Abstract
Rationale: Urokinase-type plasminogen activator (uPA) regulates extracellular proteolysis in lung injury and repair. Although alveolar expression of uPA increases, procoagulant activity predominates.
Objectives: This study was designed to investigate whether uPA alters the expression of tissue factor (TF), the major initiator of the coagulation cascade, in lung epithelial cells (ECs).
Methods: Bronchial, primary airway ECs and C57B6 wild-type, uPA-deficient (uPA−/−) mice were exposed to phosphate-buffered saline, uPA, or LPS. Immunohistochemistry, protein, cellular, and molecular techniques were used to assess TF expression and activity.
Measurements and Main Results: uPA enhanced TF mRNA and protein expression, and TF-dependent coagulation in lung ECs. uPA-induced expression of TF involves both increased synthesis and enhanced stabilization of TF mRNA. uPA catalytic activity had little effect on induction of TF. By contrast, deletion of the uPA receptor binding growth factor domain from uPA markedly attenuated the induction of TF, suggesting that uPA receptor binding is sufficient for TF induction. Lung tissues of uPA-deficient mice expressed less TF protein and mRNA compared with wild-type mice. In addition, intratracheal instillation of mouse uPA increased TF mRNA and protein expression and accelerated coagulation in lung tissues. uPA−/− mice exposed to LPS failed to induce TF.
Conclusions: uPA increased TF expression and TF-dependent coagulation in the lungs of mice. We hypothesize that uPA-mediated induction of TF occurs in lung ECs to promote increased fibrin deposition in the airways during acute lung injury.
Keywords: urokinase, tissue factor, lung epithelial cells, idiopathic pulmonary fibrosis
AT A GLANCE COMMENTARY.
Scientific Knowledge on the Subject
Urokinase-type plasminogen activator (uPA) regulates extracellular proteolysis in lung injury and repair. Although alveolar expression of uPA increases, procoagulant activity predominates.
What This Study Adds to the Field
uPA increased tissue factor (TF) expression and TF-dependent coagulation in the lungs of mice. We hypothesize that uPA-mediated induction of TF occurs in lung epithelial cells to promote increased fibrin deposition in the airways during acute lung injury.
Increased procoagulant and decreased fibrinolytic activities are commonly found in the lungs of patients with acute and chronic inflammatory lung diseases, such as acute respiratory distress syndrome (ARDS), and fibrotic lung diseases, such as idiopathic pulmonary fibrosis (1). In these disorders, the elevated procoagulant activity found in the alveolar compartment is mainly attributable to TF-induced activation of coagulation (2). TF, a 47-kD transmembrane glycoprotein, initiates extrinsic coagulation by binding and activating coagulation factor VIIa. The resultant TF-VIIa complex activates factor X to generate factor Xa, triggering the generation of thrombin, which converts soluble fibrinogen to insoluble fibrin (3).
Fibrin degradation in the airways and alveolar space is mediated primarily through urokinase-type plasminogen activator (uPA)-mediated plasminogen activation (4). Although relatively high levels of uPA and low levels of TF are found in bronchoalveolar lavage (BAL) fluids of healthy individuals, elevated levels of TF-VIIa complexes, factor Xa, thrombin, uPA, and plasminogen activator inhibitor (PAI)-1 are generally found in BAL of patients with acute inflammatory and fibrotic lung diseases (1). Intraalveolar fibrin deposition is invariably present in these diseases (1) and serves as a provisional matrix for leukocyte migration and fibroblast proliferation and activation. Although uPA is induced by proinflammatory agents, fibrinolytic activity is depressed in BAL of patients with acute or chronic lung injury, which is largely attributable to a disproportionate increase in PAI-1 (5).
Previous studies have shown that induction of uPA, uPA receptor (uPAR), and PAI-1 may occur in lung ECs through a uPA-mediated feedback pathway (6–8). Given the findings that uPA, PAI-1, and TF are all elevated in lung ECs during lung injury (9), we suspected that alveolar and airway ECs that were exposed to increased levels of uPA would demonstrate a concomitant increase in TF expression. Such an effect could fine-tune fibrin turnover within the alveolar compartment. In this way the equilibrium between uPA-mediated TF induction and uPA-mediated fibrinolysis could help to set the balance between physiological and pathological remodeling at the sites of alveolar damage. However, to our knowledge, regulation of TF expression by uPA has not been previously demonstrated.
In addition to its role in thrombin generation, TF-initiated signal transduction might be involved in lung injury and repair associated with endotoxemia, pneumonia, or severe sepsis-induced acute lung injury (ALI). An increase in TF expression has been identified in tumors that overexpress uPA; the increased interaction of TF with integrins promotes tumor growth and signal transduction and can modify uPAR–integrin interactions (9, 10). Conversely, TF-VIIa has been reported to activate plasminogen, further linking the functionality of the coagulation and fibrinolytic pathways (11). The studies reported herein demonstrate a new paradigm through which TF expression by lung epithelium is regulated by uPA. This pathway may provide a coordinated link between the coagulation and fibrinolytic systems that is operative after exposure to LPS and other forms of inflammatory lung injury. Some of the results of these studies have been previously reported in the form of an abstract (12).
METHODS
Materials
Anti-TF and anti-uPA antibodies were obtained from American Diagnostica (Greenwich, CT). The uPA antagonist B428 was the generous gift of Dr. Andrew Mazar (Angstrom Pharmaceuticals, San Diego, CA). Full-length single-chain uPA and uPA deletion mutants were cloned and the recombinant proteins were expressed in S2 cells and purified, including the amino-terminal fragment (ATF) (amino acids 1–135), low molecular weight (LMW) uPA fragments (amino acids 136–411), the deletion mutants δ-growth factor domain (GFD)-single chain uPA (scuPA) (amino acids 4–43), and δkringle-scuPA (amino acids 47–135), as described (13).
Cell Culture
Human bronchial ECs (Beas2B) were maintained in LHC-9 medium containing 1% antibiotics as previously described (14). Primary cultures of human small airway ECs (SAECs) were cultured in SAGM medium, as described (8).
Mice
Transgenic mice with a deletion in the uPA gene (uPA−/−) and control mice on the same genetic background (C57B6/129) were described earlier (15). The mice were kept on a 12:12 hour light–dark cycle with free access to food and water. All experiments were conducted in accordance with institutional review board–approved protocols.
Total Protein Extraction and Western Blotting
Cells were incubated with or without recombinant human two-chain uPA (tcuPA) or other agents for specified times as described earlier (8, 14). The cell lysates were separated by sodium dodecylsulfate (SDS)-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes. The membranes were blocked with 1% bovine serum albumin (BSA) followed by overnight hybridization with anti-TF and β-actin monoclonal antibodies. TF and β-actin proteins were detected by enhanced chemiluminescence. Because of high basal TF expression by lung ECs, we used 3 μg total protein for immunoblotting to detect the changes in TF expression in response to different treatment conditions. However, we noticed that stripping and reprobing the membranes containing 3 μg/lane total protein with anti–β-actin antibody failed to readily detect β-actin proteins by enhanced chemiluminescence. Therefore, for selected experiments 3 μg and 25 μg/lane of total protein from the same sample was used to assess the changes in TF and β-actin proteins, respectively.
Clotting Time
We measured uPA-mediated clotting and expression of TF procoagulant activity in a single-stage clotting assay using a Coag-a-mate XM (BioMericux, Durham, NC) photo-optical clot detector. The clotting time was measured photo-optically to the nearest 0.1second. The amount of TF in each sample was determined with reference to clotting times obtained using a standard curve prepared with human TF relipidated in phospholipid vesicles (90% phosphotidyl choline [PC], 10% phosphotidyl serine [PS]). One unit of TF activity is defined as the amount that yielded a 50-second clotting time.
Overexpression of uPA in Beas2B Cells Transfected with uPA cDNA
Beas2B cells were transfected with the uPA cDNA subcloned into the eukaryotic expression vector pRc/CMV2 (Invitrogen, Carlsbad, CA) containing the cyto megalo virus promoter at Hind III/Not I sites using LipofectAMINE (Invitrogen). Stable cell lines were analyzed for uPA expression by Western blotting using a uPA monoclonal antibody. The effect of endogenous uPA overexpression on TF induction was then measured by Western blotting as described above (14).
Plasmid Construction, In Vitro Transcription, and Random Priming
Human TF cDNAs corresponding to the full-length CDR and 3′ untranslated region (UTR) were subcloned to PCRII-TOPO vector (Invitrogen). Linearized plasmids containing the human TF mRNA transcriptional templates of TF cDNA corresponding to TF coding region (CDR) and 3′UTR were separately transcribed in vitro with T7 or Sp6 polymerase using [32P]UTP (Ambion, Austin, TX). The TF CDR cDNA fragment was labeled with [32P]dCTP using a Rediprime labeling kit (Promega, Madison, WI) and used as probe for Northern blotting.
