Abstract
Study Objective:
Recent studies have found increased autoantibodies against Tribbles homolog 2 (anti-TRIB2) and anti-streptolysin O (ASO) in narcolepsy. In this study, we replicated this finding with a primary focus on recent onset cases.
Participants and Methods:
Participants included (1) 90 cases with cataplexy, (2) 57 cases without cataplexy, and (3) 156 age-sex matched controls, including 73 human leukocyte antigen (HLA)-DQB1*0602 allele carriers. A radioligand binding assay was used to detect anti-TRIB2 antibodies.
Results:
Anti-TRIB2 antibodies were prevalent in HLA-DQB1*0602 positive cases with cataplexy (25.0% of 76) and rare in cases without cataplexy (3.5% of 57, OR = 9.2, 95% CI = 2.5 - 33.5, P = 6.0 × 10−4) or controls (4.5% of 156, OR = 7.1, 95% CI = 3.1 - 16.2, P = 9.3 × 10−6). Anti-TRIB2 positivity in controls was not associated with DQB1*0602. In DQB1*0602 narcolepsy-cataplexy cases, the presence of anti-TRIB2 was associated with short disease duration (2.3 years from cataplexy onset), with 41.0% positive in this group (OR = 7.4 versus cases with onset > 2.3 years, 95% CI = 1.9 - 28.5, P = 9.0 × 10−4). Anti-TRIB2 positivity in 39 DQB1*0602 positive recent onset cases was associated with increased ASO antibody (> 200 IU) (OR = 6.2, 95% CI = 1.6 - 24.6, P = 0.01), but did not correlate with age, gender, or body mass index.
Conclusion:
Anti-TRIB2 autoantibodies are strongly associated with narcolepsy close to cataplexy onset (≤ 2.3 years). Anti-TRIB2 was rarely found in cases without cataplexy or with distant onset.
Citation:
Kawashima M; Lin L; Tanaka S; Jennum P; Knudsen S; Nevsimalova S; Plazzi G; Mignot E. Anti-Tribbles homolog 2 (TRIB2) autoantibodies in narcolepsy are associated with recent onset of cataplexy. SLEEP 2010;33(7):869-874.
Keywords: Human narcolepsy, Tribbles homolog 2 (TRIB2), autoantibody, anti-streptolysin O (ASO)
NARCOLEPSY IS TIGHTLY ASSOCIATED WITH THE HUMAN LEUKOCYTE ANTIGEN (HLA) CLASS II REGION, WITH 95% OF NARCOLEPSY PATIENTS WITH cataplexy carrying the HLA-DQB1*0602 allele.1 In most human narcolepsy cases with cataplexy, hypocretin-1 concentration is reduced or undetectable in the cerebrospinal fluid (CSF).2,3 The lack of CSF hypocretin (HCRT) is due to a 90% to 95% loss of HCRT neurons in postmortem brains of narcolepsy patients, without apparent alteration of adjacent neurons, such as those expressing melanin-concentrating hormone.4 As a lack of HCRT or HCRT receptors causes narcolepsy in animal models,5,6 the loss of HCRT transmission is the likely cause of the symptoms in human narcolepsy.
Although the tight HLA association suggests a potential autoimmune destruction of HCRT neurons as the etiology of most narcolepsy cases, the proof for such a mechanism has remained elusive.7 A recent genome-wide association study found a strong additional association between narcolepsy and polymorphisms in T cell receptor alpha gene (TCRA).8 Further, Cvetkovic-Lopes et al.9 found increased Tribbles homolog 2 (TRIB2) autoantibodies in narcolepsy, previously reported to be associated with autoimmune uveitis in 3 of 10 cases.10 Detection of the autoantibodies was directed against the C-terminal end of the protein, and used an enzyme-linked immunosorbent assay (ELISA) method.
In the present study, we confirmed the association of anti-TRIB2 autoantibodies in Caucasians with narcolepsy, extending the study to include patients with and without documented HCRT deficiency. Further, unlike the Cvetkovic-Lopes et al. study,9 we used a radioligand binding assay (RLA) rather than an ELISA. Prior work has shown this technique to be more sensitive and to better conserve epitope specificity.11,12
MATERIALS AND METHODS
Patients and Controls
All included cases and controls were Caucasians. Narcoleptic patients all met International Classification of Sleep Disorders, 2nd edition13 for narcolepsy with cataplexy (typical cataplexy), or without cataplexy (no or atypical cataplexy but with a positive multiple sleep latency test). Cataplexy severity was scored from 0 - 8 using the Stanford Sleep Inventory, as described in Hong et al.14 Most narcolepsy cases were enrolled by the Stanford Center for Narcolepsy, with the exception of 5 Italian, 10 Czech, and 16 Danish patients. Special attention was made to include the largest possible number of cases with recent cataplexy onset (< 3 years). CSF hypocretin-1 was available in 48 cases with cataplexy (93.8% with low CSF hypocretin-1) and 52 cases without cataplexy (11.5% with low CSF hypocretin-1). Patients with cataplexy and DQB1*0602 positivity were considered to have HCRT deficiency, based on prior data indicating a higher than 90% correspondence.15 We also included a small number of extremely rare atypical cases that were DQB1*0602 negative with low CSF hypocretin-1 (< 110 pg/mL) (n = 4), DQB1*0602 positive with normal CSF hypocretin-1 (> 200 pg/mL) (n = 3), and DQB1*0602 negative with normal CSF hypocretin-1 (n = 10). Controls were matched to each patient based on age, gender, and geographic location. Daytime sleepiness was assessed using the Epworth Sleepiness Scale (ESS); mean score (± SEM) among controls was 5.7 ± 0.3 (narcolepsy with cataplexy: 16.8 ± 0.4, narcolepsy without cataplexy: 15.6 ± 0.6). Nine controls with documented normal CSF hypocretin-1 were also included. The presence or absence of DQB1*0602 was determined using DQB1 exon2 sequence specific primers8. Demographics and DQB1*0602 positivity for each subgroup are reported in Table 1. Local institutional review boards at each institution approved human protocols for the study. Written informed consent was obtained from all study participants.
