Abstract
Study Objectives:
Narcolepsy is a sleep disorder characterized by excessive daytime sleepiness and cataplexy. The association with human leukocyte antigen (HLA)-DQB1*0602 and T-cell receptor alpha locus suggests that autoimmunity plays a role in narcolepsy. A recent study reported an increased prevalence of autoantibodies against Tribbles homolog 2 (TRIB2) in patients with narcolepsy. To replicate this finding, we examined anti-TRIB2 autoantibodies in Japanese patients with narcolepsy.
Design:
We examined anti-TRIB2 autoantibodies against a full-length [35S]-labeled TRIB2 antigen in Japanese patients with narcolepsy-cataplexy (n = 88), narcolepsy without cataplexy (n = 18), and idiopathic hypersomnia with long sleep time (n = 11). The results were compared to Japanese healthy controls (n = 87). Thirty-seven healthy control subjects were positive for HLA-DRB1*1501-DQB1*0602. We also examined autoantibodies against another Tribbles homolog, TRIB3, as an experimental control.
Measurements and Results:
Autoantibodies against TRIB2 were found in 26.1% of patients with narcolepsy-cataplexy, a significantly higher prevalence than the 2.3% in healthy controls. We found that anti-TRIB3 autoantibodies were rare in patients with narcolepsy and showed no association with anti-TRIB2 indices. No significant correlation was found between anti-TRIB2 positivity and clinical information.
Conclusions:
We confirmed the higher prevalence and specificity of anti-TRIB2 autoantibodies in Japanese patients with narcolepsy-cataplexy. This suggests a subgroup within narcolepsy-cataplexy might be affected by an anti-TRIB2 autoantibody-mediated autoimmune mechanism.
Citation:
Toyoda H; Tanaka S; Miyagawa T; Honda Y; Tokunaga K; Honda M. Anti-Tribbles homolog 2 autoantibodies in Japanese patients with narcolepsy. SLEEP 2010;33(7):875-878.
Keywords: Narcolepsy, autoantibody, Tribbles homolog 2
NARCOLEPSY IS A SLEEP DISORDER CHARACTERIZED BY EXCESSIVE DAYTIME SLEEPINESS AND CATAPLEXY. NARCOLEPSY AFFECTS 0.16% TO 0.18% of the general population in Japan and 0.02% to 0.06% of the population in the United States and Europe.1 Several reports have demonstrated that the level of hypocretin (HCRT) is reduced to undetectable levels in the cerebrospinal fluid (CSF) of more than 90% of patients with narcolepsy-cataplexy2–4 and the reduction of HCRT neurons in the postmortem hypothalami of patients with narcolepsy.5,6 Narcolepsy is associated with the human leukocyte antigen (HLA)-DQB1*0602 allele7 and a single-nucleotide polymorphism located in the T-cell receptor alpha-locus,8 suggesting an immunological abnormality in narcolepsy. Therefore, several studies exploring the pathophysiology of narcolepsy have been based on the hypothesis that an autoimmune-mediated process targets hypothalamic HCRT neurons. However, no definitive answers have been obtained so far.9
Recently, genetically engineered mice were utilized to identify the expression profiling of genes enriched in mice hypothalamic Hcrt neurons.10 Twenty-three genes were found to be specifically expressed in Hcrt neurons. Among them, Tribbles homolog 2 (TRIB2) was the only gene found to be an autoantigen. TRIB2 was previously reported to be associated with human autoimmune uveitis.11 Using an enzyme-linked immunosorbent assay (ELISA) method, higher anti-TRIB2 titers were detected in sera from patients with narcolepsy compared to healthy controls.10
This finding prompted us to re-evaluate the presence of anti-TRIB2 autoantibodies in Japanese narcolepsy patients. We have developed a radioligand binding assay that uses recombinant full-length human [35S]-TRIB2 produced by in vitro transcription/translation as an antigen. The radioligand binding assay is well known to detect autoantibodies more effectively than the ELISA system, especially autoantibodies against conformational epitopes.12,13
MATERIALS AND METHODS
Subjects
