Abstract
Study Objectives:
To examine whether current and/or history of marital/cohabitation status are associated with sleep, independent of demographic and general health risk factors.
Design:
Longitudinal, observational study of women, with sleep measured via multi-night in-home polysomnography and up to 35 nights of actigraphy.
Setting:
Participants' homes.
Participants:
Caucasian (n = 170), African American (n = 138), and Chinese women (n = 59); mean age 51 years.
Interventions:
None.
Measurements:
Sleep quality was assessed via questionnaire. Sleep duration, continuity, and architecture were calculated using in-home polysomnography (PSG). Sleep continuity was also assessed by actigraphy. Categories of marital/cohabiting status or changes in status were inclusive of women who were legally married or living as married as well as transitions into or out of those partnership categories.
Results:
Partnered (married or cohabiting) women at the time of the sleep study had better sleep quality and PSG and actigraphy-assessed sleep continuity than unpartnered women; however, with covariate adjustment, most of these associations became non-significant. Analyses of women's relationship histories over the 6-8 years prior to the sleep study showed advantages in sleep for women who were consistently partnered versus women who were unpartnered throughout this interval, or those who had lost or gained a partner over that time course. These results persisted after adjusting for potential confounders.
Conclusions:
The stable presence of a partner is an independent correlate of better sleep quality and continuity in women.
Citation:
Troxel WM; Buysse DJ; Matthews KA; Kravitz HM; Bromberger JT; Sowers M; Hall MH. Marital/cohabitation status and history in relation to sleep in midlife women. SLEEP 2010;33(7):973-981.
Keywords: Marriage, marital transition, women, menopause, sleep
EPIDEMIOLOGIC RESEARCH HAS SUGGESTED THAT DIVORCED INDIVIDUALS, PARTICULARLY WOMEN, HAVE HIGHER RATES OF SLEEP DISTURBANCE THAN their married counterparts.1–4 Importantly, these studies typically measure marital status at a single point in time, which precludes determination of whether something specific about having a stable partner is beneficial for sleep or whether dissolution of a committed relationship is detrimental for sleep. For example, is the risk attributable to divorce due to the absence of a partner or to the psychosocial sequelae of ending the relationship? Selection bias may also contribute to the effects: healthier people, including those with healthier sleep profiles, may be more likely to enter into and stay in committed relationships. Although directionality cannot be inferred from cross-sectional studies, examination of the association between sleep and the course of relationship status over time, including transitions into or out of committed relationships, provides stronger evidence to support the direction of association linking stable relationships to sleep.
The limited extant literature suggests that it is the aftermath of marital dissolution or the affective response of bereavement that is most detrimental for sleep, rather than the absence of the partner per se.5,6 For instance, Reynolds and colleagues6 documented an increased risk for sleep disturbances among bereaved older adults but only among those who were also depressed. Similarly, in a study of the effects of divorce on sleep, Cartwright and Wood demonstrated significant reductions in latency to REM sleep and elevations in the percentage of REM only among divorced individuals who were also depressed.7 As well, participants currently undergoing a divorce had significant reductions in delta sleep (stage 3+4 NREM) compared to those for whom the divorce was finalized.7 In addition, an emerging literature has shown that marital happiness is associated with higher sleep quality in women,8,9 whereas anxiety in close relationships is associated with poorer subjective sleep quality10,11 and indicators of poorer objective sleep, such as percentage of stage 3+4 sleep.12 Taken together, these findings suggest that relationship status per se may be less salient for sleep than are the affective responses to relationship dissolution and/or the underlying qualitative dimensions of high quality relationships, including the degree to which the relationship affords a sense of stability and security. The stability of a marital or cohabiting relationship may be another marker of relationship quality (albeit an imperfect one) that could relate to sleep more strongly than a single, static examination of relationship status.
Examining how both relationship status and history contributes to sleep in women is important for several reasons. First, women are more likely to have insomnia than men.13 In particular, midlife is a time of increased risk for sleep disturbances14 and of significant life transitions in women.15 In addition, some evidence suggests that women tend to be more negatively affected, both psychologically and physiologically, by distressed relationships than are men.16 Thus, relationship dissolution may heighten the risk for sleep problems among women by negatively influencing mood and affect and leading to endocrine and autonomic dysregulation, all of which are associated with sleep disturbances, including insomnia.17–20 Sociodemographic factors that have been linked with sleep, including African American race and low socioeconomic status,4,21–23 may also play a role in explaining the sleep benefits of marriage/cohabitation, as unpartnered women are disproportionately represented by African Americans and those in lower socioeconomic strata as compared to partnered women.24 Women who lose a partner due to separation, divorce, or widowhood, often experience a precipitous decline in socioeconomic resources stemming from the loss of the primary or a contributing wage-earner in the family.25 Thus, the stable presence of a partner as well as the gain of a new partner may confer benefits for sleep in women, by increasing a woman's socioeconomic position and access to resources.
