Abstract
Attention deficit/hyperactivity disorder (ADHD) is characterized by symptoms of inattention, impulsivity, and locomotor hyperactivity. Recent advances in neurobiology, imaging, and genetics have led to a greater understanding of the etiology and treatment of ADHD. Studies have found that ADHD is associated with weaker function and structure of prefrontal cortex (PFC) circuits, especially in the right hemisphere. The prefrontal association cortex plays a crucial role in regulating attention, behavior, and emotion, with the right hemisphere specialized for behavioral inhibition. The PFC is highly dependent on the correct neurochemical environment for proper function: noradrenergic stimulation of postsynaptic alpha-2A adrenoceptors and dopaminergic stimulation of D1 receptors is necessary for optimal prefrontal function. ADHD is associated with genetic changes that weaken catecholamine signaling and, in some patients, with slowed PFC maturation. Effective pharmacologic treatments for ADHD all enhance catecholamine signaling in the PFC and strengthen its regulation of attention and behavior. Recent animal studies show that therapeutic doses of stimulant medications preferentially increase norepinephrine and, to a lesser extent, dopamine, in the PFC. These doses reduce locomotor activity and improve PFC regulation of attention and behavior through enhanced catecholamine stimulation of alpha-2A and D1 receptors. These findings in animals are consistent with improved PFC function in normal human subjects and, more prominently, in patients with ADHD. Thus, a highly cohesive story is emerging regarding the etiology and treatment of ADHD.
Attention deficit/hyperactivity disorder (ADHD) is characterized by symptoms of inattention, poor impulse control, and increased motor activity.1 In the last 20 years, advances in the fields of neuroscience and genetics have provided new insights into this common disorder. We have learned how genetic alterations can affect neural circuits and lead to the symptoms of ADHD, and how correcting these alterations can lead to rational treatments. Much of the research on ADHD has pointed to weaknesses in the prefrontal cortex (PFC), the most highly evolved of the association cortices. The PFC regulates attention and behavior through its widespread connections to sensory and motor cortices, and to subcortical structures such as the basal ganglia and cerebellum. Imaging studies have demonstrated that patients with ADHD have alterations in PFC circuits and demonstrate weaker PFC activation while trying to regulate attention and behavior. The PFC requires optimal levels of norepinephrine (NE) and dopamine (DA) for proper functioning. Genetic studies have consistently noted alterations in genes involved in catecholamine transmission in patients with ADHD. All pharmacologic treatments for ADHD strengthen catecholamine signaling in the PFC and ameliorate symptoms. This article provides a brief summary of the neurobiology of ADHD.
OVERVIEW OF THE PREFRONTAL CORTEX
The PFC is highly developed in humans and consists of the cortex anterior to the motor and premotor cortices in the frontal lobe. The functions of the PFC are specialized by region. In right-handed individuals, portions of the left hemisphere are involved with the generation of language (e.g., Broca’s area), and the right hemisphere is particularly important for the regulation of attention, behavior, and emotion.2 The dorsal and lateral portions of the PFC regulate attention and motor responses, and the ventral and medial portions regulate emotion.2, 3 The PFC has extensive connections throughout the brain to orchestrate thoughts and responses4 and to provide intelligent decision making, insight, and judgment.5, 6 The PFC is essential for the so-called executive functions, allowing us to organize and plan for the future and to inhibit responses to distractions in order to achieve a goal.3 Not surprisingly, the PFC is the brain structure that is last to mature, with full maturation occurring only in late adolescence.7–9 The PFC is also especially sensitive to its neurochemical environment: like Goldilocks, it needs to have everything “just right” for proper function.10 Thus, this brain region is particularly vulnerable to environmental and genetic insults.
Regulation of Attention
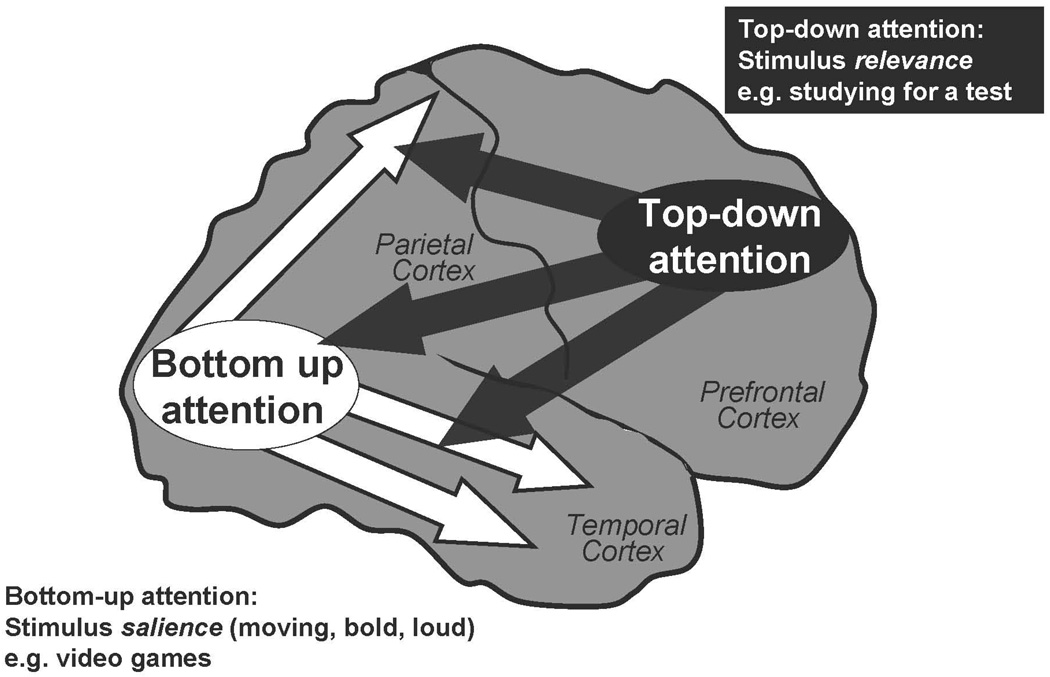
The PFC mediates “top-down” attention, regulating our attention so that we devote our resources to that which is relevant to our goals and plans.11–15 The PFC allows us to concentrate and sustain our attention, especially under “boring conditions” such as long delays between stimuli (e.g., a teacher who talks slowly).16 The PFC helps us to focus on material that is important but not inherently salient (e.g., studying for a test, reading homework) and to inhibit internal and external distractions.17–21 The PFC allows us to divide and shift our attention as appropriate with task demands (so-called multi-tasking)2, 22 and to plan and organize for the future23 As described above, many of the attentional functions of the PFC are the purview of the right hemisphere, and lesions to this hemisphere induce distractibility and poor concentration.24 The PFC accomplishes top-down attentional regulation through its extensive connections back to the sensory cortices for gating of sensory inputs (Figure 1)4, 25 The PFC is able to suppress processing of irrelevant stimuli and enhance the processing of relevant stimuli through these extensive connections.
Figure 1.
The PFC regulates “top-down” attention, allocating and directing attentional resources based on stimulus relevance. Top-down attention includes stimulus gating, reducing distractibility and sustaining attention on relevant information. These operations are thought to arise from PFC projections back to the sensory cortices. In contrast, the posterior sensory cortices mediate “bottom-up” attention, processing sensory characteristics based on stimulus salience. Most patients with ADHD have difficulties with top-down attention regulation.
Attention problems in children with ADHD are diagnosed using the Inattention scale in the Diagnostic and Statistical Manual of Mental Disorders (DSM), Fourth Edition.26 These symptoms of inattention generally refer to problems with top-down attention, as exemplified in children who are easily distracted, have difficulty sustaining attention on “boring” material but are readily captivated by more salient stimuli, e.g., they are able to attend to video games but are not able to listen to their teacher. Most children with ADHD have these problems with attention regulation. However, there are a few children who are truly unable to pay attention (usually diagnosed with attention deficit disorder [ADD], rather than ADHD), and these individuals may have problems with posterior attention systems in the parietal and temporal lobes.
The parietal and temporal sensory cortices mediate “bottom-up” aspects of attention.15, 27 These cortical systems process stimuli according to inherent salience (e.g., are the stimuli bold, loud, brightly colored, moving), rather than their relevance. Research over the last 20 years has been particularly successful in discovering how visual stimuli are processed and perceived: the ventral stream through the temporal association cortices evaluates visual features, such as lines and colors, to determine what things are.13, 28, 29 Thus, lesions to the inferior temporal cortices cause agnosias (not knowing what something is).11 In contrast, the dorsal stream culminating in the parietal cortices determines where things are, and whether they are moving.30, 31 The parietal association cortex is essential for orienting our attention,32, 33 with the right hemisphere specialized for orienting attention to parts of visual space, and the left hemisphere marshalling our attention to a point in time, e.g., if we are expecting an important event to occur.34 Lesions to the right parietal cortex induce a striking syndrome known as contralateral neglect, where patients have no conscious experience of stimuli in the left visual field.35, 36
Although most children with ADHD or ADD (attention problems without hyperactivity or impulsivity) have problems with attention consistent with PFC deficits, there are likely some children who have weakness in the parietal or temporal cortices, or both, and truly have difficulties paying attention, e.g., a child who is not engaged even by video games. Unfortunately, the term “inattention” does not distinguish between these scenarios, and the current DSM criteria are not helpful in this regard. It will be important that we create better evaluation scales in the future to discern PFC vs. posterior cortical weakness, as the optimal medications for treating PFC deficits may not be ideal for treating posterior cortical problems.
