three independent experiments were perfomed with similar results.
Bcl-xL rescues autophagy in bax-expressing cells
Then we asked whether anti-apoptotic Bcl-2/Bcl-xL antagonizes the ability of Bax to reduce autophagy. Interestingly, Fig 6(a) shows that Bcl-xL (column 3) dramatically increased autophagosome number in cells where Bax is overexpressed, compared to the cells overexpressing Bax but no Bcl-xL (column 2). However, Bcl-xL (column 4) decreased autophagosome number in cells without overexpression of Bax, in comparison with the cells without overexpression of Bax and Bcl-xL (column 1). However, we also observed that Bax (column 3) restored autophagosome number that is inhibited by Bcl-xL (column 4). This effect may be due to Bax disrupting the Bcl-xL-Beclin 1 interaction (Fig 1(a)), similar to the effect of BH3-only proteins on autophagy 31. The pictures of autophagosomes in these cells are shown in Fig 6(b). Fig 6(c) shows LC3-II levels in the cells where vector, Bax, Bax-Bcl-xL, or Bcl-xL overexpressed. The LC3-II levels are consistent with autophagosome numbers in the cells overexpressing Bax, Bax-Bcl-xL or Bcl-xL (Fig 6(a), (b)). Finally, we observed that Bcl-xL rescued both the loss of Beclin 1 and the increased cell death caused by Bax overexpression (Fig 6(d), Fig S12).
Fig 6. Bcl-xL rescues the inhibitory effects of Bax on autophagosome formation.
(a) GFP-LC3 was transfected with empty vector, Bax, Bax-Bcl-xL or Bcl-xL into HeLa cells. After 20 hours, cells were fixed and the numbers of GFP puncta per cell were scored. *: P<0.05.
(b) Pictures were taken under fluorescent microcope (100X). Bar: 5um.
(c) HeLa cells were transfected with empty vector, Bax, Bax-Bcl-xL or Bcl-xL in duplicate. After 20 hours, one set of cells were treated with Baf (lanes 5-8). Cells were harvested and blots were probed with anti-LC3, Bcl-xL, Bax and tubulin respectively. Note that endogenous LC3-I gives a very weak signal in HeLa cells compared to LC3-II.
(d) HeLa cells were transfected with empty vector, Bax, Bax-Bcl-xL or Bcl-xL. After 20 hours, cells were harvested and blots were probed with anti-Beclin 1 and tubulin respectively.
Discussion
We find that autophagosome synthesis is inhibited after caspase activation, and this appears to be predominantly mediated by caspase cleavage of Beclin 1. Our finding that Beclin 1 is a caspase substrate is consistent with a recent study by Cho et al. that made the same initial observation 32, but did not identify the cleavage site 32. However, the main conclusion of our study is that caspases/apoptosis inhibit autophagy. This is novel and has not been shown in the Cho paper, which reported that Atg6/Beclin 1 modulates sensitivity of cells to TRAIL- induced cell death, an issue distinct from our study. Furthermore, Cho et al 32did not examine if the caspase cleavage of Beclin 1 was critical for autophagy inhibition (as caspases could potentially cleave many other autophagy modulators). While caspase-cleavage of Beclin 1 appears to be a major determinant of the effects we have observed (since they are abrogated by non-caspase cleavable Beclin 1 mutants and pan-caspase or caspase 3 inhibitors), we cannot exclude additional factors influencing Beclin 1 expression under these conditions.
We believe that our data provide new and important insights into the relationship between apoptosis and autophagy. Since autophagic flux will be downregulated in cells after caspase activation, the antiapoptotic effects of autophagy will not compete with apoptosis once the latter process is established, and may explain why autophagic and apoptotic morphologies generally do not coexist.