Nuclear Run-on Transcription Activation Assay
Beas2B cells treated with PBS, LPS (10 μg/ml), or uPA (1 μg/ml) for 3 hours at 37°C was analyzed using the transcription activation assay, as described (8).
TF mRNA Assessment by Northern Blotting
A Northern blotting assay was used to assess the steady-state level of TF mRNA in Beas2B as well as mouse lung tissues. The total RNA (20 μg) from Beas2B cells incubated with uPA for 0 to 24 hours and mouse lung tissues were analyzed for TF mRNA expression as described earlier (16). The intensity of the bands was measured by densitometry and normalized against that of β-actin. TF mRNA stability was assessed by transcription chase experiments. In these experiments, cells stimulated with PBS or uPA for 6 hours were then treated with 5,6-dichloro-1-β-D-ribofuranosylbenz-imidazole to inhibit ongoing transcription, after which total RNA was isolated at specific time points. TF mRNA was measured by Northern blot.
Gel Mobility Shift Assay
Binding assays were performed using uniformly 32P-labeled transcripts corresponding to the TF CDR or 3′UTR regions. Reactions were performed by incubating these transcripts (20,000 cpm) with cytosolic extracts of Beas2B cells that had been incubated with uPA for 0 to 24 hours (50 μg) in the presence of Escherichia coli tRNA (200 ng/μl) in a total volume of 20 μl at 30°C for 30 minutes. The reaction mixtures were treated with 50 units of RNase T1 and incubated for an additional 30 minutes at 37°C. To avoid nonspecific protein binding, 5 mg/ml heparin was added and the mixture was incubated at room temperature for an additional 10 minutes. Samples were then separated by electrophoresis on 5% native polyacrylamide gels in 0.25 × Tris Borate EDTA running buffer. The gels were dried and autoradiographed at −70°C using Kodak X-ray film.
In a separate experiment, cytosolic extracts were incubated with 0 to 400-fold excess of unlabeled TF sense 3′UTR mRNA at 30°C for 30 minutes and then treated with RNase T1 and heparin as described above. The reaction mixtures were run on 5% native gels, dried, and autoradiographed. To determine the specificity of the RNA–protein complex, the cytoplasmic extract was pretreated with a 200-fold molar excess of ribonucleotide poly (A), poly (C), poly (G), or poly (U) for 30 minutes at 30°C before the 32P-labeled TF mRNA and RNase T1 steps.
In an additional experiment, cytosolic extract was treated with SDS (0.1%) or proteinase K (2.5 mg/ml) for 30 minutes at 30°C before subjecting them to the gel mobility shift assays described above. 32P-labeled TF 3′UTR mRNA was predigested with RNase T1 (50 units) for 30 minutes at 37°C before subjecting to RNA binding of cytosolic extract to confirm the specificity of the interaction.
Northwestern Assay
To confirm the molecular weight of TF 3′UTR mRNA binding proteins, Beas2B cell lysates incubated with uPA for 0 to 24 hours were subjected to TF 3′UTR mRNA-TF mRNABps interaction by Northwestern assay as we described earlier (17). The cytosolic extracts were separated on 8% SDS–polyacrylamide gel electrophoresis and then blotted onto nitrocellulose membranes. The membranes were incubated with 32P-labeled TF 3′UTR mRNA (2 × 105 cpm/ml) in a gel shift buffer containing 1% BSA and 20 μg ribosomal RNA for 1 hour at room temperature. The membranes were later washed and exposed to X-ray film. The same membranes were later stripped and analyzed by Western blotting for β-actin with anti–β-actin antibody as a loading control.
Treatment of uPA in Mice
Mice were given a single intratracheal injection of scuPA or tcuPA (60 μg/mouse) using a 100-μl microsprayer; control mice received saline. After an incubation period of 24 hours, the mice were killed by an intraperitoneal injection of a lethal dose of Beuthanasia-D and blood in the lung vasculature was flushed out with 10 ml PBS via right ventricular perfusion, after which the whole lung was harvested, rinsed in PBS, blotted, and stored at −80°C until further use. In a separate series of experiments, wild-type (wt) and uPA−/− mice were given an intratracheal injection of 6 μg of LPS (Sigma-Aldrich, St. Louis, MO) or saline as a negative control. Twenty-four hours later, the mice were sacrificed and the lung homogenates were analyzed for expression of TF by Western blotting and clotting times as described below.
Preparation of Crude Extract from Mouse Lung and Western Blotting
Extracts from mouse lungs were prepared as described (15, 18). Briefly, mouse lungs were separated into small pieces with fine scissors and rinsed three times with PBS. The tissues were then homogenized in a 1-ml volume of extraction buffer (25 mM Tris–HCl, pH 7.9; 0.5 mM ethylenediaminetetraacetic acid and 0.1 mM phenylmethylsulfonyl fluoride). The homogenate was freeze–thawed three times and centrifuged at 12,000 × g for 15 minutes at 4°C. The supernatant was used to measure the clotting time.
As a second approach, lung tissues were homogenized in lysis buffer containing β-D-glucopyranoside. The homogenate was allowed to rotate at 4°C for 2 hours (6). The homogenate was then centrifuged at 12,000 × g for 30 minutes. The clarified supernatant was analyzed for TF expression by Western blotting using anti-TF antibody.
In a final alternate approach, alveolar type (AT) II cells were isolated from wt and uPA−/− mice that were treated with PBS or LPS. Lysates were analyzed for TF and surfactant protein C (SPC) antigens by Western blot to confirm the uPA involvement in LPS-induced TF expression in ATII cells.
Immunohistochemistry
After killing, lungs from saline- and uPA-treated mice as well as LPS-treated wt or uPA−/− mice were inflated by an intratracheal instillation of 10% formalin in PBS and then fixed in 3.7% formalin overnight at room temperature. Five-micrometer sections cut from paraffin-embedded lung tissues were studied for immunohistochemistry (IHC) after incubation with rabbit anti-mouse TF antibody (American Diagnostica, Greenwich, CT) or anti-SPC antibody or normal rabbit IgG as negative control using a commercially available kit (Lab Vision, Fremont, CA) as described before (15).
Statistical Analyses
We evaluated the data for differences between different treatment conditions using Student t test.
RESULTS
Time-dependent Induction of TF by tcuPA
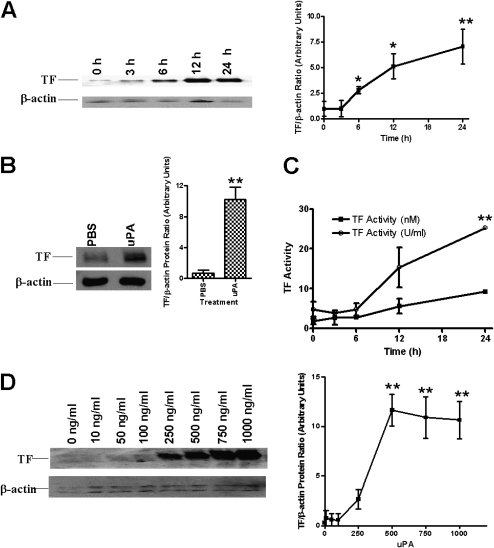
In an earlier study, we reported that tcuPA regulates proteases involved in lung EC fibrinolysis and that this process is in part uPAR dependent (6, 8, 14). Based on this observation, we first sought to determine whether tcuPA could initiate the coagulation protease cascade by inducing the expression of TF in nonmalignant Beas2B cells. To address this question, we incubated these cells with tcuPA for 0 to 24 hours and analyzed several indicators of TF expression using cell lysates. First, total TF protein was analyzed by Western blotting using an anti-TF antibody. The data in Figure 1A demonstrate that tcuPA induced TF protein expression in Beas2B cells in a time-dependent manner. The induction was detectable by 6 hours after the addition of tcuPA and was maintained for up to 24 hours. TF expression declined to basal level around 48 hours after uPA exposure (data not shown). Because Beas 2B cells are SV40 transformed, we also used primary SAECs. A similar induction of TF protein was also observed in primary SAECs (Figure 1B). The TF-dependent clotting activity of these samples paralleled the increase in TF protein expression (Figure 1C).
Figure 1.
Time- and concentration-dependent induction of tissue factor (TF) expression by two-chain urokinase-type plasminogen activator (tcuPA) in lung epithelial cells (ECs). (A) Human bronchial ECs (Beas2B) were incubated with or without recombinant human tcuPA (1 μg/ml) for 0 to 24 hours. Total protein (3 μg/lane) from the cell lysates were immunoblotted with anti-TF antibody. Same membranes were stripped and later developed with anti–β-actin monoclonal antibody to assess loading. The data illustrated are integrated from at least four independent experiments, and mean densities of the individual bands are presented in the line graph. (B) Analysis of TF protein expression in primary small airway ECs (SAECs) treated with phosphate-buffered saline or tcuPA for 24 hours. Total lysates (3 μg protein/lane) from SAECs were analyzed for TF expression by Western blotting. Total protein (25 μg/lane) from the same samples were blotted with β-actin monoclonal antibody for assessment of loading. The mean and SD of densitometric values of individual bands of three experiments from B are represented as a bar graph. (C) Beas2B cells incubated with tcuPA as described in A were lysed in Hanks' balanced salt solution and analyzed for TF activity using a single-stage clotting assay. The experiments were repeated at least three times and the results are shown as nM TF and U/ml determined as described in Methods. (D) Beas2B cells grown to confluence were incubated with 0 to 1 μg/ml of tcuPA for 24 hours. The total proteins from cell lysates were subjected to immunoblotting as described in A. The blots are representative of three separate experiments. The figure (line graph) shows the mean band densities ± SD of four independent experiments. The differences in TF are significant (*P < 0.005 or **P < 0.001) when compared with its level at 0 hours or 0 ng/ml.