Table 1.
Cases and controls used in the study
| Number | Age (mean ± SD) | Female/Male | Number of Positive Reaction (%) | HCRT Deficiency | HLA-DQB1*0602 | Number | Age (mean ± SD) | Female/Male | Number of Positive Reaction (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Narcolepsy with cataplexy | 90a | 29.9 ± 19.3 | 46/44 | 19 (21.1) | + | + | 73 | 28.0 ± 19.6 | 37/36 | 17 (23.3)b,c | |
| + | − | 4 | 34.9 ± 25.6 | 2/2 | 0 (0.0) | ||||||
| − | + | 3 | 42.7 ± 15.8 | 2/1 | 2 (66.7)b,c | ||||||
| − | − | 10 | 37.5 ± 14.4 | 5/5 | 0 (0.0) | ||||||
| Narcolepsy with cataplexy | 57a,b,e | 30.2 ± 12.6 | 26/31 | 2 (3.5) | + | + | 6 | 32.2 ± 20.9 | 2/4 | 0 (0.0) | |
| + | − | 0 | − | − | 0 (0.0) | ||||||
| − | + | 10 | 29.4 ± 11.8 | 9/1 | 0 (0.0) | ||||||
| − | − | 41 | 32.3 ± 10.7 | 15/26 | 2 (4.9) | ||||||
| Age-Sex Matched Ctrl | 156a,c,e | 30.8 ± 16.1 | 75/81 | 7 (4.5) | no data | + | 73 | 34.1 ± 16.0 | 37/36 | 4 (5.5)d | |
| no data | − | 74 | 26.7 ± 16.2 | 35/39 | 3 (4.1)d | ||||||
| Normal HCRT Ctrl | − | − | 9 | 36.9 ± 6.7 | 3/6 | 0 (0.0)d | |||||
Narcolepsy with and without cataplexy are defined as per the International Classification of sleep disorders 2.13 Mean age and gender of patient groups do not differ significantly from controls.
Odds ratio (OR) = 3.5, 95% confidence interval (CI) = 1.5 - 8.3, P = 0.005,
OR = 9.2, 95% CI = 2.5 - 33.5, P = 6.0 × 10−4,
OR = 7.1, 95% CI = 3.1 - 16.2, P = 9.3 × 10−6,
P = 0.7,
P = 1. Ctrl, control; SD, standard deviation.
Construction of the TRIB2 Expression Vector
The open reading frame of the human TRIB2 was obtained by RT-PCR amplification of poly(A)+ RNA extracted from human hypothalamus (Takara Bio, Kyoto, Japan). cDNA was synthesized using ReverTraAce (TOYOBO, Tokyo, Japan) with random hexamer primers, according to the manufacturer's instructions. The PCR amplification primers used were: 5′-CGCGGATCCATGAACATACACAGGTCTA-3′ and 5′-CCGCTCGAGATTCTTGGCCAACTGTTCCTT-3′ (BamHI and XhoI sites are underlined). The TRIB2 PCR product was sub-cloned into a pET28a (+) expression vector (Novagen, Madison, WI, USA) to generate the TRIB2 expression vector (TRIB2/pET28a).
Recombinant [35S]-TRIB2 Radioligand Binding Assay for Anti-TRIB2 Autoantibody Detection
Recombinant [35S]-Methionine labeled TRIB2 was generated as described previously.16 Briefly, the TNT quick couples system (Promega, Madison, WI, USA) was used to transcribe and translate labeled TRIB2 (with verification of a size of 36 kDa) to be used as the antigen for the detection of serum autoantibodies in RLA. An anti-Trib2 mouse monoclonal IgG (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) was used as a positive control. Serum from controls and patients (1 μL, 1:30, duplicate for each sample) was added to [35S]-TRIB2 for overnight incubation at 4°C. Protein G was then added, and the reaction mixtures were filtered, allowing the retention of Protein G-antigen-autoantibody complexes on the filter (for counting) if present. Samples showing > 10% coefficient of variation (CV) within duplicates were re-assayed. Final intra-assay CV was 0.0% to 9.4% and inter-assay CV was 1.3% to 8.8%. To reduce inter-assay variation, results were expressed as follows: cpm of each serum sample / cpm of 100 pooled healthy control sera, a measure we call the anti-TRIB2 autoantibody index. A measure of 1.0 thus represents the mean reactivity of a large number of control sera, with higher numbers indicating increased presence of TRIB2 autoantibodies.
Measurement of Anti-Streptolysin O Antibodies
Anti-streptolysin O (ASO) antibody titers, a semi-quantitative measure of recent Streptococcus pyogenes infection,17 were assayed in serum according to the manufacturer'Os instructions (Apogent, Lenexa, KS, USA). We retested all old samples used in the previous study18 and tested more samples.