We assayed autophagosome numbers both by western blotting of LC3-II and by counting LC3 dots in cells. The reductions of either parameter induced by Bax were similar (about 50% - see Fig 1e and Fig 6a), although perhaps the effects on LC3 dots were slightly more obvious. One possible explanation for the slightly larger magnitude of effect on Bax on GFP-LC3 dots than on LC3-II expression as measured by western blot analysis is that Atg6 and Beclin 1 may not be directly required for Atg8/LC3 lipidation as an isolated process, although they are required for the localisation of lipidated LC3 to autophagosomal membranes. Therefore, the assays used in this study may be underrepresenting the inhibitory effects of Bax overexpression on autophagy.
Our data also reveal another role for Bcl-2 family members in autophagy regulation. In contrast with BH3-only proteins that can act to enhance autophagy by relieving the inhibition of the inhibitory effects of the Bcl-2/Bcl-xL interaction with Beclin 1, Bax/caspase induction results in Beclin 1 cleavage and decreased autophagy (Fig S13). This model is also supported by the observation that while autophagosome numbers are decreased by either Bax (by cleaving Beclin 1), or Bcl-xL (by binding to Beclin 1 20), co-transfection of Bcl-xL with Bax normalizes autophagosome numbers (Fig 6, Fig S13). This may occur because Bcl-xL antagonizes the proapoptotic effects of Bax, while Bax, in turn, disrupts the Beclin 1-Bax interaction (see Fig 1(a)). Thus, there are two scenarios that appear to be operating with Bax overexpression, depending on the anti-apoptotic state of the cells (Fig 1(a) versus Fig 6). In cells without increased anti-apoptotic capacity (e.g. without Bcl-xL overexpression or caspase inhibitor treatment), Bax overexpression will lead to decreased Beclin 1 levels by caspase cleavage and this will decrease autophagic flux. However, in cells with sufficient anti-apoptotic capacity (e.g. with Bcl-xL overexpression or caspase inhibitor treatment) then Bax will disrupt the Bcl-xl-Beclin interaction and induce autophagy. Another important factor that will regulate the differential effects of Bcl-2 family members is subcellular localisation. For instance, ER-localised, but not mitochondrial Bcl-2 appears to be relevant for autophagy inhibition 20. Conversely, mitochondrial Bcl-2 is relevant for its anti-apoptotic effects. Since these protective roles of Bcl-2 would reduce caspase activity after pro-apoptotic insults, they would also be predicted to preserve autophagy in cells exposed to various toxic agents. Thus, in certain circumstances, Bcl-2/Bcl-xL may have opposite effects on autophagy, depending on whether it is associated with mitochondria or the ER.
Materials and Methods
DNA construction
Beclin 1 or its truncated forms were generated by PCR and subcloned into pCMV-5a vector (C-terminal Flag tagged expression vector), pCMV-6M (N-terminal Myc-tagged expression vector), pEGFP-N3 (C-terminal GFP tagged expression vector). Beclin 1 point mutants were generated with Stratagene Quikchange Mutagenesis kit. Mouse Bad was purchased from Cell Signaling and subcloned into pcDNA3. Bax, Bcl-xL were the products from Addgene and cloned into pcDNA3. Vps34 was cloned into pcDNA3. All the DNA constructs were confirmed by DNA sequencing.
Antibodies and reagents
Rabbit polyclonal antibodies: anti-Beclin 1 (C-) (1:1000) (Sigma); anti-Beclin 1 (N-) (1:1000) (Sigma); anti-Myc (1:1,000) (Sigma); anti-PARP p85 (1:1000) (Promega). Anti-mouse monoclonal antibodies: anti-Flag (M2) (1:1,000), anti-tubulin (1:5,000) (Sigma), anti-Myc (9E10) (1:1,000) (Sigma). Anti-Flag M2-agarose affinity gel Sigma). TNF-α, recombinant caspase-3 were purchased from Chemicon International. Cycloheximide was from Sigma. Bafilomycin A1 (Baf) was purchased from Millipore (400nM). Caspase-3 inhibitor (Ac-DEVD-CHO) and Pan-caspase inhibitor (z-VAD-fmk) were from Calbiochem. Cells were treated with 20uM Ac-DEVD-CHO and 20uM z-VAD-fmk. Both 3-methyladenine (3-MA) and trehalose were from Sigma.