Given the dynamic nature of marriages and that roughly 40% of marriages end in divorce, with even higher rates among African Americans,26 a critical question that remains unanswered is how relationship history, specifically, the stability of relationship status over time versus changes in relationship status, contributes to sleep quality in women. In addition, the bulk of the extant literature has focused exclusively on marital status as a correlate of subjective sleep outcomes, often assessed using single or few-item measures of sleep with questionable validity and lacking information on normative values.27 Such reliance on subjective sleep outcomes exclusively is particularly problematic given that the unmarried are also disproportionately more likely to be clinically depressed or have elevated depressive symptoms as compared to their married counterparts.28 Thus, observed relationships between marital status and sleep disturbances may be due to general negative affect or reporting bias among the unmarried. Moreover, given the considerable overlap between marital status and other sociodemographic risk factors known to covary with sleep, including race and indicators of SES,4,25 it remains unclear whether marriage is merely a proxy for these known risk factors, or whether having a partner is an independent correlate of better sleep.
The current study of sleep, nested in a larger longitudinal study of women's health, examined how marital/cohabitation status (hereafter referred to as marital status) measured concurrently with sleep measures, as well as marital/cohabitation history (hereafter referred to as marital history) assessed over approximately 8 years of study follow-up, were related to sleep in women at midlife. By analyzing marital history over time, we were able to address the question of whether sleep differed according to marital role continuity (consistently married or consistently unmarried over 8 years of study follow-up) as well as marital role transitions, including the gain as well as the loss of a partner. Marital history data was drawn from the longitudinal Study of Women's Health Across the Nation (SWAN), the parent study for the SWAN Sleep Study; a cross-sectional evaluation that involved a multi-modal assessment of sleep, including subjective sleep quality, as measured by the well-validated Pittsburgh Sleep Quality Index (PSQI)29; indices of sleep duration, continuity, and architecture, as measured by multi-night in-home polysomnography (PSG); and behavioral measures of participant's usual sleep duration and continuity, as measured by up to 35 days of actigraphy in participants' usual sleeping environments. Such a multimodal assessment of sleep provided an ideal opportunity to understand whether women's marital status and/or marital history related to well-validated measures of subjective sleep (PSQI) and objective sleep (PSG), as well as their sleep-wake patterns in their habitual sleep environments over an extended period of time (actigraphy).
We hypothesized that consistently married/cohabiting women (hereafter referred to as married) would have better sleep, in terms of subjective sleep quality, continuity, and duration as compared to consistently unpartnered women or those who had lost a partner, and that these effects would persist after statistical adjustment for other known risk factors for sleep disturbance. Moreover, consistent with limited evidence linking marital transitions, specifically the loss of a partner due to divorce or widowhood with anomalies in sleep architecture,7,30 we further predicted that women who had lost a partner would have decreased stage 3+4 sleep as compared to consistently married women. We expected that the sleep of women who gained a partner over the course of follow-up would not differ from the consistently married women, as the gain of a partner is generally perceived as a positive life transition31 and also generally positively affects a woman's socioeconomic status.25 Finally, we expected that marital status measured concurrently with sleep assessments would also correlate with sleep outcomes, with the unmarried women having greater sleep disturbances than the married women. However, we expected that sleep differences observed among the married versus unmarried at the univariate level would largely be accounted for by other factors known to covary with both sleep and marital status; specifically, racial and socioeconomic differences between the married and unmarried4 as well as higher rates of depressive symptomatology among the unmarried.28
METHODS
Study Participants
As reported previously in detail22 the SWAN Sleep Study is a cross-sectional substudy of sleep from a multiracial sample of midlife women participating in the Study of Women's Health across the Nation (SWAN). The Core SWAN Study is a longitudinal community-based study of the menopausal transition conducted at 7 clinical sites in the United States.32 Participants in the SWAN Sleep Study were 370 Caucasian, African American and Chinese women from 4 study sites in Chicago, IL; Detroit area, MI; Oakland, CA; and Pittsburgh, PA. SWAN Sleep Study exclusion criteria were: current hormone therapy (HT) use; current chemotherapy or radiation; current oral corticosteroid use; regular nocturnal shiftwork; regular consumption of > 4 alcoholic drinks/day; and noncompliance with Core SWAN procedures (missed > 50% of annual visits, refused annual visit blood draw). Written informed consent was obtained in accordance with approved protocols and guidelines of the Institutional Review Board at each participating institution, which also approved the study protocol. Participants were remunerated for their participation.