Statistical Analyses
The cutoff point was set at mean + 2SD in 156 controls (1.22) for anti-TRIB2 autoantibody index values, as used by Cvetkovic-Lopes et al.9 We also used quality receiver operating characteristic curve (QROC) analysis19 to determine the best diagnostic cutoff point for narcolepsy versus control, leading to a value ≥ 1.22, which, by chance, is also equal to the mean + 2SD. Anti-TRIB2 autoantibody positivity was then compared between cases and controls using Fisher's exact test.
Predictors of anti-TRIB2 autoantibody positivity (≥ 1.22) were next studied by using backward elimination stepwise regression with a probability of 0.05 and/or univariate analysis in all subjects (age, gender, body mass index [BMI], ASO, and rs1154155 genotype [marking the TCRA locus association]) and within narcolepsy-cataplexy subjects only (cataplexy status [presence/absence], DQB1*0602 positivity, HCRT deficiency, age, gender, BMI, ASO, ESS, rs1154155 genotype, and disease duration).
As disease duration below 3 years of onset was associated with higher anti-TRIB2 levels in Cvetkovic-Lopes et al.,9 QROC was also conducted in these cases to determine the best cutoff point between 1 - 3 years of onset in predicting presence of anti-TRIB2 autoantibodies in narcolepsy.
Group parameters such as mean disease duration or % positive were compared using t-test or Fisher's exact test, respectively. Associations were considered statistically significant at < 0.05 (2-tailed test).
RESULTS
Efficiency of the RLA in Detection of Anti-TRIB2 Autoantibodies
As mentioned above, a value of 1.0 represents the mean value of 100 controls. Any positive deviation above this signal represents either noise, or signal, or both. Our mouse monoclonal anti-Trib2 antibody showed a positive reaction, with an anti-TRIB2 autoantibody index of 4.31 on 1:50 dilutions and 1.37 on 1:500 dilutions (mean > 10 experiments), respectively, suggesting any value > 1.37 is definitively positive. Of note, this assay was designed to detect antibodies cross-reactivity directed to any position along the TRIB2 protein.
Anti-TRIB2 Autoantibodies Are Prevalent in Narcolepsy
The presence of anti-TRIB2 autoantibodies was first compared between all cases versus controls. As previously Cvetkovic-Lopes et al. reported,9 we found anti-TRIB2 autoantibody positivity to be higher in narcolepsy (14.3%) versus controls (4.5%) (odds ratio [OR] = 3.5, 95% confidence interval [CI] = 1.5 - 8.3, P = 0.005) (Table 1). No differences were detected for age, gender, BMI, ASO, or rs1154155 genotype.
Presence of Anti-TRIB2 Autoantibodies in Controls Is Not Associated with HLA-DQB1*0602 or ASO Positivity
Our control sample was age and gender matched, but also artificially enriched in DQB1*0602 positivity (carrier frequency in white Americans: 12.7%1) to test whether anti-TRIB2 autoantibody positivity was associated with DQB1*0602 positivity in control group. As shown in Table 1, anti-TRIB2 autoantibodies were found with a similar frequency in DQB1*0602 positive (5.5%) and negative (3.6%) controls (OR = 1.5, 95% CI = 0.3 - 7.1, P = 0.7). Similarly, the prevalence of ASO antibodies was similar in anti-TRIB2 positive (2/7) and negative (50/149) controls (OR = 0.8, 95% CI = 0.1 - 4.2, P = 1).
Prevalence of Anti-TRIB2 Autoantibodies Is Highest in HLA-DQB1*0602 Positive Narcolepsy with Cataplexy
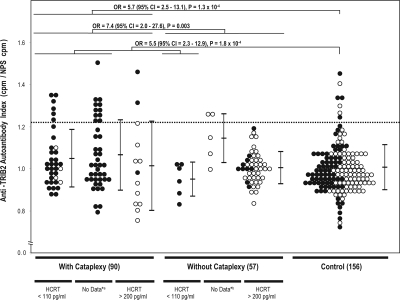
In our univariate models, the best predictors for anti-TRIB2 autoantibody positivity in 147 narcolepsy cases was cataplexy status (OR = 7.4, 95% CI = 1.6 - 32.9, x2 = 10.5, P = 0.001), followed by DQB1*0602 positivity (OR = 6.9, 95% CI = 1.5 - 30.9, c2 = 9.7, P = 0.002) and HCRT deficiency (OR = 3.9, 95% CI = 1.2 - 12.1, x2 = 6.5, P = 0.01). Further analysis found that almost all samples with detected anti-TRIB2 autoantibodies (n = 19) were from DQB1*0602 positive narcolepsy-cataplexy cases (Table 1 and Figure 1). Indeed, in our models, the interaction term, DQB1*0602 and cataplexy status, was the best predictor of anti-TRIB2 positivity in narcolepsy patients (OR = 11.5, 95% CI = 2.6 - 51.5, c2 = 16.9, P = 4.0 × 10−5). The odds ratio of anti-TRIB2 positivity in these cases versus cases of narcolepsy without cataplexy was 9.2 (95% CI = 2.5 - 33.5, P = 6.0 × 10−4) or versus controls was 7.1 (95% CI = 3.1 - 16.2, P = 9.3 × 10−6). Interestingly, two of these cases were DQB1*0602 positive with normal CSF hypocretin-1 level. In addition, 14 narcolepsy-cataplexy cases without DQB1*0602 allele and 16 narcolepsy without cataplexy with DQB1*0602 allele were negative for anti-TRIB2 autoantibodies.
