Abstract
Study Objectives:
The main energy reserve of the brain is glycogen, which is almost exclusively localized in astrocytes. We previously reported that cerebral expression of certain genes related to glycogen metabolism changed following instrumental sleep deprivation in mice. Here, we extended our investigations to another set of genes related to glycogen and glucose metabolism. We also compared the effect of instrumentally and pharmacologically induced prolonged wakefulness, followed (or not) by 3 hours of sleep recovery, on the expression of genes related to brain energy metabolism.
Design:
Sleep deprivation for 6–7 hours.
Setting:
Animal sleep research laboratory.
Participants:
Adults OF1 mice.
Interventions:
Wakefulness was maintained by “gentle sleep deprivation” method (GSD) or by administration of the wakefulness-promoting drug modafinil (MOD) (200 mg/kg i.p.).
Measurements and Results:
Levels of mRNAs encoding proteins related to energy metabolism were measured by quantitative real-time PCR in the cerebral cortex. The mRNAs encoding protein targeting to glycogen (PTG) and the glial glucose transporter were significantly increased following both procedures used to prolong wakefulness. Glycogenin mRNA levels were increased only after GSD, while neuronal glucose transporter mRNA only after MOD. These effects were reversed after sleep recovery. A significant enhancement of glycogen synthase activity without any changes in glycogen levels was observed in both conditions.
Conclusions:
These results indicate the existence of a metabolic adaptation of astrocytes aimed at maintaining brain energy homeostasis during the sleep-wake cycle.
Citation:
Petit, JM; Tobler I; Kopp C; Morgenthaler F; Borbély AA; Magistretti PJ. Metabolic response of the cerebral cortex following gentle sleep deprivation and modafinil administration. SLEEP 2010;33(7):901–908.
Keywords: Energy metabolism, astrocytes, glycogen, glucose, psychostimulant, wakefulness
THERE IS GROWING EVIDENCE INDICATING THAT NEURONAL MECHANISMS THAT CONTROL THE SLEEP-WAKING CYCLE ARE INTERCONNECTED WITH the mechanisms that regulate energy homeostasis.1,2 Moreover, the homeostatic regulation of sleep fits the canonical view that the role of sleep to restore brain energy homeostasis, most recently reformulated by Benington and Heller.3 In this view, sleep, and in particular slow wave sleep (SWS), might serve to replenish glycogen stores depleted during the waking period, thus defining an anabolic state of the brain during sleep. Glycogen constitutes the main brain energy reserve under the form of branched chains of glycosyl units.4 In the cerebral cortex, glycogen is exclusively localized in astrocytes and its levels are tightly regulated by neuronally derived signals, such as the neurotransmitters vasoactive intestinal peptide (VIP) and noradrenaline.5 These modalities of regulation represent one of the many examples of neuron-glia metabolic coupling.6 We have previously observed the existence of a metabolic plasticity for certain genes related to glycogen metabolism.7 Thus we have shown that the level of expression of genes encoding glycogen synthase (GSynt), glycogen phosphorylase (GPhos) and protein targeting to glycogen (PTG) were modulated throughout the sleep-waking cycle and after 6 h of “gentle” sleep deprivation (GSD) in the cerebral cortex. The GSynt and GPhos are the enzymes responsible for glycogen synthesis and degradation respectively. PTG is a regulatory subunit of the protein phosphatase1 known to activate GSynt and to inactivate GPhos in peripheral tissues8,9 as well as in primary culture of astrocytes.10 Here we have extended our analysis to the gene encoding glycogenin, the protein that acts as primer for glycogen granules,11 as well as to other genes involved in neuron-glia metabolic coupling such as glucose transporters (Glut1 and Glut3) and Hexokinase 1 (HeK1). We also examined whether the changes in neuron-glia metabolic induced by GSD can be generalized to pharmacological induction of sleep deprivation, such as for example with the one induced by the wakefulness-promoting drug Modafinil. Moreover, we also assessed the effect of sleep recovery on the expression level of the same set of genes. Because different factors such as animal activity, blood glucose, and stress can affect peripheral as well as brain glycogen levels,12,13 we determined cerebrocortical glycogen levels, locomotor activity, plasma cortisol levels and glycemia in the same experimental conditions. Results indicate that sleep deprivation, independent of the method used, leads to a similar pattern of gene activation functionally characterized by an orientation of glycogen metabolism towards its synthesis and to the facilitation of glucose entry into the brain parenchyma.
MATERIALS AND METHODS
Animals
Experiments were performed using adult male OF1 mice (8–9 weeks of age, 39.5 ± 2.7g at the time of experiment) (Charles River, St Genis/l'Arbresle, France). Mice were kept individually in standard conditions (23 ± 2.0°C; 12:12h L:D; light on at 06:00 or 07:00) with access to food and water ad libitum. After 2 weeks of habituation to these conditions, they were randomly divided into 3 groups. The experiments were carried out according to the European Community's Council Directive (86/609/EEC) and with the authorization of the veterinary services of the Canton of Zurich and Geneva.
Drugs
Modafinil was a gift from Cephalon-France (Maisons-Alfort, France). It was prepared according to the protocol given by the supplier. A suspension was obtained in sterile water after dissolution in 0.25% of Arabic gum (Sigma-Aldrich, Buchs, Switzerland). The suspension was injected i.p. just after its preparation in a volume of 10 mL/kg of body weight. Based on the results of a pilot study indicating that a dose of 200 mg/kg i.p. was appropriate to induce 6–7 h of waking in mice, this dosage was used in all experiments.
Sleep Deprivation Procedure
For all experiments, drug injection and gentle sleep deprivation started at light onset, corresponding to zeitgeber time zero (ZT0). The control group (CTL) corresponds to mice which remained undisturbed in their home cage. Mice belonging to the gentle sleep-deprived group (GSD) or to the modafinil (MOD)-treated one, were moved to an adjacent room and continuously observed. GSD was performed according to the method previously described,7,14 According to this method, the animals were kept wake by providing new nesting material or new objects into their home cage when they adopted a sleeping posture and immobility. Mice were not handled, touched, or stimulated by cage moving. They were not disturbed when they were drinking or eating. In order to optimize comparisons between the experimental conditions in the MOD and GSD groups, mice from the GSD group also received an injection of solvent at the beginning of the sleep deprivation period. When an animal from the MOD group fell asleep (i.e., when the mouse remained immobile for 2–3 min in a sleeping posture), it was rapidly sacrificed within 2–3 min. Then, a mouse from the GSD group and a mouse from the CTL group were immediately sacrificed. In this way, a similar range of sleep deprivation time was obtained in the MOD and GSD groups. A similar protocol was followed for sleep recovery study. In this case, 3 groups of animals were used: (1) Gently sleep deprived mice left undisturbed in their cage for 3 h from the moment they fell asleep (GSD-SR group), (2) Modafinil treated mice left undisturbed in their cage for 3 h from the moment they fell asleep (MOD-SR group), and (3) mice left undisturbed throughout the experiment and sacrificed at the same time as animals of the GSD-SR or MOD-SR groups (CTL-SR). This group corresponds to the circadian control between ZT9 and ZT10. To assess the sleep recovery efficiency, locomotor activity of the GSD-SR and MOD-SR animals was recorded throughout the recovery period.