Study Protocol
The SWAN Sleep Study protocol was conducted across one menstrual cycle or 35 days, whichever was shorter. For regularly cycling women, the protocol was initiated within 7 days of the start of menstrual bleeding. For irregularly cycling and non-cycling women, the protocol was scheduled at the participants' convenience. Unattended, in-home polysomnography (PSG) sleep studies were conducted on the first 3 nights of the protocol. Actigraphs were worn and sleep diaries were completed for the entire menstrual cycle or 35-day period, whichever was shorter (range = 1-35 days; mean [SD] number of days of actigraphy = 28.8 [7.7]).
MEASURES
Marital Status and Marital History
Participants reported their marital/cohabitation status at the baseline Core SWAN interview and annually at each visit up to the sleep study (years 6-8). For cross-sectional analyses, women who reported being “married or living as married” at the visit closest to the Sleep Study were coded as 0 (58% of sample; referred to as married) and women who reported being single (13%), divorced (15%), separated (3%), or widowed (3%) were coded as 1 (referred to as unmarried). For the women's 6-8 year marital/cohabitation histories, those who reported being married or living as married at each annual visit are identified as the consistently married group (the referent group; coded as 0); women who reported being divorced, widowed, separated or single at all annual visits are identified as the consistently unmarried group; and women who reported one or more changes in marital/cohabitation status over the course of follow-up, are identified as having lost a partner (transition from married to unmarried category) or gained a partner (transition from unmarried to married category) based on the transition occurring closest to the sleep study. Two-thirds of marital transitions (gain or loss) occurred at least 2 years prior to the sleep study.
Sleep Outcomes
Sleep data include subjective sleep quality; PSG-assessed indices of sleep duration (total sleep time, TST) and continuity (sleep latency, SL; and wakefulness after sleep onset; WASO, arousal index), sleep architecture (% stage, 1, 2, 3+4, and REM), sleep disordered breathing; and actigraphic measures of sleep duration and continuity (TST, SL, WASO, and fragmentation index).
Global subjective sleep quality was assessed using the 19-item Pittsburgh Sleep Quality Index (PSQI)29 and referenced the month prior to the SWAN Sleep Study. Higher scores on the PSQI represent more sleep complaints within the past month, with a possible range of 0-21.
Polysomnographic (PSG) sleep data were collected in participants' homes with Vitaport-3 (TEMEC VP3) ambulatory monitors. Study staff visited participants in their homes on each night of sleep studies to apply electrodes and calibrate monitors. Participants slept in their own beds, under their usual circumstances, and at their usual sleep and wake times. Participants removed study equipment and turned off the recorder upon awakening in the morning. PSG signals collected on each study night included bilateral central referential EEG channels (C3 and C4, referenced to A1 linked to A2), electro-oculogram (EOG), submentalis electromyogram (EMG), and electrocardiogram (EKG). In addition, on the first sleep study night, information was collected for the assessment of sleep disordered breathing (SDB) with nasal pressure cannula, oral-nasal thermistors, fingertip oximetry, and abdominal and thoracic respiratory effort, as measured by inductance plethysmography.
Visual sleep stage scoring in 20-sec epochs was conducted by trained PSG technologists with established reliability, using standard scoring criteria33 and American Academy of Sleep Medicine recommendations34 for scoring the apnea-hypopnea index (AHI). With the exception of AHI, which was measured on night 1 only, all other summary sleep variables were averaged over nights 2 and 3 to reduce adaptation effects related to SDB measures and first-night effects35.
PSG sleep continuity measures included SL (time from beginning of the recording period to the first of 10 consecutive minutes of stage 2 or stage 3 + 4 sleep interrupted by ≤ 2 min of stage 1 or wakefulness), WASO; (total minutes of wakefulness between sleep onset and good morning time), arousal index; (defined as an abrupt increase in EEG frequency lasting 3-15 sec per hour of sleep); and TST. Measures of sleep architecture included percent of time spent asleep in NREM stages 1, 2, and 3+4, as well as REM sleep. Sleep disordered breathing was assessed using the AHI.
Wrist actigraphy was measured with the Minimitter Actiwatch-64 device (Respironics, Inc., Murrysville, PA), which was worn concurrently with the collection of sleep diaries for one menstrual cycle or 35 days (whichever was shorter) to provide a behavioral assessment of participants' sleep duration and continuity in their usual sleeping environments. Actigraphy data were collected in 1-min epochs and analyzed with the Actiware Version 5.04 software program. Sleep diary data for bed time and rise time were entered for calculation of sleep-wake variables. Actigraphy outcome variables included SL, WASO, TST, and fragmentation index (higher values indicate greater mobility during the sleep period).