Inhibition of Inappropriate Behaviors
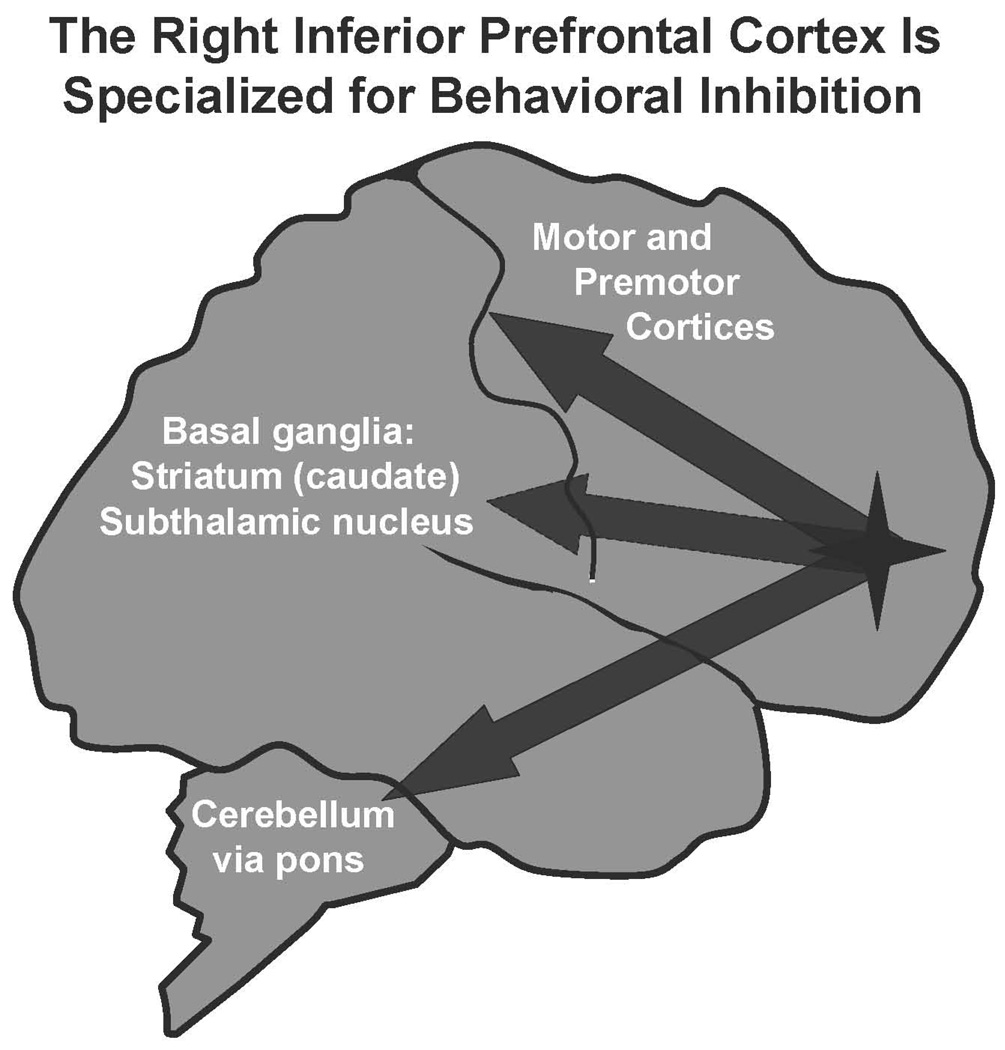
The PFC is also essential for the regulation of behavior, for planning future actions, and for the inhibition of inappropriate responses. For example, lesions to the PFC in monkeys induce locomotor hyperactivity and impulsive responding, similar to what is observed in children with ADHD.37–39 The PFC can guide behavioral output through its massive projections to the motor and premotor cortices, to basal ganglia structures such as the caudate and subthalamic nucleus, and to the cerebellum by way of the pons (Figure 2).40, 41 Thus, lesions in areas such as the caudate or cerebellum can sometimes mimic lesions in the PFC, as they are part of a circuit needed to guide behavioral response. In humans, the right inferior PFC is specialized for behavioral inhibition.42 Functional imaging studies have shown that the right inferior PFC is active when subjects successfully inhibit or stop movements2, 42, 43 Conversely, lesions or weakness to this area impairs the ability to inhibit inappropriate responses.44 A recent study45 showed that manipulations that weaken the right inferior PFC in normal subjects impaired the ability to stop an ongoing motor response (this study used a technology called transmagnetic stimulation, where magnetic pulses are directed at the brain to alter the electrical activity of the cortex beneath the skull). As described below, imaging studies have often shown that the right inferior PFC is underactive in patients with ADHD.46
Figure 2.
The PFC regulates behavior and inhibits inappropriate impulses. In humans, the right inferior PFC is specialized for behavioral inhibition. Projections from this area to the premotor and motor cortices, the basal ganglia (striatum and subthalamic nucleus), and the cerebellum (by way of the pontine nuclei) are likely involved in the inhibition of inappropriate movements and impulses. In monkeys, blockade of alpha-2A receptors in the PFC induces a pattern of impulsive responding and locomotor hyperactivity.68,69
Regulation of Emotion
Whereas the dorsal and lateral portions of the PFC regulate attention and behavior, the ventral and medial portions of the PFC regulate emotion.2, 47 The ventral surface of the PFC is often referred to as the orbital cortex, as it sits just above the orbits of the eyes. The ventromedial PFC monitors and inhibits emotions and emotional habits through extensive projections to the amygdala, hypothalamus, and nucleus accumbens, as well as to brainstem nuclei mediating the stress response.48–51 Weakness in ventromedial PFC function (especially in the right hemisphere) leads to emotional dysregulation, including disinhibited aggressive impulses.52–54 Symptoms of aggression and oppositionality (e.g., conduct disorder) are often comorbid with ADHD, particularly in boys.
Neuronal Networks Representing Goals and Rules
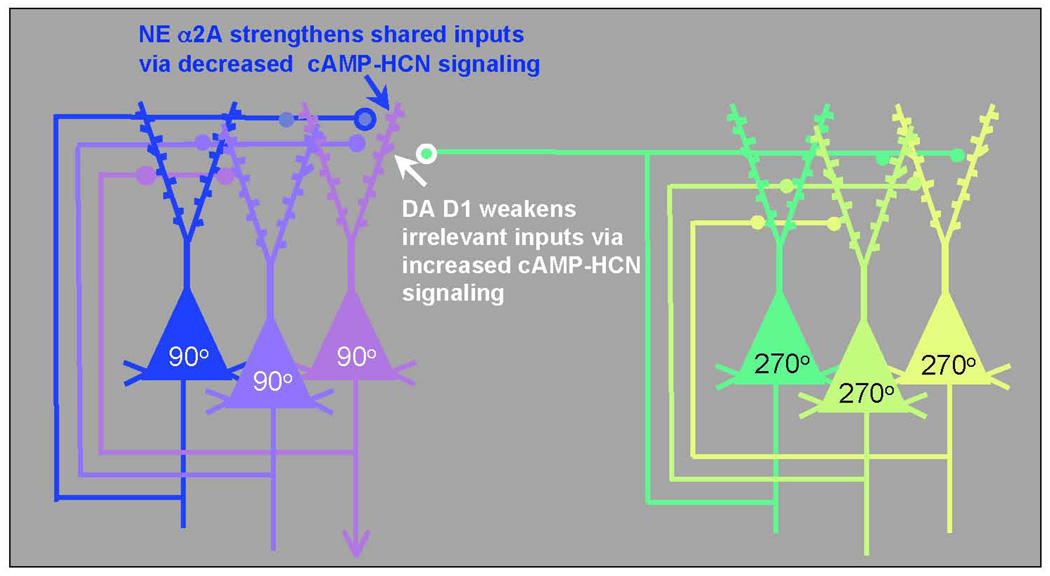
The PFC regulates attention, actions, and emotion through networks of PFC neurons. These networks consist of pyramidal cells that use glutamate as their neurotransmitter (schematically illustrated in Figure 3) and are able to excite each other to maintain firing even in the absence of environmental stimulation.55 These networks are able to “keep in mind” information to help guide attention and behavior in a thoughtful manner. For example, they can keep in mind information about where you just left a book you were reading or your reading glasses (e.g., “the book is 90° away from the couch”, as illustrated by the network of 90° cells in Figure 3). Higher-order networks appear to be able to represent goals and plans for the future (e.g., “Sit in your seat!”, “Do your homework now so you can play tonight”). The neurons in these networks interact with other pyramidal cells through synapses on dendritic spines.55 These spines contain NE alpha-2A receptors56 or DA D1 receptors,57 which dynamically alter the strength of incoming network connections and are essential to PFC function.
Figure 3.
The PFC guides attention, behavior, and emotion through networks of pyramidal cells. These pyramidal cells engage in recurrent excitation to represent stimuli (e.g., the spatial positions 90° or 270°, as shown here) or to represent goals or rules. The networks interconnect through synapses on dendritic spines that contain NE alpha-2A receptors or DA D1 receptors. Network connectivity is powerfully modulated by the catecholamines: NE alpha-2A receptor stimulation strengthens network inputs from cells with shared network properties by reducing the production of cAMP, thus closing HCN channels and enhancing synaptic inputs to the spine (increasing “signals”). Conversely, optimal levels of DA D1 receptor stimulation weaken irrelevant inputs to the neuron by increasing the production of cAMP, opening HCN channels near the synapse, and shunting incoming information (decreasing “noise”). Thus, for the network representing 90°, alpha-2A receptor stimulation increases the strength of connections from other 90° neurons, and D1 receptor stimulation weakens the connections from neurons with dissimilar characteristics (e.g., 270°). Adapted with permission from Arnsten AF 2007.10
CATECHOLAMINE MODULATION OF PREFRONTAL CORTEX
Optimal Catecholamine Levels Are Needed for Proper Function
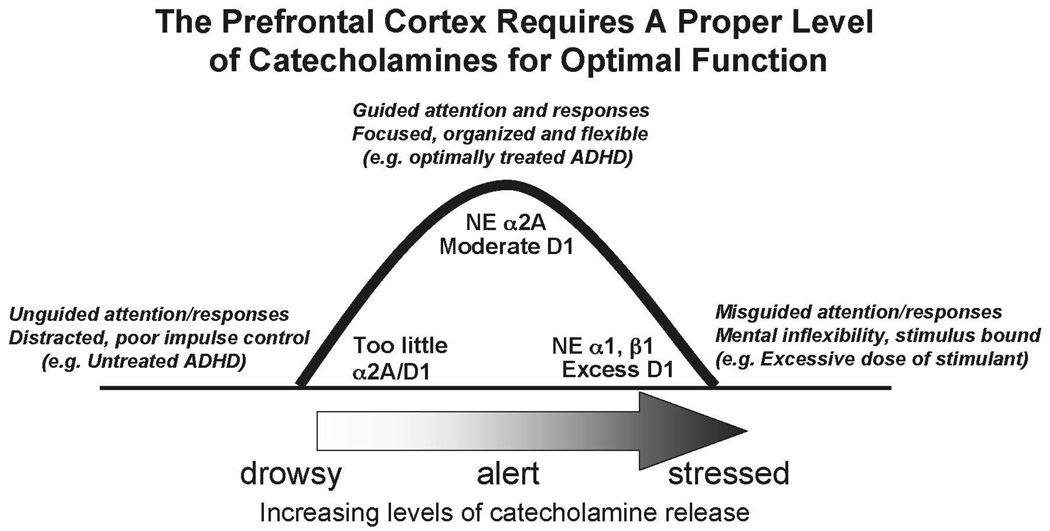
NE and DA are important components of the arousal systems that arise from the brainstem and project across the entire cortical mantle, including the PFC.58–60 The PFC requires an optimal level of NE and DA for proper function: either too little (as when we are drowsy or fatigued) or too much (as when we are stressed) markedly impairs PFC regulation of behavior and thought.10 This is often called the inverted U dose response, as illustrated in Figure 4. Indeed, NE and DA are so critical to PFC function that depleting them is as detrimental as removing the cortex itself.61 As described below, genetic and imaging studies suggest that many patients with ADHD have inadequate transmission of NE or DA, or both. Treatments for ADHD all enhance NE or DA function, or both. Thus, understanding catecholamine actions in the PFC is essential to our understanding of ADHD. The receptor and intracellular mechanisms by which NE and DA influence PFC networks have now been characterized and are summarized here. In brief, NE stimulation of alpha-2A receptors enhances PFC function by strengthening appropriate network connections (increasing “signals”), and DA stimulation of D1 receptors exerts its beneficial effects by weakening inappropriate connections (decreasing “noise”).10
Figure 4.