Beas2B cells were later incubated with 0 to 1 μg/ml (0–20 nM) of tcuPA for 24 hours and then TF protein was measured by Western blotting. Figure 1D shows that tcuPA induced TF expression in a concentration-dependent manner. The effect becomes apparent at levels as low as 0.25 μg/ml (5 nM) tcuPA, with TF expression peaking between 0.75 and 1.0 μg/ml tcuPA.
LPS is known to induce TF expression (19, 20). To determine if LPS contaminating the tcuPA preparation was responsible for the induction of TF, we measured LPS content by the Limulus Amebocyte lysate ELISA method. We found that the tcuPA preparation used contains minimal amounts (∼1 pg/ml) of LPS. To determine if this residual contamination accounts for the induction of TF, we next incubated Beas2B cells for up to 24 hours with 1 pg/ml LPS and compared the results with exposure to 20 μg/ml LPS as a positive control. Low concentrations (1 pg/ml) of LPS failed to induce TF, whereas the higher concentration (20 μg/ml) augmented TF expression, as predicted based on prior findings (20). Therefore, the induction of TF expression in Beas2B cells by tcuPA could not be explained by LPS contamination.
The ability of endogenous uPA to induce TF expression in Beas2B cells was next examined by overexpressing uPA and measuring TF levels. Beas2B cells transfected with a uPA cDNA construct expressed greater amounts of uPA than vector cDNA-transfected or nontransfected control cells (14). TF protein expression in the uPA-overexpressing cells was also elevated compared with vector-transfected and nontransfected cells (data not shown). These cells showed a comparable increase in TF protein expression to those cells exposed to exogenous uPA.
Induction of TF mRNA Expression by tcuPA in Lung Epithelial Cells
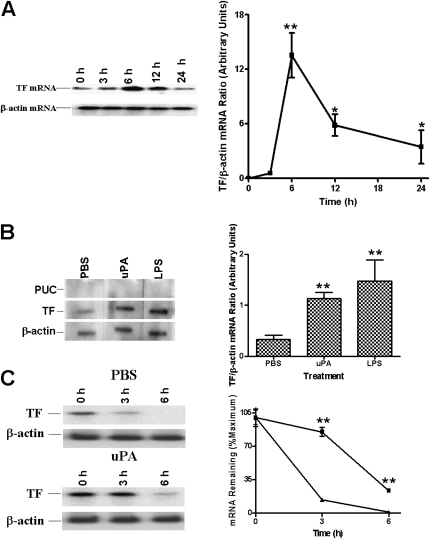
We next evaluated the possibility that the uPA-mediated increase in TF protein expression is in part mediated through an increase in TF mRNA expression. The steady-state levels of TF mRNA in tcuPA-treated Beas2B cells were measured by Northern blotting using a TF cDNA probe. As shown in Figure 2A, tcuPA induces TF mRNA in Beas2B cells, with the induction observed between 6 and 12 hours after treatment. This mRNA expression profile is consistent with that of TF protein expression by Beas2B cells. The level of TF mRNA was quantified by densitometric scanning and normalized against β-actin loading controls. As shown by the composite data, resting Beas2B cells express a small amount of TF mRNA. After addition of tcuPA, the level of TF mRNA was increased by 6 hours and remained elevated over a 12-hour time period.
Figure 2.
Regulation of tissue factor (TF) mRNA expression by two-chain urokinase-type plasminogen activator (tcuPA) in human bronchial epithelial cells (Beas2B). (A) Time-dependent induction of TF mRNA by tcuPA. The cells were treated as described in the legend to Figure 1. Total RNA (20 μg/lane) was subjected to Northern blotting using 32P-labeled TF cDNA. Same blots were stripped and probed for β-actin mRNA. The blot is representative of three separate experiments. The line graph depicts the integrated data (mean ± SD) of four individual experiments. (B) Effect of tcuPA on the rate of transcription of TF mRNA. Nuclei isolated from Beas2B cells, incubated with phosphate-buffered saline (PBS) or tcuPA for 3 hours as described in Figure 1, were subjected to the transcription reaction in the presence of [32P]UTP at 30°C for 30 minutes. 32P-labeled nuclear RNA was hybridized with TF cDNA immobilized on nitrocellulose membrane. β-actin and plasmid from the University of California cDNAs were used as positive and negative loading controls, respectively. (C) Effect of tcuPA on TF mRNA decay stability. Beas2B cells were incubated with PBS or tcuPA for 6 hours, after which 5,6-dichloro-1-β-D-ribofuranosylbenz-imidazole (20 μg/ml) was added for various periods of time. TF mRNA was analyzed by Northern blotting. Same membranes were stripped and developed for β-actin mRNA. In B and C the blots are representative of three separate experiments. The bar and line graphs show the mean and SD of integrated data from three independent experiments. The increased expression of TF mRNA is significant (*P < 0.05 or **P < 0.001) when compared with its level at 0 hours.
Nuclear run-on experiments indicated that addition of either tcuPA or LPS to Beas2B cells induced TF mRNA within 3 hours (Figure 2B). These data indicate that both tcuPA and LPS induce TF in part via transcriptional activation.
We examined whether tcuPA influenced TF mRNA stability by incubating the Beas2B cells with PBS or tcuPA for 6 hours and then inhibiting ongoing transcription by the addition of 20 μg/ml of 5,6-dichloro-1-β-D-ribofuranosylbenz-imidazole for varying lengths of time. TF mRNA was analyzed by Northern blotting using 32P-labeled TF cDNA. As shown in Figure 2C, TF mRNA expressed by PBS-treated Beas2B cells has a very short half-life (t1/2∼30 min). However, exposure to tcuPA stabilized TF mRNA, increasing its half life to more than 3 hours.
Effects of Phosphatase and Phospho-Tyrosine Kinase Inhibitors on tcuPA-mediated TF Induction
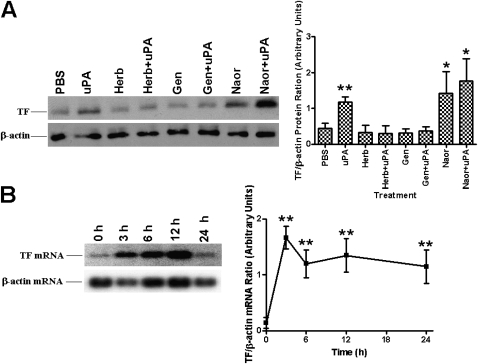
We next sought to determine if tcuPA-mediated TF expression involves one or more pathways that are implicated in tcuPA signaling (8, 14, 20, 21) and if they are the same ones that are responsible for the observed increase in mRNA stability. To address this possibility we pretreated Beas2B cells with herbimycin A and genistein separately or in combination with tcuPA. The results in Figure 3A suggest that herbimycin A and genistein alone do not induce TF expression. However, tyrosine kinase inhibitors totally reversed tcuPA-mediated TF expression in Beas2B cells. Conversely, treatment of cells with sodium orthovanadate (a tyrosine phosphatase inhibitor) alone or with tcuPA induced TF expression. To further confirm that sodium orthovanadate-induced TF expression involves induction of TF mRNA, we treated Beas2B cells with sodium orthovanadate and found a time-dependent increase in TF mRNA (Figure 3B).
Figure 3.
Effect of tyrosine kinase and phosphatase inhibitors on two-chain urokinase-type plasminogen activator (tcuPA)-mediated tissue factor (TF) expression. (A) Human bronchial epithelial cells (Beas2B) grown to confluence were treated with or without herbimycin A (Herb), genistein (Gen), and sodium orthovanadate (Naor) for 2 hours followed by tcuPA (1 μg/ml) for 24 hours at 37°C and subjected to immunoblotting with anti-TF and β-actin antibodies as described in Figure 1B. (B) Beas2B cells treated with sodium orthovanadate for 0 to 24 hours were analyzed for the expression of TF mRNA by Northern blotting. Same membranes were stripped and developed for β-actin mRNA. In A and B the blots are representative of four and three experiments, respectively, and the bar graphs illustrate the mean band densities ± SD of three independent experiments. The increase in the level of TF expression is significant (*P < 0.05 or **P < 0.001) when compared with its level in cells treated with phosphate-buffered saline or Naor at 0 hours.