Cell culture
HeLa, SK-N-SH cells and Atg5 knockout mouse embryonic fibroblasts (MEFs) were cultured with standard methods in DMEM supplemented with 10% FCS (Sigma). Transfection was performed with Lipofectamine 2000 (Invitrogen) according to standard methods. In cellular Beclin 1 cleavage assays, cells were treated with 10ng/ml TNF-α and 30ug/ml cycloheximide (CHX) or Staurosporine (STS, 1uM) for indicated times. To inhibit autophagy, cells were treated with 10mM 3-MA for 24 hours following transfection. To stimulate autophagy, we treated cells with 100mM trehalose for the indicated times.
siRNA transfection
HeLa cells were split 1 day prior to transfection to 50% confluence and left overnight in antibiotic-free DMEM containing 10% FBS. Beclin 1 siRNAs (Ambion) were transfected with DharmaFect 1 according to the manufacturer's instructions. Non-targeting siRNA (Daharmacon, cat no: D-001210-01) was the control siRNA. HeLa cells were maintained in 10% FBS DMEM containing no antibiotics for 48 hours after transfection.
In vitro caspase cleavage
Cells were lysed in lysis buffer (50mM HEPES, 5mM DTT, 0.1mM EDTA, 0.1% CHAPS, pH 7.4, supplied by Calbiochem in Caspase-3 activity assay kit). Beclin 1 in the lysates was then cleaved with 1 U caspase-3 in cleavage buffer (50mM HEPES, 50mM NaCl, 0.1% CHAPS, 10mM DTT, 1mM EDTA, 5% Glycerol) for 1 hour at 37°C. The cleaved products were subjected to western blot analysis.
Caspase-3 activity assay
Caspase-3 activity assay was performed according to the instructions in the Caspase-3 cellular activity assay kit (Calbiochem, Cat No: 235419). Briefly, caspase substrate Ac-DEVD-pNA in assay buffer was added to the same amount of cellular lysates (15ul) as for in vitro caspase cleavage and the mixtures were incubated for 1 hour at 37°C. Absorbance was read at 405nm.
Estimation of aggregates
To measure aggregates, approximately 200 transfected cells were counted in multiple random visual fields per slide. All coverslips were scored with the observer blinded to the identity of the slides. Cells were analysed using a fluorescent microscope (Eclipse E600, Nikon, Japan). The figures show data from representative experiments in triplicate. Cells were counted as aggregate-positive if one or several aggregates were visible within a cell.
Estimation of numbers of autophagosome per cell
GFP-LC3 puncta were scored under a fluorescent microscope (100X). Approximately 50 GFP-positive cells were counted in multiple random visual fields per slide. All coverslips were scored with the observer blinded to the identity of the slides. The figures show data from experiments in triplicate, at least.
Quantification of autoradiographs
To quantify western blot band densitometry, the relevant specified bands were analysed using ChemiImager (Alpha Innotech Co.).
Statistics
T-test was used and P-values were determined by unconditional logistical regression analysis by using the general loglinear option of SPSS 9.1 software (SPSS, Chicago, IL).
Immunoprecipitation
Immunoprecipitation (IP) was performed using Buffer A (20mM Tris-HCl, pH 7.2, 2mM MgCl2, 150mM NaCl, 5mM NaF, 1mM Na3VO4, 0.5% NP-40, protease inhibitor cocktail [Roche]). Cells were lysed in Buffer A for 20 min on ice, followed by centrifugation at 13,000 × g for 15 min. 500ug-1mg total protein were used as the starting material for IPs. Anti-Flag M2-agarose affinity gel was added to a final concentration of 5ug/ml and incubated for 2 hours to overnight at 4°C. IP products were either directly boiled in Laemmli buffer and subjected to PVDF membrane transfer and western blot.
Supplementary Material
Acknoledgements
We are grateful to Brinda Ravikumar for helpful comments and to the MRC (Programme grant) and the Wellcome Trust (Senior Clinical Fellowship) for funding.