Sample Collections
Mice were sacrificed by decapitation, the brain rapidly removed, and the cerebral cortex dissected on ice. The samples were placed in 3.5 mL of chilled TRIzol reagent (Life Technologies, Basel, Switzerland) and homogenized samples were stored at −80°C until RNA extraction. About one-fourth of the cortex was put into 300 μL of homogenization buffer (for GSynt activity) and stored at −80°C until assays were performed. For glycogen determination experiments, mice were rapidly anesthetized with isoflurane (4.5%) and were sacrificed using a focused microwave fixation device irradiating the brain at 4 kW for 0.6 s (Gerling Applied Engineering, Inc., Modesto, CA, USA). This device allows inactivating cerebral enzymes in 2 s and therefore recovering of labile metabolites such as glycogen. Then the brain was removed and cerebral cortex was dissected out, cut sagittally in 2 parts, frozen in liquid nitrogen, and stored at −80°C until glycogen and protein assays were performed. Trunk blood was collected into heparinized microtubes (Microvettes, Sarstedt, Germany) for glycemia determination. Plasma was separated by centrifugation (6000 rpm for 5 min at room temperature), and frozen at −80° for corticosterone assays.
Quantitative RT-PCR experiments
Total RNA was extracted from tissue samples according to the manufacturer's (TRIzol) protocol. The concentration of each sample was measured with NanoDrop (NanoDrop Technologies, Wilmington, DE, USA), and the purity was assessed using the ratios of optic density at (260/280) nm and (260/230) nm. Finally, the RNA integrity was assessed using the 2100-Bioanalyzer (Agilent Technologies, Waldbronn, Germany). The first strand of cDNA was synthesized from 200ng of total RNA using TaqMan RT-reagents (Applied Biosystem, Foster City, USA) after incubation during 45 min at 48°C followed by 5 min at 95°C and finally stored at 4°C. Then, 2 μL of RT-reagents were added to 0.5 μL of forward and reverse primers and to 23 μL of Sybr-Green PCR MasterMix (Applied Biosystem, Foster City, USA) following manufacturer's instructions. Forty cycles of amplification were then performed in an ABI Prism 7900 (Applied Biosystem, Foster City, USA) with 384-well plate, which allowed the analysis of 4 genes simultaneously including β-actin for normalization in each plate. Each RT (from the cortex of one animal) was tested in triplicate. Primer sequences were designed using Primer Express (3.0) software (Applied Biosystem, Foster City, USA). Oligonucleotides primers were synthesized by Microsynth (Balgach, Switzerland) and designed according to the published cDNA sequences (NCBI database) (primer sequences are shown in Table 1). Finally, data were obtained using the SDS sequence detector software (Applied Biosystem, Foster City, USA). To determine the expression of the different genes, the mean of the ΔCt of each gene was divided by the mean of ΔCt obtained for the β-actin, a housekeeping gene classically used in sleep deprivation experiments to normalize data. Final results were expressed as percentage of change relative to the control group.
Table 1.
Gene primers sequences
| Genes (nomenclature name) | GeneBank Accession No. | Sequences |
|---|---|---|
| Actin-beta (Actb) | NM007393 | Forward: 5′-GCTTCTTTGCAGCTCCTTCGT- |
| Reverse: 3′-CAAGCGGTACCTACTGCTATA- | ||
| GSynt (Gys1) | NM030678 | Forward: 5′ GCTGGACAAGGAGGACTTCACT- |
| Reverse: 3′-CAGAAAGGGTGGTCACACGT- | ||
| GPhos (Pygb) | NM153781 | Forward: 5′-GCTGCTCAACTGCCTACACATT- |
| Reverse: 3′-GGAAACACGGGTCCTGACAA- | ||
| Glgn (Gyg) | NM013755 | Forward: 5′-ACACCTTCACCACCAACGTCTT- |
| Reverse: 3′-TACTACCTTGTACAGAGTCCTCG- | ||
| PTG (Ppp1r3c) | NM016854 | Forward: 5′-TGCCTCTCGGTCCAATGAG- |
| Reverse: 3′-AACTGTTCAAGGCAGTACGG- | ||
| Glut1 (Slc2a1) | NM011400 | Forward: 5′-CCAGCTGGGAATCGTCGTT- |
| Reverse: 3′-AGGTAGTACCCGTTACGTC- | ||
| Glut3 (Slc2a3) | M75135 | Forward: 5′-TCAGCAGCTCTCTGGGATCA |
| Reverse: 3′-CCGCGTCCTTGAAGATTCC | ||
| HeK1 (Hk1) | NM010438 | Forward: 5′-ACAGTGTGAAGTCGGCCTGAT- |
| Reverse: 3′-TACCTCCTTGACGCTGTGTAG- | ||
| c-fos (Fos) | V00727 | Forward: 5′-CGGAGGAGGGAGCTGACA |
| Reverse: 3′-CTGCAACGCAGACTTCTCATCT | ||
Primers used for qRT-PCR experiments. Each amplicon generated extends over two exons to avoid genomic DNA interference.
Glycogen Synthase Activity Assay
The activity of GSynt was measured using the method of Thomas et al. based on the UDP-14Cglucose incorporation into glycogen.15 Briefly, the samples were centrifuged and enzyme activity was determined in the supernatant. Following incorporation of UDP-14Cglucose into glycogen at 30°C for one hour, the reaction mixture was deposed onto a small piece of paper (Whatman E31), and the 14Cglucose incorporated into glycogen was finally determined with a β counter. The total activity of the GSynt corresponding to the sum of the unphosphorylated active form of GSynt (GSynt a) and the phosphorylated inactive form of GSynt (GSynt b) was determined using saturating concentration of G6P (10 mM) in the reaction mixture. The activity of the non-phosphorylated active form (GSynt a) was determined without G6P. The ratio GSynt a/(GSynt a + GSynt b) was used to indicate the activation state of the GSynt in the sample.
