Abstract
Rationale: Inhaled granulocyte/macrophage–colony stimulating factor (GM-CSF) is a promising therapy for pulmonary alveolar proteinosis (PAP) but has not been adequately studied.
Objectives: To evaluate safety and efficacy of inhaled GM-CSF in patients with unremitting or progressive PAP.
Methods: We conducted a national, multicenter, self-controlled, phase II trial at nine pulmonary centers throughout Japan. Patients who had lung biopsy or cytology findings diagnostic of PAP, an elevated serum GM-CSF antibody level, and a PaO2 of less than 75 mm Hg entered a 12-week observation period. Those who improved (i.e., alveolar–arterial oxygen difference [A–aDO2] decreased by 10 mm Hg) during observation were excluded. The rest entered sequential periods of high-dose therapy (250 μg Days 1–8, none Days 9–14; × six cycles; 12 wk); low-dose therapy (125 μg Days 1–4, none Days 5–14; × six cycles; 12 wk), and follow-up (52 wk).
Measurements and Main Results: Fifty patients with PAP were enrolled in the study. During observation, nine improved and two withdrew; all of these were excluded. Of 35 patients completing the high- and low-dose therapy, 24 improved, resulting in an overall response rate of 62% (24/39; intention-to-treat analysis) and reduction in A–aDO2 of 12.3 mm Hg (95% confidence interval, 8.4–16.2; n = 35, P < 0.001). No serious adverse events occurred, and serum GM-CSF autoantibody levels were unchanged. A treatment-emergent correlation occurred between A–aDO2 and diffusing capacity of the lung, and high-resolution CT revealed improvement of ground-glass opacity. Twenty-nine of 35 patients remained stable without further therapy for 1 year.
Conclusions: Inhaled GM-CSF therapy is safe, effective, and provides a sustained therapeutic effect in autoimmune PAP.
Clinical trial registered with www.controlled-trials.com/isrctn (ISRCTN18931678), www.jmacct.med.or.jp/english (JMA-IIA00013).
AT A GLANCE COMMENTARY.
Scientific Knowledge on the Subject
Studies in granulocyte/macrophage–colony stimulating factor (GM-CSF) knockout mice and series of patients with pulmonary alveolar proteinosis (PAP) showed that inhaled GM-CSF is promising as therapy for PAP.
What This Study Adds to the Field
This national, multicenter study demonstrates that inhaled GM-CSF therapy of PAP is safe, efficacious, and provides a durable treatment effect in many patients.
Pulmonary alveolar proteinosis (PAP) is a rare disorder in which surfactant accumulates within pulmonary alveoli causing progressive respiratory insufficiency (1). Autoimmune PAP is specifically associated with high levels of autoantibodies against granulocyte/macrophage–colony stimulating factor (GM-CSF) (2). These autoantibodies neutralize the biologic activity of GM-CSF (3) and are presumed to cause the lung manifestations in these patients (4) by impairing alveolar macrophage-mediated pulmonary surfactant clearance, which requires GM-CSF in mice and humans (5–8). The incidence and prevalence of autoimmune PAP in Japan are 0.49 and 6.04 cases per million, respectively, in the general population (9). The disorder is commonly treated by whole-lung lavage (10), a procedure involving general anesthesia in which each lung is infused with up to 50 L of saline while mechanically percussing the chest to physically remove the accumulated sediment. Although this treatment improves lung function in most patients (11), surfactant often accumulates again and periodically repeated treatments are usually required (12). Furthermore, the highly invasive nature of the procedure remains a concern, especially in patients with severe disease.
Based on studies in GM-CSF knockout mice demonstrating that inhaled but not extrapulmonary delivery of GM-CSF corrected PAP (13), a phase I pilot study of inhaled GM-CSF was conducted in three patients with PAP (14). Improvement was noted in clinical, physiologic, radiologic, cytological, and biochemical parameters in all three patients and none experienced adverse events. We report here on the extension of this treatment approach in a national, prospective, multicenter, phase II trial evaluating inhaled GM-CSF in patients with unremitting or progressive PAP. These results were first presented at the Annual Conference of the American Thoracic Society in San Francisco, May 2007 (15).
METHODS
Participants
Patients were enrolled at nine hospitals covering all regions of Japan, including Hokkaido University Hospital, Tohoku University Hospital, Niigata University Medical and Dental Hospital, Chiba University Hospital, Kitasato University Hospital, Aichi Medical University, National Hospital Organization (NHO) Kinki-Chuo Chest Disease Center, NHO Yamaguchi-Ube Medical Center (formerly NHO Sanyo Hospital), and Nagasaki University Institute of Tropical Medicine. The institutional review board of each hospital approved the study and all participants gave written informed consent before enrollment and reconfirmed the consent before the high-dose therapy.
Patients with PAP between 20 and 80 years of age were eligible if they had lung biopsy or cytology findings diagnostic of PAP, an elevated serum GM-CSF antibody level (>3 μg/ml) (16), a PaO2 of less than 75 mm Hg, and agreed to brief hospitalization to initiate treatment. A diagnosis of PAP was established in participants based on transbronchial lung biopsy (n = 13), open-lung biopsy (n = 5), cytology findings of bronchial lavage fluid (n = 50), and a positive serum GM-CSF autoantibody test (n = 50). Individuals were excluded if they had received lung lavage therapy within 6 months before enrollment, previous GM-CSF or other cytokine therapy, leukocytosis greater than 12,000/μl, fever of 38°C or higher, severe edema, hematologic malignancy, primary or metastatic lung cancer, severe asthma, congestive heart failure, angina, bleeding diathesis, or any medical condition likely to interfere with participation in the trial as judged by the investigator. Women who were pregnant or planned to become pregnant during the study period or were lactating were excluded. The eligibility criteria for all participants were centrally reviewed at Niigata University.
Study Design
This was a national, multicenter, self-controlled, phase II trial. The alveolar-arterial oxygen difference (A–aDO2) served as the primary outcome variable. The trial comprised three sequential 12-week periods: observation, high-dose therapy, and low-dose therapy. Study visits occurred at 1, 12, 24, and 36 weeks. Thereafter, patients were followed for 1 year. All participants entered an initial observation period during which disease severity and progression were evaluated (Figure 1). Participants in whom the A–aDO2 decreased by 10 mm Hg or more were defined as having undergone spontaneous improvement and were excluded from enrollment into the treatment group. Patients with worsening or unchanging A–aDO2 were defined as having progressive/unremitting PAP and were enrolled into the treatment group. Importantly, the observation period provided an untreated baseline for all treated patients, thus permitting each treated patient to serve as their own, paired nontreated control.
Figure 1.
Profile of the study cohort. GM-CSF = granulocyte/macrophage–colony stimulating factor; PAP = pulmonary alveolar proteinosis.
The primary endpoint was a change in the A–aDO2 between successive periods. A clinical response was defined as a reduction in A–aDO2 by at least 10 mm Hg at the end of the low-dose period compared with that at the start of the high-dose therapy period, as described previously (17, 18). Efficacy of GM-CSF inhalation was also evaluated using secondary endpoints, including pulmonary function tests, serum biomarkers of PAP, and safety. The clinical protocol received an International Standard Randomized Controlled Trial Number (ISRCTN18931678).