Figure 1.
Anti-TRIB2 autoantibody Index in narcolepsy and control sera. Anti-TRIB2 autoantibody reactivity index is shown in narcolepsy with cataplexy, narcolepsy without cataplexy, and age-gender matched controls. Each dot represents one individual. The cutoff value at mean + 2SD is shown as the dotted line (1.22). Values above this line are considered positive. P values were calculated using Fisher's exact tests. HLA-DQB1*0602 positive, ● negative, ○; * alikely HCRT deficiency (< 110 pg/mL); *blikely normal HCRT (> 200 pg/mL).
Presence of Anti-TRIB2 Autoantibodies Is Associated with Duration of Cataplexy
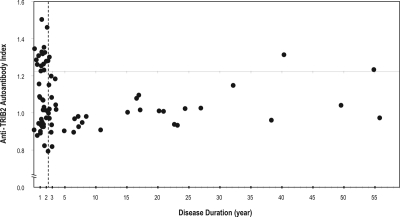
We next compared demographics (cataplexy severity, DQB1*0602 positivity, HCRT deficiency, age, gender, BMI, ASO, ESS, rs1154155 genotype, and disease duration) for the 19 narcolepsy-cataplexy DQB1*0602 positive cases that had anti-TRIB2 autoantibodies versus 57 such cases with no anti-TRIB2 autoantibodies, with special emphasis on recent onset (defined as time from cataplexy onset to blood sampling, Figure 2). As found in Cvetkovic-Lopes et al.,9 disease duration was shorter in cases with detected anti-TRIB2 autoantibodies (mean ± SEM: 6.3 ± 3.4 years) compared to those without autoantibodies (8.6 ± 1.6 years), although this was not significant (P = 0.5). We next performed QROC analysis in DQB1*0602 positive narcolepsy-cataplexy cases to define the best cutoff point for anti-TRIB2 autoantibody detection, which indicated a disease duration ≤ 2.3 years (sensitivity = 0.84, specificity = 0.61). Using this cutoff point as a dichotomous variable defined as “recent onset” in further logistic regression, we found that DQB1*0602 positive narcolepsy-cataplexy cases with recent onset (≤ 2.3 years) were 7.4 times more likely to carry anti-TRIB2 autoantibodies (16/39: 41.0%) than those with more distant onset (3/37: 8.1%) (95% CI = 1.9 - 28.5, x2 = 11.0, P = 9.0 × 10−4).
Figure 2.
The presence of anti-TRIB2 autoantibodies is strongly associated with disease duration. Seventy-six narcolepsy-cataplexy patients were tested. Each dot corresponds to one individual. Anti-TRIB2 indices are plotted as a function of disease duration (age at blood sample collection minus age at onset of cataplexy). Positives have values above 1.22 (see Figure 1). Note that all but 2 positives are disease duration ≤ 2.3 years (best discriminant value using quality receiver operating characteristic curve). The dotted line delineates 2.3 years. Subjects with disease duration below 2.3 years are called recent onset.
Prevalence of Anti-TRIB2 Autoantibodies Is Highest in ASO Antibody Positive Recent Onset Cases
We finally explored whether anti-TRIB2 autoantibodies in 39 recent onset cases were associated with presence of ASO antibodies, a marker of streptococcal infection that was recently found at increased prevalence in the cases close to onset,18 or with DQB1 genotypes other than DQB1*0602 (Tables S1 and S2, availalbe online only at www.journalsleep.org). Sample size was small but we found that presence of anti-TRIB2 autoantibodies was not predicted by a single DQB1 genotype (Table S2). In contrast, recent onset cases with anti-TRIB2 autoantibodies were more frequently ASO positive by Fisher's exact test (OR = 6.2, 95% CI = 1.6 - 24.6, P = 0.01) (Table S1). This effect was independent of disease duration (mean ± SEM: 1.3 ± 0.2 years in 16 recent onset cases with anti-TRIB2 autoantibodies versus 1.3 ± 0.1 years in 23 recent onset cases without anti-TRIB2 autoantibody, P = 1). Age, gender, BMI, cataplexy severity, and ESS were also identical across the two groups. Genotype at the recent TCRA associated marker rs1154155 was also compared across groups but did not show a significant effect (OR = 2.8, 95% CI = 0.7 - 10.9, P = 0.2).
Table S1.