Locomotor Activity Recording
Locomotor activity was assessed in a selection of mice belonging to all 3 experimental groups used to measure glycemia, corticosterone, and glycogen. Measurements were made using an infrared sensor placed above each cage. These sensors detect lateral movements with a magnitude greater than 1–2 cm and rearing along the walls. Grooming is not detected. Data from sensors were collected and summed over a period of 15 min with the “NEPAAL” device made by the electronic workshop of the Department of Physiology of the Lausanne University (Switzerland). Locomotor activity was recorded during the first 6 h, corresponding to the sleep deprivation period (CTL: n = 4; MOD: n = 3; GSD: n = 4). Visual observation of sleep deprived mice was also performed during the sleep deprivation period.
Glycemia and Plasma Corticosterone Determination
Glucose concentration was determined in 10 μL of blood using a Precision-Xtra glucometer (Abbott-Medisense, Baar, Switzerland). After plasma was separated by centrifugation (6000 rpm for 5 min at room temperature), it was deep frozen at −80° until corticosterone assay. Plasma corticosterone concentration was determined using an EIA kit according to the protocol provided by the supplier (Assay Designs, Ann Arbor, USA). All samples were run on the same assay in order to avoid inter-assay variability.
Cortical Glycogen Level Determination
Cortical samples were disrupted by sonication in 500 μL of 0.1N NaOH. Then 50 μL of homogenate was taken for protein determination and kept at −20°C until assays were performed. The homogenate was boiled for 10 min and centrifuged at 4°C at 13000 rpm for 5 min. The supernatant was divided in four 50 μL aliquots which were neutralized by 50 μL of HCl 0.1N. Two sets of two 100 μL aliquots for each sample were processed in parallel for glycogen assay as previously described.16 Thus, 300 μL of acetate buffer (0.1 M, pH 4.65) was added to the first set of duplicate, and 300 μL of the same acetate buffer containing 1% (v/v) amyloglucosidase (140 U/mL) was added to the second set of duplicate. After incubation (30 min at room temperature) 2 mL of Tris-HCl buffer (0.1 mM, pH 8.1) containing 3.3 mM MgCl2, 0.2 mM ATP, 25 μg/mL NADP, 0.7 U/mL hexokinase, and 0.35 U/mL glucose-6-phosphate dehydrogenase were added to all duplicates, and the mixture was incubated for 30 min at room temperature. The fluorescence of the NADPH formed was read at 340/450 nm (excitation/emission) with a fluorometer with an appropriate standard curve (using glucose as a standard). The fluorescent signal in the first set of aliquots corresponds to the amounts of glucose and glucose-6-phosphate, while the fluorescent signal in the second set of aliquots corresponds to the amounts of glycosyl units from glycogen plus glucose and glucose-6-phosphate. The amount of glycogen is determined by the difference between the 2 sets of aliquots. Protein quantity was determined in each sample using the method of Bradford.17 The glycogen content in cortical samples was expressed as micromol of glycosyl units per grams of protein.
Statistics
GraphPad-Instat 3.0 or Prism 5.0 for Windows was used for the statistical analysis. The change in gene expression was analyzed with one-way ANOVA. Significant differences between groups were assessed using a Dunnett t-test as post hoc test. Significance was accepted at P < 0.05. If the data within each group did not follow a Gaussian distribution or if their standard deviation was too different (assessed by the method of Barlett), they were statistically analyzed using the Kruskal-Wallis test (nonparametric ANOVA) followed by post hoc by a Dunn multiple comparisons test. In the results section, all values are expressed as mean ± SEM.
RESULTS
Sleep Deprivation
In groups tested for gene expression and GSynt activity assays, the mean waking duration obtained for each sleep deprived group was: MOD 429 ± 21 min, n = 12; and GSD 394 ± 21 min, n = 8. Only animals with sleep latency greater than 275 min were selected for gene expression analysis and GSynt activity assay. Both sleep deprivation protocols induced a large increase in cortical c-fos gene expression: 445% ± 32% in the GSD group and 374% ± 42% in the MOD group (P < 0.001 for CTL vs. MOD and CTL vs. GSD, n = 8 in each group). For the sleep deprived animals used for glycemia, glycogen levels and locomotor activity measurements, the mean waking durations in Mod and GSD groups were: MOD (380 ± 15 min, n = 9) and GSD (382 ± 14 min, n = 8). In sleep recovery experiments, the mean waking duration was 475 ± 33 min (n = 7) for the MOD-SR and 475 ± 42 min (n = 7) for the GSD-SR group. Locomotor activity recordings indicated that time without movements corresponded to 94% ± 3% and 98% ± 1% of the 3 h of sleep recovery period for the MOD-SR and the GSD-SR groups, respectively.
Expression of Genes Related to Glycogen Metabolism
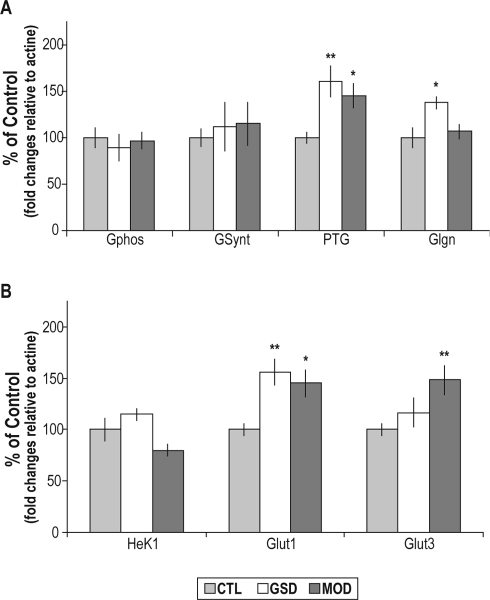
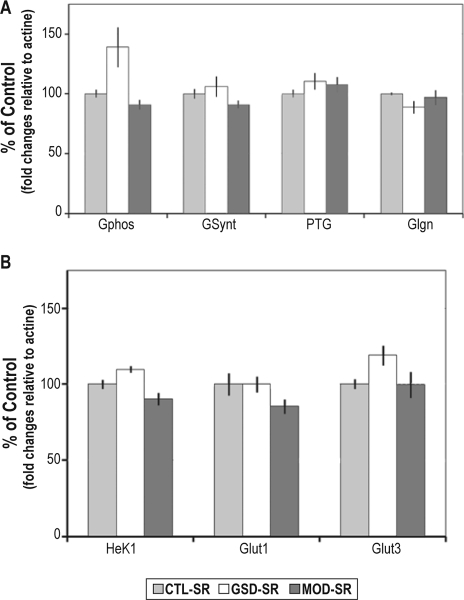
A similar profile of induction of PTG mRNA levels was found in MOD (P < 0.05) and GSD (P < 0.01) groups in reference to the control group. Glycogenin mRNA levels were significantly increased following GSD (P < 0.05) but not after modafinil administration. No significant changes in the GSynt mRNA levels were observed following modafinil administration or GSD (Figure 1A). The GPhos mRNA levels remained unchanged following modafinil administration or following GSD (Figure 1A). The levels of mRNA encoding the glucose transporter Glut1 were significantly increased in the GSD (P < 0.01) and in the MOD (P < 0.05) groups relative to the control group (Figure 1B), whereas Glut3 mRNA levels were only induced following modafinil administration (+48%, P < 0.01) (Figure 1B). Hexokinase 1 mRNA levels were not significantly modified by sleep deprivation protocols. After a period of 3 hours of sleep recovery, the levels of mRNA encoding metabolic genes exhibited no significant change relative to the circadian control group (Figure 2).