Effects of uPA Fragments on TF Expression
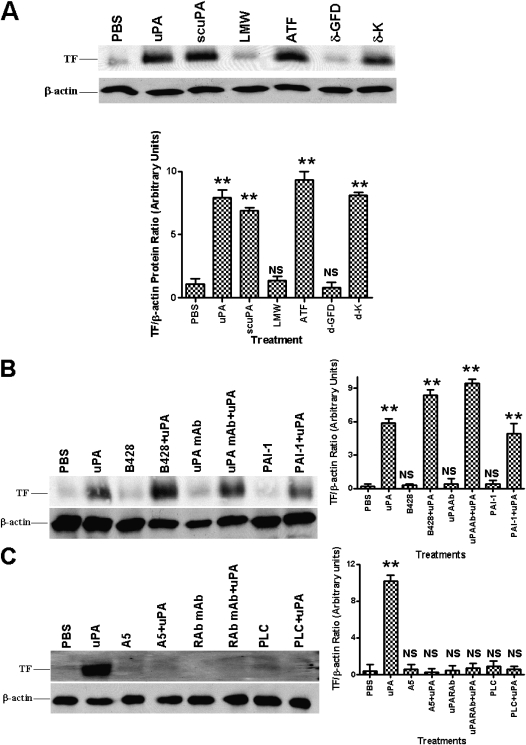
The uPA protein molecule is composed of the uPAR-binding ATF and a catalytically active LMW region. To ascertain whether either or both of these domains are required for the induction of TF expression, we incubated cells with either tcuPA, ATF, scuPA, LMW uPA, or scuPA mutants lacking the GF or the kringle domain. tcuPA, scuPA, and uPAR-binding ATF each were found to induce TF, whereas catalytically active LMW, which lacks the ability to bind to uPAR, failed to induce TF (Figure 4A). Deletion of the uPAR-binding GFD almost totally abolished the inductive effect, whereas deletion of the kringle domain failed to affect TF expression. These data suggest that uPA GFD-binding to uPAR is required for the observed induction of TF.
Figure 4.
Effect of urokinase-type plasminogen activator (uPA) enzymatic activity and its receptor interaction on tissue factor (TF) expression in human bronchial epithelial cells (Beas2B). (A) Effect of different fragments of uPA on TF expression. Beas2B cells were incubated with phosphate-buffered saline (PBS) or 1 μg/ml each of active two-chain uPA (tcuPA), single chain uPA (scuPA), the amino-terminal fragments (ATF) or low molecular weight (LMW) fragments of uPA, scuPA growth factor domain deletion (δ-GFD), or kringle domain deletion (δ-K) mutant for 24 hours. Cellular proteins (3 μg and 25 μg/lane) were immunoblotted as described above (Figure 1B) with anti-TF and anti–β-actin antibodies, respectively. The data illustrated are representative of the findings of four independent experiments. Composite densitometric analyses of the effect of deletion fragments of uPA on TF induction in Beas2B cells shown as the mean ± SD of four experiments. (B) Effect of uPA enzymatic activity on TF expression. Beas2B cells were treated with or without B-428 (0.02 mM), anti-uPA monoclonal antibody (uPA mAb) (2 μg/ml), plasminogen activator inhibitor-1 (4 μg/ml) for 2 hours followed by tcuPA (1 μg/ml) for 24 hours at 37°C in a basal media, and the cell lysates were analyzed for TF and β-actin expression as described in A. (C) Effect of uPA interaction with its receptor on TF expression. Beas2B cells were incubated with or without anti-uPAR antibody (RAb, 2 μg/ml), A5 (1 μg/ml) compound, or phosphatidylinositol-phospholipase C (10 units/ml) for 2 hours followed by uPA (1 μg/ml) for 24 hours at 37°C in basal medium. The cell lysates (3 μg and 25 μg total protein/lane) were analyzed for the expression of TF and β-actin, respectively, by Western blotting using anti-TF and β-actin antibodies. In B and C, the blots are representative of three independent experiments and bar graphs show the data from those experiments expressed as the mean ± SD. The increased level of TF expression is significant (**P < 0.001) when compared with phosphate-buffered saline (PBS)-treated cells. NS = not significant compared with PBS-treated controls.
We next evaluated the possibility that uPA enzymatic activity is necessary for inducing TF expression using three independent inhibitors of uPA activity: the small molecule inhibitor B428, an anti-uPA antibody, and PAI-1, all of which block uPA activity but not binding to uPAR (Figure 4B). In each case, uPA was preincubated with the inhibitor before the addition of the inhibitor–uPA mixture to the cells. In each of the three experiments the treated uPA retained the ability to induce TF expression in cells, whereas inhibitor by itself showed no effect. These data demonstrate that uPA catalytic activity is not required for uPA-mediated induction of TF.
To confirm the involvement of uPAR in uPA-mediated TF expression, we next preincubated Beas2B cells for 2 hours with selected agents that interfere with the uPA–uPAR interaction before exposing the cells to uPA for 24 hours. As shown in Figure 4C, preincubation of Beas2B cells with an anti-uPAR antibody blocked the induction of TF expression in response to uPA addition. This was confirmed by pretreatment of Beas2B cells with A5 peptide (an uPAR agonist), which also inhibited TF induction by uPA. In a third approach, we incubated the cells with phosphatidylinositol-phospholipase C, which almost completely removes uPAR from the cell surface (8). Phosphatidylinositol-phospholipase C treatment completely abrogated uPA-mediated TF expression. These combined observations indicate that tcuPA-mediated induction of TF involves a uPA-specific interaction with uPAR that is mediated by the uPA GFD region.
Role of Proteases and Protease Inhibitors on uPA-mediated TF Expression
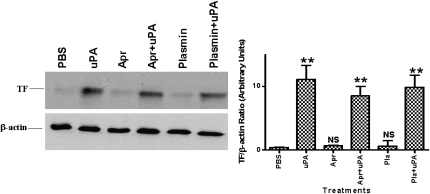
In the next series of experiments, we determined whether the induction of TF by tcuPA requires selected proteases that are expressed during remodeling of extracellular matrix. Beas2B cells were treated with uPA in the presence of aprotinin. Aprotinin alone did not induce TF expression, nor did it interfere with uPA-induced TF expression (Figure 5). Plasmin added alone or in combination with uPA likewise failed to alter TF expression.
Figure 5.
Effect of inhibitors of two-chain urokinase-type plasminogen activator (tcuPA) activity on uPA-mediated tissue factor (TF) expression by human bronchial epithelial cells (Beas2B). Beas2B cells were incubated with or without uPA, aprotinin (1 μg/ml), or plasmin (1 μg/ml) alone or in combination with uPA for 24 hours at 37°C. Total proteins from cell lysates were isolated and subjected to immunoblotting as described in Figure 4. The blots are representative of four to five independent experiments and bar graphs show the data from those experiments expressed as the mean ± SD. The increased expression of TF is significant (**P < 0.001) when compared with its level in phosphate-buffered saline (PBS)-treated cells. Apr = aprotinin; NS = not significant compared with PBS treated controls.
Role of uPA in TF Expression in Mice
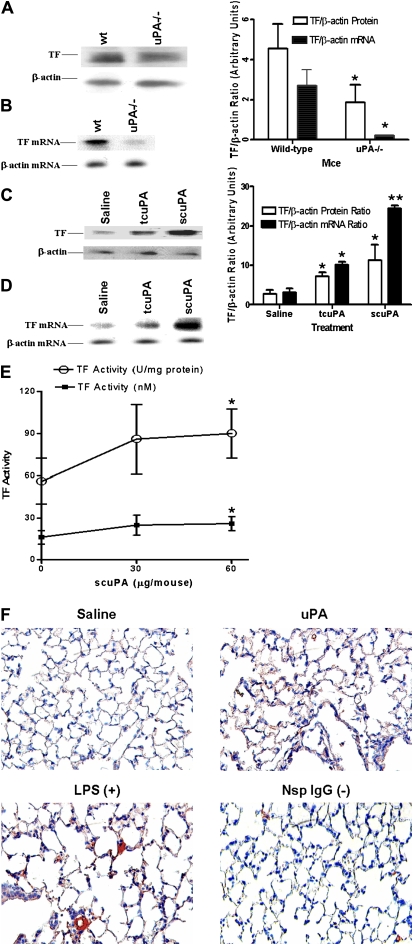
To examine the role of uPA on TF expression in vivo, we analyzed lung lysates from wt and uPA-deficient mice for TF protein and mRNA expression. As shown in Figure 6A, lung lysates from uPA-deficient mice expressed less TF protein compared with those from wt mice. TF mRNA expression was also reduced in uPA−/− mice compared with wt mice (Figure 6B). In addition, uPA-treated wt mice showed increased expression of TF protein (Figure 6C) and mRNA (Figure 6D), and TF-dependent coagulation (Figure 6E) compared with saline-treated controls. These results indicate that uPA induces TF expression in uninjured lungs in vivo.