The regulatory functions of the PFC are highly dependent on its neurochemical state. The catecholamines NE and DA are released based on our state of arousal. Either too little or too much catecholamine release is detrimental to PFC function: there is an inverted U dose-response relationship. Inadequate catecholamine release is associated with fatigue and ADHD, and excessive catecholamine release occurs during uncontrollable stress or very high doses of stimulant medications. NE has its highest affinity for alpha-2A receptors and has lower affinity for alpha-1 and beta-1 receptors. Thus, different receptors are engaged based on the amount of NE released in the PFC. Therapeutic doses of stimulants, atomoxetine, or guanfacine likely normalize catecholamine transmission in patients with inadequate DA or NE levels, or both, thus bringing PFC function to more optimal levels at the top of the inverted U.
Role of Norepinephrine
The beneficial effects of moderate doses of NE occur at postsynaptic alpha-2A receptors on PFC neurons.56, 62, 63 Research on alpha-2 actions conducted in the 1970s focused on presynaptic alpha-2 receptors on NE cells and terminals that serve as negative feedback to reduce NE cell firing and NE release.64 However, it is now known that the majority of alpha-2 receptors in the brain are actually postsynaptic to NE cells,65 situated, for example, on the dendritic spines of PFC pyramidal cells.56 There are 3 subtypes of alpha-2 receptors: the A, B, and C subtypes,66 and it is the A subtype that is most important to NE’s beneficial actions in the PFC.67
NE alpha-2A receptor stimulation improves PFC regulation of attention, behavior, and emotion by strengthening network connections between neurons with shared inputs.56 This is illustrated in Figure 3, which shows that stimulation of alpha-2A receptors on the spines of a 90° neuron increases the strength of inputs from other neurons that respond to 90°. Thus, alpha-2A receptor stimulation increases “signals” within PFC networks. Alpha-2A receptor stimulation strengthens network connections by closing “leaky” ion channels near the synapses on dendritic spines. These hyperpolarization-activated cyclic nucleotide-gated (HCN) ion channels pass both sodium and potassium when they are opened by cyclic adenosine monophosphate (cAMP), thus shunting nearby inputs. Stimulation of alpha-2A receptors near the HCN channels stops the production of cAMP, closing the channels and increasing the strength of nearby synaptic inputs.56
Stimulation of alpha-2A receptors is essential to PFC function, and blockade of these receptors with yohimbine induces a profile similar to ADHD. In monkeys, infusion of yohimbine directly into the PFC increases locomotor hyperactivity68 and impulsivity,69 similar to lesions of the same area (Figure 2). Infusion of yohimbine in to prefrontal cortex also weakens working regulation of memory and attention.70 Conversely, stimulation of alpha-2A receptors with guanfacine lessens distractibility and strengthens behavioral regulation.56, 67, 71–75 Thus, conditions that lead to inadequate NE stimulation of alpha-2A receptors (including genetic insults in ADHD, as described below) lead to marked PFC dysfunction.
In contrast to the essential effects of moderate levels of NE, high levels of NE, such as those occurring during stress or excessive stimulant doses, impair PFC function.76 These detrimental actions occur through engagement of alpha-1 receptors (and possibly beta-1 receptors) that have lower affinity for NE.77–79 Stimulation of alpha-1 receptors impairs PFC function by engaging the phosphotidyl inositol intracellular signaling pathway,80 the pathway that is altered in bipolar disorder.81, 82 Overactivation of this pathway suppresses PFC cell firing and markedly impairs PFC function.80
Role of Dopamine
As with NE, DA is essential to PFC function.83 DA acts at the D1 family of receptors (D1 and D5) and the D2 family of receptors (D2, D3, D4). Studies of DA actions at the D2 family are just emerging. D2 receptors appear to modulate response-related firing of PFC neurons,84 and D4 receptors are concentrated on gamma aminobutyric acid (GABA)-ergic interneurons.85 D4 receptor stimulation appears to suppress these inhibitory GABAergic interneurons and thus allow pyramidal neurons to fire.86 Genetic weakness in the D4 receptor (e.g., the 7-repeat that is more common in ADHD) should lead to excessive GABAergic inhibition and inadequate activity of PFC pyramidal cells. It is important to note that the D4 receptor can be stimulated by both NE and DA, and that NE has higher affinity for D4 receptors than for adrenoceptors.87 Thus, medications that increase NE availability likely influence D4 receptor transmission. However, relatively little research has been done on D4 receptor actions in PFC, and they likely have more complex actions than described here. Instead, most research has focused on the D1 family of receptors, as these are most abundant in the PFC 88. Currently, no drugs distinguish D1 from D5 receptors; thus, it should be understood that reference to D1 in this review could apply to actions at either of these receptors.
Moderate levels of DA D1 receptor stimulation improve PFC functions by decreasing “noise”.89 D1 receptors appear to be on a different set of spines than alpha-2A receptors; the D1 receptors appear to gate incoming inputs, screening out those that are irrelevant to the present task demands.10 This is schematically illustrated in Figure 3. D1 receptor stimulation prevents inputs from the 270° neurons from entering the 90° cell. D1 receptors weaken irrelevant inputs to the neuron by increasing the production of cAMP, opening HCN channels near the synapse and shunting the incoming information. Thus, DA and NE have complementary beneficial actions.
However, excessive D1 receptor stimulation (such as occurs during stress) impairs PFC function by weakening too many network connections. Under these conditions, network activity collapses, and responding becomes inflexible.89 This may explain the problems with mental flexibility when children take excessive doses of stimulant medication.
ALTERED PREFRONTAL AND CATECHOLAMINE FUNCTION IN ADHD
ADHD and Deficits in PFC Function
Patients with ADHD have symptoms similar to those caused by lesions to the right PFC.44, 90–92 Imaging studies have shown reduced size and reduced functional activity of the right PFC in patients with ADHD.46, 93–97 Recent studies have also reported more disorganized white matter tracks emanating from the PFC in patients with ADHD, consistent with weaker prefrontal connectivity.98, 99 Other brain regions connected to the PFC, e.g., the caudate and cerebellum, have also been reported to be smaller in some studies of children with ADHD.100 There is also evidence of slower prefrontal maturation in some patients with ADHD.101 However, for many patients, ADHD is a lifelong disorder, as supported by results from imaging studies showing evidence of weakened PFC function and reduced right PFC volume in adults with ADHD symptoms.102, 103 Supporting the notion of ADHD as a highly heritable disorder are imaging studies showing disruptions in prefrontal white matter tracts in both parents and their children when both have ADHD.98
Genetic Changes in Catecholamine Transmission
As is typical in mental illness, multiple genes contribute a small risk to ADHD symptomology.104 Many studies report alterations in the genes encoding for molecules involved in catecholamine signaling, e.g., the DA D1 and D5 receptors,105–108 the DA and NE transporters,105, 108–110 the D4 receptor,106, 107, 111 the alpha-2A receptor,112–114 and dopamine beta hydroxylase (the enzyme needed for the synthesis of NE).105, 115, 116 There are also associations with the catabolic enzyme, monoamine oxidase, and some serotonergic genes.104 Recent studies have begun to relate genotype to symptomology. For example, genetic variation in the gene encoding for dopamine beta hydroxylase is related to executive function and the ability to sustain attention.117, 118 Thus, patients with two copies of the Taq I polymorphism in ADHD have poorer sustained attention.117 These studies suggest that weaker NE production may impair the PFC circuits mediating the regulation of attention and behavior.
Imaging Studies Show Changes in Catecholamine Transmission
Neuroreceptor imaging also supports weakened catecholamine transmission in ADHD. These studies have all been done in adults with ADHD, given the necessity of using radioactive tracers in positron emission tomography or single photon emission computed tomography. The vast majority of this work has focused on DA mechanisms in the striatum, as there are currently no good tracers to image NE or DA levels in the cortex. There have been mixed results with studies of the DA transporter, with many studies showing increased levels in the striatum,119–121 but other studies found no effect122 or reported decreases,123 possibly reflecting genetic heterogeneity in the DA transporter gene. Recent imaging studies have assessed DA release in the striatum and found evidence of decreased DA release in adult patients with ADHD.124 It is likely that this reflects global reductions in DA release throughout the brain, as earlier studies have suggested reduced catecholamine levels in the PFC as well.125 Reduced DA in the striatum is associated with slowed motor activity, as in Parkinsonism,126 and reduced DA in the PFC produces locomotor hyperactivity in animals.127 Such findings suggest that it is the loss of catecholamines in the PFC that is most important for ADHD symptoms.
ADHD TREATMENTS AND THE NORMALIZATION OF CATECHOLAMINE TRANSMISSION
Therapeutic doses of either stimulant or nonstimulant medications potentiate catecholamine transmission in the PFC. Thus, these agents would normalize catecholamine transmission in patients with genetic abnormalities in these pathways.