Figure 1.
Cortical levels of mRNAs encoding genes directly involved in A) glycogen metabolism and B) glucose transport and phosphorylation, measured by qRT-PCR from undisturbed animals (CTL), or sleep deprived animals by the gentle sleep deprivation method (GSD) or by administration of modafinil (MOD). Significant increases were observed for PTG mRNA levels in the MOD group (145% ± 13%) and in the GSD group (161% ± 16%), glycogenin mRNA levels in the GSD group (137% ± 6%), Glut1 mRNA levels in the GSD and MOD groups ([156% ± 14%] and [145% ± 12%], respectively), and for Glut3 mRNA levels in MOD group (148% ± 14%). Bars represent the mean of the fold change relative to actin mRNA levels of each group expressed as a percentage of the mean value of the CTL group. Bars represent mean values and SEM. (n = 8 per group). **P < 0.01; *P < 0.05, comparison of MOD and GSD groups with the CTL group; Dunnett t-test after significance in a one-way ANOVA.
Figure 2.
Cortical levels of mRNAs encoding genes directly involved in (A) glycogen metabolism and (B) glucose transport and phosphorylation, measured by qRT-PCR from animals maintained awake with modafinil administration followed by 3 h of sleep recovery (MOD-SR), from animals that underwent a gentle sleep deprivation followed by 3 h of sleep recovery (GSD-SR), and from undisturbed animals sacrificed at the same time point (CTL-SR). No significant change was observed for any gene analyzed. Bars represent the mean of the fold change relative to actin mRNA levels of each group expressed as a percentage of the mean value of the CTL group. Bars represent mean values and SEM (n = 8 or 7 per group). Comparison of MOD-SR and GSD-SR groups with the CTL-SR group: Dunnett t-test after significance in a one-way ANOVA.
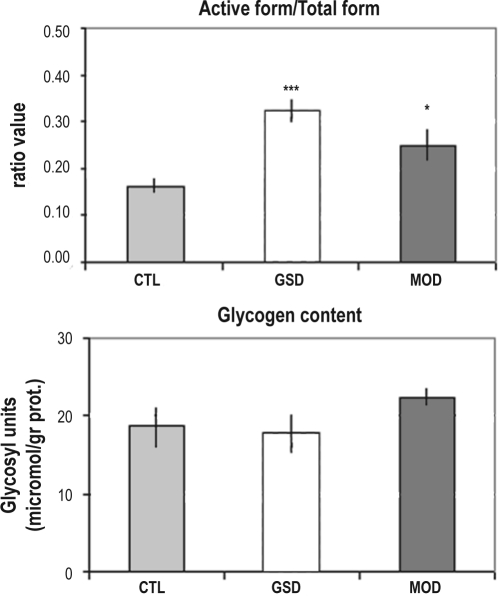
Glycogen Synthase Activity Assay
As mentioned earlier, the GSynt a/(GSynt a + GSynt b) ratio is an index of the activation state of the GSynt. Samples from the same animals were used for the mRNAs and GSynt assays; the latter were processed in duplicate (n = 7 in each group). The ratio GSynt a/(GSynt a + GSynt b) was significantly increased by both sleep deprivation methods (Figure 3A). A significant increase (153% ± 23%) was observed in the modafinil treated mice, and a more pronounced induction (197% ± 16%) was observed in the GSD group (P < 0.05 and P < 0.01 for MOD vs. CTL and GSD vs. CTL, respectively). No significant variation in the total form of the GSynt; i.e., (GSynta + GSyntb) was observed in either treatment.
Figure 3.
(A) Activity of the glycogen synthase measured at the end of the sleep deprivation period from undisturbed animals (CTL), or animals sleep deprived by the gentle sleep deprivation method (GSD) or by administration of modafinil (MOD). The GSynt activity was measured as the ratio between the active form of the GSynt and the total form of the enzyme. The ratio was significantly increased in GSD group (0.32 ± 0.03) and in MOD group (0.25 ± 0.04). Bars represent the mean of this ratio in each group. Vertical lines represent the SEM. n = 7 in each group. **P < 0.01; *P < 0.05. The statistical significance was determined by a Dunnett t-test after a one-way ANOVA. MOD and GSD groups were compared to CTL group. (B) Cortical glycogen levels expressed as μmol of glycosyl units per gram of protein. Bars represent the mean of this ratio in each group. Vertical lines represent the SEM n = 5 for CTL and GSD groups and n = 6 for MOD group. The statistical significance was determined by a Kruskal-Wallis test.
Locomotor Activity
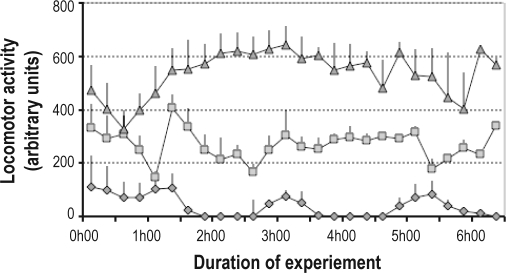
A significant increase in locomotor activity was observed following modafinil treatment compared to the locomotor activity displayed by the GSD group (Kruskal-Wallis test P < 0.001) (Figure 4). Direct visual observation of the mice after both treatments confirmed that the increased locomotion was not due to stereotypical or other abnormal behaviors.