Figure 6.
Effect of urokinase-type plasminogen activator (uPA) on tissue factor (TF) expression by lung tissues in vivo. (A) The lung lysates (20 μg/lane) of wt and uPA-deficient (uPA−/−) mice were analyzed for TF protein expression by Western blotting (three mice per group). The same membranes were stripped and analyzed for β-actin to assess equal loading. (B) Total RNA isolated from lung homogenates of wt and uPA-deficient mice were analyzed for TF mRNA (three mice per group). (C) Saline or uPA (two-chain [tc] uPA or single-chain [sc] uPA) was injected intratracheally into wt mice and the lung lysates (20 μg/ lane) were analyzed for tissue factor expression by Western blotting as described in A (three mice per group). (D) Total RNA isolated from lung homogenates of mice treated with saline or uPA were analyzed for expression of TF and β-actin mRNAs by Northern blotting. The bar represents densitometric values (mean ± SD) of three independent experiments. (E) The lung tissues of saline and scuPA-treated wt mice (n = 3–6) as described in D were lysed in Hanks' balanced salt solution and analyzed for TF activity using a single-stage clotting assay. The results are shown as nM TF and U/ml determined as described in Methods. The asterisks represent differences that are significant (*P < 0.05 and **P < 0.001) versus the saline-treated control. (F) Immunohistochemical staining for TF antigen was performed on lung tissue (5 μm) from mice exposed to saline or murine scuPA using anti-TF antibody. Plates are representative fields from three mice for each condition at 200× magnification. Lung sections from LPS-exposed mice incubated with an anti-TF antibody were used as a positive control (+) and sections incubated with nonimmune rabbit IgG are shown as the negative control (−).
TF Expression in Lung after Exposure to uPA
Lung sections from saline- and uPA-treated mice were also analyzed by IHC using an anti-TF antibody. Lung sections from wt mice exposed to intratracheal LPS were used as a positive control. Microscopic examination of stained lung sections indicated that uPA induced TF expression in both alveolar and airway epithelial cells of wt mice. In contrast, no significant increase in TF staining was observed in saline-treated wt mice (Figure 6F). Lung homogenates from uPA-treated mice express increased TF activity compared with saline-treated controls, consistent with the finding of increased TF protein expression by IHC and Western blotting.
Effect of LPS on TF Expression in uPA−/− and wt Mouse Lungs
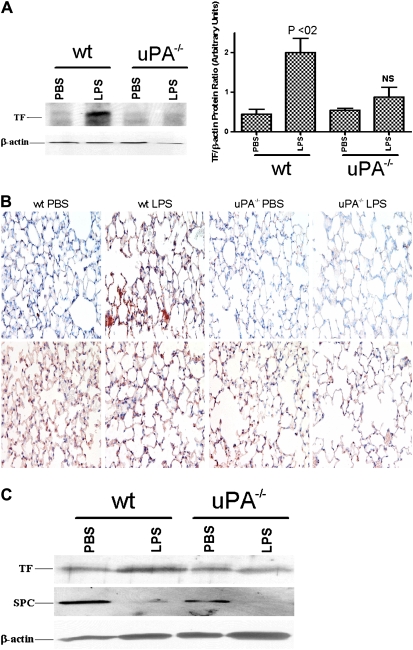
To ascertain whether uPA is crucial for TF expression during sepsis in vivo, uPA−/− mice were exposed to intratracheal LPS or saline, and the tissue homogenates were analyzed for TF protein. LPS induced TF expression in wt mice to a greater extent than was seen in saline-treated controls (P < 0.02). Minimal TF expression was detected in uPA−/− mice exposed to saline. In contrast to wt mice, LPS failed to induce TF in uPA−/− mice (Figure 7A). To confirm that uPA expression is required for TF induction in mice exposed to LPS, lung sections from the saline- or LPS-treated wt and uPA−/− mice were analyzed by IHC using an anti-TF antibody. Microscopic evaluation of randomly selected fields from the stained sections of LPS-exposed wt mice demonstrated a marked increase in TF (red) staining in airway and ATII cells as compared with wt control mice that had been given saline alone. In contrast, we found little staining for TF in uPA−/− mice that were exposed to saline or LPS (Figure 7B). These observations support the notion that uPA is crucial for expression of TF as a result of lung injury induced by LPS. To determine if ATII cells induce TF in response to LPS exposure and, if so, whether the process involves uPA expression by these cells, lung sections from wt and uPA−/− mice exposed to PBS or LPS were subjected to IHC for SPC antigens. Microscopic examination of TF and SPC-stained lung sections showed increased TF expression by ATII cells in wt mice exposed to LPS as compared with ATII cells of LPS treated uPA−/− mice. Finally, Western blot analyses of isolated ATII cells from wt and uPA−/− mice (Figure 7C) corroborated the IHC findings showing that LPS induced ATII cell TF expression only in wt mice. We found focal areas of pulmonary inflammation and alveolar edema in the lungs of WT and uPA−/− mice harvested at 24 hours after LPS, but there was no appreciable difference in the distribution of these lesions between the groups (not shown).
Figure 7.
Role of urokinase-type plasminogen activator (uPA) in LPS-induced tissue factor (TF) expression in mouse lungs. (A) Total proteins (20 μg/ lane) isolated from the lung homogenates of phosphate-buffered saline– or LPS-challenged wt or uPA−/− mice were separated by sodium dodecylsulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under nonreducing conditions and transferred to nitrocellulose membranes. The membranes were immunoblotted using an anti-TF antibody. The same membranes were later stripped and developed for β-actin to assess loading equality. LPS significantly (P < 0.02) induced TF expression in wt mice when compared with the saline-treated counterparts. In contrast, the difference in TF levels between saline- versus LPS-treated uPA−/− mice did not reach statistical significance (NS). (B) Lung sections (5 μm) from wt and uPA−/− mice exposed to saline or LPS were subjected to IHC staining for TF (upper panel) and SPC (lower panel) antigen as described in Figure 6F. Representative fields for each condition at 200× magnification are presented. (C) Alveolar type (AT)II cells isolated from the lungs of wt and uPA−/− mice exposed to PBS or LPS as described in A were lysed, and the lysates (20 μg/ lane) were separated by SDS-PAGE and transferred to nitrocellulose membranes. The membranes were immunoblotted using an anti-TF antibody. The same membranes were later stripped and developed for surfactant protein C to confirm the isolation of ATII cells and β-actin to assess loading equality.
Identification of TF mRNA-binding Protein
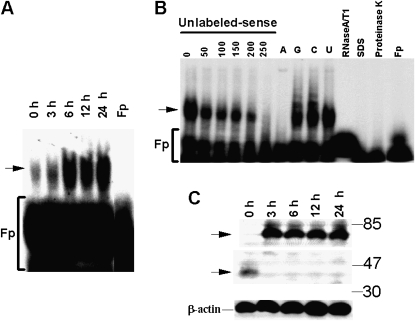
Induction of TF expression by uPA is mediated partly by slowing posttranscriptional mRNA turnover, which in general involves interaction of the mRNA with specific mRNA-binding proteins. To analyze if the stability of TF mRNA is regulated by uPA and involves interaction of specific mRNA-binding proteins with TF mRNA, Beas2B cells were incubated with uPA for 0 to 24 hours and cell lysates were tested for the ability to interact with the TF mRNA CDR and 3′UTR by gel mobility shift assay. Lysates of Beas2B cells failed to bind to 32P-labeled TF mRNA CDR (data not shown), whereas they formed a specific RNA–protein complex with TF mRNA 3′UTR. Furthermore, the TF-3′UTR mRNA–mRNA binding protein (mRNABp) interaction increased with uPA exposure (Figure 8A).
Figure 8.
Identification of tissue factor (TF) 3′untranslated region (3′UTR) mRNA binding protein by gel mobility shift assay. (A) Lysates from human bronchial epithelial cells (Beas2B) treated with urokinase-type plasminogen activator (uPA) were incubated with the 32P-labeled TF mRNA 3′UTR. The mRNA-binding protein complexes were analyzed by gel mobility shift assay. Fp = buffer alone. Arrow indicates RNA–protein complex. (B) Determination of specificity of TF mRNABp-TF mRNA 3′UTR interaction by gel mobility shift assay. Beas2B cell lysate was incubated with 32P-labeled probe TF 3′UTR mRNA in the presence of 0 to 250-fold molar excess of unlabeled sense transcript or with a 200-fold molar excess of unlabeled poly (A), poly (C), poly (G), and poly (U) polyribonucleotides. The 32P-labeled TF mRNA probe was added, and the mixtures were digested with RNase T1 and analyzed by gel mobility shift assay. Beas2B cell lysates were treated with proteinase K (2.5 mg/ml) and 0.1% sodium dodecyl sulfate (SDS) for 30 minutes at 37°C and subjected to TF 3′UTR binding by gel mobility shift assay as described above. 32P-labeled TF 3′UTR mRNA predigested with RNase A/T1 mixture before exposure to TF mRNA. Fp = probe alone. (C) Identification of TF mRNA binding protein by Northwestern assay. Lysates from Beas2B cells incubated with 1 μg/ml of uPA for 0 to 24 hours were separated on 8% SDS-polyacrylamide gel electrophoresis, transferred to nitrocellulose membranes. The membranes were later washed and exposed to X-ray film. The same membrane was stripped and analyzed for β-actin by Western blotting.