Characteristics of 39 recent onset Caucasian narcolepsy-cataplexy cases
| Anti-TRIB2 Index | Gender | BMI | Age | Cataplexy Onset Age | Disease Duration | Cataplexy Severity (0-8) | Epworth Sleepiness Scale | HCRT (pg/mL) | ASO | rs1154155 (TCR alpha) Risk Allele: C | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Anti-TRIB2 Negative | 0.79 | M | 30.2 | 15.3 | 13 | 2.3 | 7 | 17 | ND | − | AA |
| 0.82 | F | ND | 7.8 | 6 | 1.8 | 8 | 16 | ND | − | AA | |
| 0.88 | F | 18.9 | 7.6 | 7 | 0.6 | 3 | 15 | 37.9 | − | AC | |
| 0.89 | F | 30.7 | 10.1 | 9 | 1.1 | 5 | 13 | 10.0 | + | AA | |
| 0.90 | F | 29.1 | 10.1 | 9 | 1.1 | 8 | 18 | ND | − | AC | |
| 0.91 | M | 28.1 | 48.1 | 48 | 0.1 | ND | 16 | ND | − | AA | |
| 0.93 | M | 19.7 | 12.4 | 11 | 1.4 | 5 | 19 | ND | − | AA | |
| 0.93 | F | 16.2 | 11.6 | 10 | 1.6 | 6 | 21 | 45.6 | − | AA | |
| 0.94 | F | 18.7 | 18.2 | 17 | 1.2 | 8 | 22 | ND | − | AC | |
| 0.94 | F | 22.4 | 13.6 | 12 | 1.6 | 6 | 8 | ND | + | AC | |
| 0.94 | M | 26.4 | 26.8 | 26 | 0.8 | 8 | 23 | ND | − | AA | |
| 0.95 | F | 20.3 | 12.3 | 11 | 1.3 | 7 | 15 | ND | + | AA | |
| 0.96 | F | 24.6 | 16.2 | 15 | 1.2 | 3 | 16 | ND | − | AC | |
| 0.97 | M | 30.4 | 27.0 | 25 | 2.0 | 7 | 16 | ND | − | AA | |
| 1.01 | F | ND | 15.2 | 13 | 2.2 | ND | 14 | ND | − | AC | |
| 1.01 | M | 35.4 | 11.5 | 10 | 1.5 | ND | 10 | ND | + | AA | |
| 1.01 | F | 19.6 | 13.8 | 12 | 1.8 | 4 | 18 | ND | − | AA | |
| 1.03 | F | 36.7 | 12.7 | 11 | 1.7 | 7 | 17 | 0.0 | − | AA | |
| 1.07 | F | 20.0 | 18.5 | 17 | 1.5 | ND | 16 | ND | + | AA | |
| 1.07 | M | 22.9 | 21.3 | 17 | 1.4 | 8 | 16 | ND | − | AA | |
| 1.08 | M | 17.4 | 13.0 | 12 | 1.0 | 7 | 8 | 41.6 | + | AA | |
| 1.09 | F | 18.9 | 17.9 | 17 | 0.9 | 5 | 15 | ND | − | AA | |
| 1.16 | F | 20.7 | 32.1 | 28 | 0.9 | 4 | 16 | ND | − | AA | |
| Mean ± SEM / %Positivity | 0.97 ± 0.02 | F: 65.2% | 24.2 ± 1.3 | 17.1 ± 1.9 | 15.5 ± 1.9 | 1.3 ± 0.1 | 6.1 ± 0.4 | 15.9 ± 0.8 | - | 26.1% | 26.1% |
| Anti-TRIB2 Positive | 1.22 | M | 24.2 | 17.1 | 16 | 1.1 | 8 | 12 | ND | − | AA |
| 1.23 | F | 25.8 | 14.7 | 13 | 1.7 | 7 | 16 | ND | + | AC | |
| 1.25 | M | 18.4 | 13.2 | 12 | 1.2 | 8 | 12 | ND | + | AC | |
| 1.26 | F | 18.2 | 14.5 | 14 | 0.5 | 8 | 15 | 17.0 | + | AA | |
| 1.26 | F | 29.1 | 78.6 | 77 | 1.6 | 4 | 23 | ND | − | AC | |
| 1.28 | M | 21.9 | 31.0 | 29 | 2.0 | 7 | 15 | ND | + | AC | |
| 1.28 | M | 29.0 | 17.3 | 15 | 2.3 | 8 | 20 | ND | + | AA | |
| 1.28 | F | 20.5 | 20.4 | 20 | 0.4 | 8 | 18 | 100.8 | + | AA | |
| 1.31 | F | 24.9 | 6.8 | 6 | 0.8 | 7 | 11 | ND | + | AC | |
| 1.32 | F | ND | 13.4 | 12 | 1.4 | 7 | 18 | 22.0 | + | AC | |
| 1.33 | M | 31.7 | 17.8 | 16 | 1.8 | 7 | 14 | ND | − | AA | |
| 1.33 | M | 28.4 | 15.3 | 14 | 1.3 | 8 | 22 | ND | + | AA | |
| 1.35 | F | 23.3 | 9.1 | 9 | 0.1 | 5 | 17 | 30.1 | + | AC | |
| 1.35 | F | 18.2 | 61.6 | 60 | 1.6 | 5 | 7 | 77.1 | − | CC | |
| 1.46 | F | 30.9 | 30.2 | 28 | 2.2 | 6 | 19 | 353.5 | + | AA | |
| 1.50 | M | ND | 17.3 | 16 | 1.3 | 3 | 15 | ND | − | AA | |
| Mean ± SEM / %Positivity | 1.31 ± 0.02 | F: 56.3% | 24.6 ± 1.2 | 23.7 ± 4.9 | 22.3 ± 4.8 | 1.3 ± 0.2 | 6.6 ± 0.4 | 15.9 ± 1.0 | - | 68.8% | 50.0% |
There were no differences between anti-TRIB2 autoantibodies positive recent onset cases (n = 16) and anti-TRIB2 autoantibodies negative recent onset cases (n = 23) other than ASO positivity (OR = 6.2, 95% CI = 1.6 - 24.6, P = 0.01).