Covariates
Variables known to be related to sleep (age, body mass index, use of medications that could affect sleep, menopausal status) and/or to both sleep and marital status, including race, financial strain (SES indicator), and depressive symptoms were included as covariates in models to determine the independent associations between marital status and marital history and sleep.
Sociodemographics
Participants' race was categorized as Caucasian, African American, or Chinese based on self-identification. In the analysis, Caucasians served as the referent group. Age at the time of the sleep study was self-reported. Financial strain36was measured using a single-item, 3-level variable from the Core SWAN screening questionnaire, in which participants were asked participants to rate how difficult it was to pay for “basics,” like food, housing, medical care, and heating. This 3-level response was dichotomized as “somewhat hard” to “very hard” versus “not hard at all.”
Mental and physical health characteristics
Body mass index (BMI; kg/m2) was measured in the Core SWAN protocol. Self-reported depressive symptoms were measured concurrently with in-home sleep studies using the 16-item Inventory of Depressive Symptomatology (IDS)37 with sleep items removed. Participants' menopausal status was analyzed categorically as pre- or early perimenopausal (referent) versus late perimenopausal, postmenopausal, prior use of hormone therapy, or hysterectomy. Use of prescription or over-the-counter medications that could influence sleep (including opioids, antiepileptics, anxiolytics, hypnotics and sedatives, antidepressants, and antihistamines), was recorded at the start of the sleep study protocol and coded as “present” or “absent.”
Statistical Analysis
Sleep variables with skewed distributions were transformed prior to analysis. Descriptive statistics were used to characterize the study sample according to primary study variables (marital status and marital history) and covariates. To reduce the number of comparisons, preliminary analyses examining group differences in PSG and actigraphy outcomes according to marital status (married versus unmarried) and marital histories (consistently married, consistently unmarried, lost a partner, or gained a partner) used multivariate analysis of variance (MANOVA) with outcomes grouped according to modality and dimension of sleep. Specifically, the following groups of outcomes were included in 3 separate MANOVAs: (1) PSG sleep continuity variables including SL, WASO, arousal index, and TST; (2) PSG sleep architecture including % stage 1, 2, 3+4, and REM sleep; and (3) actigraphy SL, WASO, TST, and fragmentation index. Significant MANOVA results were followed by individual ANOVAs. Marital group differences in PSQI and AHI were assessed in separate t-tests (marital status) or F-tests (marital history).
Significant group differences in sleep outcomes from the unadjusted models were followed by linear regression analyses which regressed each of the sleep outcomes on marital status or marital history (in separate models), with adjustment for covariates. For the marital history models, 3 dummy codes were entered to examine the following contrasts: (1) consistently married to consistently unmarried; (2) consistently married to lost a partner; and (3) consistently married to gained a partner. In models for current marital status, the married group was the referent in comparison to the unmarried group. Covariates in the regression models were age, race, financial strain, BMI, menopausal status, sleep medications, and depressive symptoms.
RESULTS
Sample Characteristics (Table 1)
Table 1.
Background characteristics for full sample (N = 367)
| Age (years), Mean (SD) | 51.3 (2.1) |
| Race, No. (%) | |
| Caucasian | 170 (46) |
| African American | 138 (38) |
| Chinese | 59 (16) |
| Marital status at time of sleep study | |
| Married | 233 (63.6) |
| Unmarried | 134 (36.4) |
| Marital history, No. (%) during 8-year observation period | |
| Consistently unmarried | 87 (23.7) |
| Consistently married | 200 (54.5) |
| Lost partner during 8-year observation period | 47 (12.8) |
| Gained partner during 8-year observation period | 33 (9.0) |
| Difficulty paying for basics, No. (%) | |
| Not difficult at all | 263 (73.5) |
| Somewhat to very difficult | 95 (26.5) |
| Menopausal status, No. (%) | |
| Pre- or early perimenopausal | 228 (61.6) |
| Late peri- or postmenopausal or using HT | 142 (38.4) |
| Taking medications that affect sleep | 99 (27.3) |
| BMI (kg/m2), mean (SD) | 29.9 (7.5) |
| Depressive symptoms (IDS), mean (SD) | 4.8 (3.0) |
By study design, of the 367 women, 46% of the sample was Caucasian, 38% African American, and 16% Chinese and the majority of women were pre- or early perimenopausal. The mean age of the sample was 51 years. On average, depressive symptoms were infrequent in this sample. Average BMI was in the overweight range, according to standard criteria38. Roughly one-quarter (27%) of the sample was taking medications that could affect sleep. At the time of the sleep study, 64% of women were married and 36% were unmarried. According to marital histories aggregated over the 8 years of study follow-up, 55% were consistently married, 24% were consistently unmarried, 13% lost a partner, and 9% gained a partner over the course of study follow-up.