Figure 4.
Locomotor activity measured by infrared sensors during the 6 h of a separate sleep deprivation experiment. Each point represents a mean 15-min value and SEM for each group. Diamonds: CTL group (undisturbed control animals; n = 4), Triangles: MOD group (mice treated with modafinil; n = 3); Squares: GSD group (mice sleep deprived by the gentle sleep deprivation method; n = 4).
Glycemia and Plasma Corticosterone
No significant effect of GSD or modafinil on glycemia was observed at the end of the sleep deprivation period (Table 2). A two-fold increase in plasma corticosterone concentration was observed in the GSD group but not in the MOD group (Table 2).
Table 2.
Plasma parameters
| Plasma glucose | CTL | GSD | MOD |
|---|---|---|---|
| mean ± SEM (mM) | 9.5 ± 0.3 | 10.1 ± 0.3 | 10 ± 0.3 |
| Plasma corticosterone | |||
| mean ± SEM (pg/mL) | 44.8 ± 3.9 | 94.5 ± 9.9 (**) | 41 ± 4.6 |
Plasma levels of (A) glucose and (B) corticosterone, measured at the end of the sleep deprivation period. CTL: undisturbed animals (n = 9); GSD: sleep deprived animals by “gentle sleep deprivation” method (n = 9 for glucose determination and n = 8 for corticosterone determination) and MOD: sleep deprived animals by administration of modafinil (n = 9).
P < 0.01 for CTL vs GSD, Dunnett t-test after significance in a one-way ANOVA.
Glycogen Levels
Glycogen levels were measured in the cerebral cortex. For this brain region, a mix of samples from right and left hemispheres was used to avoid any effect of lateralization. No significant change in glycogen levels was observed following sleep deprivation induced by either GSD or modafinil. The mean levels of glycogen expressed as micromol of glycosyl units per grams of protein were 18.6 ± 2.8 in CTL group, 22.4 ± 1.5 in MOD group, and 17.7 ± 2.5 in GSD group (Figure 3B).
DISCUSSION
A first conclusion of this study is the common impact of wakefulness induced by modafinil or GSD on cortical glycogen metabolism. Both treatments also elicited an increase in cortical levels of c-fos mRNA, consistent with previous reports.18,19
Effects of Modafinil and GSD on Plasma Corticosterone Levels and Glycemia
The plasma corticosterone levels were not increased at the end of the wakefulness induced by modafinil. Interestingly, as previously reported in young and adult rats20 as well as in mice21 submitted to a similar GSD protocol, plasma corticosterone was enhanced in our gently sleep-deprived mice compared to undisturbed ones. However, the magnitude of this increase was moderate in comparison to the effect of an intense stressor, such as immobilization.21 Since modafinil and GSD exerted a similar influence on gene expression and glycogen levels, it is unlikely that the mild stress induced by GSD was involved in these effects. The unchanged levels of glycemia following GSD or modafinil suggest that the parameters related to brain glycogen metabolism measured here were not influenced by peripheral glucose concentration. These results also suggest that the induction of glucose transporter mRNAs was likely driven by a central signal rather than changes in circulating glucose.
Effects of Modafinil and GSD on the Expression of Genes Encoding Glucose Transporters
Our results corroborate the increase in cortical levels of Glut1 mRNA observed after sleep deprivation or a spontaneous waking period in the rat.22 Notably, Glut1 mRNA enhancement was observed independent of the sleep deprivation method, indicating that an increase in Glut1mRNA might be part of the metabolic response triggered by prolonged wakefulness. Indeed, in our study, cortical Glut1 and Glut3 mRNA levels were enhanced by modafinil, while only Glut1 gene expression was increased after GSD. It has been shown that Glut1 is highly expressed at the blood brain barrier and in astrocytes, whereas Glut3 is enriched on neuronal membranes.23 This observation suggests a specific metabolic action of modafinil at the neuronal level which is of particular interest since modafinil displays cognitive enhancing properties in sleep deprived as well as non–sleep deprived human or animals.24,25 Interestingly, induction of Glut1 and Glut3 mRNAs was not maintained after the 3-h sleep recovery period, suggesting that it is likely linked to the energy need created by the sustained cortical activity associated with prolonged wakefulness.
Effect of GSD on Cortical Glycogen Metabolism
As we have reported previously, 6 h of GSD induced an increase in cortical PTG mRNA levels accompanied by an increase in GSynt activity. PTG is a non-catalytic subunit of protein phosphatase 1 that binds GSynt and GlyP and targets this molecular scaffold to glycogen.26,27 Overexpression of PTG in hepatocytes or in human muscle cells activates glycogen synthesis through an increase in the active form of GSynt.28,29 A similar role in the brain is largely suggested by results obtained in primary cultures of astrocytes.10 In the present study, GSynt and GlyP mRNA were unaffected, while we reported a moderate decrease in our initial study,7 where the mRNAs levels were assayed by Northern blot, a technique less sensitive than the quantitative RT-PCR. Moreover, since the total form of the GSynt reflects the quantity of this enzyme, the stability of its level reported here is coherent with the absence of change in the GSynt mRNA. Interestingly, glycogenin (Glgn) mRNA was also induced by GSD, whereas modafinil administration failed to change its mRNA levels. In skeletal muscle, Glgn mRNA and protein increase together to facilitate glycogen resynthesis following prolonged intense exercise.30 Indeed, Glgn is required to form new glycogen particles named proglycogen (Mr 400kDa), in astrocytes.31 Thus, the induction of Glgn mRNA levels could result in an increase in number of glycogen particles in cortical astrocytes during GSD. As we observed for the mRNA encoding the glucose transporters, the levels of mRNAs encoding glycogen related genes returned to baseline following the sleep recovery period subsequent to GSD. This is in agreement with our previous observation where a similar pattern was displayed by the GSynt activity.6 Therefore, when sensorimotor activities end, glycogen metabolism stops to be directed towards a synthetic mode.