This study was approved by the ethics committee of all collaborative organizations. Written informed consent was obtained from all study participants. Diagnosis was made according to the International Classification of Sleep Disorders, 2nd edition1 at the Neuropsychiatric Research Institute (Tokyo, Japan). Blood samples and data related to sleep conditions were collected at the Tokyo Institute of Psychiatry (Tokyo, Japan) and Neuropsychiatric Research Institute (Tokyo, Japan). Control subjects were excluded if they had excessive daytime sleepiness or any signs of immunologic abnormalities based on responses to a questionnaire at the time of blood collection. Five milliliters of venous blood were drawn, and separated sera were stored at −80°C until use. Genotyping for HLA-DRB1 and DQB1 loci were done at NPO HLA Laboratory (Kyoto, Japan) using polymerase chain reaction (PCR)-sequence specific oligonucleotide probes coupled with the Luminex bioassay detection system (Wakunaga, Hiroshima, Japan). Patients with narcolepsy-cataplexy were all positive for the HLA-DRB1*1501-DQB1*0602 allele. Thirty-seven of 87 healthy controls, 9 of 18 patients with narcolepsy without cataplexy, and 2 of 11 patients with idiopathic hypersomnia with long sleep time were positive for the HLA-DRB1*1501-DQB1*0602 allele. All subjects were unrelated Japanese individuals living in Tokyo or in neighboring areas. We collected the following data to analyze potential relationships with the autoantibody index: age, sex, body mass index, disease duration, Epworth Sleepiness Scale at the time of blood collection, presence of nocturnal sleep disruption (subjective report), and past history of autoimmune disorders. CSF was not collected from the subjects for analysis. The mean age and sex distributions are summarized in Table 1. Distribution of age and sex of the patient groups was not significantly different from that of the healthy controls by the Mann-Whitney U test.
Table 1.
Age, sex, and anti-TRIB2 positive reaction in patients with hypersomnia and healthy controls
| Subjects | Number examined | Age (years) (mean ± SD) | Female/Male | Number of positive reaction (%) over mean+2SD | Number of positive reaction (%) over mean+3SD | TRIB2 index (mean ± SD) |
|---|---|---|---|---|---|---|
| Narcolepsy with cataplexy | 88 | 43.5 ± 18.3 | 40/48 | 23 (26.1)a | 14 (15.9)b | 16.0 ± 3.6c |
| Narcolepsy without cataplexy | 18 | 34.8 ± 10.0 | 7/11 | 1 (5.6)† | 0 (0.0)† | 13.0 ± 2.0† |
| Idiopathic hypersomnia | 11 | 34.3 ± 7.1 | 6/5 | 0 (0.0)† | 0 (0.0)† | 14.0 ± 1.9† |
| Healthy control | 87 | 38.6± 10.3 | 42/45 | 2 (2.3) | 0 (0.0) | 14.7 ± 1.5 |
Distribution of age and sex of the patient groups was not significantly different from those of healthy controls.
P = 4.8 × 10−6.
P = 7.0 × 10−5.
P = 0.01.
No significant differences between healthy controls and patients with narcolepsy without cataplexy or healthy controls and patients with idiopathic hypersomnia were found. SD, standard deviation.
Construction of TRIB2 and TRIB3 Expression Vectors
The open-reading frame of TRIB2 or TRIB3 was obtained by reverse transcription-polymerase chain reaction (RT-PCR) amplification using the human hypothalamic poly-A RNA (Takara Bio, Kyoto, Japan) or human lymphocytic total RNA.14 TRIB3 expression was found in human hypothalamus, though its expression was weak (Figure S1, available online only at www.journalsleep.org). Therefore, we also examined anti-TRIB3 autoantibodies as experimental controls. The following primer pairs with either a BamHI or a XhoI site for TRIB2 or an EcoRI or a XhoI site for TRIB3, 5′-CGCGGATCCATGAACATACACAGGTCTA-3′ (TRIB2 forward) and 5′-CCGCTCGAGATTCTTGGCCAACTGTTCCTT-3′ (TRIB2 reverse) (the BamHI and XhoI sites are underlined) or 5′-GGAATTCATGCGAGCCACCCCTCTGGCTGC-3′ (TRIB3 forward) and 5′-CCGCTCGAGACAGACCCTGCCTAATCCTGT (TRIB3 reverse) (the EcoRI and XhoI sites are underlined), were used for each amplification. TRIB2 or TRIB3 amplification product was ligated into the pET28a (+) expression vector (Novagen, Madison, WI, USA).