We further confirmed the specificity of TF mRNA 3′UTR–protein interaction by a series of cold competition experiments. Preincubation of cytosolic extract with a molar excess of unlabeled TF 3′UTR sense transcripts resulted in dose-dependent inhibition of 32P-labeled TF 3′UTR mRNA–mRNABp interaction (Figure 8B). Pretreatment of Beas2B cytosolic proteins with molar excess of homopoly (G), (C), or (U) ribonucleotides likewise failed to affect TF 3′UTR mRNA–mRNABp interaction, whereas addition of a molar excess of homopoly (A) ribonucleotide inhibited the binding interaction, indicating that TF mRNABp binds to specific A-rich nucleotide sequences on the TF mRNA 3′UTR. Treatment of Beas2B cytosolic protein with proteinase-K or SDS abolished its ability to interact with the TF mRNA 3′UTR. We next characterized the TF mRNA binding interaction by Northwestern blotting, as shown in Figure 8C. These analyses revealed that two protein species with approximate molecular weight of 80 kD and 37 kD interact with the TF mRNA 3′UTR. uPA enhances the binding of an 80-kD protein with TF mRNA 3′UTR, whereas it abolishes its interaction with the 37-kD protein. Although identification of these proteins is currently underway, these observations clearly suggest that uPA-mediated control of TF mRNA stabilization is tightly regulated by specific regulatory interactions with these mRNABps.
DISCUSSION
In this study, we show that uPA increases the expression of TF and TF procoagulant activity in human lung ECs. This response is germane to homeostatic control of fibrin turnover in the normal lung. TF and uPA are both induced by diverse hormones, cytokines, and growth factors. In the lung, TF is expressed at the surface of injured lung ECs, which also elaborate uPA. In the context of lung injury and its repair, this pathway may also provide a feedback mechanism to regulate the extent of fibrin accretion in the airspaces and interstitium. Extravascular fibrin serves as a provisional matrix promoting leukocyte migration, in part mediated through uPA/uPAR–integrin interactions (10) that help to confront microbial and other provocative stimuli. The fibrin matrix also supports physiological small airway and alveolar remodeling (6).
Although excess fibrinolysis can damage and weaken lung ultrastructure, excess coagulation can lead to alveolar fibrin deposition, thus resulting in stiffening of the lung and impairment of function. Excessive fibrin deposition impairs gas exchange in alveoli, as in ARDS, and promotes bronchospasm (22) and interstitial lung diseases (1, 5, 10, 23). Therefore, regulation of the delicate balance between coagulation and fibrinolysis is crucial for maintaining proper lung structure and function, and a slight alteration in this balance can dictate whether observed fibrin turnover in the alveolar compartment is homeostatic or aberrant (1, 2, 10). The balance between enzymes involved in proteolysis and coagulation is also critical in the regulation of tissue remodeling and normal angiogenesis (6, 9–11). Thus, the temporal and geographic balance and interplay between the procoagulant and fibrinolytic systems is critical for the lung to adapt to diverse forms of environmental injury. The possibility that this balance is achieved through a process that is initiated by uPA has not been previously addressed, to our knowledge. Therefore, we sought to determine whether uPA contributed to coagulation by regulating TF expression by lung epithelial cells.
uPA-dependent proteolysis contributes to tissue remodeling in inflammation (23–25). Binding of uPA to uPAR mediates proteolysis locally and thereby assists in remodeling transitional fibrin in the airspaces (10). This interaction also regulates the viability of lung ECs (14, 16), representing a potentially important mechanism that can help to restore damaged airway epithelium. Our data show that the system contributes to homeostatic fibrin turnover in the uninjured lung. Thus, the uPA-TF induction pathway we describe may provide a versatile regulatory system through which the airway epithelium might be protected from the potentially injurious effects of excessive pericellular proteolysis caused by high local concentrations of uPA in the lung microenvironment mediated by cytokines such as tumor necrosis factor-α (18, 23) and other stimuli (24). However, the key independent contributions of uPA and TF to the pathogenesis of lung injury and repair suggest that this interaction could likewise limit clearance of aberrant fibrin deposition after acute lung injury.
In the normal lung, uPA is present in BAL fluids and could induce TF, but in the absence of coagulation substrates, activation of coagulation does not occur. In the setting of ALI, uPA-mediated induction of TF could restrict excessive clearance of fibrin at the sites of injury. The concentrations of uPA we studied are biologically relevant and are within the range of those found in the BAL of healthy individuals and patients with ARDS (26–31). In fact, as the concentrations of uPA in BAL may actually be higher than reported because of dilution by saline during recovery, the effects we observed may in fact be enhanced in pathophysiological settings. In addition, diverse types of pulmonary cells may be stimulated to express uPA during sepsis and pneumonia and expression of uPAR and other receptors likely enhances the concentrations of uPA at the epithelial surface during resolution of ALI.
Multiple reports have indicated that administration of uPA mitigates bleomycin-induced lung fibrosis (32). We speculate that under such scenarios the relatively high level of exogenous uPA that is administered readily overwhelms the reciprocal effect of TF induction that occurs locally at the epithelial surface, thus favoring fibrinolysis. The inability of LPS to markedly induce TF expression in uPA−/− mice and the resistance exhibited by these mice to ALI caused by exposure to bacterial endotoxin (1, 15) demonstrate an intricate link between procoagulant and fibrinolytic pathways in vivo and potentially substantial pathophysiological significance. On the other hand, it is interesting to note that uPA-deficient mice develop worse fibrosis after bleomycin-induced injury. These contrasting responses by uPA−/− mice to acute injury induced by LPS and chronic injury caused by the profibrogenic bleomycin probably involve differences in neutrophil activation and cytokine production by macrophages in the two models (1) and are worthy of continued study.
IHC and morphometric analyses of lung sections for SPC and TF expression indicate that ATII cells are primarily responsive to uPA. Western blot analyses for TF and SPC antigens of freshly isolated ATII cells from wt and uPA-deficient mice treated with LPS further demonstrate that uPA expression is crucial for induction of TF. Potentiation of LPS-induced neutrophil activation by uPA administration and the early (within 6 h) resistance of uPA−/− mice to neutrophil influx and edema formation in the lungs (1) suggested that the differences in TF expression between uPA−/− and wt mice could have caused by reduced influx of inflammatory cells in the uPA-deficient mice. Although the present study does not exclude this possibility, induction of TF protein and mRNA by uPA in cultured ECs and comparable amounts of focal neutrophil influx, edema formation, and fibrin accumulation in LPS-treated wt or uPA-deficient mice demonstrate that it is unlikely that cytokines released from the inflammatory cells are solely responsible for the up-regulation of TF in the EC. We attribute the focal distribution of ALI we identified in this study to the low concentrations of LPS that were administered intratracheally, with assessment of the lesions 24 hours later.
Lung epithelial cells express increased levels of TF in response to cytokines implicated in the pathogenesis of ALI and its repair (31, 33). The expression of TF in lower respiratory tract fluids under control conditions and in ALI is likely to derive from multiple resident lung cell populations, including the airway and alveolar epithelium and alveolar macrophages (6, 31). Our study extends these observations and demonstrates that lung EC expression of TF is also subject to induction by uPA. uPA could therefore regulate TF expression and initiation of coagulation at sites of epithelial injury. uPA could otherwise modulate repair after ALI via TF-mediated signaling through protease activated receptors (PARs) cleaved by Xa, thrombin, and plasmin at the epithelial cell surface (34).
Identification of the signaling pathway responsible for uPA-mediated TF expression is currently in progress. Involvement of tyrosine phosphorylation in TF induction by uPA is supported by the observation that inhibition of tyrosine kinases abolishes uPA-mediated TF expression. Conversely, suppression of tyrosine phosphatase activity augments TF expression, indicating that the uPA-mediated effect could involve phosphorylation of other intermediaries, including mRNABps and/or transcription factor(s), consistent with uPA-mediated uPAR expression (17, 35–39).
LPS induces TF expression through both transcriptional and posttranscriptional mechanisms in THP-1 cells (20, 21). Transcriptional regulation of TF has been studied extensively and involves several transcription factors, including activated protein-1 (AP-1), nuclear factor (NF)-κB, and Egr-1 (20, 21, 36, 37). By contrast, the mechanisms that govern post-transcriptional regulation of TF expression have not been studied. Our findings suggest that the uPA GFD, which lacks catalytic activity, mimics tcuPA in terms of inducing TF expression. Furthermore, and unlike uPA-mediated uPA–uPAR–PAI-1 expression, induction of TF expression by uPA is mediated at both the transcriptional and post-transcriptional levels.