Abbreviations
- Baf
Bafilomycin A1
- BH
Bcl-2 homology
- CHX
cycholoheximide
- GFP-74Q
GFP-tagged exon 1 mutant htt fragment
- htt
huntingtin
- MEFs
mouse embryonic fibroblasts
- 3MA
3-methyl adenine
- PE
phosphatidylethanolamine
- STS
staurosporin
- TNF-α
tumor necrosis factor alpha
References
- 1.Klionsky DJ. The molecular machinery of autophagy: unanswered questions. J Cell Sci. 2005;118:7–18. doi: 10.1242/jcs.01620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levine B, Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell. 2004;6:463–77. doi: 10.1016/s1534-5807(04)00099-1. [DOI] [PubMed] [Google Scholar]
- 3.Mizushima N. The pleiotropic role of autophagy: from protein metabolism to bactericide. Cell Death Differ. 2005;12(Suppl 2):1535–41. doi: 10.1038/sj.cdd.4401728. [DOI] [PubMed] [Google Scholar]
- 4.Rubinsztein DC, Gestwicki JE, Murphy LO, Klionsky DJ. Potential therapeutic applications of autophagy. Nat Rev Drug Discov. 2007;6:304–12. doi: 10.1038/nrd2272. [DOI] [PubMed] [Google Scholar]
- 5.Jahreiss L, Menzies FM, Rubinsztein DC. The itinerary of autophagosomes: from peripheral formation to kiss-and-run fusion with lysosomes. Traffic. 2008;9:574–87. doi: 10.1111/j.1600-0854.2008.00701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang WP, Klionsky DJ. Autophagy in yeast: a review of the molecular machinery. Cell Struct Funct. 2002;27:409–20. doi: 10.1247/csf.27.409. [DOI] [PubMed] [Google Scholar]
- 7.Mizushima N, Noda T, Yoshimori T, Tanaka Y, Ishii T, George MD, et al. A protein conjugation system essential for autophagy. Nature. 1998;395:395–8. doi: 10.1038/26506. [DOI] [PubMed] [Google Scholar]
- 8.Ravikumar B, Imarisio S, Sarkar S, O'Kane CJ, Rubinsztein DC. Rab5 modulates aggregation and toxicity of mutant huntingtin through macroautophagy in cell and fly models of Huntington disease. J Cell Sci. 2008;121:1649–60. doi: 10.1242/jcs.025726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H, et al. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature. 1999;402:672–6. doi: 10.1038/45257. [DOI] [PubMed] [Google Scholar]
- 10.Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. Embo J. 2000;19:5720–8. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levine B, Yuan J. Autophagy in cell death: an innocent convict? J Clin Invest. 2005;115:2679–88. doi: 10.1172/JCI26390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lum JJ, DeBerardinis RJ, Thompson CB. Autophagy in metazoans: cell survival in the land of plenty. Nat Rev Mol Cell Biol. 2005;6:439–48. doi: 10.1038/nrm1660. [DOI] [PubMed] [Google Scholar]
- 13.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–75. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yorimitsu T, Klionsky DJ. Eating the endoplasmic reticulum: quality control by autophagy. Trends Cell Biol. 2007;17:279–85. doi: 10.1016/j.tcb.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 15.Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–19. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 16.Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–30. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Willis SN, Fletcher JI, Kaufmann T, van Delft MF, Chen L, Czabotar PE, et al. Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science. 2007;315:856–9. doi: 10.1126/science.1133289. [DOI] [PubMed] [Google Scholar]
- 18.Certo M, Del Gaizo Moore V, Nishino M, Wei G, Korsmeyer S, Armstrong SA, et al. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell. 2006;9:351–65. doi: 10.1016/j.ccr.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 19.Oberstein A, Jeffrey PD, Shi Y. Crystal structure of the Bcl-XL-Beclin 1 peptide complex: Beclin 1 is a novel BH3-only protein. J Biol Chem. 2007;282:13123–32. doi: 10.1074/jbc.M700492200. [DOI] [PubMed] [Google Scholar]