Cross-Sectional Marital Status and Sleep
Table 2 shows the results comparing cross-sectionally defined married women to unmarried women for each of the sleep domains. Unmarried women reported worse sleep quality than married women (PSQI; t364 = 3.4, P < 0.001). MANOVAs revealed significant group differences for PSG and actigraphy continuity measures (P's < 0.01). Univariate analyses for these outcomes revealed that married women had shorter PSG and actigraphy-assessed SL and WASO, as well as less actigraphy-assessed sleep fragmentation (P's < 0.05), but no significant differences were observed in arousal index or PSG or actigraphy- assessed TST. MANOVAs revealed no group differences in PSG-assessed sleep architecture or AHI according to marital status.
Table 2.
Analyses of variance and multivariate analyses of variance (MANOVAS) of the association between concurrent marital status and sleep domains
| Married | Unmarried | ||
|---|---|---|---|
| Mean (SD) | Mean (SD) | t or F- statistic | |
| PSQI | 5.4 (3.0) | 6.6 (3.6) | t364 = 3.4, P < 0.001 |
| AHI† | 10.5 (16.2) | 10.5 (13.0) | t346 = 0.675, P = 0.50 |
| PSG continuity | |||
| MANOVA | F3, 359 = 3.8, P = 0.005 | ||
| SL† | 17.7 (17.4) | 23.1 (21.3) | F1, 361 = 8.6, P = 0.004 |
| WASO† | 48.0 (26.4) | 59.6 (44.7) | F1, 361 = 3.9, P = 0 .05 |
| Arousal index† | 8.6 (5.0) | 9.5 (6.8) | F1, 361 = 2.5, P = 0.12 |
| TST | 386.1 (54.9) | 374.3 (65.3) | F1, 361 = 3.3, P = 0.07 |
| Sleep Architecture | |||
| MANOVA | F4, 358 = 1.8, P = 0.14 | ||
| Actigraphy | |||
| MANOVA | F4, 327 = 4.1, P = 0.003 | ||
| SL† | 22.3 (23.2) | 35.6 (45.6) | F1, 330 = 9.6, P = 0.002 |
| WASO† | 48.8 (23.6) | 59.0 (27.4) | F1, 330 = 11.6, P = 0.001 |
| TST | 362.0 (56.2) | 351.1 (57.5) | F1, 330 = 2.8, P = 0.09 |
| Fragmentation Index+ | 28.3 (11.5) | 32.9 (11.8) | F1, 330 = 12.2, P = 0.001 |
Transformations used in analyses. To facilitate interpretation, means (SDs) are raw values, represented in minutes. Sleep architecture outcomes include: %stage 1, 2, 3+4, and REM sleep. ANOVA results not reported for sleep architecture due to nonsignificant MANOVA.
Higher scores reflect greater movement during the sleep period (range 0-150).
After adjustment for covariates, unmarried women showed significantly higher actigraphy-assessed WASO (β = 0.13; ΔR2 = 0.02; P = 0.02) than married women (Table 3). However, for all of the remaining outcomes (PSQI, PSG SL and WASO, and actigraphy SL and fragmentation index), the association between marital status and sleep was reduced to nonsignificance, indicating that variation in sleep attributed to current marital status in unadjusted models was accounted for by other known risk factors. Specifically, older age (PSQI, PSG SL, and PSG WASO), African American race (PSQI, PSG SL, PSG WASO, and actigraphy SL, WASO, and fragmentation index), postmenopausal status (PSQI, actigraphy SL), use of medications that could affect sleep (PSQI), financial strain (PSG SL, actigraphy WASO and fragmentation index), and greater depressive symptoms (PSQI, PSG WASO, and actigraphy fragmentation index) were significant covariates (all Ps < 0.05) in the models.
Table 3.
Adjusted linear regression models examining the cross-sectional relationships between marital status (at time of sleep study) and sleep outcomes
| SELF-REPORT | Married | Unmarried‡ | P-value |
|---|---|---|---|
| Sleep Quality | |||
| Complaints (PSQI) | REF | 0.06 (0.39) | 0.22 |
| POLYSOMNOGRAPHY | |||
| SL (minutes) † | REF | 0.10 (0.16) | 0.09 |
| WASO (minutes) † | REF | 0.02 (0.02) | 0.75 |
| ACTIGRAPHY | |||
| SL (minutes) † | REF | 0.04 (0.08) | 0.45 |
| WASO (minutes) † | REF | 0.13 (0.15) | 0.02 |
| Fragmentation Index+ | REF | 0.08 (1.94) | 0.15 |
Values are: Standardized β coefficients (Unstandardized β coefficients). Model adjusted for age, race, financial strain, menopausal status, use of medications that affect sleep, body mass index, and depressive symptoms.