In previous studies, we found that a post-transcriptional pathway influences levels of uPA, uPAR, and PAI-1 mRNA in lung cancer–derived cell lines and nonmalignant lung ECs (38–40). Similar findings were previously reported in cells exposed to phorbol 12-myristate 13-acetate, insulin, insulin-like growth factor, and cyclic nucleotide analogs (40–43). Cytokines expressed in the setting of ALI or in the tumor microenvironment increase TF expression (31, 44). Previous studies have indicated that TF 3′UTR contains several AU-rich elements, including AUUUA, poly U tracks, and UUAUUUAAU, that are known to control the decay of several mRNA species (7, 45–48). Studies are in progress to determine the responsible mechanism and to identify the responsible regulatory factors involved in the induction of TF by uPA.
Based on this information, we confirmed by gel shift assay that specific mRNA binding protein(s) interact directly with TF mRNA 3′UTR to regulate its stability. Northwestern assay indicated involvement of two specific protein species, with approximate molecular weights of 80 kD and 37 kD. uPA increased the interaction of 80-kD protein with TF 3′UTR, indicating that this protein may be involved in the stabilization. Interaction of the 37-kD protein was attenuated in lung epithelial cells in a time-dependent manner after uPA exposure, indicating this protein may be involved in the destabilization of TF mRNA. Specificity and competition experiments indicate that the interaction of TF mRNABp with TF mRNA 3′UTR requires a unique A-rich sequence. Purification and characterization of the TF 3′UTR mRNA binding proteins are underway to define its role in the regulatory mechanism.
The mechanism by which tcuPA induces TF in Beas2B cells appears to be largely independent of its proteolytic activity. Inhibitors of the uPA catalytic site or its proteolytic inactivation caused little loss of TF-inducing activity. Moreover, essentially identical amounts of TF were induced by full-length single- and two-chain uPA and by a kringle domain deletion variant. In contrast, little or no TF was induced when the growth factor domain was deleted from the full-length molecule. Thus, binding of the GFD to uPAR appears to be necessary and sufficient to induce TF. This also implies that inactive uPA–PAI-1 complexes that retain interaction with uPAR could signal increased TF expression. This is important because tumor cells express high levels of uPA, uPAR, and PAI-1, and increased circulating and tissue concentrations of PAI-1 are present during severe infection and other causes of lung inflammation, accompanied by other signals that induce TF, resulting in conditions that may augment fibrin deposition by stimulating this TF-mediated pathway and thereby exacerbate lung injury.
In summary, we demonstrate that uPA stimulates expression of TF by lung ECs in culture and in vivo. This newly identified pathway is, to our knowledge, the first observation that uPA regulates the expression of components involved in coagulation in any cell type. The physiological and pathological implications of uPA-mediated TF expression will require additional study, but our data show that the pathway is operative in normal lung tissue. Induction of TF by uPA regulates fibrin turnover in the alveolar compartment of the normal lung and might contribute to local overexpression of TF associated with inflammatory lung diseases (1). Identification of the intracellular mediators of uPA-mediated TF expression may provide a new understanding of how regulation of this pathway may help to limit aberrant fibrin turnover associated with lung injury.
Acknowledgments
The authors thank Brad Low and M.B. Harish for their technical assistance.
Supported by National Heart, Lung, and Blood Institute grant P01-HL076406 and by clinical innovator award from Flight Attendant Medical Research Institute (ID-082380), HL76206 (to E.A.).
Originally Published in Press as DOI: 10.1164/rccm.200901-0015OC on March 1, 2010
Conflict of Interest Statement: S.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. Y.P.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.K.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. T.V. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. P.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. K.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.R.G. has received sponsored grants from the Department of Veterans Affairs (more than $100,000). D.B.C. has received a NIH grant ($250,000). S.I. has received advisory board fees from Kluwers (Clinical Pulmonary Medicine) ($1,001–$5,000); he has received lecture fees from Brahms ($5,001–$10,000); he has received industry-sponsored grants from Attenuon, LLC ($10,001–$50,000); he has received fees from Williams and Wilkens Publishers for CPM Editorial Board activities ($1,001–$5,000), Hodder Arnold Publishers for a book chapter (up to $1,000), and Wolters Kluwer Publishers for a book chapter (up to $1,000); he has received consultancy fees from NIH (up to $1,000), sponsored grants from NIH (more than $100,000), sponsored grants from FAMRI (more than $100,000), and sponsored grants from the U.S. Army ($10,001–$50,000). P.F.N. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. C.R. has received consultancy fees from GlaxoSmithKline (up to $1,000). A.G. has received advisory board fees from GlaxoSmithKline ($1,001–$5,000), Actelion ($1,001–$5,000), Activaero ($1,001–$5,000), and Nycomed ($10,001–$50,000); he has received lecture fees from Pfizer ($1,001–$5,000) and Actelion ($1,001–$5,000); he has received expert witness fees from Nycomed ($1,001–$5,000); he has received industry-sponsored grants from Sanofi ($10,001–$50,000). E.A. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. R.S.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Abraham E, Gyetko MR, Kuhn K, Arcaroli J, Strassheim D, Park JS, Shetty S, Idell S. Urokinase-type plasminogen activator potentiates lipopolysaccharide-induced neutrophil activation. J Immunol 2003;170:5644–5651. [DOI] [PubMed] [Google Scholar]
- 2.Julie AB, Ling W, Thomas G, Zhengming W, Kurt HA, Michael AM, Lorraine BW. The alveolar epithelium can initiate the extrinsic coagulation cascade through expression of tissue factor. Thorax 2007;62:608–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altman R, Scazziota AS, Herrera MDL, Gonzalez C. Thrombin generation by activated factor VII on platelet activated by different agonists. Extending the cell-based model of hemostasis. Thromb J 2006;4:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hattori N, Sisson TH, Xu Y, Simon RH. Upregulation of fibrinolysis by adenovirus-mediated transfer of urokinase-type plasminogen activator genes to lung cells in vitro and in vivo. Hum Gene Ther 1999;10:215–222. [DOI] [PubMed] [Google Scholar]
- 5.Chua F, Sly PD, Laurent GJ. Pediatric lung disease: from proteinases to pulmonary fibrosis. Pediatr Pulmonol 2005;39:392–401. [DOI] [PubMed] [Google Scholar]
- 6.Chapman HA. Disorders of lung matrix remodeling. J Clin Invest 2004;113:148–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dixon DA, Kaplan CD, McIntyre TM, Zimmerman GA, Prescott SM. Post-transcriptional control of cyclooxygenase-2 gene expression. The role of the 3′-untranslated region. J Biol Chem 2000;275:11750–11757. [DOI] [PubMed] [Google Scholar]
- 8.Shetty S, Pendurthi UR, Halady PK, Azghani AO, Idell S. Urokinase induces its own expression in Beas2B lung epithelial cells. Am J Physiol 2002;283:L319–L328. [DOI] [PubMed] [Google Scholar]
- 9.Versteeg HH, Schaffner F, Kerver M, Peterson HH, Ahamed J, Habermann BF, Takada Y, Mueller BM, Ruf W. Inhibition of tissue factor signaling suppresses tumor growth. Blood 2008;111:190–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Idell S, Mazar AP, Bitterman P, Mohla S, Harabin AL. Fibrin turnover in lung inflammation and neoplasia. Am J Respir Crit Care Med 2001;163:578–584. [DOI] [PubMed] [Google Scholar]
- 11.Fan Z, Larson PJ, Bognacki J, Raghunath PN, Tomaszewski JE, Kuo A, Canziani G, Chaiken I, Cines DB, Higazi AA. Tissue factor regulates plasminogen binding and activation. Blood 1998;15:91:1987–1998. [PubMed] [Google Scholar]