Synthesis of Recombinant [35S]-TRIB2 and [35S]-TRIB3 Antigens and the Radioligand Binding Assay
The antigen-antibody reaction was conducted with 1 γL of serum sample obtained from subjects and 20,000 cpm of [35S]-labeled recombinant antigen (50 γL in total). The precise methods for synthesis of recombinant [35S]-antigens and radioligand binding assay were described previously.15 To avoid inter-assay variation, the results were expressed by an autoantibody index calculated as follows: 100 × cpm of the sample serum / cpm of mouse monoclonal anti-TRIB2 or anti-TRIB3 antibody (diluted in 1:500). As a result, the intra-assay coefficient of variation ranged from 2.6% to 9.6%, and the inter-assay coefficient of variation ranged from 1.5% to 9.0%. The cut-off value was set at the mean + 2SD in healthy controls. The antibodies used as an internal control were commercially available [mouse anti-TRIB2 (sc-100878) or mouse anti-TRIB3 (sc-100879), Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA].
Statistical Analyses
The positive reactions against TRIB2 and TRIB3 were compared between patient groups and healthy controls by Fisher exact test. Distribution of TRIB2/3 antibody indices was compared by the Mann-Whitney U test (one-tailed test) between patient groups and healthy controls. The Pearson correlation coefficient was used to calculate correlations between the antibody indices and the following clinical information: body mass index, disease duration, and Epworth Sleepiness Scale as an indicator for severity of sleepiness. A P-value < 0.05 was considered statistically significant.
RESULTS
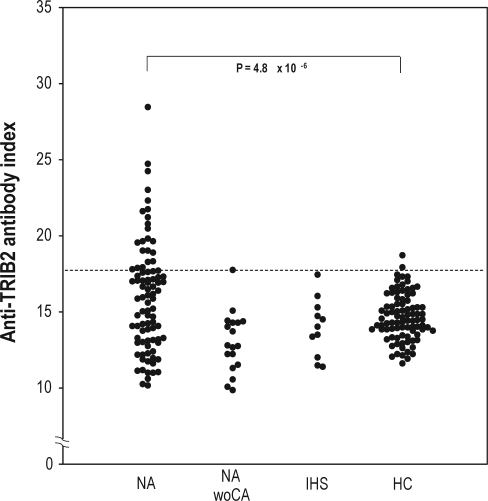
Using a radioligand binding assay, we found autoantibodies against TRIB2 in sera from Japanese patients and compared the levels with those of healthy controls. Demographic data and percentages of positive reactions are summarized in Table 1. With the cutoff value at 2 SD above the mean in healthy controls, sera from 23 of 88 patients with narcolepsy-cataplexy (26.1%) and 2 of 87 healthy controls (2.3%) showed a positive reaction (Fisher exact test, P = 4.8 × 10-6) against TRIB2 antigen (Figure 1, Table 1). One of 18 patients with narcolepsy without cataplexy and no patients with idiopathic hypersomnia showed a positive reaction against TRIB2 antigen in their sera. Even with the cutoff value at 3SD, the anti-TRIB2 autoantibody positive rate was significantly higher in patients with narcolepsy-cataplexy (14 of 88 patients with narcolepsy-cataplexy versus 0 of 87 healthy controls; Fisher's exact test, P = 7.0 × 10-5) (Table 1). Indices of anti-TRIB2 autoantibodies in patients with narcolepsy-cataplexy were also significantly higher compared to healthy controls (Mann-Whitney U test, P = 0.01) (Table 1).
Figure 1.
Autoantibody index of human TRIB2. Indices of autoantibodies in patients with narcolepsy-cataplexy, narcolepsy without cataplexy, and idiopathic hypersomnia with long sleep time, and healthy controls are represented. Each dot corresponds to data from one subject. The dotted line indicates the mean + 2SD levels in healthy controls. The indices with subjects above this line were considered to be positive. NA, narcolepsy with cataplexy; NAwoCA, narcolepsy without cataplexy; IHS, idiopathic hypersomnia with long sleep time; HC, healthy control.
We also examined autoantibodies against TRIB3 using the same subjects as experimental controls. While a positive reaction against TRIB3 antigen was detected in one patient with narcolepsy-cataplexy, in contrast to TRIB2, no significant differences between groups were found (Figure S2, available online only at www.journalsleep.org).
We analyzed correlations between anti-TRIB2 positive reactions and clinical information, body mass index, disease duration, and Epworth Sleep Scale score. No significant correlation was found when examining either the entire patient cohort, or just the 26.1% of the narcolepsy-cataplexy patients with high titers (> 2 SD) of anti-TRIB2 antibodies.