Natural log transformed prior to analyses. For dummy-coded variables (i.e., marital status), the unstandardized β coefficient indicates the value of the increase or decrease in the predicted outcome relative to the referent group (married), after adjustment for covariates.
Higher scores reflect greater movement during the sleep period (range 0-150).
Marital History and Sleep
Table 4 shows the results for ANOVAs and MANOVAs for each of the sleep domains according to marital history category. There were significant group differences in PSQI scores according to marital history (F3, 364 = 6.6, P < 0.001). MANOVAs revealed significant group differences in PSG and actigraphy-assessed sleep continuity (P's < 0.01). Analysis of the individual outcomes within these domains revealed significant differences in PSG- assessed SL and TST, and actigraphy-assessed SL, WASO, TST, and fragmentation index (P's < 0.01). No group differences were observed in sleep architecture or AHI (P's > 0.10).
Table 4.
Analyses of variance (ANOVA) and multivariate analyses of variance (MANOVA) of the associations between marital history and sleep domains
| Consistently Married | Consistently Unmarried | Lost a Partner | Gained a Partner | ||
|---|---|---|---|---|---|
| ANOVAS | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | F- statistic |
| PSQI | 5.4 (3.1) | 6.2 (3.2) | 7.6 (3.9) | 5.5 (2.9) | F3, 364 = 6.6, P < 0.001 |
| AHI† | 9.6 (13.9) | 10.6 (13.6) | 10.1 (11.9) | 15.8 (26.1) | F3, 346 = 0.85, P = 0.47 |
| PSG Continuity | |||||
| MANOVA | F9, 358 = 2.3, P = 0.007 | ||||
| SL† | 17.6 (17.7) | 23.7 (19.5) | 22.3 (24.5) | 18.8 (15.9) | F3, 362 = 3.3, P = 0.02 |
| WASO† | 47.0 (25.9) | 57.1 (42.3) | 63.2 (48.9) | 52.3 (29.9) | F3, 362 = 1.9, P = 0.13 |
| Arousal Index† | 8.3 (4.7) | 9.6 (6.8) | 9.5 (6.7) | 9.9 (6.0) | F3, 362 = 1.7, P = 0.17 |
| TST | 389.6 (54.1) | 380.1 (60.5) | 364.8 (73.2) | 365.1 (59.1) | F3, 362 = 3.3, P = 0.02 |
| Sleep Architecture | |||||
| MANOVA | F12, 237 = 1.4, P = 0.18 | ||||
| Actigraphy | |||||
| MANOVA | F12, 325 = 3.2, P < 0.001 | ||||
| SL† | 21.2 (21.9) | 34.4 (40.7) | 38.1 (54.5) | 30.3 (30.5) | F3, 330 = 4.5, P = 0.004 |
| WASO† | 47.3 (23.1) | 61.3 (26.8) | 54.0 (28.5) | 59.1 (25.5) | F3, 330 = 6.9, P < 0.001 |
| TST | 365.7 (53.0) | 355.6 (53.0) | 341.2 (65.0) | 338.7 (71.7) | F3, 330 = 3.5, P = 0.02 |
| Fragmentation Index + | 27.1 (10.7) | 33.0 (10.2) | 32.5 (14.6) | 35.7 (14.0) | F3, 330 = 8.9, P < 0.001 |
Natural log transformation used in analyses. To facilitate interpretation, means (SDs) are based on raw values (represented in minutes). SL, Sleep Latency; WASO, wakefulness after sleep onset; TST, total sleep time.
Higher scores reflect greater movement during the sleep period (range 0-150).