Stimulants
The stimulants amphetamine (Adderall® [amphetamine], Vyvanse™ [lisdexamphetamine dimesylate], Shire US Inc., Wayne, Penn.) and methylphenidate (Ritalin®[methylphenidate], Novartis Pharmaceuticals, East Hanover, NJ; Concerta® [methylphenidate extended release], McNeil Pediatrics, Ft. Washington, Penn.) block both catecholamine transporters, the transporter for DA and that for NE. Because there are low levels of DA transporters in the PFC, NE transporters thus clear both NE and DA in this brain region.128 Previous biochemical studies of amphetamine and methylphenidate in rodents used excessively high doses that increased locomotor activity, impaired PFC function, and had sensitizing effects on pathways involved with, for example, drug abuse.129 Recently, more appropriate, lower doses have been identified which produce blood levels in rats similar to those observed in patients with ADHD who are treated with stimulant medication.130, 131 These therapeutic doses of stimulants reduce locomotor activity and improve PFC cognitive function in rats just as they do in humans.130–132 Biochemical analyses of these more relevant stimulant doses revealed that they substantially increase both DA and NE release in the PFC but have little effect on catecholamine levels in subcortical areas.131 These data are consistent with those showing that therapeutic doses of stimulants incur little abuse potential when taken properly. In the rat PFC, therapeutic doses of stimulants increase NE release more than they increase DA release,131 thus, it is inaccurate to refer to these agents as simply dopaminergic. Consistent with dual actions on both NE and DA, the cognitive-enhancing effects of these agents in rodents are blocked by either NE alpha-2 or DA D1 receptor antagonists.132 However, higher doses of stimulants impair function of the PFC and induce an inflexible pattern of responding similar to that seen following uncontrollable stress.131, 132 These findings with high doses of methylphenidate are likely relevant to the cognitive inflexibility that can occur with excessive doses of stimulant medication.133
Therapeutic doses of stimulants improve PFC functions and enhance the efficiency of PFC activity in normal, young adult subjects.134, 135 A similar, but much more pronounced profile is observed in subjects with ADHD.136–139 Thus, stimulant actions in ADHD are not paradoxical, but simply more apparent.134, 135
Nonstimulants
Atomoxetine
Atomoxetine (Strattera® [atomoxetine], Eli Lilly, Indianapolis, Ind.) selectively blocks the NE transporter. Administration of atomoxetine increases both NE and DA in the rat PFC,140 indicating the importance of the NE transporter for clearing DA as well as NE in the PFC. Preliminary data indicate that moderate doses of atomoxetine, as with methylphenidate, improve PFC functions through both NE alpha-2 and DA D1 actions, and higher doses can impair PFC function in some animals (Arnsten, unpublished). Recent studies in humans have shown that therapeutic doses of atomoxetine can strengthen response inhibition in normal controls141 as well as in patients with ADHD.142 The therapeutic effects of atomoxetine are consistent with results of previous studies showing that desipramine, a tricyclic antidepressant with high selectivity for the NE transporter, is helpful in treating ADHD-related symptoms, although it has cardiovascular side effects.143, 144
Guanfacine
Guanfacine acts directly at postsynaptic, alpha-2A receptors in the PFC, where it mimics the beneficial effects of NE and strengthens PFC regulation of attention and behavior.56 Animal studies have shown that guanfacine improves a wide range of PFC functions.71, 72, 74, 145–147 As described above, guanfacine improves PFC functions by inhibiting cAMP-HCN channel signaling in dendritic spines, thus strengthening synaptic inputs onto pyramidal neurons and strengthening PFC network connectivity.56 The beneficial effects of guanfacine on PFC function are independent of the drug’s sedating actions,71, 90 which likely occur at all 3 alpha-2 receptor subtypes (the A, B, and C subtypes). For example, the thalamus is rich in alpha-2B receptors,66 and this structure is key for regulating state of arousal,148 The sedating actions of alpha-2 agonists also likely occur at presynaptic alpha-2A receptors on NE cell bodies and terminals; guanfacine has relatively lower affinity for these presynaptic receptors.149 Guanfacine is currently used in both children and adults with ADHD. It has been shown to improve ratings on both the Inattention and Hyperactivity/Impulsivity scales, consistent with its widespread beneficial effects on many PFC functions.150–152 It is especially helpful in patients who cannot take stimulant medications because of tics, aggressive impulses, or drug abuse liability.150 As with the stimulants, guanfacine also can improve normal subjects,153, 154 but it is far more effective in individuals with impaired prefrontal abilities and inadequate catecholamine function.71, 90 In view of the fact that it works directly at the receptor to mimic NE, it can be used in subjects having marked catecholamine depletion, as an intact catecholamine system is not required for its actions.
Clonidine
Clonidine has a very rapid onset of action that can be helpful in treating emergent situations. However, it has significant sedative and hypotensive actions that limit its clinical utility.155, 156 Clonidine is less selective than guanfacine for the alpha-2A receptor. It has high affinity for the alpha-2B and alpha-2C subtypes as well as the alpha-2A receptor,157, 158 and it also has high affinity for imidazoline I1 receptors.159 Clonidine has potent actions at presynaptic alpha-2A receptors, being 10 times more effective than guanfacine at these sites.149 This nonselective profile and potent presynaptic actions likely contribute to clonidine’s potent sedating effects. In addition, clonidine’s actions at imidazoline I1 receptors in the brainstem are thought to contribute to its marked hypotensive actions.159, 160
In summary, successful pharmacological treatments for ADHD mimic or enhance the beneficial effects of catecholamines on PFC function.161
DISCUSSION
Over the last 20 years, our understanding of higher cortical function has evolved so that we can now begin to explain the etiology and treatment of ADHD. We have learned that the PFC plays a crucial role in regulating attention, behavior, and emotion. Weaknesses in PFC structure and function, including alterations in catecholamine transmission, likely contribute to the etiology of ADHD symptoms. Effective treatments for ADHD optimize catecholamine signaling in the PFC and normalize PFC regulation of attention and behavior, thus reducing ADHD symptoms.
Abbreviations
- ADD
Attention deficit disorder
- ADHD
Attention deficit/hyperactivity disorder
- cAMP
cyclic Adenosine MonoPhosphate
- DA
Dopamine
- GABA
Gamma aminobutyric acid
- HCN
Hyperpolarization-activated Cyclic Nucleotide-gated
- NE
Norepinephrine
- PFC
Prefrontal cortex
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Disclosure
Amy F.T. Arnsten, PhD has received consulting fees from Shire Pharmaceuticals, Inc, has contracted research with Shire Pharmaceuticals, Inc, and has a license agreement with Marinus Pharmaceuticals, Inc and Shire Pharmaceuticals, Inc.
References
- 1.Solanto MV. Attention-Deficit/Hyperactivity Disorder: Clinical features. In: Solanto MV, Arnsten AFT, Castellanos FX, editors. Stimulant Drugs and ADHD: Basic and Clinical Neuroscience. New York: Oxford University Press; 2001. pp. 3–30. [Google Scholar]
- 2.Robbins TW. Shifting and stopping: fronto-striatal substrates, neurochemical modulation and clinical implications. Philos Trans R Soc Lond B Biol Sci. 2007;362:917–932. doi: 10.1098/rstb.2007.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stuss DT, Knight RT, editors. New York: Oxford University Press; 2002. Principles of Frontal Lobe Function. [Google Scholar]
- 4.Goldman-Rakic PS. Circuitry of the primate prefrontal cortex and the regulation of behavior by representational memory. In: Plum F, editor. Handbook of Physiology, The Nervous System, Higher Functions of the Brain. Bethesda: American Physiological Society; 1987. pp. 373–417. [Google Scholar]
- 5.Knight RT, Staines WR, Swick D, Chao LL. Prefrontal cortex regulates inhibition and excitation in distributed neural networks. Acta Psychologica. 1999;101:159–178. doi: 10.1016/s0001-6918(99)00004-9. [DOI] [PubMed] [Google Scholar]
- 6.Bunge SA, Kahn I, Wallis JD, Miller EK, Wagner AD. Neural circuits subserving the retrieval and maintenance of abstract rules. J Neurophysiology. 2003;90:3419–3428. doi: 10.1152/jn.00910.2002. [DOI] [PubMed] [Google Scholar]
- 7.Goldman-Rakic PS. Development of cortical circuitry and cognitive function. Child Development. 1987;58:601–622. [PubMed] [Google Scholar]
- 8.Rakic P. The development of the frontal lobe. A view from the rear of the brain. Advances in Neurology. 1995;66:1–8. [PubMed] [Google Scholar]
- 9.Lewis DA. Development of the prefrontal cortex during adolescence: Insights into vulnerable neural circuits in schizophrenia. Neuropsychopharmacology. 1997;16:385–398. doi: 10.1016/S0893-133X(96)00277-1. [DOI] [PubMed] [Google Scholar]
- 10.Arnsten AF. Catecholamine and second messenger influences on prefrontal cortical networks of ″representational knowledge″: a rational bridge between genetics and the symptoms of mental illness. Cerebral Cortex. 2007;17(Suppl 1):i6–i15. doi: 10.1093/cercor/bhm033. [DOI] [PubMed] [Google Scholar]