DISCUSSION
A recent study identified elevated levels of autoantibodies against TRIB2 in patients with narcolepsy-cataplexy.10 In the present study, we re-evaluated this finding by testing for autoantibodies against TRIB2 in Japanese patients with narcolepsy-cataplexy. A significant number of patients with narcolepsy-cataplexy showed a positive reaction for anti-TRIB2 autoantibodies compared to healthy controls.
We also conducted semi-quantitative RT-PCR using human hypothalamic and hippocampus cDNAs (Takara Bio, Kyoto, Japan) and found that TRIB2 expression in the human hypothalamus is much higher than that in the human hippocampus (Figure S1). The specific reactions against TRIB2 in narcolepsy and its high expression in the hypothalamus are consistent with the hypothesis that anti-TRIB2 autoantibodies target hypothalamic HCRT neurons, resulting in HCRT loss in narcolepsy.
To further confirm the specificity for anti-TRIB2 autoantibodies in patients with narcolepsy, we examined the reactivity against another Tribbles homolog expressed in human hypothalamus, TRIB3 (Figure S1), using the same sera samples. Although the amino acid sequences of human TRIB2 share 52% identity with human TRIB3 overall, the 28 C-terminal amino acids of TRIB2 used as an ELISA antigen10 have only a low identity with TRIB3. We found a significant incidence of anti-TRIB2 positivity using a full-length TRIB2 antigen containing the C-terminal region, and only one patient with narcolepsy-cataplexy showed a positive reaction against TRIB3. These results suggest that anti-TRIB2 autoantibodies might recognize the TRIB2 C-terminal region, which has a low sequence identity with TRIB3.
Unlike recent reports,10,16 the relationship between disease duration and anti-TRIB2 autoantibody levels was not replicated in the present study (Figure S3, available online only at www.journalsleep.org). We had a limited number of narcolepsy patients within 5 years of disease onset compared to other groups, although 2 of our 5 patients with recent onset showed higher anti-TRIB2 autoantibody indices (> 2 SD). The number of narcolepsy patients with recent onset might not be sufficient to discuss the relationship between disease duration and anti-TRIB2 autoantibody in our Japanese group.
In conclusion, we confirmed the presence of highly specific autoantibodies against TRIB2 antigen in Japanese patients with narcolepsy-cataplexy as reported in a recent study.10
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
We thank all of the participants in this study. We also thank Ms. Miyuki Fukazawa (Tokyo Institute of Psychiatry) for processing the blood samples and Dr. Simon Christopher Warby (Stanford University) for proofreading our manuscript.
This work was supported by Grants-in-Aid for Scientific Research (No. 20790868 to S. Tanaka, No. 19390310 to M. Honda) from the Ministry of Education, Science, and Culture of Japan, and in part by grants from the Mitsubishi Pharma Research Foundation (to S. Tanaka) and the “Comprehensive Genomics” Priority Area from the Ministry of Education, Science and Culture of Japan (to M. Honda and to K. Tokunaga). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
A commentary on this article appears in this issue on page 857.
Supplementary Material
Expression of TRIB2 and TRIB3 in human hypothalamus and hippocampus. Expression of genes were analyzed by RT–PCR. GAPDH and ACTB were used as loading controls. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; ACTB, actin-beta.
Autoantibody index of human TRIB3. Indices of autoantibodies in patients with narcolepsy-cataplexy, narcolepsy without cataplexy, and idiopathic hypersomnia with long sleep time and healthy controls are represented. Each dot corresponds to data from one subject. The dotted line indicates the mean + 2SD levels in healthy controls. The indices with subjects above this line were considered to be positive. NA, narcolepsy with cataplexy; NAwoCA, narcolepsy without cataplexy; IHS, idiopathic hypersomnia with long sleep time; HC, healthy control.
Indices of anti-TRIB2 autoantibodies in Japanese patients are not associated with disease duration. Indices of autoantibodies in patients with narcolepsy-cataplexy are plotted. Each dot corresponds to data from one subject. The dotted line indicates the mean + 2SD levels in healthy controls.