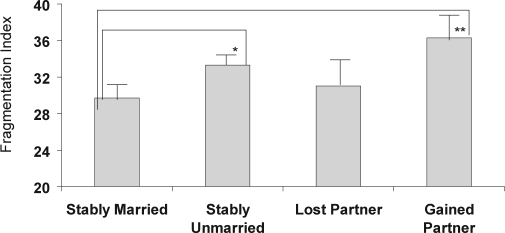
Follow-up linear regression models tested the hypothesis that consistently married women would show relative advantages in sleep as compared to the other marital history categories even after covariate adjustment (Table 5). The step entering marital history contrasts was significant for PSQI (ΔR2 = 0.01, P < 0.05). Analysis of the individual contrasts revealed a significant difference between the consistently married and the lost a partner group (β = 0.12; P < 0.05), indicating worse sleep quality among the women who had lost a partner. The marital history contrasts were not significant for PSG-assessed TST; African American race was the only significant covariate associated with shorter TST (P < 0.05) in the model (as has been previously reported22). For PSG-assessed SL, the step entering the marital group contrasts was nonsignificant (ΔR2 = 0.01, P > 0.10); however, the contrast comparing the consistently married to the consistently unmarried group was statistically significant (β = 0.12; P < 0.05), indicating longer SL for the consistently unmarried. For actigraphy outcomes, the marital history contrasts contributed a significant additional percentage of explained variability for WASO and fragmentation index (ΔR2 = 0.04, P < 0.01 and ΔR2 = 0.03, P = 0.01, respectively), over and above the variance accounted for by covariates. Specifically, both the consistently unmarried and the gained a partner group had greater actigraphy-assessed WASO (β = 0.20, P < 0.001 and β = 0.11, P < 0.05, respectively; Figure 1) and greater sleep fragmentation (β = 0.13, P < 0.05 and β = 0.15, P < 0.01, respectively; Figure 2) than the consistently married group. There were no significant differences in actigraphy-assessed TST or SL according to marital history.
Table 5.
Adjusted linear regression models comparing consistently married women to consistently unmarried women and those who have lost or gained a partner during the 8-year follow-up period
| SELF-REPORT | Consistently Married | Consistently Unmarried‡ | Lost a Partner‡ | Gained a Partner‡ |
|---|---|---|---|---|
| Sleep Quality Complaints (PSQI) | REF | 0.01 (0.10) | 0.11* (1.02) | −0.02 (−0.26) |
| POLYSOMNOGRAPHY | ||||
| SL (minutes)† | REF | 0.12* (0.22) | 0.03 (0.06) | −0.02 (−0.06) |
| TST (minutes) | REF | −0.04 (−5.54) | −0.08 (−14.96) | −0.09 (−18.40) |
| Actigraphy | ||||
| SL (minutes)† | REF | 0.09 (0.20) | −0.02 (−0.06) | 0.06 (0.23) |
| WASO (minutes)† | REF | 0.20*** (0.24) | 0.02 (0.02) | 0.11* (0.21) |
| TST (minutes) | REF | 0.00 (0.30) | −0.02 (−2.71) | −0.08 (−15.73) |
| Fragmentation Index+ | REF | 0.13* (3.63) | 0.04 (1.32) | 0.15** (6.59) |
Values are: Standardized β coefficients (Unstandardized β coefficients). Model adjusted for age, race, financial strain, menopausal status, use of medications that could affect sleep, body mass index, and depressive symptoms.
Natural log transformed prior to analyses. For dummy-coded variables (i.e., marital history), the unstandardized β coefficient indicates the value of the increase or decrease in the predicted outcome relative to the referent group (consistently married), after adjustment for covariates.
Higher scores reflect greater movement during the sleep period (range 0-150);
P ≤ 0.05;
P ≤ 0.01;
P ≤ 0.001
Figure 1.
Actigraphy-assessed WASO according to marital history category, with covariate adjustment. Values represent means (SEs). *P < 0.05; **P < 0.01
Figure 2.