Modafinil Exerts Effects on Cortical Glycogen Metabolism Similar to GSD
Interestingly, our results indicate that modafinil, which acts on EEG slow wave activity in sleep in a manner similar to GSD,32 also triggers the expression of cortical PTG mRNA and an increase in GSynt activity. The similarity in the two wakefulness-promoting manipulations on expression of certain genes was also observed in a transcriptome analysis. Furthermore, similarly to GSD, genes upregulated at the end of the wakefulness period induced by modafinil returned to their basal levels after 3 hours of sleep recovery. Different hypotheses have been proposed for the wakefulness-promoting action of modafinil including increase in serotonin or glutamate release in various brain regions,33,34 activation of orexinergic neurons35 and potentiation of noradrenergic transmission.36 Moreover, a stimulation of the dopaminergic system has been also suggested by different studies37–39 but still inconclusive concerning the exact mechanism. As a result, it is unknown if a neuronal mechanism involving modafinil could increase glycogenesis in astrocytes, although the increase in noradrenergic transmission could play this role, since noradrenaline markedly stimulates PTG mRNA expression and glycogen levels in primary cultures of mouse astrocytes.10,41
Changes in Energy Metabolism as a Signature of Sleep Deprivation
At the end of the sleep deprivation period, cortical glycogen levels exhibited no change in the present experiments. This fits with results on glycogen content observed following a 6-h gentle sleep deprivation in flies, rats, and in different strains of mice.42–44 Indeed, brain glycogen regulation depends on the time-scale that is considered. In rats, 15 minutes of sensory stimulation induced glycogenolysis in the corresponding somatosensory cortex45 indicating a role of astrocytic glycogen as an energy buffer during sustained neuronal activity. One would therefore predict that prolonging sensorimotor stimulation by sleep deprivation should induce a massive glycogen mobilization in the entire cortex. Contrary to this prediction, the fact that cortical glycogen levels are unchanged following GSD suggests that another type of regulation overrides this role for glycogen. Indeed, in vitro studies indicate that certain neurotransmitters such as noradrenaline, adenosine, or VIP are able, in addition to their rapid (within seconds and minutes) glycogenolytic action, to initiate a series of transcriptional and translational events leading to a massive glycogen re-synthesis.41,46 During wakefulness this mechanism might progressively change the balance between synthesis and degradation of cortical glycogen in favor of its synthesis, as suggested by the fact that highest levels of PTG are reached at the end of the waking period.7 The unchanged cortical glycogen levels at the end of the sleep deprivation period in the OF1 mice used here, but also in Akr and BalbC mice,42 could suggest that the increase in glycogen synthesis balances the increase in glycogen degradation driven by the sensorimotor stimulation evoked by wakefulness and by sleep deprivation. Thus cortical glycogen turnover appears to increase during sleep deprivation. This notion is consistent with recent data reported by Morgenthaler et al. indicating an increase in glycogen turnover rate as revealed by 13C-MRS following prolonged wakefulness.47
In summary, the present study indicates that a mild sleep deprivation (6 h) induces a specific metabolic response in the cerebral cortex of mice, independent of the method used. This metabolic response is characterized by an induction of the expression of Glut1 and PTG genes and an increase in the activity of GSynt. These changes constitute a coordinated mechanism whereby the entry of glucose into the brain parenchyma can be increased in parallel with an enhancement of the mechanisms that favor glycogen synthesis. This metabolic response to sleep deprivation pushes astrocytes towards a metabolic profile in which glycogen levels are maintained constant in spite of an increased glycogen turnover. Results from sleep recovery indicate that this metabolic response is transient and parallel to sensorimotor activation. Altogether, results reported here would tend to contradict the view according to which sleep serves to replenish glycogen stores used during wakefulness. They rather suggest that transcriptionally regulated metabolic adaptations are triggered during wakefulness and by sleep deprivation which allow maintaining glycogen levels. This mechanism would ensure that sufficient glycogen levels are available to meet the energetic needs of sustained neuronal activity. At the end of wakefulness, the metabolic profile of astrocytes is geared towards glycogen resynthesis. This is consistent with observations made by Karnowsky et al.,48 showing that glycogen levels are increased already after a period of sleep as short as 5–11 minutes. This observation could reflect the existence of a “supercompensation” induced by the sudden loss of balance between the decrease in neuronal activation and the metabolic process already directed towards glycogen synthesis in astrocytes.
Based on in vitro data obtained in cultured astrocytes,41,46 it is likely that the signal for such behaviorally induced changes in glycogen metabolism may be provided by those neurotransmitters which induce massive glycogen resynthesis, namely VIP, adenosine and noradrenaline. Interestingly these neurotransmitters have been shown to play a role in sleep regulation in a variety of experimental models.49
These results also stress the importance of astrocyte-neuron metabolic coupling and plasticity to maintain brain energy homeostasis during the sleep-waking cycle. Together with the recent work of Halassa et al. indicating that purinergic gliotransmission has an impact on the sleep homeostasis,40 these results suggest that astrocytes could play a important role in the regulation of the sleep-wake cycle through their direct action on synaptic plasticity mechanisms and their role on energy metabolism accompanying them. Finally, the induction of GLUT3 mRNA, which is exclusively expressed in neurons, points to an additional cellular target in modafinil-mediated wakefulness.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
Research in PJM's laboratory is supported by the Asterion Foundation Chair; this work was supported by FNRS grant 3100AO-108336/1 to PJM.
We thank C. Haeberli for the construction of the NEPAAL device, E. Dunand and J. Gyger for their excellent technical assistance, and S. Lengacher for his help in the primer design. The support of Laboratoires Lafon (now Cephalon-France, Maisons-Alfort, France) for initial experiments and for providing modafinil is kindly acknowledged.