- 20.Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, et al. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–39. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 21.Maiuri MC, Le Toumelin G, Criollo A, Rain JC, Gautier F, Juin P, et al. Functional and physical interaction between Bcl-X(L) and a BH3-like domain in Beclin-1. Embo J. 2007;26:2527–39. doi: 10.1038/sj.emboj.7601689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sarkar S, Davies JE, Huang Z, Tunnacliffe A, Rubinsztein DC. Trehalose, a novel mTOR-independent autophagy enhancer, accelerates the clearance of mutant huntingtin and alpha-synuclein. J Biol Chem. 2007;282:5641–52. doi: 10.1074/jbc.M609532200. [DOI] [PubMed] [Google Scholar]
- 23.Rozman-Pungercar J, Kopitar-Jerala N, Bogyo M, Turk D, Vasiljeva O, Stefe I, et al. Inhibition of papain-like cysteine proteases and legumain by caspase-specific inhibitors: when reaction mechanism is more important than specificity. Cell Death Differ. 2003;10:881–8. doi: 10.1038/sj.cdd.4401247. [DOI] [PubMed] [Google Scholar]
- 24.Waterhouse NJ, Finucane DM, Green DR, Elce JS, Kumar S, Alnemri ES, et al. Calpain activation is upstream of caspases in radiation-induced apoptosis. Cell Death Differ. 1998;5:1051–61. doi: 10.1038/sj.cdd.4400425. [DOI] [PubMed] [Google Scholar]
- 25.Hsu H, Huang J, Shu HB, Baichwal V, Goeddel DV. TNF-dependent recruitment of the protein kinase RIP to the TNF receptor-1 signaling complex. Immunity. 1996;4:387–96. doi: 10.1016/s1074-7613(00)80252-6. [DOI] [PubMed] [Google Scholar]
- 26.Qu X, Yu J, Bhagat G, Furuya N, Hibshoosh H, Troxel A, et al. Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. J Clin Invest. 2003;112:1809–20. doi: 10.1172/JCI20039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ravikumar B, Duden R, Rubinsztein DC. Aggregate-prone proteins with polyglutamine and polyalanine expansions are degraded by autophagy. Hum Mol Genet. 2002;11:1107–17. doi: 10.1093/hmg/11.9.1107. [DOI] [PubMed] [Google Scholar]
- 28.Ravikumar B, Vacher C, Berger Z, Davies JE, Luo S, Oroz LG, et al. Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat Genet. 2004;36:585–95. doi: 10.1038/ng1362. [DOI] [PubMed] [Google Scholar]
- 29.Shibata M, Lu T, Furuya T, Degterev A, Mizushima N, Yoshimori T, et al. Regulation of intracellular accumulation of mutant Huntingtin by Beclin 1. J Biol Chem. 2006;281:14474–85. doi: 10.1074/jbc.M600364200. [DOI] [PubMed] [Google Scholar]
- 30.Liang XH, Yu J, Brown K, Levine B. Beclin 1 contains a leucine-rich nuclear export signal that is required for its autophagy and tumor suppressor function. Cancer Res. 2001;61:3443–9. [PubMed] [Google Scholar]
- 31.Maiuri MC, Criollo A, Tasdemir E, Vicencio JM, Tajeddine N, Hickman JA, et al. BH3-only proteins and BH3 mimetics induce autophagy by competitively disrupting the interaction between Beclin 1 and Bcl-2/Bcl-X(L) Autophagy. 2007;3:374–6. doi: 10.4161/auto.4237. [DOI] [PubMed] [Google Scholar]
- 32.Cho DH, Jo YK, Hwang JJ, Lee YM, Roh SA, Kim JC. Caspase-mediated cleavage of ATG6/Beclin-1 links apoptosis to autophagy in HeLa cells. Cancer Lett. 2008 doi: 10.1016/j.canlet.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 33.Mizushima N, Yoshimori T. How to interpret LC3 immunoblotting. Autophagy. 2007;3:542–5. doi: 10.4161/auto.4600. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.