Study Procedures
Recombinant human GM-CSF (rhGM-CSF; sargramostim, Leukine, lyophilized formulation; Berlex, Seattle, WA) was administered to patients included in the treatment group by inhalation as previously described (14). Briefly, 125 μg of lyophilized Leukine dissolved in 2 ml of sterile saline was inhaled as an aqueous aerosol using an LC-PLUS nebulizer with a manual interrupter valve connected to a PARI Turbo BOY compressor (PARI GmbH, Starnberg, Germany), for which aqueous aerosol lung deposition characteristics have been reported (19). This previous study on the lung deposition showed that 11.8 mg of the initial 80 mg of tobramycin would be deposited in the lungs in normal adults. Furthermore, the aerosol output of the device is 20% as per the requirement of the CEN (Comité Européen de Normalization, European Committee of Standardization) standard EN 13544–1, according to the manufacturer. The drug and nebulizers were paid for as a study expense by the funding agency. Treatments included high-dose GM-CSF administration (125 μg twice daily on Days 1–8, none on Days 9–14) for six 2-week cycles, then low-dose administration (125 μg once daily on Days 1–4, none on Days 5–14) for six 2-week cycles. These two treatment periods were intended to serve as induction and maintenance therapy, respectively, which were designed based on the results from our previous phase II study and considering the high cost of the study drug (see online data supplement). The study was designed and monitored for data quality and safety by a steering committee composed of the principal investigator at each participating site. Toxicity was graded according to the National Cancer Institute's Common Terminology Criteria for Adverse Events, Version 3.0 (20).
Assessments
Study visits during the observation period included a history, adverse events, and physical examination, arterial blood gas (ABG) analysis, pulmonary function testing, and a posterior-anterior chest radiograph. Data obtained during the observation period permitted each patient to serve as their own paired, nontreated control. Visits during the treatment period also included a history and physical examination, ABG analysis, pulmonary function testing, a posterior-anterior chest radiograph, and measurement of serum biomarkers of PAP, including lactate dehydrogenase, carcinoembryonic antigen, KL-6, a mucin-like glycoprotein, surfactant protein (SP)-A, SP-D, and GM-CSF antibody (all by ELISA) (3, 8, 9, 14, 17).
We performed ABG testing with patients breathing room air for at least 15 minutes and in the supine position for at least 5 minutes and confirmed that oxygen saturation had stabilized using a pulse oximeter before obtaining the blood sample. The local barometric pressure was used in calculating the A–aDO2 according to the alveolar gas equation (Table 1).
TABLE 1.
CLINICAL CHARACTERISTICS OF PATIENTS WITH PULMONARY ALVEOLAR PROTEINOSIS
| All Patients (n = 50) |
Progressive/Unremitting Disease (n = 40) |
Spontaneous Improvement (n = 9) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | n | % | Median (I.Q. range)* or Mean ± SE | n | % | Median (I.Q. range) or Mean ± SE | n | % | Median (I.Q. range) or Mean ± SE | P Value† |
| Age, y | 50 | 56 (45–59) | 40 | 56 (46–63) | 9 | 58 (43–59) | 0.63‡ | |||
| Sex | 0.32§ | |||||||||
| Female | 20 | 40 | 18 | 45 | 2 | 22 | ||||
| Male | 30 | 60 | 22 | 55 | 7 | 78 | ||||
| Duration of symptoms, mo | 50 | 20 (9–59) | 40 | 20 (11–62) | 9 | 16 (6–37) | 0.27‡ | |||
| Symptoms | ||||||||||
| Dyspnea | 48 | 96 | 38 | 95 | 9 | 100 | 0.49§ | |||
| Cough | 46 | 20 | 50 | 3 | 33 | 0.26§ | ||||
| Sputum | 16 | 32 | 14 | 35 | 2 | 22 | 0.38§ | |||
| Smoking status | 0.29§ | |||||||||
| Current smoker | 15 | 30 | 12 | 30 | 3 | 33 | ||||
| Ex-smoker | 13 | 26 | 9 | 23 | 4 | 44 | ||||
| Never smoker | 22 | 44 | 19 | 48 | 2 | 22 | ||||
| Dust exposure | 48 | 38 | 9 | 0.73§ | ||||||
| Yes | 19 | 40 | 16 | 42 | 3 | 33 | ||||
| No | 29 | 60 | 22 | 58 | 6 | 67 | ||||
| Past lung lavage (>6 mo before study) | 0.60§ | |||||||||
| Yes | 13 | 26 | 11 | 28 | 2 | 22 | ||||
| No | 37 | 74 | 29 | 72 | 7 | 78 | ||||
| Pulmonary function | ||||||||||
| FVC, % predicted | 41 | 81.9 ± 2.2 | 37 | 81.0 ± 2.4 | 3 | 95.3 ± 4.4 | 0.10‖ | |||
| VC, % predicted | 48 | 82.2 ± 2.1 | 39 | 81.9 ± 2.4 | 8 | 84.6 ± 4.8 | 0.63‖ | |||
| DlCO, % predicted | 41 | 55.6 ± 2.7 | 37 | 55.0 ± 2.8 | 3 | 58.0 ± 10.8 | 0.78‖ | |||
| PaCO2, mm Hg¶ | 50 | 38.4 ± 0.5 | 40 | 38.8 ± 0.6 | 9 | 37.2 ± 0.7 | 0.25‖ | |||
| PaO2, mm Hg¶ | 50 | 60.9 ± 1.2 | 40 | 61.7 ± 1.4 | 9 | 57.9 ± 3.1 | 0.25‖ | |||
| A–aDO2, mm Hg** | 50 | 42.6 ± 1.4 | 40 | 41.5 ± 1.5 | 9 | 47.1 ± 3.2 | 0.12‖ | |||
| GM-CSF autoantibody, μg/ml | 50 | 23.1 (13.5–34.9) | 40 | 22.8 (9.2–33.4) | 9 | 30.4 (19.3–52.6) | 0.13‡ | |||
Definition of abbreviations: A–aDO2 = alveolar-arterial oxygen difference; DlCO = diffusing capacity of carbon monoxide; GM-CSF = granulocyte/macrophage–colony stimulating factor; PB = barometric pressure measured by local observatories; PH20 = partial pressure of water vapor in inspired air (assumed to be 47 mm Hg); R = respiratory quotient (assumed to be 0.8).
Interquartile (I.Q.) range is the range from the 25th to the 75th percentiles of the distribution.
Comparison between patients with progressive/unremitting disease and those with spontaneous improvement.
Calculated using the Wilcoxon rank sum test.
Calculated using the χ2 test.
Calculated using Student t test.
Measured with patient in a supine position and breathing room air.
Calculated using the following equation: A–aDO2 = (PB − PH20) × Fio2 − Paco2/R + { Paco2 × Fio2 × (1 − R)/R} − Pao2
High-resolution computed tomography (HRCT) of the chest was obtained before and after GM-CSF therapy and evaluated in blinded fashion as previously described (21) with minor modifications by a board-certified radiologist. Briefly, the extent of ground-glass opacification (GGO) was quantified visually in HRCT scan slices representing three lung regions: upper (just above the aortic arch), middle (at the main carina), and lower (at the bifurcation of the lingular and lower lobe bronchi). Scans were scored as follows: 0 = no GGO, 1 = less than 5% GGO, 2 = 5–24% GGO, 3 = 25–49% GGO, 4 = 50–74% GGO, and 5 = 75% or more GGO. The zonal HRCT score was determined as a single value representing the right and left lungs in upper, middle, and lower lung zones for each scan. The total HRCT score was calculated as the sum of all HRCT score values for each scan.