- 11.Mesulam MM. From sensation to cognition. Brain. 1998;121:1013–1052. doi: 10.1093/brain/121.6.1013. [DOI] [PubMed] [Google Scholar]
- 12.Knight RT, Grabowecky MF, Scabini D. Role of human prefrontal cortex in attention control. Adv Neurol. 1995;66:21–34. [PubMed] [Google Scholar]
- 13.Desimone R. Visual attention mediated by biased competition in extrastriate visual cortex. Philos Trans R Soc Lond B Biol Sci. 1998;353:1245–1255. doi: 10.1098/rstb.1998.0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gazzaley A, Rissman J, Cooney J, Rutman A, Seibert T, Clapp W, et al. Functional interactions between prefrontal and visual association cortex contribute to top-down modulation of visual processing. Cereb Cortex. 2007;17(sp 1):i125–i135. doi: 10.1093/cercor/bhm113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buschman TJ, Miller EK. Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science. 2007;315:1860–1862. doi: 10.1126/science.1138071. [DOI] [PubMed] [Google Scholar]
- 16.Wilkins AJ, Shallice T, McCarthy R. Frontal lesions and sustained attention. Neuropsychologia. 1987;25:359–365. doi: 10.1016/0028-3932(87)90024-8. [DOI] [PubMed] [Google Scholar]
- 17.Bartus RT, Levere TE. Frontal decortication in rhesus monkeys: A test of the interference hypothesis. Brain Res. 1977;119:233–248. doi: 10.1016/0006-8993(77)90103-2. [DOI] [PubMed] [Google Scholar]
- 18.Knight RT, Scabini D, Woods DL. Prefrontal cortex gating of auditory transmission in humans. Brain Res. 1989;504:338–342. doi: 10.1016/0006-8993(89)91381-4. [DOI] [PubMed] [Google Scholar]
- 19.Chao LL, Knight RT. Human prefrontal lesions increase distractibility to irrelevant sensory inputs. Neuroreport. 1995;6:1605–1610. doi: 10.1097/00001756-199508000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Bunge SA, Ochsner KN, Desmond JE, Glover GH, Gabrieli JD. Prefrontal regions involved in keeping information in and out of mind. Brain. 2001;124:2074–2086. doi: 10.1093/brain/124.10.2074. [DOI] [PubMed] [Google Scholar]
- 21.Moore T, Armstrong KM. Selective gating of visual signals by microstimulation of frontal cortex. Nature. 2003;421:370–373. doi: 10.1038/nature01341. [DOI] [PubMed] [Google Scholar]
- 22.Godefroy O, Rousseaux M. Divided and focused attention in patients with lesion of the prefrontal cortex. Brain and Cognition. 1996;30:155–174. doi: 10.1006/brcg.1996.0010. [DOI] [PubMed] [Google Scholar]
- 23.Manes F, Sahakian BJ, Clark L, Rogers R, Antoun N, Aitken M, et al. Decision-making processes following damage to the prefrontal cortex. Brain. 2002;125:624–639. doi: 10.1093/brain/awf049. [DOI] [PubMed] [Google Scholar]
- 24.Woods DL, Knight RT. Electrophysiological evidence of increased distractability after dorsolateral prefrontal lesions. Neurology. 1986;36:212–216. doi: 10.1212/wnl.36.2.212. [DOI] [PubMed] [Google Scholar]
- 25.Barbas H, Medalla M, Alade O, Suski J, Zikopoulos B, Lera P. Relationship of prefrontal connections to inhibitory systems in superior temporal areas in the rhesus monkey. Cereb Cortex. 2005;15:1356–1370. doi: 10.1093/cercor/bhi018. [DOI] [PubMed] [Google Scholar]
- 26.4th edition. American Psychiatric Association; DSM I. Diagnostic and statistical manual of mental disorders. [Google Scholar]
- 27.Knudsen EI. Fundamental components of attention. Annu Rev Neurosci. 2007;30:57–78. doi: 10.1146/annurev.neuro.30.051606.094256. [DOI] [PubMed] [Google Scholar]
- 28.Desimone R, Albright TD, Gross CG, Bruce C. Stimulus-selective properties of inferior temporal neurons in the macaque. J Neurosci. 1984;4:2051–2062. doi: 10.1523/JNEUROSCI.04-08-02051.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ungerleider LG. The corticocortical pathways for object recognition and spatial perception. In: Chagas C, Gattass R, Gross C, editors. Pattern Recognition Mechanisms. Vatican City: The Pontifical Academy of Sciences; 1985. pp. 21–37. [Google Scholar]
- 30.Snyder LH, Grieve KL, Brotchie P, Andersen RA. Separate body-and world-referenced representations of visual space in parietal cortex. Nature. 1998;394:887–891. doi: 10.1038/29777. [DOI] [PubMed] [Google Scholar]
- 31.Rudolph K, Pasternak T. Transient and permanent deficits in motion perception after lesions of cortical areas MT and MST in the macaque monkey. Cereb Cortex. 1999;9:90–100. doi: 10.1093/cercor/9.1.90. [DOI] [PubMed] [Google Scholar]
- 32.Posner MI, Walker JA, Friedrich FJ, Rafal RD. Effects of parietal injury on covert orienting of visual attention. J. Neurosci. 1984;4:1863–1874. doi: 10.1523/JNEUROSCI.04-07-01863.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crowe DA, Chafee MV, Averbeck BB, Georgopoulos AP. Neural activity in primate parietal area 7a related to spatial analysis of visual mazes. Cereb Cortex. 2004;14:23–34. doi: 10.1093/cercor/bhg088. [DOI] [PubMed] [Google Scholar]
- 34.Coull JT, Nobre AC. Where and when to pay attention: The neural systems for directing attention to spatial locations and to time intervals as revealed by both PET and fMRI. J. Neuroscience. 1998;18:7426–7435. doi: 10.1523/JNEUROSCI.18-18-07426.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mesulam MM. A cortical network for directed attention and unilateral neglect. Ann Neurol. 1981;10:309–325. doi: 10.1002/ana.410100402. [DOI] [PubMed] [Google Scholar]
- 36.Ghacibeh GA, Shenker JI, Winter KH, Triggs WJ, Heilman KM. Dissociation of neglect subtypes with transcranial magnetic stimulation. Neurology. 2007;69:1122–1127. doi: 10.1212/01.wnl.0000276950.77470.50. [DOI] [PubMed] [Google Scholar]
- 37.Gross CG. Locomotor activity following lateral frontal lesions in rhesus monkeys. J Comp Physiol Psychol. 1963;56:232–236. doi: 10.1037/h0048041. [DOI] [PubMed] [Google Scholar]
- 38.French GM. Locomotor effects of regional ablation of frontal cortex in rhesus monkeys. J Comp Physiol Psychol. 1959;52:18–24. doi: 10.1037/h0042491. [DOI] [PubMed] [Google Scholar]
- 39.Petrides M. The effect of periarcuate lesions in the monkey on the performance of symmetrically and asymmetrically reinforced visual and auditory go, no-go tasks. J. Neuroscience. 1986;6:2054–2063. doi: 10.1523/JNEUROSCI.06-07-02054.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goldman-Rakic PS, Bates JF, Chafee MV. The prefrontal cortex and internally generated motor acts. Current Opinion in Neurobiology. 1992;2:830–835. doi: 10.1016/0959-4388(92)90141-7. [DOI] [PubMed] [Google Scholar]
- 41.Middleton FA, Strick PL. Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Research-Brain Research Reviews. 2000;31:236–250. doi: 10.1016/s0165-0173(99)00040-5. [DOI] [PubMed] [Google Scholar]
- 42.Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 43.Rubia K, Russell T, Overmeyer S, Brammer MJ, Bullmore ET, Sharma T, et al. Mapping motor inhibition: conjunctive brain activations across different versions of go/no-go and stop tasks. Neuroimage. 2001;13:250–261. doi: 10.1006/nimg.2000.0685. [DOI] [PubMed] [Google Scholar]
- 44.Clark L, Blackwell AD, Aron AR, Turner DC, Dowson J, Robbins TW, et al. Association between response inhibition and working memory in adult ADHD: a link to right frontal cortex pathology? Biol Psychiatry. 2007;61:1395–1401. doi: 10.1016/j.biopsych.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 45.Chambers CD, Bellgrove MA, Stokes MG, Henderson TR, Garavan H, Robertson IH, et al. Executive ″brake failure″ following deactivation of human frontal lobe. Journal of Cognitive Neuroscience. 2006;18:444–455. doi: 10.1162/089892906775990606. [DOI] [PubMed] [Google Scholar]
- 46.Rubia K, Overmeyer S, Taylor E, Brammer M, Williams SCR, Simmons A, et al. Hypofrontality in Attention Deficit Hyperactivity Disorder during higher-order motor control: A study with functional MRI. Am. J. Psychiatry. 1999;156:891–896. doi: 10.1176/ajp.156.6.891. [DOI] [PubMed] [Google Scholar]
- 47.Dias R, Roberts A, Robbins TW. Dissociation in prefrontal cortex of affective and attentional shifts. Nature. 1996;380:69–72. doi: 10.1038/380069a0. [DOI] [PubMed] [Google Scholar]
- 48.Arnsten AFT, Goldman-Rakic PS. Selective prefrontal cortical projections to the region of the locus coeruleus and raphe nuclei in the rhesus monkey. Brain Res. 1984;306:9–18. doi: 10.1016/0006-8993(84)90351-2. [DOI] [PubMed] [Google Scholar]
- 49.Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Ann. Rev. Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- 50.Floyd NS, Price JL, Ferry AT, Keay KA, Bandler R. Orbitomedial prefrontal cortical projections to distinct longitudinal columns of the periaqueductal gray in the rat. J. Comp. Neurol. 2000;422:556–578. doi: 10.1002/1096-9861(20000710)422:4<556::aid-cne6>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 51.Ghashghaei HT, Barbas H. Pathways for emotion: interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience. 2002;115:1261–1279. doi: 10.1016/s0306-4522(02)00446-3. [DOI] [PubMed] [Google Scholar]
- 52.Stuss DT, Gow CA, Hetherington CR. ″No longer Gage″: Frontal lobe dysfunction and emotional changes. J. Consult. Clin. Psychol. 1992;60(3):349–359. doi: 10.1037//0022-006x.60.3.349. [DOI] [PubMed] [Google Scholar]
- 53.Anderson SW, Bechara A, Damasio H, Tranel D, Damasio AR. Impairment of social and moral behavior related to early damage in human prefrontal cortex. Nature Neuroscience. 1999;2:1032–1037. doi: 10.1038/14833. [DOI] [PubMed] [Google Scholar]