Table S2.
The distribution of second HLA-DQB1 allele in 39 recent onset narcolepsy cases
| Anti-TRIB2 Negative (n = 23) | % | Anti-TRIB2 Positive (n = 16) | % | Fisher exact test P value | |
|---|---|---|---|---|---|
| 0201 | 4 | 17.4 | 1 | 6.3 | 0.63 |
| 0202 | 1 | 4.3 | 2 | 12.5 | 0.56 |
| 0301 | 10 | 43.5 | 7 | 43.8 | - |
| 0302 | 1 | 4.3 | 1 | 6.3 | - |
| 0402 | 1 | 4.3 | 0 | 0.0 | - |
| 0501 | 1 | 4.3 | 0 | 0.0 | - |
| 0503 | 1 | 4.3 | 0 | 0.0 | - |
| 0601 | 1 | 4.3 | 0 | 0.0 | - |
| 0602 | 2 | 8.7 | 4 | 25.0 | 0.21 |
| 0604 | 1 | 4.3 | 1 | 6.3 | - |
All 39 recent onset narcolepsy with cataplexy cases carry at least one HLA-DQB1*0602 allele.
DISCUSSION
We have confirmed the presence of anti-TRIB2 autoantibodies in Caucasian narcolepsy-cataplexy patients. This, together with a companion paper by Toyoda et al. showing similar results in a Japanese sample20 provides for a strong replication of the work of Cvetkovic-Lopes et al.9 across ethnic groups. Most of the narcolepsy subjects carrying anti-TRIB2 autoantibodies had disease onset within 3 years, as previously reported (compare our Figure 2 with Figure 2 in Cvetkovic-Lopes et al.9). Conducting QROC analysis, we further determined that a cutoff point of 2.3 years following cataplexy onset is a strong predictor for presence of the anti-TRIB2 autoantibodies. Using this optimized cutoff, 41.0% of recent onset DQB1*0602 positive cases with cataplexy were anti-TRIB2 positive versus 4% to 8 % in all other groups. This strong difference is however likely to be overestimated since the 2.3-year cutoff for positive value was calculated using the same dataset.
An interesting aspect of this study is the inclusion of 100 cases with documented CSF hypocretin-1 levels. Many of these cases were selected purposefully to encompass the widest range of presentation, explaining the inclusion of rare DQB1*0602 negative cases with cataplexy and normal (n = 10) or low (n = 4) CSF hypocretin-1. In this study, cataplexy rather than low CSF hypocretin-1 correlated with the presence of anti-TRIB2 autoantibodies, but this result should be interpreted cautiously. Indeed, only 6 patients with low CSF hypocretin-1 but without cataplexy were tested. Two of these subjects were young and recent onset, yet were anti-TRIB2 autoantibody negative. Similarly, we tested 4 DQB1*0602 negative narcolepsy-cataplexy cases with low CSF hypocretin-1, and found all to be anti-TRIB2 autoantibody negative (one with recent onset). Finally, we found that 2 out of 3 narcolepsy-cataplexy cases that were DQB1*0602 positive but with normal CSF hypocretin-1 levels were anti-TRIB2 autoantibody positive (one with recent onset), again suggesting a higher correlation with cataplexy and DQB1*0602 positivity than with HCRT deficiency.
A remaining question of interest is why ∼59% of cases are anti-TRIB2 negative, even in recent onset cases. Presence of autoantibodies in other autoimmune diseases is often related to HLA and time since disease onset. However 100% autoantibody positivity is rarely achieved even in selected subgroups.21–23 To address this question, we studied various clinical and genetic features in 39 recent onset DQB1*0602 positive cases with cataplexy (23 anti-TRIB2 negative versus 16 anti-TRIB2 positive cases, see Table S1). Clinically and in terms of disease duration and cataplexy severity, these cases did not differ. A non-significant increase in the prevalence of the rs1154155C in TCRA was found in anti-TRIB2 positive recent onset cases (26.1% carrying rs1154155C in anti-TRIB2 negative, versus 50.0% rs1154155C in anti-TRIB2 positive, P = 0.2). No difference in the occurrence of the second HLA allele located in trans of DQB1*0602 was found (Table S2). Rather, the only significant association was with the presence of ASO antibodies (≥ 200 IU) (26.1% versus 68.8%, P = 0.01), a marker of recent streptococcus infection.17 Even in the 17 ASO positive recent onset subjects however, only 64.7% of cases were anti-TRIB2 positive, thus the TRIB2 association with ASO in narcolepsy-cataplexy is weak and needs replication. Further, we found no association of ASO with anti-TRIB2 autoantibodies in control subjects.