During and after the treatment, disease severity of each participant was evaluated using PAP disease severity score (DSS) based on the presence of symptoms and degree of reduction in PaO2 as previously described (9). Briefly, the categories included DSS 1: no symptoms and PaO2 ≥70 mm Hg; DSS 2: symptomatic and PaO2 ≥70 mm Hg; DSS3: PaO2 ≥60 mm Hg and <70 mm Hg; DSS 4: PaO2 ≥50 mm Hg and <60 mm Hg; DSS 5: PaO2 <50 mm Hg.
Statistical Analysis
Numeric results are presented as the mean ± SE or the median ± interquartile range. In the primary analysis, A–aDO2 values for treated patients (i.e., with worsening/unchanging A–aDO2 during observation) were compared with corresponding paired data for each patient in the different trial periods (observation, high-dose treatment, and low-dose treatment). The χ2 test was used to evaluate proportions for variables between the progressive/unremitting disease group and the spontaneous improvement group. The paired t test was used for comparisons between normally distributed data from the observation and therapy periods. Comparisons of nonparametric data were made using the Wilcoxon signed-rank test. For group comparisons, unpaired t test and Wilcoxon rank-sum tests were used. The level of significance for multiple comparisons was determined using Bonferroni correction; hence, a P value of 0.017 was considered significant for three tests. The zonal CT scores were compared with the Wilcoxon rank-sum tests and the contingency-table analyses for ordered variables using the χ2 test on the platform of the JMP software. All P values reported are two-sided. Analysis was performed using JMP software version 6.0.3.
In preliminary studies, paired analysis (i.e., before and after aerosol GM-CSF therapy) was done in 13 patients and included evaluation of the effects of GM-CSF on A–aDO2, serum biomarkers of PAP, and other parameters (online supplement). On this basis of A–aDO2, the target sample size was 25, chosen to give 90% power to detect a mean change in A–aDO2 of 11 mm Hg allowing for a 5% type-I error. Considering the other outcome measures and participant drop-out, including spontaneous remission, the study size was increased to 50 patients with the anticipation of 40 participants completing therapy.
RESULTS
Patients
Between February 2004 and October 2007, 50 patients were enrolled into the study. Baseline patient characteristics (Table 1) were similar to those with moderate to severe PAP in a large Japanese cohort (9). During the observation period, five patients had progressive disease (i.e., A–aDO2 increased more than 10 mm Hg), and 35 patients had unremitting disease (i.e., change of A–aDO2 less than 10 mm Hg). Nine patients had spontaneous improvement (i.e., A–aDO2 decreased by 10 mm Hg), 1 patient failed to return and was lost to follow-up, and 1 patient with unremitting disease withdrew consent after completing the observation period; these 11 were excluded from the treatment group (Figure 1). The baseline characteristics of patients with spontaneous improvement were not different from patients with progressive or unremitting disease (Table 1). Thirty-nine patients with progressive/unremitting disease, whose A–aDO2 increased by 1.7 ± 1.1 (95% confidence interval [CI], −0.4 to 3.9) mm Hg during the observation period (41.8 ± 1.5 to 43.6 ± 1.5; n = 39; P = 0.11; paired t test), were entered into the treatment phase of the study. Of those, 39 completed the high-dose period and 35 completed the low-dose period (Figure 1). Four patients did not finish the low-dose treatment due to noncompliance, pneumonia, or tuberculous lymphadenitis, or the decision to pursue alternative therapy (one each). Overall compliance with study procedures was excellent.
Primary Endpoint
Among 39 patients completing high-dose treatment (125 μg twice daily on Days 1–8, none on Days 9–14, for six 2-wk cycles), the A–aDO2 changed by −8.3 ± 1.7 (95% CI, −11.7 to −5.0) mm Hg during this treatment period (weeks 12–24; A–aDO2 43.6 ± 1.5 to 35.3 ± 2.1; n = 39; P < 0.001; paired t test).
Among 35 patients completing both high-dose treatment (125 μg twice daily on Days 1–8, none on Days 9–14, for six 2-wk cycles) and subsequent low-dose treatment (125 μg once daily on Days 1–4, none on Days 5–14, for six 2-wk cycles), 24 patients (69% of patients completing GM-CSF therapy) had a clinical response, resulting in an overall response rate of 62% (24/39, intention-to-treat analysis). The overall change in A–aDO2 was −12.3 ± 1.9 (95% CI, −16.2 to −8.4) mm Hg between the end of observation period and the end of low-dose therapy (weeks 12–36; n = 35; P < 0.0001). Improvement was greater during high-dose therapy (weeks 12–24; −9.0 ± 1.7 mm Hg; n = 35) than during low-dose therapy (weeks 24–36; −3.3 ± 1.3 mm Hg; n = 35; P = 0.026) (Figure 2A). The mean A–aDO2 differed significantly among weeks 1, 12, and 36, corresponding to enrollment, the end of observation period, and the completion of low-dose therapy, respectively, (n = 50, 49, 35, respectively; P < 0.0001; analysis of variance). Multiple group comparisons revealed significant improvement in A–aDO2 between weeks 1 and 36 (enrollment and the completion of therapy, n = 50 and 35; P < 0.0001), and between week 12 and week 36 (the end of observation and the completion of therapy, n = 49 and 35; P = 0.0012).
Figure 2.
Alveolar-arterial oxygen difference (A–aDO2) of the response to inhaled granulocyte/macrophage–colony stimulating factor (GM-CSF) in patients with pulmonary alveolar proteinosis (PAP). (A) The overall mean (± SE) A–aDO2 for all participants receiving inhalation therapy with GM-CSF. *P < 0.05; **P < 0.001. Thirty-nine patients completed the high-dose induction therapy period (weeks 1, 12, and 24; n = 39) and 35 patients completed subsequent low-dose maintenance therapy period (week 36; n = 35). (B) Change in A–aDO2 in patients who responded to inhaled GM-CSF during each trial period (n = 24). A responder was defined as a participant who had improvement in A–aDO2 of at least 10 mm Hg during the treatment period (weeks 12–36). Each bar represents the mean (± SE) for the improvement of A–aDO2 during the designated period. *P < 0.05; **P < 0.01.
Among the 24 responders, improvement occurred during the early, high-dose period (early responders) in 17 patients and during the late, low-dose period (late responders) in 7 patients. The overall improvement of A–aDO2 in responders was −18.2 ± 1.7 mm Hg (n = 24; P < 0.001) (Figure 2B). This consisted of improvement during high- and low-dose periods of −13.3 ± 1.6 mm Hg (n = 24; P < 0.001) and −4.9 ± 1.6 mm Hg (n = 24; P = 0.009), respectively (Figure 2B). During the low-dose treatment period, only 2 of the early responders continued to improve, whereas 15 remained stable and none worsened. Among those not responding during the early, high-dose treatment period, 4 improved during the low-dose period, 13 remained stable, and only 1 had an increase in A–aDO2 (of 10.6 mm Hg).