- 54.Davidson RJ, Putnam KM, Larson CL. Dysfunction in the neural circuitry of emotion regulation--a possible prelude to violence. Science. 2000;289:591–594. doi: 10.1126/science.289.5479.591. [DOI] [PubMed] [Google Scholar]
- 55.Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- 56.Wang M, Ramos B, Paspalas C, Shu Y, Simen A, Duque A, et al. Alpha2A-adrenoceptor stimulation strengthens working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell. 2007;129:397–410. doi: 10.1016/j.cell.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 57.Smiley JF, Levey AI, Ciliax BJ, Goldman-Rakic PS. D1 dopamine receptor immunoreactivity in human and monkey cerebral cortex: predominant and extrasynaptic localization in dendritic spines. Proc Natl Acad Sci U S A. 1994;91(12):5720–5724. doi: 10.1073/pnas.91.12.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Levitt P, Rakic P, Goldman-Rakic P. Region-specific distribution of catecholamine afferents in primate cerebral cortex: A fluorescence histochemical analysis. J. Comp. Neurol. 1984;227:23–36. doi: 10.1002/cne.902270105. [DOI] [PubMed] [Google Scholar]
- 59.Lewis DA, Cambell MJ, Foote SL, Goldstein M, Morrison JH. The distribution of tyrosine hydroxylase-immunoreactive fibers in primate neocortex is widespread but regionally specific. J. Neurosci. 1987;282:317–330. doi: 10.1523/JNEUROSCI.07-01-00279.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lewis DA, Morrison JH. Noradrenergic innervation of monkey prefrontal cortex: a dopamine-beta-hydroxylase immunohistochemical study. J. Comp. Neurol. 1989;282:317–330. doi: 10.1002/cne.902820302. [DOI] [PubMed] [Google Scholar]
- 61.Brozoski T, Brown RM, Rosvold HE, Goldman PS. Cognitive deficit caused by regional depletion of dopamine in prefrontal cortex of rhesus monkey. Science. 1979;205:929–931. doi: 10.1126/science.112679. [DOI] [PubMed] [Google Scholar]
- 62.Arnsten AFT, Goldman-Rakic PS. Alpha-2 adrenergic mechanisms in prefrontal cortex associated with cognitive decline in aged nonhuman primates. Science. 1985;230:1273–1276. doi: 10.1126/science.2999977. [DOI] [PubMed] [Google Scholar]
- 63.Cai JX, Ma Y, Xu L, Hu X. Reserpine impairs spatial working memory performance in monkeys: Reversal by the alpha-2 adrenergic agonist clonidine. Brain Res. 1993;614:191–196. doi: 10.1016/0006-8993(93)91034-p. [DOI] [PubMed] [Google Scholar]
- 64.Cedarbaum JM, Aghajanian GK. Catecholamine receptors on locus coeruleus neurons: pharmacological characterization. Eur. J. Pharmacol. 1977;44:375–385. doi: 10.1016/0014-2999(77)90312-0. [DOI] [PubMed] [Google Scholar]
- 65.U'Prichard DC, Bechtel WD, Rouot BM, Snyder SH. Multiple apparent alpha-noradrenergic receptor binding sites in rat brain: effect of 6-hydroxydopamine. Mol. Pharmacol. 1979;16:47–60. [PubMed] [Google Scholar]
- 66.MacDonald E, Kobilka BK, Scheinin M. Gene targeting-Homing in on alpha-2-adrenoceptor subtype function. Trends in Pharmacolog Sci. 1997;18:211–219. doi: 10.1016/s0165-6147(97)01063-8. [DOI] [PubMed] [Google Scholar]
- 67.Franowicz JS, Kessler L, Dailey-Borja CM, Kobilka BK, Limbird LE, Arnsten AFT. Mutation of the alpha2A-adrenoceptor impairs working memory performance and annuls cognitive enhancement by guanfacine. J Neurosci. 2002;22:8771–8777. doi: 10.1523/JNEUROSCI.22-19-08771.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ma C-L, Arnsten AFT, Li B-M. Locomotor hyperactivity induced by blockade of prefrontal cortical alpha-2-adrenoceptors in monkeys. Biological Psychiatry. 2005;57:192–195. doi: 10.1016/j.biopsych.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 69.Ma C-L, Qi X-L, Peng J-Y, Li B-M. Selective deficit in no-go performance induced by blockade of prefrontal cortical alpha2-adrenoceptors in monkeys. Neuroreport. 2003;14:1013–1016. doi: 10.1097/01.wnr.0000070831.57864.7b. [DOI] [PubMed] [Google Scholar]
- 70.Li B-M, Mei Z-T. Delayed response deficit induced by local injection of the alpha-2 adrenergic antagonist yohimbine into the dorsolateral prefrontal cortex in young adult monkeys. Behav. Neural. Biol. 1994;62:134–139. doi: 10.1016/s0163-1047(05)80034-2. [DOI] [PubMed] [Google Scholar]
- 71.Arnsten AFT, Cai JX, Goldman-Rakic PS. The alpha-2 adrenergic agonist guanfacine improves memory in aged monkeys without sedative or hypotensive side effects. J Neurosci. 1988;8:4287–4298. doi: 10.1523/JNEUROSCI.08-11-04287.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rama P, Linnankoski I, Tanila H, Pertovaara A, Carlson S. Medetomidine, atipamezole, and guanfacine in delayed response performance of aged monkeys. Pharmacol. Biochem. Behav. 1996;54:1–7. doi: 10.1016/s0091-3057(96)00111-6. [DOI] [PubMed] [Google Scholar]
- 73.Avery RA, Franowicz JS, Studholme C, van Dyck CH, Arnsten AFT. The alpha-2A-adenoceptor agonist, guanfacine, increases regional cerebral blood flow in dorsolateral prefrontal cortex of monkeys performing a spatial working memory task. Neuropsychopharmacology. 2000;23:240–249. doi: 10.1016/S0893-133X(00)00111-1. [DOI] [PubMed] [Google Scholar]
- 74.O'Neill J, Fitten LJ, Siembieda DW, Ortiz F, Halgren E. Effects of guanfacine on three forms of distraction in the aging macaque. Life Sciences. 2000;67:877–885. doi: 10.1016/s0024-3205(00)00681-0. [DOI] [PubMed] [Google Scholar]
- 75.Ramos B, Stark D, Verduzco L, van Dyck CH, Arnsten AFT. Alpha-2A-adrenoceptor stimulation improves prefrontal cortical regulation of behavior through inhibition of cAMP signaling in aging animals. Learning and Memory. 2006;13:770–776. doi: 10.1101/lm.298006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Arnsten AFT. The biology of feeling frazzled. Science. 1998;280:1711–1712. doi: 10.1126/science.280.5370.1711. [DOI] [PubMed] [Google Scholar]
- 77.Birnbaum SG, Gobeske KT, Auerbach J, Taylor JR, Arnsten AFT. A role for norepinephrine in stress-induced cognitive deficits: Alpha-1-adrenoceptor mediation in prefrontal cortex. Biol. Psychiatry. 1999;46:1266–1274. doi: 10.1016/s0006-3223(99)00138-9. [DOI] [PubMed] [Google Scholar]
- 78.Mao Z-M, Arnsten AFT, Li B-M. Local infusion of alpha-1 adrenergic agonist into the prefrontal cortex impairs spatial working memory performance in monkeys. Biol. Psychiatry. 1999;46:1259–1265. doi: 10.1016/s0006-3223(99)00139-0. [DOI] [PubMed] [Google Scholar]
- 79.Ramos B, Colgan L, Nou E, Ovadia S, Wilson SR, Arnsten AFT. The beta-1 adrenergic antagonist, betaxolol, improves working memory performance in rats and monkeys. Biological Psychiatry. 2005;58:894–900. doi: 10.1016/j.biopsych.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 80.Birnbaum SB, Yuan P, Bloom A, Davis D, Gobeske K, Sweatt D, et al. Protein kinase C overactivity impairs prefrontal cortical regulation of working memory. Science. 2004;306:882–884. doi: 10.1126/science.1100021. [DOI] [PubMed] [Google Scholar]
- 81.Manji HK, Lenox RH. Protein kinase C signaling in the brain: Molecular transduction of mood stabilization in the treatment of manic-depressive illness. Biological Psychiatry. 1999;46:1328–1351. doi: 10.1016/s0006-3223(99)00235-8. [DOI] [PubMed] [Google Scholar]
- 82.Arnsten AFT, Manji HK. Mania: A rational neurobiology. Future Neurology. 2008;3 in press. [Google Scholar]
- 83.Sawaguchi T, Goldman-Rakic PS. D1 dopamine receptors in prefrontal cortex: Involvement in working memory. Science. 1991;251:947–950. doi: 10.1126/science.1825731. [DOI] [PubMed] [Google Scholar]
- 84.Wang M, Vijayraghavan S, Goldman-Rakic PS. Selective D2 receptor actions on the functional circuitry of working memory. Science. 2004;303:853–856. doi: 10.1126/science.1091162. [DOI] [PubMed] [Google Scholar]
- 85.Mrzljak L, Bergson C, Pappy M, Levenson R, Huff R, Goldman-Rakic PS. Localization of dopamine D4 receptors in GABAergic neurons of the primate brain. Nature. 1996;381:245–248. doi: 10.1038/381245a0. [DOI] [PubMed] [Google Scholar]
- 86.Wang X, Zhong P, Yan Z. Dopamine D4 receptors modulate GABAergic signaling in pyramidal neurons of prefrontal cortex. J Neuroscience. 2002;22:9185–9193. doi: 10.1523/JNEUROSCI.22-21-09185.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Van Tol HHM, Bunzow JR, Guan H-C, Sunahara RK, Seeman P, Niznik HB, et al. Cloning of the gene for a human dopamine D4 receptor with high affinity for the antipsychotic clozapine. Nature. 1991;350:610–614. doi: 10.1038/350610a0. (18 Apr) [DOI] [PubMed] [Google Scholar]
- 88.Lidow MS, Goldman-Rakic PS, Gallager DW, Rakic P. Distribution of dopaminergic receptors in the primate cerebral cortex: Quantitative autoradiographic analysis using [3H]raclopride, [3H]spiperone, and [3H]SCH 23390. Neurosci. 1991;40:657–671. doi: 10.1016/0306-4522(91)90003-7. [DOI] [PubMed] [Google Scholar]
- 89.Vijayraghavan S, Wang M, Birnbaum SG, Bruce CJ, Williams GV, Arnsten AFT. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nature Neuroscience. 2007;10:376–384. doi: 10.1038/nn1846. [DOI] [PubMed] [Google Scholar]
- 90.Arnsten AFT, Steere JC, Hunt RD. The contribution of alpha-2 noradrenergic mechanisms to prefrontal cortical cognitive function: potential significance to Attention Deficit Hyperactivity Disorder. Arch Gen Psychiatry. 1996;53:448–455. doi: 10.1001/archpsyc.1996.01830050084013. [DOI] [PubMed] [Google Scholar]
- 91.Loo SK, Humphrey LA, Tapio T, Moilanen IK, McGough JJ, McCracken JT, et al. Executive functioning among Finnish adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2007;46:1594–1604. doi: 10.1097/chi.0b013e3181575014. [DOI] [PubMed] [Google Scholar]
- 92.Rubia K, Smith AB, Taylor E. Performance of children with attention deficit hyperactivity disorder (ADHD) on a test battery of impulsiveness. Child Neuropsychol. 2007;13:276–304. doi: 10.1080/09297040600770761. [DOI] [PubMed] [Google Scholar]