The potential role of Streptococcus pyogenes in triggering narcolepsy has been suggested by two studies.18,24 In a population-based case-control study identifying prevalent cases in King County (Washington), narcolepsy with cataplexy was 5.4-fold more common among people reporting a physician-diagnosed strep throat before the age of 21 years.24 Similarly, Aran et al. recently found increased ASO and anti-DNAse B levels in Caucasian narcoleptic patients within 1-3 years of onset.18 Increased co-occurrence of ASO and anti-TRIB2 autoantibodies could be explained either by molecular mimicry between TRIB2 and subtypes of Streptococcus, or by the existence of other infections frequently co-occurring with Streptococcus then mediating the anti-TRIB2 autoantibodies positive autoimmune reaction. The association of various autoimmune diseases with various infections is increasingly recognized.25
Although the presence of anti-TRIB2 autoantibodies is clearly associated with recent cataplexy onset, it is unclear if anti-TRIB2 is causing HCRT cell death. TRIB2 has widespread brain and glial expression, and is not solely expressed in HCRT cells.9 Anti-TRIB2 autoantibodies may be the by-product of a co-occurring infection or be generated as an indirect non-specific immune response to neuronal damage. In most autoimmune diseases, multiple target antibodies are produced due to epitope spreading, and are usually non-pathogenic. Alternatively, HCRT cells may be preferentially vulnerable to anti-TRIB2 autoantibodies, explaining primary damage to these cells but not others. Active and passive immune transfer studies of anti-TRIB2 in animals may help answer this question.
The potential of anti-TRIB2 autoantibodies as a diagnostic marker for narcolepsy also warrants further exploration. Even in recent onset cases, however, only 41.0% positivity was documented. Technical advances may reveal higher positivity rates, either by better documentation of the involved epitope(s), or by increasing sensitivity/specificity of the assay.21–23 The study of other groups of patients is also needed. Studies to date have only studied hypersomnia, multiple sclerosis, and other inflammatory neurological diseases.9,20 Cases with neuronal damage such as post–head trauma or severe neurodegeneration should be explored. In an earlier study, anti-TRIB2 autoantibody positivity was found in 3 out of 10 cases of autoimmune (anterior) uveitis.10 Whether or not transient uveitis is more frequent around narcolepsy onset will need exploration, together with a confirmation of anti-TRIB2 in a larger sample of uveitis patients. Uveitis is characterized by attacks of pain, watering, redness, blurred vision, and photophobia, and is typically only diagnosed once multiple attacks have occurred.26 As autoimmune uveitis is also related to other autoimmune diseases, most typically ankylosing spondylitis, Behçcet's disease, and Vogt-Koyanagi-Harada syndrome, testing serum of patients with these conditions may be helpful.
One may wonder whether anti-TRIB2 is the first of many other narcolepsy-associated autoantibodies that will be identified in the near future. Tanaka and Honda recently reported IgG abnormalities in narcolepsy and other hypersomnia patients, suggesting a broader reaction.27 Although narcolepsy is associated with HLA and TCRA polymorphisms, autoantibodies specific to HCRT cells have been difficult to detect reliably using immunocytochemistry, western blots and other assays.16,28,29 Jackson et al.30 suggested the presence of autoantibodies interfering with colonic migrating contractions and bladder contractile responses using functional assays in animals, but this work awaits replication. The fact anti-TRIB2 antibodies are primarily found in recent onset cases, suggests the need for searching new autoimmune markers in this population.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Mignot has consulted for Jazz, Actelion, Roche, and Cephalon; is on the advisory board of Eli Lilly; has participated in lectures for Roche; has received research support from Jazz; and has financial interests in Resmed. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
We thank patients and families for participation in this study, Anna Voros and Jing Zhang for sample preparation, Juliette Faraco and Tom Rico for edits, and Mali Einen for her assistance in recruiting. The study was funded by NIH grant NS-23724 to Dr. Emmanuel Mignot, MSM 0021620848 to Dr. Sona Nevsimalova, and Minae Kawashima is a JSPS Postdoctoral Fellow for Research Abroad.
Experiments—Informed consent: institute 1, 2, 3, 4, 5; Autoantibody measurement: institute 1; Constructed vectors: institute 1, 2; Radioligand binding assay: institute 1; Statistical analyses: institute 1.
Footnotes
A commentary on this article appears in this issue on page 857.
REFERENCES
- 1.Mignot E, Lin L, Rogers W, et al. Complex HLA-DR and -DQ interactions confer risk of narcolepsy-cataplexy in three ethnic groups. Am J Hum Genet. 2001;68:686–99. doi: 10.1086/318799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nishino S, Ripley B, Overeem S, Lammers GJ, Mignot E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet. 2000;355:39–40. doi: 10.1016/S0140-6736(99)05582-8. [DOI] [PubMed] [Google Scholar]
- 3.Mignot E, Lammers GJ, Ripley B, et al. The role of cerebrospinal fluid hypocretin measurement in the diagnosis of narcolepsy and other hypersomnias. Arch Neurol. 2002;59:1553–62. doi: 10.1001/archneur.59.10.1553. [DOI] [PubMed] [Google Scholar]