REFERENCES
- 1.Laposky AD, Bass J, Kohsaka A, Turek FW. Sleep and circadian rhythms: Key components in the regulation of energy metabolism. FEBS Lett. 2008;582:142. doi: 10.1016/j.febslet.2007.06.079. [DOI] [PubMed] [Google Scholar]
- 2.Penev PD. Sleep deprivation and energy metabolism: to sleep, perchance to eat? Curr Opin Endocrinol Diabetes Obes. 2007;14:374–81. doi: 10.1097/MED.0b013e3282be9093. [DOI] [PubMed] [Google Scholar]
- 3.Benington JH, Heller HC. Restoration of brain energy metabolism as the function of sleep. Prog Neurobiol. 1995;45:347–60. doi: 10.1016/0301-0082(94)00057-o. [DOI] [PubMed] [Google Scholar]
- 4.Brown AM, Ransom BR. Astrocyte glycogen and brain energy metabolism. Glia. 2007;55:1263–71. doi: 10.1002/glia.20557. [DOI] [PubMed] [Google Scholar]
- 5.Sorg O, Magistretti PJ. Characterization of the glycogenolysis elicited by vasoactive intestinal peptide, noradrenaline and adenosine in primary cultures of mouse cerebral cortical astrocytes. Brain Res. 1991;563:227–33. doi: 10.1016/0006-8993(91)91538-c. [DOI] [PubMed] [Google Scholar]
- 6.Magistretti PJ. Neuron-glia metabolic coupling and plasticity. J Exp Biol. 2006;209:2304–11. doi: 10.1242/jeb.02208. [DOI] [PubMed] [Google Scholar]
- 7.Petit J-M, Tobler I, Allaman I, Borbély AA, Magistretti PJ. Sleep deprivation modulates brain mRNAs encoding genes of glycogen metabolism. Eur J Neurosci. 2002;16:1163–7. doi: 10.1046/j.1460-9568.2002.02145.x. [DOI] [PubMed] [Google Scholar]
- 8.O'Doherty RM, Jensen PB, Anderson P, et al. Activation of direct and indirect pathways of glycogen synthesis by hepatic overexpression of protein targeting to glycogen. J Clin Invest. 2000;105:479–88. doi: 10.1172/JCI8673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brady MJ, Printen JA, Mastick CC, Saltiel AR. Role of protein targeting to glycogen (PTG) in the regulation of protein phosphatase-1 activity. J Biol Chem. 1997;272:20198–204. doi: 10.1074/jbc.272.32.20198. [DOI] [PubMed] [Google Scholar]
- 10.Allaman I, Pellerin L, Magistretti PJ. Protein targeting to glycogen mRNA expression is stimulated by noradrenaline in mouse cortical astrocytes. Glia. 2000;30:382–91. [PubMed] [Google Scholar]
- 11.Alonso MD, Lomako J, Lomako WM, Whelan WJ. A new look at the biogenesis of glycogen. FASEB J. 1995;9:1126–37. doi: 10.1096/fasebj.9.12.7672505. [DOI] [PubMed] [Google Scholar]
- 12.Gip P, Hagiwara G, Sapolsky RM, Cao VH, Heller HC, Ruby NF. Glucocorticoids influence brain glycogen levels during sleep deprivation. Am J Physiol Regul Integr Comp Physiol. 2004;286:R1057–62. doi: 10.1152/ajpregu.00528.2003. [DOI] [PubMed] [Google Scholar]
- 13.Lei H, Gruetter R. Effect of chronic hypoglycaemia on glucose concentration and glycogen content in rat brain: a localized 13C NMR study. J Neurochemistry. 2006;99:260–8. doi: 10.1111/j.1471-4159.2006.04115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deboer T, Tobler I. Vigilance state episodes and cortical temperature in the Djungarian hamster: the influence of photoperiod and ambient temperature. Pflugers Arch. 1997;433:230–7. doi: 10.1007/s004240050272. [DOI] [PubMed] [Google Scholar]
- 15.Thomas JA, Schlender KK, Larner J. A rapid filter paper assay for UDPglucose-glycogen glucosyltransferase, including an improved biosynthesis of UDP-14C-glucose. Anal Biochem. 1968;25:486–99. doi: 10.1016/0003-2697(68)90127-9. [DOI] [PubMed] [Google Scholar]
- 16.Allaman I, Pellerin L, Magistretti PJ. Glucocorticoids modulate neurotransmitter-induced glycogen metabolism in cultured cortical astrocytes. J Neurochem. 2004;88:900–8. doi: 10.1046/j.1471-4159.2003.02235.x. [DOI] [PubMed] [Google Scholar]
- 17.Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;7:248–54. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 18.Basheer R, Sherin JE, Saper CB, Morgan JI, McCarley RW, Shiromani PJ. Effects of sleep on wake-induced c-fos expression. J Neurosci. 1997;17:9746–50. doi: 10.1523/JNEUROSCI.17-24-09746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Semba K, Pastorius J, Wilkinson M, Rusak B. Sleep deprivation-induced c-fos and junB expression in the rat brain: effects of duration and timing. Behav Brain Res. 2001;120:75–86. doi: 10.1016/s0166-4328(00)00362-4. [DOI] [PubMed] [Google Scholar]
- 20.Hairston IS, Ruby NF, Brooke S, et al. Sleep deprivation elevates plasma corticosterone levels in neonatal rats. Neurosci Lett. 2001;315:29. doi: 10.1016/s0304-3940(01)02309-6. [DOI] [PubMed] [Google Scholar]
- 21.Palchykova S, Winsky-Sommerer R, Meerlo P, Durr R, Tobler I. Sleep deprivation impairs object recognition in mice. Neurobiol Learn Mem. 2006;85:263. doi: 10.1016/j.nlm.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 22.Cirelli C, Gutierrez CM, Tononi G. Extensive and divergent effects of sleep and wakefulness on brain gene expression. Neuron. 2004;41:35. doi: 10.1016/s0896-6273(03)00814-6. [DOI] [PubMed] [Google Scholar]
- 23.Simpson IA, Carruthers A, Vannucci SJ. Supply and demand in cerebral energy metabolism: the role of nutrient transporters. J Cereb Blood Flow Metab. 2007;27:1766. doi: 10.1038/sj.jcbfm.9600521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wesensten N. Effects of modafinil on cognitive performance and alterness during sleep deprivation. Curr Pharm Des. 2006;12:2457–71. doi: 10.2174/138161206777698819. [DOI] [PubMed] [Google Scholar]
- 25.Shuman T, Wood SC, Anagnostaras SG. Modafinil and memory: Effects of modafinil on Morris water maze learning and Pavlovian fear conditioning. Behav Neurosci. 2009;123:257–66. doi: 10.1037/a0014366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fong NM, Jensen TC, Shah AS, Parekh NN, Saltiel AR, Brady MJ. Identification of binding sites on protein targeting to glycogen for enzymes of glycogen metabolism. J Biol Chem. 2000;275:35034–9. doi: 10.1074/jbc.M005541200. [DOI] [PubMed] [Google Scholar]