Secondary Endpoints
GM-CSF inhalation therapy was associated with improvement in secondary outcome measures, including dyspnea, supplemental oxygen use, exercise tolerance, pulmonary function test (Table 2), and chest HRCT (Figure 3A). The diffusing capacity of carbon monoxide (DlCO) did not correlate with A–aDO2 at baseline (R2 = 0.06; P = 0.17) but correlated strongly after GM-CSF inhalation therapy (R2 = 0.352; P = 0.0002). Serum biomarkers of disease severity in patients with PAP (9) also improved after GM-CSF therapy, compared with pretreatment values (Table 2). Chest HRCT scans obtained before therapy in 35 patients revealed more extensive GGO infiltration in lower and middle than upper lung regions by visual (Figure 3A) and by quantitative assessment (Figure 3B). Interestingly, middle and lower zones showed greater improvement of the HRCT score after inhaled GM-CSF therapy than the upper lung zone (Figure 3B). The total HRCT score correlated very well with PaO2, A–aDO2, DlCO, and serum biomarkers (except GM-CSF autoantibody) before and after GM-CSF inhalation therapy (Table 3). In contrast, serum GM-CSF autoantibody levels were unaffected during observation (weeks 1–12; 25.4 ± 3.0 to 24.0 ± 3.2; n = 34; P = 0.15; paired t test) as well as during inhalation period in both responders (weeks 12–36; 22.3 ± 4.0 to 22.4 ± 3.3; n = 24; P = 0.94) and nonresponders (weeks 12–36; 28.2 ± 5.3 to 27.2 ± 4.2; n = 10; P = 0.71) (Figure 4).
TABLE 2.
SYMPTOM, OXYGEN SUPPLEMENT, EXERCISE TOLERANCE, PULMONARY FUNCTION, SERUM BIOMARKERS, AND HEMATOLOGIC INDICES IN PATIENTS WITH PULMONARY ALVEOLAR PROTEINOSIS BEFORE AND AFTER INHALED GRANULOCYTE/MACROPHAGE–COLONY STIMULATING FACTOR THERAPY
| Before Therapy |
After Therapy |
||||||
|---|---|---|---|---|---|---|---|
| Characteristic | n | % | Mean ± SE | n | % | Mean ± SE | P Value |
| Dyspnea | <0.0001* | ||||||
| Yes | 34 | 97 | 15 | 43 | |||
| No | 1 | 3 | 20 | 57 | |||
| Oxygen supplement | 0.034* | ||||||
| Yes | 14 | 40 | 6 | 17 | |||
| No | 21 | 60 | 29 | 83 | |||
| 6-min walking test† | |||||||
| Walking distance, m | 22 | 393 ± 27 | 22 | 444 ± 24 | 0.0046‡ | ||
| Minimal SpO2 (%) | 22 | 84.6 ± 1.2 | 22 | 89.0 ± 1.2 | 0.0017‡ | ||
| Pulmonary function | |||||||
| FVC, % predicted | 35 | 80.5 ± 2.5 | 35 | 84.2 ± 3.0 | 0.29‡ | ||
| VC, % predicted | 35 | 81.0 ± 2.5 | 35 | 86.8 ± 2.9 | 0.0007‡ | ||
| FEV1/FVC | 35 | 86.4 ± 1.6 | 35 | 85.3 ± 1.3 | 0.54‡ | ||
| DlCO,% predicted | 33 | 53.7 ± 2.9 | 34 | 61.4 ± 3.1 | 0.0008‡ | ||
| Serum biomarkers of PAP | |||||||
| LDH, IU/L | 35 | 300 ± 15.4 | 35 | 265 ± 11.9 | 0.009‡ | ||
| CEA, ng/ml | 35 | 6.8 ± 0.8 | 35 | 3.7 ± 0.5 | 0.0001‡ | ||
| KL-6, U/L | 35 | 9,831 ± 1224 | 35 | 4,663 ± 632 | 0.0001‡ | ||
| SP-A, ng/ml | 35 | 136 ± 12 | 35 | 97 ± 10 | 0.0003‡ | ||
| SP-D, ng/ml | 35 | 249 ± 21 | 35 | 216 ± 29 | 0.094‡ | ||
| Hematologic indices | |||||||
| White blood count, cells/μl | 35 | 5,865 ± 222 | 35 | 5,414 ± 249 | 0.023‡ | ||
| Neutrophils, cells/μl | 34 | 3,487 ± 160 | 34 | 2,984 ± 162 | 0.0007‡ | ||
| Monocytes, cells/μl | 34 | 362 ± 17 | 34 | 327 ± 24 | 0.088‡ | ||
| Lymphocytes, cells/μl | 34 | 1,869 ± 121 | 34 | 1,896 ± 109 | 0.76‡ | ||
| Eosinophils, cells/μl | 34 | 139 ± 23 | 34 | 176 ± 33 | 0.029‡ | ||
| Hemoglobin, g/dl | 35 | 15.1 ± 0.3 | 35 | 14.6 ± 0.2 | 0.0047‡ | ||
| Platelets, ×103 cells/μl | 35 | 240 ± 8.2 | 35 | 222 ± 7.4 | 0.0032‡ | ||
Definition of abbreviations: CEA = carcinoembryonic antigen; DlCO = diffusing capacity of carbon monoxide; KL-6 = a mucin-like glycoprotein; LDH = lactate dehydrogenase; PAP = pulmonary alveolar proteinosis; SP = surfactant protein; SpO2 = oxygen saturation measured by pulse oximetry.
Calculated using the χ2 test.
Optional evaluation including 17 responders and 5 nonresponders, in whom change in A–aDO2 was −14.5 ± 2.8 and did not significantly differ from that of the total 35 patients.
Calculated using Student t test.
Figure 3.
Changes in computed tomography of the chest in response to inhaled granulocyte/macrophage–colony stimulating factor (GM-CSF) in patients with pulmonary alveolar proteinosis (PAP). (A) High-resolution computed tomography (HRCT) of the chest of a representative patient before (left) and after (right) treatment with inhaled GM-CSF therapy for 24 weeks. (B) Effect of inhaled GM-CSF therapy on the severity of PAP lung disease measured by the zonal HRCT score (21) as described in the Methods. Shown are pre (open boxes) and post (shaded boxes) regional HRCT score values for upper, middle, and lower lung regions in 35 patients with PAP before and after completing both high- and low-dose GM-CSF treatment periods. Box plots show the median (dashed line), 25th (box bottom), and 75th (box top) percentiles, 10th percentile (lower T bars), 90th percentile (upper T bars). *P < 0.05; **P < 0.01; calculated by the contingency-table analysis for ordered variables using the χ2 test on the platform of the JMP software.
TABLE 3.