- 4.Peyron C, Faraco J, Rogers W, et al. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med. 2000;6:991–7. doi: 10.1038/79690. [DOI] [PubMed] [Google Scholar]
- 5.Lin L, Faraco J, Li R, et al. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–76. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- 6.Chemelli RM, Willie JT, Sinton CM, et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–51. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- 7.Scammell TE. The frustrating and mostly fruitless search for an autoimmune cause of narcolepsy. Sleep. 2006;29:601–2. doi: 10.1093/sleep/29.5.601. [DOI] [PubMed] [Google Scholar]
- 8.Hallmayer J, Faraco J, Lin L, et al. Narcolepsy is strongly associated with the T-cell receptor alpha locus. Nat Genet. 2009;41:708–11. doi: 10.1038/ng.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cvetkovic-Lopes V, Bayer L, Dorsaz S, et al. Elevated Tribbles homolog 2-specific antibody levels in narcolepsy patients. J Clin Invest. 2010;120:713–9. doi: 10.1172/JCI41366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y, Davis JL, Li W. Identification of tribbles homolog 2 as an autoantigen in autoimmune uveitis by phage display. Mol Immunol. 2005;42:1275–81. doi: 10.1016/j.molimm.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 11.Kimura M, Tatsumi K, Tada H, et al. Anti-CYP2D6 antibodies detected by quantitative radioligand assay and relation to antibodies to liver-specific arginase in patients with autoimmune hepatitis. Clinica Chimica Acta. 2002;316:155–64. doi: 10.1016/s0009-8981(01)00747-1. [DOI] [PubMed] [Google Scholar]
- 12.Kerkar N, Ma Y, Hussain M, et al. A novel assay for detecting antibodies to cytochrome P4502D6, the molecular target of liver kidney microsomal antibody type 1. J Immunol Methods. 1999;223:227–35. doi: 10.1016/s0022-1759(98)00213-0. [DOI] [PubMed] [Google Scholar]
- 13.American Academy of Sleep Medicine. International Classification of Sleep Disorders, 2nd ed, Diagnostic & Coding Manual. Westchester, IL: 2005. [Google Scholar]
- 14.Hong SC, Lin L, Jeong JH, et al. A study of the diagnostic utility of HLA typing, CSF hypocretin-1 measurements, and MSLT testing for the diagnosis of narcolepsy in 163 Korean patients with unexplained excessive daytime sleepiness. Sleep. 2006;29:1429–38. doi: 10.1093/sleep/29.11.1429. [DOI] [PubMed] [Google Scholar]
- 15.Bourgin P, Zeitzer JM, Mignot E. CSF hypocretin-1 assessment in sleep and neurological disorders. Lancet Neurol. 2008;7:649–62. doi: 10.1016/S1474-4422(08)70140-6. [DOI] [PubMed] [Google Scholar]
- 16.Tanaka S, Honda Y, Inoue Y, Honda M. Detection of autoantibodies against hypocretin, hcrtrl, and hcrtr2 in narcolepsy: anti-Hcrt system antibody in narcolepsy. Sleep. 2006;29:633–8. doi: 10.1093/sleep/29.5.633. [DOI] [PubMed] [Google Scholar]
- 17.Todd EW. Antigenic streptococcal hemolysin. J Exp Med. 1932;55:267–80. doi: 10.1084/jem.55.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aran A, Lin L, Nevsimalova S, et al. Elevated anti-streptococcal antibodies in patients with recent narcolepsy onset. Sleep. 2009;32:979–83. doi: 10.1093/sleep/32.8.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kraemer HC. Evaluating Medical Tests. SAGE Publications Inc. 1992 [Google Scholar]
- 20.Toyoda H, Tanaka S, Miyagawa T, Honda Y, Tokunaga K, Honda M. Anti-Tribbles homolog 2 autoantibodies in Japanese patients with narcolepsy. Sleep. 2010;33:875–878. doi: 10.1093/sleep/33.7.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahler M, Fritzler MJ. Epitope specificity and significance in systemic autoimmune diseases. Ann N Y Acad Sci. 2010;1183:267–87. doi: 10.1111/j.1749-6632.2009.05127.x. [DOI] [PubMed] [Google Scholar]
- 22.Castro C, Gourley M. Diagnostic testing and interpretation of tests for autoimmunity. J Allergy Clin Immunol. 2010;125:S238–47. doi: 10.1016/j.jaci.2009.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bingley PJ. Clinical applications of diabetes antibody testing. J Clin Endocrinol Metab. 2010;95:25–33. doi: 10.1210/jc.2009-1365. [DOI] [PubMed] [Google Scholar]
- 24.Koepsell T, Longstreth W, Ton T. Medical exposures in youth and the frequency of narcolepsy with cataplexy: a population-based case-control study in genetically predisposed people. J Sleep Res. 2010;19:80–6. doi: 10.1111/j.1365-2869.2009.00756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sfriso P, Ghirardello A, Botsios C, et al. Infections and autoimmunity: the multifaceted relationship. J Leukoc Biol. 2010;87:385–95. doi: 10.1189/jlb.0709517. [DOI] [PubMed] [Google Scholar]
- 26.Agrawal RV, Murthy S, Sangwan V, Biswas J. Current approach in diagnosis and management of anterior uveitis. Indian J Ophthalmol. 2010;58:11–9. doi: 10.4103/0301-4738.58468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanaka S, Honda M. IgG abnormality in narcolepsy and idiopathic hypersomnia. PLoS One. 2010;5:e9555. doi: 10.1371/journal.pone.0009555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Honda M, Eriksson KS, Zhang S, et al. IGFBP3 colocalizes with and regulates hypocretin (orexin) PLoS One. 2009;4:e4254. doi: 10.1371/journal.pone.0004254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Overeem S, Black JL, 3rd, Lammers GJ. Narcolepsy: immunological aspects. Sleep Med Rev. 2008;12:95–107. doi: 10.1016/j.smrv.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jackson MW, Spencer NJ, Reed JH, Smith AJ, Gordon TP. Potentiation of a functional autoantibody in narcolepsy by a cholinesterase inhibitor. Lab Invest. 2009;89:1332–9. doi: 10.1038/labinvest.2009.108. [DOI] [PubMed] [Google Scholar]