- 93.Casey BJ, Castellanos FX, Giedd JN, Marsh WL, Hamburger SD, Schubert AB, et al. Implication of right frontostriatal circuitry in response inhibition and attention-deficit/hyperactivity disorder. J. Amer. Acad. Child Adolescent Psychiatry. 1997;36:374–383. doi: 10.1097/00004583-199703000-00016. [DOI] [PubMed] [Google Scholar]
- 94.Sowell ER, Thompson PM, Welcome SE, Henkenius AL, Toga AW, Peterson BS. Cortical abnormalities in children and adolescents with attention-deficit hyperactivity disorder. Lancet. 2003;362:1699–1707. doi: 10.1016/S0140-6736(03)14842-8. [DOI] [PubMed] [Google Scholar]
- 95.Seidman LJ, Valera EM, Makris N. Structural brain imaging of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1263–1272. doi: 10.1016/j.biopsych.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 96.Bush G, Valera EM, Seidman LJ. Functional neuroimaging of attention-deficit/hyperactivity disorder: a review and suggested future directions. Biol Psychiatry. 2005;57:1273–1284. doi: 10.1016/j.biopsych.2005.01.034. [DOI] [PubMed] [Google Scholar]
- 97.Sheridan MA, Hinshaw S, D'Esposito M. Efficiency of the prefrontal cortex during working memory in attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2007;46:1357–1366. doi: 10.1097/chi.0b013e31812eecf7. [DOI] [PubMed] [Google Scholar]
- 98.Casey BJ, Epstein JN, Buhle J, Liston C, Davidson MC, Tonev ST, et al. Frontostriatal connectivity and its role in cognitive control in parent-child dyads with ADHD. Am J Psychiatry. 2007;164:1729–1736. doi: 10.1176/appi.ajp.2007.06101754. [DOI] [PubMed] [Google Scholar]
- 99.Makris N, Buka SL, Biederman J, Papadimitriou GM, Hodge SM, Valera EM, et al. Attention and Executive Systems Abnormalities in Adults with Childhood ADHD: A DT-MRI Study of Connections. Cereb Cortex. 2007 doi: 10.1093/cercor/bhm156. epub ahead of print:Sep 30. [DOI] [PubMed] [Google Scholar]
- 100.Castellanos FX, Lee PP, Sharp W, Jeffries NO, Greenstein DK, Clasen LS, et al. Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. JAMA. 2002;288:1740–1748. doi: 10.1001/jama.288.14.1740. [DOI] [PubMed] [Google Scholar]
- 101.Shaw P, Eckstrand K, Sharp W, Blumenthal J, Lerch JP, Greenstein D, et al. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc Natl Acad Sci U S A. 2007;104:19649–19654. doi: 10.1073/pnas.0707741104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Seidman LJ, Valera EM, Makris N, Monuteaux MC, Boriel DL, Kelkar K, et al. Dorsolateral prefrontal and anterior cingulate cortex volumetric abnormalities in adults with attention-deficit/hyperactivity disorder identified by magnetic resonance imaging. Biol Psychiatry. 2006;60:1071–1080. doi: 10.1016/j.biopsych.2006.04.031. [DOI] [PubMed] [Google Scholar]
- 103.Makris N, Biederman J, Valera EM, Bush G, Kaiser J, Kennedy DN, et al. Cortical thinning of the attention and executive function networks in adults with attention-deficit/hyperactivity disorder. Cereb Cortex. 2007;17:1364–1375. doi: 10.1093/cercor/bhl047. [DOI] [PubMed] [Google Scholar]
- 104.Faraone SV, Perlis RH, Doyle AE, Smoller JW, Goralnick JJ, Holmgren MA, et al. Molecular genetics of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1313–1323. doi: 10.1016/j.biopsych.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 105.Daly G, Hawi Z, Fitzgerald M, Gill M. Mapping susceptibility loci in attention deficit hyperactivity disorder: preferential transmission of parental alleles at DAT1, DBH and DRD5 to affected children. Mol Psychiatry. 1999;4(2):192–196. doi: 10.1038/sj.mp.4000510. [DOI] [PubMed] [Google Scholar]
- 106.Tahir E, Yazgan Y, Cirakoglu B, Ozbay F, Waldman I, Asherson PJ. Association and linkage of DRD4 and DRD5 with attention deficit hyperactivity disorder (ADHD) in a sample of Turkish children. Mol Psychiatry. 2000;5(4):396–404. doi: 10.1038/sj.mp.4000744. [DOI] [PubMed] [Google Scholar]
- 107.Kustanovich V, Ishii J, Crawford L, Yang M, McGough JJ, McCracken JT, et al. Transmission disequilibrium testing of dopamine-related candidate gene polymorphisms in ADHD: confirmation of association of ADHD with DRD4 and DRD5. Mol Psychiatry. 2004;9:711–717. doi: 10.1038/sj.mp.4001466. [DOI] [PubMed] [Google Scholar]
- 108.Bobb AJ, Addington AM, Sidransky E, Gornick MC, Lerch JP, Greenstein DK, et al. Support for association between ADHD and two candidate genes: NET1 and DRD1. Am J Med Genet B Neuropsychiatr Genet. 2005;134:67–72. doi: 10.1002/ajmg.b.30142. [DOI] [PubMed] [Google Scholar]
- 109.Durston S, Fossella JA, Casey BJ, Hulshoff Pol HE, Galvan A, Schnack HG, et al. Differential effects of DRD4 and DAT1 genotype on fronto-striatal gray matter volumes in a sample of subjects with attention deficit hyperactivity disorder, their unaffected siblings, and controls. Mol Psychiatry. 2005;10:678–685. doi: 10.1038/sj.mp.4001649. [DOI] [PubMed] [Google Scholar]
- 110.Mill J, Caspi A, Williams BS, Craig I, Taylor A, Polo-Tomas M, et al. Prediction of heterogeneity in intelligence and adult prognosis by genetic polymorphisms in the dopamine system among children with attention-deficit/hyperactivity disorder: evidence from 2 birth cohorts. Arch Gen Psychiatry. 2006;63:462–469. doi: 10.1001/archpsyc.63.4.462. [DOI] [PubMed] [Google Scholar]
- 111.Sunohara GA, Roberts W, Malone M, Schachar RJ, Tannock R, Basile VS, et al. Linkage of the dopamine D4 receptor gene and attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2000;39(12):1537–1542. doi: 10.1097/00004583-200012000-00017. [DOI] [PubMed] [Google Scholar]
- 112.Comings DE. Clinical and molecular genetics of ADHD and Tourette syndrome. Two related polygenic disorders. Ann N Y Acad Sci. 2001;931:50–83. doi: 10.1111/j.1749-6632.2001.tb05773.x. [DOI] [PubMed] [Google Scholar]
- 113.Xu C, Schachar R, Tannock R, Roberts W, Malone M, Kennedy JL, et al. Linkage study of the alpha2A adrenergic receptor in attention-deficit hyperactivity disorder families. Am J Med Genet. 2001;105:159–162. [PubMed] [Google Scholar]
- 114.Roman T, Schmitz M, Polanczyk GV, Eizirik M, Rohde LA, Hutz MH. Is the alpha-2A adrenergic receptor gene (ADRA2A) associated with attention-deficit/hyperactivity disorder? Am J Med Genet B Neuropsychiatr Genet. 2003;120:116–120. doi: 10.1002/ajmg.b.20018. [DOI] [PubMed] [Google Scholar]
- 115.Roman T, Schmitz M, Polanczyk GV, Eizirik M, Rohde LA, Hutz MH. Further evidence for the association between attention-deficit/hyperactivity disorder and the dopamine-beta-hydroxylase gene. Am J Med Genet. 2002;114(2):154–158. doi: 10.1002/ajmg.10194. [DOI] [PubMed] [Google Scholar]
- 116.Kopecková M, Paclt I, Goetz P. Polymorphisms of dopamine-beta-hydroxylase in ADHD children. Folia Biol (Praha) 2006;52:194–210. [PubMed] [Google Scholar]
- 117.Bellgrove MA, Hawi Z, Gill M, Robertson IH. The cognitive genetics of attention deficit hyperactivity disorder (ADHD): Sustained attention as a candidate phenotype. Cortex. 2005 doi: 10.1016/s0010-9452(08)70426-x. in press. [DOI] [PubMed] [Google Scholar]
- 118.Kieling C, Genro JP, Hutz MH, Rohde LA. The-1021 C/T DBH polymorphism is associated with neuropsychological performance among children and adolescents with ADHD. Am J Med Genet B Neuropsychiatr Genet. 2007 doi: 10.1002/ajmg.b.30636. Epub ahead of print:Dec 14. [DOI] [PubMed] [Google Scholar]
- 119.Cheon KA, Ryu YH, Kim YK, Namkoong K, Kim CH, Lee JD. Dopamine transporter density in the basal ganglia assessed with [123I]IPT SPET in children with attention deficit hyperactivity disorder. Eur J Nucl Med Mol Imaging. 2003;30:306–311. doi: 10.1007/s00259-002-1047-3. [DOI] [PubMed] [Google Scholar]
- 120.la Fougère C, Krause J, Krause KH, Josef Gildehaus F, Hacker M, Koch WJ, et al. Value of 99mTc-TRODAT-1 SPECT to predict clinical response to methylphenidate treatment in adults with attention deficit hyperactivity disorder. Nucl Med Commun. 2006;27:733–737. doi: 10.1097/01.mnm.0000230077.48480.68. [DOI] [PubMed] [Google Scholar]
- 121.Spencer TJ, Biederman J, Madras BK, Dougherty DD, Bonab AA, Livni E, et al. Further evidence of dopamine transporter dysregulation in ADHD: a controlled PET imaging study using altropane. Biol Psychiatry. 2007;62:1059–1061. doi: 10.1016/j.biopsych.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.van Dyck CH, Quinlan DM, Cretella L, Staley JK, Malison RT, Baldwin RM, et al. Striatal dopamine transporter availability with [123I]β-CIT SPECT is unaltered in adult Attention Deficit Hyperactivity Disorder. Am. J. Psychiatry. 2002;159:309–312. doi: 10.1176/appi.ajp.159.2.309. [DOI] [PubMed] [Google Scholar]
- 123.Volkow ND, Wang GJ, Newcorn J, Fowler JS, Telang F, Solanto MV, et al. Brain dopamine transporter levels in treatment and drug naïve adults with ADHD. Neuroimage. 2007;34:1182–1190. doi: 10.1016/j.neuroimage.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 124.Volkow ND, Wang GJ, Newcorn J, Telang F, Solanto MV, Fowler JS, et al. Depressed dopamine activity in caudate and preliminary evidence of limbic involvement in adults with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2007;64:932–940. doi: 10.1001/archpsyc.64.8.932. [DOI] [PubMed] [Google Scholar]
- 125.Ernst M, Zametkin AJ, Matochik JA, Jons PH, Cohen RM. DOPA decarboxylase activity in attention deficit disorder adults. A [fluorine-18]fluorodopa positron emission tomographic study. J. Neurosci. 1998;18:5901–5907. doi: 10.1523/JNEUROSCI.18-15-05901.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Makanjuola RO, Ashcroft GW. Behavioural effects of electrolytic and 6-hydroxydopamine lesions of the accumbens and caudate-putamen nuclei. Psychopharmacology. 1982;76:33–40. doi: 10.1007/BF00449121. [DOI] [PubMed] [Google Scholar]