- 27.Printen JA, Brady MJ, Saltiel AR. PTG, a protein phosphatase 1-binding protein with a role in glycogen metabolism. Science. 1997;275:1475–8. doi: 10.1126/science.275.5305.1475. [DOI] [PubMed] [Google Scholar]
- 28.Berman HK, O'Doherty RM, Anderson P, Newgard CB. Overexpression of protein targeting to glycogen (PTG) in rat hepatocytes causes profound activation of glycogen synthesis independent of normal hormone- and substrate-mediated regulatory mechanisms. J Biol Chem. 1998;273:26421–5. doi: 10.1074/jbc.273.41.26421. [DOI] [PubMed] [Google Scholar]
- 29.Lérin C, Montell E, Berman HK, Newgard CB, Gomez-Foix AM. Overexpression of protein targeting to glycogen in cultured human muscle cells stimulates glycogen synthesis independent of glycogen and glucose 6-phosphate levels. J Biol Chem. 2000;275:39991–5. doi: 10.1074/jbc.M006251200. [DOI] [PubMed] [Google Scholar]
- 30.Shearer J, Wilson RJ, Battram DS, et al. Increases in glycogenin and glycogenin mRNA accompany glycogen resynthesis in human skeletal muscle. Am J Physiol Endocrinol Metab. 2005;289:E508–14. doi: 10.1152/ajpendo.00100.2005. [DOI] [PubMed] [Google Scholar]
- 31.Lomako J, Lomako WM, Whelan WJ, Dombro RS, Neary JT, Norenberg MD. Glycogen synthesis in the astrocyte: from glycogenin to proglycogen to glycogen. FASEB J. 1993;7:1386–93. doi: 10.1096/fasebj.7.14.8224611. [DOI] [PubMed] [Google Scholar]
- 32.Kopp C, Petit J-M, Magistretti P, Borbely A, Tobler I. Comparison of the effects of modafinil and sleep deprivation on sleep and cortical EEG spectra in mice. Neuropharmacology. 2002;43:110–8. doi: 10.1016/s0028-3908(02)00070-9. [DOI] [PubMed] [Google Scholar]
- 33.Ferraro L, Antonelli T, O'Connor WT, Tanganelli S, Rambert FA, Fuxe K. The effects of modafinil on striatal, pallidal and nigral GABA and glutamate release in the conscious rat: evidence for a preferential inhibition of striato-pallidal GABA transmission. Neurosci Lett. 1998;253:135. doi: 10.1016/s0304-3940(98)00629-6. [DOI] [PubMed] [Google Scholar]
- 34.Ferraro L, Fuxe K, Tanganelli S, Fernandez M, Rambert FA, Antonelli T. Amplification of cortical serotonin release: a further neurochemical action of the vigilance-promoting drug modafinil. Neuropharmacology. 2000;39:1974. doi: 10.1016/s0028-3908(00)00019-8. [DOI] [PubMed] [Google Scholar]
- 35.Chemelli RM, Willie JT, Sinton CM, et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation [see comments] Cell. 1999;98:437–51. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- 36.Gallopin T, Luppi P-H, Rambert FA, Frydman A, Fort P. Effect of the wake-promoting agent modafinil on sleep-promoting neurons from the ventrolateral preoptic nucleus: an in vitro pharmacologic study. Sleep. 2004;27:19–25. [PubMed] [Google Scholar]
- 37.Korotkova TM, Klyuch BP, Ponomarenko AA, Lin JS, Haas HL, Sergeeva OA. Modafinil inhibits rat midbrain dopaminergic neurons through D2-like receptors. Neuropharmacology. 2007;52:626. doi: 10.1016/j.neuropharm.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 38.Qu W-M, Huang Z-L, Xu X-H, Matsumoto N, Urade Y. Dopaminergic D1 and D2 receptors are essential for the arousal effect of modafinil. J Neurosci. 2008;28:8462–9. doi: 10.1523/JNEUROSCI.1819-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wisor JP, Eriksson KS. Dopaminergic--adrenergic interactions in the wake promoting mechanism of modafinil. Neuroscience. 2005;132:1027. doi: 10.1016/j.neuroscience.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 40.Halassa MM, Florian C, Fellin T, et al. Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron. 2009;61:213–9. doi: 10.1016/j.neuron.2008.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sorg O, Magistretti PJ. Vasoactive intestinal peptide and noradrenaline exert long-term control on glycogen levels in astrocytes: blockade by protein synthesis inhibition. J Neurosci. 1992;12:4923–31. doi: 10.1523/JNEUROSCI.12-12-04923.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Franken P, Gip P, Hagiwara G, Ruby NF, Heller HC. Changes in brain glycogen after sleep deprivation vary with genotype. Am J Physiol Regul Integr Comp Physiol. 2003;285:R413–9. doi: 10.1152/ajpregu.00668.2002. [DOI] [PubMed] [Google Scholar]
- 43.Gip P, Hagiwara G, Ruby NF, Heller HC. Sleep deprivation decreases glycogen in the cerebellum but not in the cortex of young rats. Am J Physiol Regul Integr Comp Physiol. 2002;283:R54–9. doi: 10.1152/ajpregu.00735.2001. [DOI] [PubMed] [Google Scholar]
- 44.Kong J, Shepel PN, Holden CP, Mackiewicz M, Pack AI, Geiger JD. Brain glycogen decreases with increased periods of wakefulness: implications for homeostatic drive to sleep. J Neurosci. 2002;22:5581–7. doi: 10.1523/JNEUROSCI.22-13-05581.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Swanson RA, Morton MM, Sagar SM, Sharp FR. Sensory stimulation induces local cerebral glycogenolysis: Demonstration by autoradiography. Neuroscience. 1992;51:451. doi: 10.1016/0306-4522(92)90329-z. [DOI] [PubMed] [Google Scholar]
- 46.Allaman I, Lengacher S, Magistretti PJ, Pellerin L. A2B receptor activation promotes glycogen synthesis in astrocytes through modulation of gene expression. Am J Physiol Cell Physiol. 2003;284:C696–704. doi: 10.1152/ajpcell.00202.2002. [DOI] [PubMed] [Google Scholar]
- 47.Morgenthaler FD, Lanz BR, Petit J-M, Frenkel H, Magistretti PJ, Gruetter R. Alteration of brain glycogen turnover in the conscious rat after 5 h of prolonged wakefulness. Neurochem Int. 2009;55:45–51. doi: 10.1016/j.neuint.2009.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Karnovsky ML, Reich P, Anchors JM, Burrows BL. Changes in brain glycogen during slow-wave sleep in the rat. J Neurochem. 1983;41:1498–501. doi: 10.1111/j.1471-4159.1983.tb00853.x. [DOI] [PubMed] [Google Scholar]
- 49.Jones BE. From waking to sleeping: neuronal and chemical substrates. Trends Pharmacol Sci. 2005;26:578. doi: 10.1016/j.tips.2005.09.009. [DOI] [PubMed] [Google Scholar]