CORRELATION BETWEEN HIGH-RESOLUTION COMPUTED TOMOGRAPHY SCORE AND CLINICAL VARIABLES AND BIOMARKERS AT STUDY WEEKS 12 AND 36
| Clinical Variable or Biomarker | n | Correlation Coefficient (Week 12) | P Value | n | Correlation Coefficient (Week 36) | P Value |
|---|---|---|---|---|---|---|
| PaO2 | 35 | −0.688 | <0.001 | 35 | −0.454 | 0.006 |
| A–aDO2 | 35 | 0.698 | <0.001 | 35 | 0.416 | 0.013 |
| DlCO, % predicted | 33 | −0.594 | <0.001 | 34 | −0.482 | 0.004 |
| LDH | 34 | 0.742 | <0.001 | 35 | 0.645 | <0.001 |
| KL-6 | 32 | 0.565 | <0.001 | 35 | 0.718 | <0.001 |
| SP-D | 32 | 0.639 | <0.001 | 35 | 0.530 | 0.001 |
| SP-A | 32 | 0.610 | <0.001 | 35 | 0.551 | <0.001 |
| CEA | 32 | 0.504 | 0.003 | 35 | 0.619 | <0.001 |
| GM-CSF autoantibody | 31 | 0.081 | 0.663 | 34 | 0.251 | 0.153 |
Definition of abbreviations: A–aDO2 = alveolar-arterial oxygen difference; CEA = carcinoembryonic antigen; GM-CSF = granulocyte/macrophage–colony stimulating factor; HRCT = high-resolution computed tomography; KL-6 = a mucin-like glycoprotein; LDH = lactate dehydrogenase; SP = surfactant protein.
Correlation was calculated by comparing the total HRCT score for each individual with various clinical measures and serum biomarkers after completion of high-dose (study week 12) and low-dose (study week 36) GM-CSF inhalation therapy using Spearman correlation coefficient.
Figure 4.
Serum concentration of granulocyte/macrophage–colony stimulating factor (GM-CSF) autoantibody in patients with autoimmune pulmonary alveolar proteinosis (PAP) before and after GM-CSF inhalation therapy. The line shows the GM-CSF autoantibody titer for each patient.
Predictive Factors
No significant differences in patient characteristics were observed between responders and nonresponders except for more frequent sputum production among nonresponders (Table 4). There was no difference in the responses to GM-CSF inhalation between participants with or without whole-lung lavage within 6 months before treatment (Table 4). Interestingly, KL-6 was markedly higher before treatment in the responders compared with the nonresponders (Table 5). No differences in responses to inhaled GM-CSF therapy were observed among patients in different disease severity groups (see online supplement Table E2)(9).
TABLE 4.
CLINICAL CHARACTERISTICS OF RESPONDERS AND NONRESPONDERS TO GRANULOCYTE/MACROPHAGE–COLONY STIMULATING FACTOR INHALATION
| Responders (n = 24) |
Nonresponders (n = 11) |
||||||
|---|---|---|---|---|---|---|---|
| Characteristic | n | % | Median (I.Q. range)* or Mean (SD) | n | % | Median (I.Q. range) or Mean (SD) | P Value |
| Age, y | 24 | 56 (45–67.5) | 11 | 53 (51–55.5) | 0.35† | ||
| Sex | 0.21‡ | ||||||
| Female | 12 | 50 | 3 | 36 | |||
| Male | 12 | 50 | 8 | 64 | |||
| Duration of symptoms, mo | 24 | 22 (15.5–64.5) | 11 | 15 (7.5–59.5) | 0.36† | ||
| Symptoms | |||||||
| Dyspnea | 23 | 96 | 11 | 100 | 0.38‡ | ||
| Cough | 10 | 42 | 7 | 64 | 0.19‡ | ||
| Sputum | 5 | 21 | 7 | 64 | 0.011‡ | ||
| Smoking status | 0.79‡ | ||||||
| Current smoker | 6 | 25 | 4 | 27 | |||
| Ex-smoker | 5 | 25 | 2 | 9 | |||
| Never smoker | 13 | 50 | 5 | 55 | |||
| Dust exposure | 22 | 11 | 0.79‡ | ||||
| Yes | 7 | 32 | 3 | 27 | |||
| No | 15 | 68 | 8 | 73 | |||
| Arterial blood gas analysis | |||||||
| PaCO2, mm Hg§ | 24 | 37.5 ± 0.6 | 11 | 41.0 ± 1.4 | 0.010‖ | ||
| PaO2, mm Hg§ | 24 | 61.5 ± 1.9 | 11 | 60.4 ± 2.7 | 0.75‖ | ||
| A–aDO2, mm Hg¶ | 24 | 43.2 ± 2.2 | 11 | 40.2 ± 2.2 | 0.40‖ | ||
| GM-CSF autoantibody, μg/ml | 24 | 20.0 (8.2–32.2) | 11 | 23.7 (21.2–32.0) | 0.29† | ||
| Previous lung lavage (>6 mo before study) | 0.34‡ | ||||||
| Yes | 9 | 38 | 2 | 18 | |||
| No | 15 | 63 | 9 | 82 | |||
Definition of abbreviations: A–aDO2 = alveolar-arterial oxygen difference; GM-CSF = granulocyte/macrophage–colony stimulating factor; PB = barometric pressure measured by local observatories; PH2O = partial pressure of water vapor in inspired air (assumed to be 47 mm Hg); R = respiratory quotient (assumed to be 0.8).
Thirty-five patients completed both the high-dose period and low-dose period of GM-CSF inhalation therapy.
Interquartile (I.Q.) range is the range from the 25th to the 75th percentiles of the distribution.
Calculated using the Wilcoxon rank sum test.
Calculated using the χ2 test.
Measured with patient in a supine position and breathing room air.
Calculated using Student t test.
Calculated using the following equation: A–aDO2 = (PB − PH2O) × FiO2 − PaCO2/R + {PaCO2 × FiO2 × (1 − R)/R} − PaO2.
TABLE 5.
PULMONARY FUNCTION, RADIOLOGIC APPEARANCE, SERUM BIOMARKERS, AND HEMATOLOGIC INDICES IN PATIENTS WITH PULMONARY ALVEOLAR PROTEINOSIS OF RESPONDERS AND NONRESPONDERS
| Responder |
Nonresponder |
||||
|---|---|---|---|---|---|
| n | Mean ± SE or Median (I.Q. range)* | n | Mean ± SE or Median (I.Q. range) | P Value | |
| Pulmonary function | |||||
| VC, % predicted | 24 | 81.5 ± 3.0 | 11 | 79.9 ± 4.6 | 0.77† |
| FVC, % predicted | 24 | 81.3 ± 3.0 | 11 | 78.8 ± 4.7 | 0.66† |
| FEV1/FVC | 24 | 88.3 ± 2.1 | 11 | 82.2 ± 1.8 | 0.07† |
| DlCO, % predicted | 22 | 54.5 ± 4.0 | 11 | 52.0 ± 3.9 | 0.68† |
| HRCT scores (all patients receiving treatment) | |||||
| Upper lung region | 24 | 3 (2–5) | 11 | 3 (2–5) | 0.82‡ |
| Middle lung region | 24 | 4 (3–5) | 11 | 4 (2–4.25) | 0.59‡ |
| Lower lung region | 24 | 4 (3–5) | 11 | 4 (3.75–5) | 0.91‡ |
| Serum biomarkers of PAP | |||||
| LDH, IU/L | 24 | 307 ± 20 | 11 | 285 ± 24 | 0.51† |
| CEA, ng/ml | 24 | 7.3 ± 1.1 | 11 | 5.8 ± 1.1 | 0.38† |
| KL-6, U/L | 24 | 11,531 ± 1,576 | 11 | 6,121 ± 1,316 | 0.04† |
| SP-A, ng/ml | 24 | 136 ± 15 | 11 | 134 ± 24 | 0.92† |
| SP-D, ng/ml | 24 | 261 ± 25 | 11 | 223 ± 40 | 0.38† |
| Hematologic indices | |||||
| White blood count, cells/μl | 24 | 5,948 ± 302 | 11 | 5,685 ± 266 | 0.59† |
| Neutrophils, cells/μl | 24 | 3,465 ± 210 | 10 | 3,542 ± 214 | 0.83† |
| Monocytes, cells/μl | 24 | 375 ± 22 | 10 | 331 ± 20 | 0.24† |
| Lymphocytes, cells/μl | 24 | 1,934 ± 154 | 10 | 1,710 ± 176 | 0.41† |
| Eosinophils, cells/μl | 24 | 145 ± 25 | 10 | 126 ± 54 | 0.73† |
| Hemoglobin, g/dl | 24 | 14.9 ± 0.3 | 11 | 15.4 ± 0.4 | 0.38† |
| Platelets, ×103 cells/μl | 24 | 234 ± 9.2 | 11 | 255 ± 17 | 0.23† |
Definition of abbreviations: CEA = carcinoembryonic antigen; DlCO = diffusing capacity of carbon monoxide; HRCT = high-resolution computed tomography; KL-6 = a mucin-like glycoprotein; LDH = lactate dehydrogenase; SP = surfactant protein.