- 12.Shetty S, Bhandary YP, Gyetko MR, Cines DB, Idell S, Ruppert C, Guenther A, Abraham E and Shetty RS. Induction of tissue factor by urokinase in lung epithelial cells and in the lungs. ATS San Diego May 19, 2009; A4965. [DOI] [PMC free article] [PubMed]
- 13.Bdeir K, Kuo A, Mazar A, Sachais BS, Xiao W, Gawlak S, Harris S, Higazi AA, Cines DB. A region in domain ii of the urokinase receptor required for urokinase binding. J Biol Chem 2000;275:28532–28538. [DOI] [PubMed] [Google Scholar]
- 14.Shetty S, Idell S. Urokinase induces expression of its own receptor in Beas2B lung epithelial cells. J Biol Chem 2001;276:24549–24556. [DOI] [PubMed] [Google Scholar]
- 15.Bhandary YP, Velusamy T, Shetty P, Shetty RS, Idell S, Cines DB, Jain D, Bdeir K, Abraham E, Tsuruta Y, et al. Posttranscriptional regulation of uPAR expression in lipopolysaccharide-induced acute lung injury. Am J Respir Crit Care Med 2009;179:288–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shetty S, Gyetko MR, Mazar AP. Induction of p53 by urokinase in lung epithelial cells. J Biol Chem 2005;280:28133–28141. [DOI] [PubMed] [Google Scholar]
- 17.Shetty S, Muniyappa H, Halady PK, Idell S. Regulation of urokinase receptor expression by phosphoglycerate kinase. Am J Respir Cell Mol Biol 2004;31:100–106. [DOI] [PubMed] [Google Scholar]
- 18.Shetty S, Idell S. A urokinase receptor mRNA binding protein from rabbit lung fibroblasts and mesothelial cells. Am J Physiol 1998;274:L871–L882. [DOI] [PubMed] [Google Scholar]
- 19.Steinemann S, Ulevitch RJ, Mackman N. Role of the lipopolysaccharide (LPS)-binding protein/CD14 pathway in LPS induction of tissue factor expression in monocytic cells. Arterioscler Thromb 1994;14:1202–1209. [DOI] [PubMed] [Google Scholar]
- 20.Mackman N, Brand K, Edgington TS. Lipopolysaccharide-mediated transcriptional activation of the human tissue factor gene in THP-1 monocytic cells requires both activator protein 1 and nuclear factor kappa b binding sites. J Exp Med 1991;174:1517–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reddy KV, Bhattacharjee G, Schabbauer G, Hollis A, Kempf K, Tencati M, O'Connell M, Guha M, Mackman N. Dexamethasone enhances LPS induction of tissue factor expression in human monocytic cells by increasing tissue factor mRNA stability. J Leukoc Biol 2004;76:145–151. [DOI] [PubMed] [Google Scholar]
- 22.Dunkel B. Acute lung injury and acute respiratory distress syndrome in foals. Clinical Techniques in Equine Practice 2006;5:127–133. [Google Scholar]
- 23.Idell S, James KK, Coalson JJ. Fibrinolytic activity in bronchoalveolar lavage of baboons with diffuse alveolar damage: trends in two forms of lung injury. Crit Care Med 1992;20:1431–1440. [DOI] [PubMed] [Google Scholar]
- 24.Shetty S, Bdeir K, Cines DB, Idell S. Induction of plasminogen activator inhibitor-1 by urokinase in lung epithelial cells. J Biol Chem 2003;278:18124–18131. [DOI] [PubMed] [Google Scholar]
- 25.Shetty S, Padijnayayveetil J, Tucker T, Stankowska D, Idell S. The fibrinolytic system and the regulation of lung epithelial cell proteolysis, signaling, and cellular viability. Am J Physiol Lung Cell Mol Physiol 2008;295:L967–L975. [DOI] [PubMed] [Google Scholar]
- 26.Idell S, Thrall RS, Maunder R, Martin TR, McLarty J, Scott M, Starcher BC. Bronchoalveolar lavage desmosine in bleomycin-induced lung injury in marmosets and patients with adult respiratory distress syndrome. Exp Lung Res 1989;15:739–753. [DOI] [PubMed] [Google Scholar]
- 27.Idell S. Extravascular coagulation and fibrin deposition in acute lung injury. New Horiz 1994;2:566–574. [PubMed] [Google Scholar]
- 28.Idell S, Kumar A, Zwieb C, Holiday D, Koenig KB, Johnson AR. Effects of TGF-β and TNF-α on procoagulant and fibrinolytic pathways of human tracheal epithelial cells. Am J Physiol 1994;267:L693–L703. [DOI] [PubMed] [Google Scholar]
- 29.Idell S, Pueblitz S, Emri S, Gungen Y, Gray L, Kumar A, Holiday D, Koenig KB, Johnson AR. Regulation of fibrin deposition by malignant mesothelioma. Am J Pathol 1995;147:1318–1329. [PMC free article] [PubMed] [Google Scholar]
- 30.Idell S, Zwieb C, Boggaram J, Holiday D, Johnson AR, Raghu G. Mechanisms of fibrin formation and lysis by human lung fibroblasts: influence of TGF-β and TNF-α. Am J Physiol 1992;263:L487–L494. [DOI] [PubMed] [Google Scholar]
- 31.Idell S, Peterson BT, Gonzalez KK, Gray LD, Bach R, McLarty J, Fair DS. Local abnormalities of coagulation and fibrinolysis and alveolar fibrin deposition in sheep with oleic acid-induced lung injury. Am Rev Respir Dis 1988;138:1282–1294. [DOI] [PubMed] [Google Scholar]
- 32.Sisson TH, Hanson KE, Subbotina N, Patwardhan A, Hattori N, Simon RH. Inducible lung-specific urokinase expression reduces fibrosis and mortality after lung injury in mice. Am J Physiol Lung Cell Mol Physiol 2002;283:L1023–L1032. [DOI] [PubMed] [Google Scholar]
- 33.Idell S. Coagulation, fibrinolysis, and fibrin deposition in acute lung injury. Crit Care Med 2003; 31(4, Suppl)S213–S220. [DOI] [PubMed] [Google Scholar]
- 34.Rao VM, Pendurthi UR. Tissue factor–Factor VIIa signaling. Arterioscler Thromb Vasc Biol 2005;25:47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Atrash GA, Shetty S, Idell S, Xue Y, Kitson RP, Shetty PKH, Goldfarb RH. IL-2-mediated upregulation of uPA and uPAR in natural killer cells. Biochem Biophys Res Commun 2002;292:184–189. [DOI] [PubMed] [Google Scholar]
- 36.Tilley R, Mackman N. Tissue factor in hemostasis and thrombosis. Semin Thromb Hemost 2006;32:5–10. [DOI] [PubMed] [Google Scholar]
- 37.Guha M, Mackman N. The phosphatidylinositol 3-kinase-Akt pathway limits lipopolysaccharide activation of signaling pathways and expression of inflammatory mediators in human monocytic cells. J Biol Chem 2002;277:32124–32132. [DOI] [PubMed] [Google Scholar]
- 38.Shetty S, Idell S. Posttranscriptional regulation of plasminogen activator inhibitor-1 in human lung carcinoma cells in vitro. Am J Physiol Lung Cell Mol Physiol 2000;278:L148–L156. [DOI] [PubMed] [Google Scholar]
- 39.Shetty S, Idell S. Post-transcriptional regulation of urokinase mRNA. Identification of a novel urokinase mRNA-binding protein in human lung epithelial cells in vitro. J Biol Chem 2000;275:13771–13779. [DOI] [PubMed] [Google Scholar]
- 40.Shetty S, Kumar A, Idell S. Posttranscriptional regulation of urokinase receptor mRNA: Identification of a novel urokinase receptor mRNA binding protein in human mesothelioma cells. Mol Cell Biol 1997;17:1075–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fattal PG, Schneider DJ, Sobel BE, Billadello JJ. Post-transcriptional regulation of expression of plasminogen activator inhibitor type 1 mRNA by insulin and insulin-like growth factor 1. J Biol Chem 1992;267:12412–12415. [PubMed] [Google Scholar]
- 42.Heaton JH, Tillmann-Bogush M, Leff NS, Gelehrter TD. Cyclic nucleotide regulation of type-1 plasminogen activator-inhibitor mRNA stability in rat hepatoma cells. Identification of cis-acting sequences. J Biol Chem 1998;273:14261–14268. [DOI] [PubMed] [Google Scholar]
- 43.Tillmann-Bogush M, Heaton JH, Gelehrter TD. Cyclic nucleotide regulation of PAI-1 mRNA stability. Identification of cytosolic proteins that interact with an A-rich sequence. J Biol Chem 1999;274:1172–1179. [DOI] [PubMed] [Google Scholar]
- 44.Minamiy Y, Matsuzaki I, Sageshima M, Saito H, Taguchi K, Nakagawa T, Ogawa J. Expression of tissue factor mRNA and invasion of blood vessels by tumor cells in non-small cell lung cancer. Surg Today 2004;34:1–5. [DOI] [PubMed] [Google Scholar]
- 45.Caput D, Beutler B, Hartog K, Thayer R, Brown-Shimer S, Cerami A. Identification of a common nucleotide sequence in the 3′-untranslated region of mRNA molecules specifying inflammatory mediators. Proc Natl Acad Sci USA 1986;83:1670–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guhaniyogi J, Brewer G. Regulation of mRNA stability in mammalian cells. Gene 2001;265:11–23. [DOI] [PubMed] [Google Scholar]
- 47.Lewis T, Gueydan C, Huez G, Toulmé JJ, Kruys V. Mapping of a minimal AU-rich sequence required for lipopolysaccharide-induced binding of a 55-kDa protein on tumor necrosis factor-α mRNA. J Biol Chem 1998;273:13781–13786. [DOI] [PubMed] [Google Scholar]
- 48.Balsara RD, Xu Z, Ploplis VA. Targeting plasminogen activator inhibitor-1: role in cell signaling and the biology of domain-specific knock-in mice. Curr Drug Targets 2007;8:982–995. [DOI] [PubMed] [Google Scholar]