REFERENCES
- 1.American Academy of Sleep Medicine. Diagnostic – coding manual. 2nd ed. Westchester, IL; 2005. International classification of sleep disorders. [Google Scholar]
- 2.Mignot E, Lammers GJ, Ripley B, et al. The role of cerebrospinal fluid hypocretin measurement in the diagnosis of narcolepsy and other hypersomnias. Arch Neurol. 2002;59:1553–62. doi: 10.1001/archneur.59.10.1553. [DOI] [PubMed] [Google Scholar]
- 3.Nishino S, Ripley B, Overeem S, Lammers GJ, Mignot E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet. 2000;355:39–40. doi: 10.1016/S0140-6736(99)05582-8. [DOI] [PubMed] [Google Scholar]
- 4.Hong SC, Lin L, Jeong JH, et al. A study of the diagnostic utility of HLA typing, CSF hypocretin-1 measurements, and MSLT testing for the diagnosis of narcolepsy in 163 Korean patients with unexplained excessive daytime sleepiness. Sleep. 2006;29:1429–38. doi: 10.1093/sleep/29.11.1429. [DOI] [PubMed] [Google Scholar]
- 5.Peyron C, Faraco J, Rogers W, et al. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med. 2000;6:991–7. doi: 10.1038/79690. [DOI] [PubMed] [Google Scholar]
- 6.Thannickal TC, Moore RY, Nienhuis R, et al. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27:469–74. doi: 10.1016/s0896-6273(00)00058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mignot E, Lin L, Rogers W, et al. Complex HLA-DR and -DQ interactions confer risk of narcolepsy-cataplexy in three ethnic groups. Am J Hum Genet. 2001;68:686–99. doi: 10.1086/318799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hallmayer J, Faraco J, Lin L, et al. Narcolepsy is strongly associated with the T-cell receptor alpha locus. Nat Genet. 2009;41:708–11. doi: 10.1038/ng.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scammell TE. The frustrating and mostly fruitless search for an autoimmune cause of narcolepsy. Sleep. 2006;29:601–2. doi: 10.1093/sleep/29.5.601. [DOI] [PubMed] [Google Scholar]
- 10.Cvetkovic-Lopes V, Bayer L, Dorsaz S, et al. Elevated Tribbles homolog 2-specific antibody levels in narcolepsy patients. J Clin Invest. 2010;120:713–9. doi: 10.1172/JCI41366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y, Davis JL, Li W. Identification of tribbles homolog 2 as an autoantigen in autoimmune uveitis by phage display. Mol Immunol. 2005;42:1275–81. doi: 10.1016/j.molimm.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 12.Kimura M, Tatsumi KI, Tada H, et al. Anti-CYP2D6 antibodies detected by quantitative radioligand assay and relation to antibodies to liver-specific arginase in patients with autoimmune hepatitis. Clin Chim Acta. 2002;316:155–64. doi: 10.1016/s0009-8981(01)00747-1. [DOI] [PubMed] [Google Scholar]
- 13.Kerkar N, Ma Y, Hussain M, et al. A novel assay for detecting antibodies to cytochrome P4502D6, the molecular target of liver kidney microsomal antibody type 1. J Immunol Methods. 1999;223:227–35. doi: 10.1016/s0022-1759(98)00213-0. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka S, Honda Y, Honda M. Identification of differentially expressed genes in blood cells of narcolepsy patients. Sleep. 2007;30:974–9. doi: 10.1093/sleep/30.8.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanaka S, Honda Y, Inoue Y, Honda M. Detection of autoantibodies against hypocretin, hcrtrl, and hcrtr2 in narcolepsy: anti-hcrt system antibody in narcolepsy. Sleep. 2006;29:633–8. doi: 10.1093/sleep/29.5.633. [DOI] [PubMed] [Google Scholar]
- 16.Kawashima M, Lin L, Tanaka S, et al. Anti-Tribbles homolog 2 (TRIB2) autoantibodies in narcolepsy are associated with recent onset of cataplexy. Sleep. 2010;33:869–874. doi: 10.1093/sleep/33.7.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Expression of TRIB2 and TRIB3 in human hypothalamus and hippocampus. Expression of genes were analyzed by RT–PCR. GAPDH and ACTB were used as loading controls. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; ACTB, actin-beta.
Autoantibody index of human TRIB3. Indices of autoantibodies in patients with narcolepsy-cataplexy, narcolepsy without cataplexy, and idiopathic hypersomnia with long sleep time and healthy controls are represented. Each dot corresponds to data from one subject. The dotted line indicates the mean + 2SD levels in healthy controls. The indices with subjects above this line were considered to be positive. NA, narcolepsy with cataplexy; NAwoCA, narcolepsy without cataplexy; IHS, idiopathic hypersomnia with long sleep time; HC, healthy control.
Indices of anti-TRIB2 autoantibodies in Japanese patients are not associated with disease duration. Indices of autoantibodies in patients with narcolepsy-cataplexy are plotted. Each dot corresponds to data from one subject. The dotted line indicates the mean + 2SD levels in healthy controls.