Interquartile (I.Q.) range is the range from the 25th to the 75th percentiles of the distribution.
Calculated using Student t test.
Calculated using the Wilcoxon rank sum test.
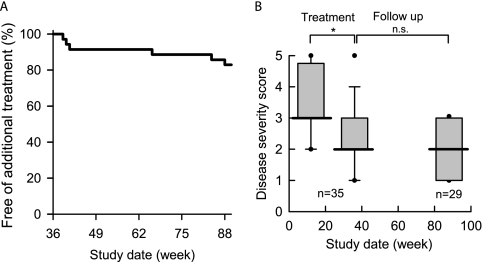
Follow-up
During the 52-week follow-up period, of the 35 patients completing both high- and low-dose treatment periods, 29 patients required no additional specific therapy for PAP and maintained the same improved disease severity score achieved during treatment (Figures 5A and 5B). Among those that did require additional specific therapy, three nonresponders received whole-lung lavage, and three patients (one responder and two nonresponders) received inhaled GM-CSF therapy; all had satisfactory improvement. Of the nine patients with spontaneous improvement during the observation period, four patients subsequently required therapy for PAP, which included GM-CSF inhalation in three and lung lavage in one patient.
Figure 5.
Durability of the response to inhaled granulocyte/macrophage–colony stimulating factor (GM-CSF) in patients with pulmonary alveolar proteinosis (PAP). (A) Kaplan-Meier plot showing individuals free of additional specific therapy of PAP. (B) Disease severity scores of patients (*P < 0.05) who completed high- and low-dose inhalation treatment (n = 35) and 1-year observation with no additional treatment (n = 29).
Adverse Events
No serious adverse events occurred during the trial. No adverse event was reported during the observation period. Adverse events were reported in 7 of 39 patients receiving inhaled GM-CSF. These included (number of patients, grade), fever (1, 1), otitis media (1, 2), gastric ulcer (1, 2), upper respiratory infection (1, 1), diarrhea (1, 1), pneumonia (1, 2), and tuberculous lymphadenitis (1, 2). All were transient and none were judged to be treatment-related except for the last two events.
While hospitalized as per protocol for a study visit in December, a 39-year-old Asian man developed a fever of 38°C with a normal chest radiograph 5 days after completing the first course of GM-CSF therapy. A chest CT revealed an infiltrate in the right upper lobe. Antibiotic therapy was instituted, the patient improved rapidly, and a presumptive diagnosis of pneumonia was made. After completing antibiotic therapy, GM-CSF therapy was resumed without further adverse events.
A 64-year-old Asian man was noted to have a left anterior chest wall nodule on a routine scheduled HRCT as per the protocol, which on surgical biopsy revealed necrotizing granuloma that cultured positive for Mycobacterium tuberculosis. The patient was treated with isoniazid, rifampin, and levofloxacin for 9 months without recurrence.
These two adverse events were judged as possibly related to the study drug and they were included in the total patient numbers in the intention-to-treat analysis but excluded from the overall efficacy analysis.
The occurrence of infection was not significantly different from the large cohort study of Japanese patients with autoimmune PAP (9). There was a minor reduction in blood neutrophil and platelet counts, although values remained within the normal range (Table 2).
DISCUSSION
This prospective, multicenter, phase II trial of inhaled GM-CSF therapy for PAP had an overall response rate of 62% (24/39, intention-to-treat analysis) during a 6-month treatment period. The overall improvement in A–aDO2 was −12.3 mm Hg for all treated patients (n = 35). A–aDO2 improved by −17.2 ± 2.1 mm Hg in responders (24/35, 69%, patients completing both high- and low-dose therapy). The treatment was safe and the response was maintained in 83% of the patients for 1 year without the need for additional therapy.
Our results agree with a small retrospective series in 12 patients with mild PAP treated with inhaled GM-CSF (250 μg twice daily on alternate weeks for 24 wk) in which the response rate was 92%, and A–aDO2 improved by −18.4 mm Hg (22). Together, these results suggest the effects of inhaled GM-CSF are dose-dependent. However, 5 of 11 responders in this previous study required additional therapy within 1 year of discontinuing therapy (22). In a recent prospective study in 21 patients with PAP receiving GM-CSF by subcutaneous administration (in escalating doses from 5 to 18 μg/kg/d), the response rate was 48%, A–aDO2 improved by −9.2 mm Hg, and 4 of 12 responders required additional therapy within 39 ± 17.3 months (18). Importantly, the total amount of GM-CSF administered per patient in this latter trial (range, 33–330 mg/patient) was higher than in our study (15 mg/patient), which is important given the high cost of pharmaceutical GM-CSF (>$600 dollars/mg at the time of the study). Furthermore, neither of these prior studies excluded individuals with spontaneous improvement, which is important for interpreting both therapeutic efficacy and durability.
Our results suggest inhaled GM-CSF may be effective as primary therapy in autoimmune PAP. Although not evaluated here, inhaled GM-CSF may promote terminal differentiation of alveolar macrophages (as it does in mice [23]), thereby increasing surfactant clearance and improving oxygen transfer. In this respect, antecedent lung lavage may be useful before GM-CSF therapy to further increase efficacy. Such an approach was successful in a 9-year-old girl with severe autoimmune PAP (24). In the present study, A–aDO2 did not fully normalize during the treatment in many patients, implying the presence of residual disease. It is possible that a higher dose and/or a longer duration of GM-CSF induction therapy or a longer duration of maintenance therapy or both may further improve efficacy (25). KL-6, a serum biomarker of PAP, improved more in responders than nonresponders. Because KL-6 appears to be related to alveolar epithelial damage, a higher KL-6 level may predict a better response to inhaled GM-CSF therapy.