- 127.Simon H. Dopaminergic A10 neurons and the frontal system. J. de Physiol. 1981;77:81–95. [PubMed] [Google Scholar]
- 128.Sesack SR, Hawrylak VA, Matus C, Guido MA, Levey AI. Dopamine axon varicosities in the prelimbic division of the rat prefrontal cortex exhibit sparse immunoreactivity for the dopamine transporter. J Neurosci. 1998;18(7):2697–2708. doi: 10.1523/JNEUROSCI.18-07-02697.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Segal DS, Kuczenski R. Escalating dose-binge treatment with methylphenidate: role of serotonin in the emergent behavioral profile. J Pharmacol Exp Ther. 1999;291:19–30. [PubMed] [Google Scholar]
- 130.Kuczenski R, Segal DS. Exposure of adolescent rats to oral methylphenidate: preferential effects on extracellular norepinephrine and absence of sensitization and cross-sensitization to methamphetamine. J Neurosci. 2002;22:7264–7271. doi: 10.1523/JNEUROSCI.22-16-07264.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Berridge CW, Devilbiss DM, Andrzejewski ME, Arnsten AFT, Kelley AE, Schmeichel B, et al. Methylphenidate preferentially increases catecholamine neurotransmission within the prefrontal cortex at low doses that enhance cognitive function. Biological Psychiatry. 2006;60:1111–1120. doi: 10.1016/j.biopsych.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 132.Arnsten AFT, Dudley AG. Methylphenidate improves prefrontal cortical cognitive function through a2 adrenoceptor and dopamine D1 receptor actions: Relevance to therapeutic effects in Attention Deficit Hyperactivity Disorder. Behavioral and Brain Functions (Biomed Central) 2005;1:2. doi: 10.1186/1744-9081-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Dyme IZ, Sahakian BJ, Golinko BE, Rabe EF. Perseveration induced by methylphenidate in children: preliminary findings. Prog Neuropsychopharmacol Biol Psychiatry. 1982;6:269–273. doi: 10.1016/s0278-5846(82)80177-2. [DOI] [PubMed] [Google Scholar]
- 134.Rapoport JL, Inoff-Germain G. Responses to methylphenidate in Attention-Deficit/Hyperactivity Disorder and normal children: update 2002. J Atten Disord. 2002;6:S57–S60. doi: 10.1177/070674370200601s07. [DOI] [PubMed] [Google Scholar]
- 135.Mehta MA, Owen AM, Sahakian BJ, Mavaddat N, Pickard JD, Robbins TW. Methylphenidate enhances working memory by modulating discrete frontal and parietal lobe regions in the human brain. J. Neuroscience. 2000;20:RC651–RC656. doi: 10.1523/JNEUROSCI.20-06-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Aron AR, Dowson JH, Sahakian BJ, Robbins TW. Methylphenidate improves response inhibition in adults with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2003;54:1465–1468. doi: 10.1016/s0006-3223(03)00609-7. [DOI] [PubMed] [Google Scholar]
- 137.Mehta MA, Goodyer IM, Sahakian BJ. Methylphenidate improves working memory and set-shifting in AD/HD: relationships to baseline memory capacity. J Child Psychol Psychiatry. 2004;45:293–305. doi: 10.1111/j.1469-7610.2004.00221.x. [DOI] [PubMed] [Google Scholar]
- 138.Turner DC, Blackwell AD, Dowson JH, McLean A, Sahakian BJ. Neurocognitive effects of methylphenidate in adult attention-deficit/hyperactivity disorder. Psychopharmacology. 2005;178:286–295. doi: 10.1007/s00213-004-1993-5. [DOI] [PubMed] [Google Scholar]
- 139.Bush G, Spencer TJ, Holmes J, Shin LM, Valera EM, Seidman LJ, et al. Functional magnetic resonance imaging of methylphenidate and placebo in attention-deficit/hyperactivity disorder during the multi-source interference task. Arch Gen Psychiatry. 2008;65:102–114. doi: 10.1001/archgenpsychiatry.2007.16. [DOI] [PubMed] [Google Scholar]
- 140.Bymaster FP, Katner JS, Nelson DL, Hemrick-Luecke SK, Threlkeld PG, Heiligenstein JH, et al. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology. 2002;27:699–711. doi: 10.1016/S0893-133X(02)00346-9. [DOI] [PubMed] [Google Scholar]
- 141.Chamberlain SR, Muller U, Blackwell AD, Clark L, Robbins TW, Sahakian BJ. Neurochemical modulation of response inhibition and probabilistic learning in humans. Science. 2006;311:861–863. doi: 10.1126/science.1121218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chamberlain SR, Del Campo N, Dowson J, Müller U, Clark L, Robbins TW, et al. Atomoxetine Improved Response Inhibition in Adults with Attention Deficit/Hyperactivity Disorder. Biol Psychiatry. 2007;62:977–984. doi: 10.1016/j.biopsych.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 143.Biederman J, Baldessarini RJ, Wright V, Knee D, Harmatz JS. A double-blind placebo controlled study of desipramine in the treatment of ADD: I. Efficacy. J Am Acad Child Adolesc Psychiatry. 1989;28:777–784. doi: 10.1097/00004583-198909000-00022. [DOI] [PubMed] [Google Scholar]
- 144.Donnelly M, Zametkin AJ, Rapoport JL, Ismond DR, Weingartner H, Lane E, et al. Treatment of childhood hyperactivity with desipramine: plasma drug concentration, cardiovascular effects, plasma and urinary catecholamine levels, and clinical response. Clin Pharmacol Ther. 1986;39:72–81. doi: 10.1038/clpt.1986.13. [DOI] [PubMed] [Google Scholar]
- 145.Arnsten AFT, Contant TA. Alpha-2 adrenergic agonists decrease distractability in aged monkeys performing a delayed response task. Psychopharmacology. 1992;108:159–169. doi: 10.1007/BF02245302. [DOI] [PubMed] [Google Scholar]
- 146.Steere JC, Arnsten AFT. The alpha-2A noradrenergic agonist, guanfacine, improves visual object discrimination reversal performance in rhesus monkeys. Behav. Neurosci. 1997;111:1–9. doi: 10.1037//0735-7044.111.5.883. [DOI] [PubMed] [Google Scholar]
- 147.Wang M, Ji JZ, Li BM. The alpha(2A)-adrenergic agonist guanfacine improves visuomotor associative learning in monkeys. Neuropsychopharmacology. 2004;29:86–92. doi: 10.1038/sj.npp.1300278. [DOI] [PubMed] [Google Scholar]
- 148.Buzsaki G, Kennedy B, Solt VB, Ziegler M. Noradrenergic control of thalamic oscillation: The role of alpha-2 receptors. Eur. J. Neuro. 1991;3:222–229. doi: 10.1111/j.1460-9568.1991.tb00083.x. [DOI] [PubMed] [Google Scholar]
- 149.Engberg G, Eriksson E. Effects of alpha-2-adrenoceptor agonists on locus coeruleus firing rate and brain noradrenaline turnover in EEDQ-treated rats. Naunyn-Schmiedebergs Arch. Pharmacol. 1991;343:472–477. doi: 10.1007/BF00169548. [DOI] [PubMed] [Google Scholar]
- 150.Scahill L, Chappell PB, Kim YS, Schultz RT, Katsovich L, Shepherd E, et al. Guanfacine in the treatment of children with tic disorders and ADHD: A placebo-controlled study. Amer. J. Psychiatry. 2001;158:1067–1074. doi: 10.1176/appi.ajp.158.7.1067. [DOI] [PubMed] [Google Scholar]
- 151.Taylor FB, Russo J. Comparing guanfacine and dextroamphetamine for the treatment of adult Attention Deficit-Hyperactivity Disorder. J. Clin. Psychopharm. 2001;21:223–228. doi: 10.1097/00004714-200104000-00015. [DOI] [PubMed] [Google Scholar]
- 152.Biederman J, Melmed RD, Patel A, McBurnett K, Konow J, Lyne A, et al. A randomized, double-blind, placebo-controlled study of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. Pediatrics. 2008;121:e73–e84. doi: 10.1542/peds.2006-3695. [DOI] [PubMed] [Google Scholar]
- 153.Franowicz JCS, Arnsten AFT. The alpha-2A noradrenergic agonist, guanfacine, improves delayed response performance in young adult rhesus monkeys. Psychopharmacology. 1998;136:8–14. doi: 10.1007/s002130050533. [DOI] [PubMed] [Google Scholar]
- 154.Jakala P, Riekkinen M, Sirvio J, Koivisto E, Kejonen K, Vanhanen M, et al. Guanfacine, but not clonidine, improves planning and working memory performance in humans. Neuropsychopharmacology. 1999;20:460–470. doi: 10.1016/S0893-133X(98)00127-4. [DOI] [PubMed] [Google Scholar]
- 155.Hunt RD, Mindera RB, Cohen DJ. Clonidine benefits children with Attention Deficit Disorder and Hyperactivity: Reports of a double-blind placebo-crossover therapeutic trial. J. Amer. Acad. Child Psychiatry. 1985;24:617–629. doi: 10.1016/s0002-7138(09)60065-0. [DOI] [PubMed] [Google Scholar]
- 156.Group TTsSS. Treatment of ADHD in children with tics: a randomized controlled trial. Neurology. 2002;58:527–536. doi: 10.1212/wnl.58.4.527. [DOI] [PubMed] [Google Scholar]
- 157.Uhlen S, Porter AC, Neubig RR. The novel alpha-2 adrenergic radioligand [3H]-MK912 is alpha-2C selective among human alpha-2A, alpha-2B and alpha-2C adrenoceptors. J. Pharmacol. Exp. Ther. 1994;271:1558–1565. [PubMed] [Google Scholar]
- 158.Uhlen S, Wikberg JES. Delineation of rat kidney alpha 2A and alpha 2B-adrenoceptors with [3H]RX821002 radioligand binding: computer modeling reveals that guanfacine is an alpha-2A-selective compound. Eur. J. Pharmacol. 1991;202:235–243. doi: 10.1016/0014-2999(91)90299-6. [DOI] [PubMed] [Google Scholar]
- 159.Coupry I, Lachaud V, Podevin RA, Koenig E, Parini A, Langbehn D. Different affinities of alpha 2-agonists for imidazoline and alpha 2-adrenergic receptors. Am J Hypertens. 1989;2:468–470. doi: 10.1093/ajh/2.6.468. [DOI] [PubMed] [Google Scholar]
- 160.van Zwieten PA, Chalmers JP. Different types of centrally acting antihypertensives and their targets in the central nervous system. Cardiovasc Drugs Ther. 1994;8:787–799. doi: 10.1007/BF00877397. [DOI] [PubMed] [Google Scholar]
- 161.Arnsten AFT. Through the looking glass: Differential noradrenergic modulation of prefrontal cortical function. Neural Plasticity. 2000;7:133–146. doi: 10.1155/NP.2000.133. [DOI] [PMC free article] [PubMed] [Google Scholar]