It is important that GM-CSF therapy was not associated with an increase in GM-CSF autoantibody levels. This suggests that exogenous GM-CSF did not induce an immune response. It is also noteworthy that GM-CSF autoantibody levels did not decline in responders, which demonstrates that the therapeutic response was not mechanistically linked to a reduction in autoantibody levels. Results demonstrated that GM-CSF improved A–aDO2, PaO2, vital capacity, and DlCO, and reduced GGO. These results are consistent with a recent report of two patients with PAP in whom inhaled GM-CSF was associated with improvement in quantitative densitometry analysis of chest CT scans, which demonstrated a reduction in GGO concurrent with an increase in airspace volume and lung inflation (26). Our data do not identify the mechanism underlying the correlation between A–aDO2 and DlCO (and with improvement of GGO), which was apparent only after the treatment. Possible mechanisms include reduction in the diffusional barrier, shunt fraction, and/or ventilation–perfusion mismatching (27). Further studies are needed to determine the precise mechanism(s) by which inhaled GM-CSF improves lung function in autoimmune PAP.
Inhaled GM-CSF therapy was well tolerated in patients with autoimmune PAP. No serious adverse effects or treatment-related early termination occurred in our study, similar to a recent retrospective study of inhaled GM-CSF therapy in patients with PAP (22). In contrast, subcutaneous administration of GM-CSF was associated with injection site reactions and other minor problems in 85% of patients with PAP in one study (18) and a “first dose” effect (i.e., fever, chills, nausea within 4 h of dosing) in 29% in another (17). Including published reports, at least 95 patients were reported to have been treated with GM-CSF without serious adverse effects (14, 17, 18, 22, 26). GM-CSF inhalation therapy did not increase serum levels of GM-CSF autoantibody, which is of importance to the autoimmune basis of the disorder in patients with the common autoimmune form of the syndrome (2). Our results are supported by other studies indicating the safety of inhaled GM-CSF therapy in humans (28, 29). A small decrease in neutrophil counts was observed during treatment but counts remained within the normal range.
Our study was designed to evaluate only patients with stable or progressive autoimmune PAP and excluded individuals with measurable spontaneous improvement and, importantly, used a design that allowed all treated patients to serve as their own untreated control. Nine of 50 participants (18%) were excluded because they underwent some degree of spontaneous improvement during the 12-week observation period. Among patients with unremitting or progressive disease, there was a trend toward worsening of the A–aDO2. We cannot exclude the possibility that some patients experienced a degree of spontaneous improvement during the treatment period. However, we believe that worsening of A–aDO2 during the 12-week observation period adequately controlled for spontaneous improvement.
The present study was limited by use of an open-label design and absence of a separate parallel placebo group. However, the use of self-control design was appropriate for a phase II trial in this rare disease without extant pharmacological therapy and is supported by several lines of reasoning. First, an equivalence or noninferiority study design comparing inhaled GM-CSF to whole-lung lavage in a multicenter setting would have been technically impractical because the latter has not been standardized and varies widely among centers. Second, a crossover design between GM-CSF and placebo treatment groups would have been inappropriate because the time course of the effects of GM-CSF therapy of PAP are unknown, and the effects of GM-CSF on lung function in PAP can be prolonged. Third, in the absence of commercial pharmaceutical industry support and an available inhaled placebo, using a placebo arm is particularly challenging for trials in very rare diseases such as PAP. Notwithstanding, our results support the need for a larger randomized controlled trial to establish the optimal dose, timing of administration, and duration of therapy for an optimal treatment effect.
We conclude that inhaled GM-CSF therapy of autoimmune PAP is safe, well-tolerated, and efficacious, has a dose effect, and results in a durable treatment effect in many patients. Inhaled GM-CSF may be an appropriate therapeutic alternative to whole-lung lavage, the current standard therapy, in some patients with autoimmune PAP.
Supplementary Material
Acknowledgments
The authors thank the investigators and patients who participated in this study; Sayoko Hattori, Yumi Ogata, and Hiroko Kanazawa for help with data management; Natsuki Totsu and Yoko Aizawa for measurement of GM-CSF autoantibody levels; and Marie Mori and Rumi Kizawa for help with preparation of data for the manuscript.
Supported by the Japanese Ministry of Education and Science, Ministry of Health, Labor, and Welfare of Japan grants H14-trans-014 (K.N.) and H21-Nanchi-Ippan−161 (Y.I.), Grant-in-Aid for Scientific Research Category B 18406031 (Y.I.), the National Center for Research Resources grant RR019498 (B.C.T.), National Institutes of Health grant HL085453 (B.C.T.), and National Hospital Organization of Japan Category Network (Y.I.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200906-0978OC on February 18, 2010
Conflict of Interest Statement: R.T. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. B.C.T. was a consultant to Boeheringer Ingelheim, received $1,001–$5,000 from MorphoSys and $1,001–$5,000 from MedImmune in consultancy fees as a lung disease specialist for trial design, $1,001–$5,000 from Lilly as an advisory board member, and $10,001–$50,000 from the Alpha 1 Foundation as a grant to support role as scientific director. Y.I. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. T.A. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. T.T. received $10,001–$50,000 from Banyu Pharmaceutical Co., Ltd and $10,001–$50,000 from Dainippon Sumitomo Pharma Co., Ltd in industry-sponsored grants for contracted research. Y.N. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. N.H. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. Y.K. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. K.T. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.H. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. H.I. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.Y. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. N.T. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. E.Y. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. R.E. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. Y.T. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. K.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.A. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.T. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.O. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.E. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. C.K. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. T.N. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.P.K. received more than $100,001 from Genzyme in sponsored grants for support for clinical trials. K.A. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. K.N. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Rosen SH, Castleman B, Liebow AA. Pulmonary alveolar proteinosis. N Engl J Med 1958;258:1123–1142. [DOI] [PubMed] [Google Scholar]
- 2.Kitamura T, Tanaka N, Watanabe J, Uchida, Kanegasaki S, Yamada Y, Nakata K. Idiopathic pulmonary alveolar proteinosis as an autoimmune disease with neutralizing antibody against granulocyte/macrophage colony-stimulating factor. J Exp Med 1999;190:875–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uchida K, Nakata K, Trapnell BC, Terakawa T, Hamano E, Mikami A, Matsushita I, Seymour JF, Oh-Eda M, Ishige I, et al. High-affinity autoantibodies specifically eliminate granulocyte-macrophage colony-stimulating factor activity in the lungs of patients with idiopathic pulmonary alveolar proteinosis. Blood 2004;103:1089–1098. [DOI] [PubMed] [Google Scholar]
- 4.Trapnell BC, Whitsett JA, Nakata K. Pulmonary alveolar proteinosis. N Engl J Med 2003;349:2527–2539. [DOI] [PubMed] [Google Scholar]
- 5.Ikegami M, Ueda T, Hull W, Whitsett JA, Mulligan RC, Dranoff G, Jobe AH. Surfactant metabolism in transgenic mice after granulocyte macrophage-colony stimulating factor ablation. Am J Physiol 1996;270:L650–L658. [DOI] [PubMed] [Google Scholar]
- 6.Dranoff G, Crawford AD, Sadelain M, Ream B, Rashid A, Bronson RT, Dickersin GR, Bachurski CJ, Mark EL, Whitsett JA, et al. Involvement of granulocyte-macrophage colony-stimulating factor in pulmonary homeostasis. Science 1994;264:713–716. [DOI] [PubMed] [Google Scholar]
- 7.Stanley E, Lieschke GJ, Grail D, Metcalf D, Hodgson G, Gall JA, Maher DW, Cebon J, Sinickas V, Dunn AR. Granulocyte/macrophage colony-stimulating factor-deficient mice show no major perturbation of hematopoiesis but develop a characteristic pulmonary pathology. Proc Natl Acad Sci USA 1994;91:5592–5596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suzuki T, Sakagami T, Rubin BK, Nogee LM, Wood RE, Zimmerman SL, Smolarek T, Dishop MK, Wert SE, Whitsett JA, et al. Familial pulmonary alveolar proteinosis caused by mutations in CSF2RA. J Exp Med 2008;205:2703–2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inoue Y, Trapnell BC, Tazawa R, Arai T, Takada T, Hizawa N, Kasahara Y, Tatsumi K, Hojo M, Ichiwata T, et al. Characteristics of a large cohort of autoimmune pulmonary alveolar proteinosis patients in Japan. Am J Respir Crit Care Med 2008;177:752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wasserman K, Blank N, Fletcher G. Lung lavage (alveolar washing) in alveolar proteinosis. Am J Med 1968;44:611–617. [DOI] [PubMed] [Google Scholar]
- 11.Beccaria M, Luisetti M, Rodi G, Corsico A, Zoia MC, Colato S, Pochetti P, Braschi A, Pozzi E, Cerveri I. Long-term durable benefit after whole lung lavage in pulmonary alveolar proteinosis. Eur Respir J 2004;23:526–531. [DOI] [PubMed] [Google Scholar]
- 12.Seymour JF, Presneill JJ. Pulmonary alveolar proteinosis: progress in the first 44 years. Am J Respir Crit Care Med 2002;166:215–235. [DOI] [PubMed] [Google Scholar]
- 13.Reed JA, Ikegami M, Cianciolo ER, Lu W, Cho PS, Hull W, Jobe AH, Whitsett JA. Aerosolized GM-CSF ameliorates pulmonary alveolar proteinosis in GM-CSF-deficient mice. Am J Physiol 1999;276:L556–L563. [DOI] [PubMed] [Google Scholar]
- 14.Tazawa R, Hamano E, Arai T, Ohta H, Ishimoto O, Uchida K, Watanabe M, Saito J, Takeshita M, Hirabayashi Y, et al. Granulocyte-macrophage colony-stimulating factor and lung immunity in pulmonary alveolar proteinosis. Am J Respir Crit Care Med 2005;171:1142–1149. [DOI] [PubMed] [Google Scholar]
- 15.Nakata T, Inoue Y, Nukiwa T, Tazawa R, Tsuchihashi K, Takada T, Terada M, Kanazawa H, Hizawa N, Trapnell BC. Multicenter phase II trial of inhaled aerosolized granulocyte-macrophage colony-stimulating factor for patients with idiopathic alveolar proteinosis; the prognosis of patients [abstract]. Am J Respir Crit Care Med 2007;175:A497. [Google Scholar]
- 16.Uchida K, Beck DC, Yamamoto T, Berclaz PY, Abe S, Staudt MK, Carey BC, Filippi MD, Wert SE, Denson LA, et al. GM-CSF autoantibodies and neutrophil dysfunction in pulmonary alveolar proteinosis. N Engl J Med 2007;356:567–579. [DOI] [PubMed] [Google Scholar]
- 17.Seymour JF, Presneill JJ, Schoch OD, Downie GH, Moore PE, Doyle IR, Vincent JM, Nakata K, Kitamura T, Langton D, et al. Therapeutic efficacy of granulocyte-macrophage colony-stimulating factor in patients with idiopathic acquired alveolar proteinosis. Am J Respir Crit Care Med 2001;163:524–531. [DOI] [PubMed] [Google Scholar]
- 18.Venkateshiah SB, Yan TD, Bonfield TL, Thomassen MJ, Meziane M, Czich C, Kavuru MS. An open-label trial of granulocyte macrophage colony stimulating factor therapy for moderate symptomatic pulmonary alveolar proteinosis. Chest 2006;130:227–237. [DOI] [PubMed] [Google Scholar]
- 19.Coates AL, Dinh L, MacNeish CF, Rollin T, Gagnon S, Ho SL, Lands LC. Accounting for radioactivity before and after nebulization of tobramycin to insure accuracy of quantification of lung deposition. J Aerosol Med 2000;13:169–178. [DOI] [PubMed] [Google Scholar]
- 20.National Cancer Institute. Common Terminology Criteria for Adverse Events, (CTCAE) v3.0. Bethesda: National Cancer Institute; 2006.
- 21.Akira M, Inoue Y, Yamamoto S, Sakatani M. Non-specific interstitial pneumonia: findings on sequential CT scans of nine patients. Thorax 2000;55:854–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wylam ME, Ten R, Prakash UB, Nadrous HF, Clawson ML, Anderson PM. Aerosol granulocyte–macrophage colony stimulating factor for pulmonary alveolar proteinosis. Eur Respir J 2006;27:585–593. [DOI] [PubMed] [Google Scholar]
- 23.Shibata Y, Berclaz PY, Chroneos ZC, Yoshida M, Whitsett JA, Trapnell BC. GM-CSF regulates alveolar macrophage differentiation and innate immunity in the lung through PU.1. Immunity 2001;15:557–567. [DOI] [PubMed] [Google Scholar]
- 24.Yamamoto H, Yamaguchi E, Agata H, Kandatsu N, Komatsu T, Kawai S, Baba K, Awaya T, Nishikomori R, Tsurusawa M, et al. A combination therapy of whole lung lavage and GM-CSF inhalation in pulmonary alveolar proteinosis. Pediatr Pulmonol 2008;43:828–830. [DOI] [PubMed] [Google Scholar]
- 25.Tazawa R, Nakata K, Inoue Y, Nukiwa T. Granulocyte-macrophage colony-stimulating factor inhalation therapy for patients with idiopathic pulmonary alveolar proteinosis: a pilot study; and long-term treatment with aerosolized granulocyte-macrophage colony-stimulating factor: a case report. Respirology 2006;41:S61–S64. [DOI] [PubMed] [Google Scholar]
- 26.Robinson TE, Trapnell BC, Goris ML, Quittell LM, Cornfield DN. Quantitative analysis of longitudinal response to aerosolized granulocyte-macrophage colony-stimulating factor in two adolescents with autoimmune pulmonary alveolar proteinosis. Chest 2009;135:842–848. [DOI] [PubMed] [Google Scholar]
- 27.Murayama J, Fukuda K, Sato T, Yano H, Ohtsuka M, Yoshizawa Y, Hasegawa S. Pulmonary alveolar proteinosis. Xe-133 scintigraphic findings before and after bronchopulmonary lavage. Clin Nucl Med 1993;18:123–125. [DOI] [PubMed] [Google Scholar]
- 28.Anderson PM, Markovic SN, Sloan JA, Clawson ML, Wylam M, Arndt CA, Smithson WA, Burch P, Gornet M, Rahman E. Aerosol granulocyte macrophage-colony stimulating factor: a low toxicity, lung-specific biological therapy in patients with lung metastases. Clin Cancer Res 1999;5:2316–2323. [PubMed] [Google Scholar]
- 29.Markovic SN, Suman VJ, Nevala WK, Geeraerts L, Creagan ET, Erickson LA, Rowland KM Jr, Morton RF, Horvath WL, Pittelkow MR. A dose-escalation study of aerosolized sargramostim in the treatment of metastatic melanoma: an NCCTG Study. Am J Clin Oncol 2008;31:573–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.