Actigraphy-assessed fragmentation index according to marital history category, with covariate adjustment. Values represent means (SEs). *P < 0.05; **P < 0.01
DISCUSSION
The present study examined the association between marital/cohabitation status and history in relation to sleep in a multi-ethnic sample of midlife women. We found that currently being married was associated with better sleep outcomes but only in unadjusted models. Consistent with prior epidemiologic studies,2,4 currently married women had better sleep quality, and PSG- and actigraphy-assessed sleep continuity than unmarried women. However, with the exception of actigraphy-assessed WASO, these results were not statistically significant in models that adjusted for relevant demographic characteristics and depressive symptoms, which are known to covary with both sleep and marital status. These findings suggest that being married, per se, may not be beneficial for sleep, but rather that marital status may serve as a proxy for these other co-occurring risk factors for sleep disturbance. On the other hand, by examining women's 6-8 year marital histories, we found that consistently married women showed relative advantages in sleep quality and continuity as compared to the other groups, and these results generally persisted in the adjusted models. In contrast to the limited extant literature, which has shown reductions in percent stage 3+4 related to divorce or widowhood,7,30 we found no differences in sleep architecture according to marital status or history. However, the broader literature on psychosocial stressors and sleep architecture is equivocal.39,40 Taken together, these findings are consistent with research and theory suggesting that the stability of attachment relationships, rather than the status of being married itself, is most salient for health and well-being, including sleep.41,42
While the extant literature on marital transitions has focused primarily on the effect of losing a partner due to divorce or widowhood, our findings shed novel insights into how a positive relationship transition—the gain of a new partner—is associated with sleep in women. Overall, we found that the women who had gained a partner were similar to the consistently married women in terms of subjective sleep quality and PSG measures of sleep continuity and architecture. However, we found important differences in measures of actigraphy-assessed sleep continuity (WASO and fragmentation), with the women who had gained a partner showing significantly greater WASO and fragmentation during sleep than the consistently married women. These findings are consistent with previous research, which shows discrepancies in the effects of sleeping with a partner on subjective versus objective sleep parameters.43,44 Specifically, the handful of studies which have examined cosleeping in romantic relationships, have shown that the presence of a bedpartner negatively affects objective measures of sleep; however, subjectively, participants prefer to sleep with the partner.45 These findings suggest that although sleeping with a bedpartner may involve objective costs, the subjective experience of sleep is enhanced by cosleeping, perhaps due to the psychological sense of safety and security derived from sleeping with a close other.42 The objective costs of sleeping with a bedpartner may be particularly evident at the early stages of a relationship, when an acclimation period may occur, involving adjusting to the presence of the bedpartner. However, given previous evidence of greater frequency of sexual intercourse in the early stages of marriage,46 an alternative explanation for these findings is that the gained a partner group were simply engaged in other activities during the defined sleep period.
These speculations must be tempered by several limitations including that our study did not specifically assess whether the participant was sleeping with or without a bedpartner during the sleep evaluation. Thus, findings suggest that stable partnership is correlated with better sleep outcomes in women, regardless of the sleeping conditions themselves. Based on the relatively small sizes of the respective marital transition groups, we could not examine the degree to which the timing of the marital transition affects sleep, as the majority of marital transitions (62%) occurred more than 2 years prior to the sleep study; nor could we examine the influence of multiple transitions. The present findings are therefore likely to reflect more enduring differences in sleep according to the gain or loss of a partner, rather than the more acute and transient effects of very recent transitions.47 The relationship groups were heterogeneous with regard to the reason for unmarried status or loss of a partner, as well as within the married or gained a partner groups, which included women who were married or living as married. Theoretically, stable unions, regardless of their legal status, should be associated with better sleep outcomes because they are indicative of an enduring social attachment to a significant other. Importantly, our characterization of marital history was based on the temporal stability of marriage or the exposure to a marital transition, but the study did not include a direct measure of relationship quality. Thus, findings reflect the influence of marital role continuity on sleep, which may be related to, but is distinct from qualitative measures of relationship functioning. Future research is needed that simultaneously examines the longitudinal course of relationship status as well as relationship quality to determine the degree to which associations between sleep and marital role stability versus transitions depends on the quality of the particular relationship. Indeed, evidence from the broader marriage and mental health literature demonstrates that marital discord preceding divorce, rather than the divorce itself, is a stronger predictor of mental health problems.48 Given previous evidence of gender differences in sleep13 and in the effects of relationships on health outcomes,16 our findings may not generalize to men, other racial/ethnic groups, or other age groups. Finally, the observational nature of the data cannot rule out the possibility of selection bias into marriage (healthier people, including those who sleep better, may be more likely to enter into marriage and stay married, or reverse causality (poor sleep leading to marital dissolution). However, our findings showing differences in actigraphy sleep parameters among women who were continuously married versus those who were newly married (gained a partner), suggests that the stability of marriage rather than entrance into marriage is most salient for sleep.
Notwithstanding these limitations, the study also has a number of strengths, including the study cohort which consisted of midlife women from three different racial groups, with well-characterized reproductive histories, longitudinal assessment of marital histories, and a multimodal assessment of sleep. Indeed, in contrast to the extant literature on marital status and sleep, which has relied primarily on subjective measures of sleep, our study not only included the “gold standard” physiologic measure of sleep (PSG), but also included the most widely used and well-validated measure of sleep quality (the PSQI), and included up to 35 days of actigraphy, to provide a behavioral measure of usual sleep habits in the participants' home environments. This multimodal approach is particularly salient for the present research questions, which aim to understand how the social context correlates with sleep.
In summary, marital history is associated with sleep quality and sleep continuity, independent of other known risk factors for sleep disturbance. Consistently married women may show relative advantages in sleep because of the enduring nature of the marriage and because of sufficient duration of the marriage to acclimate to the sleeping conditions. Research is needed that seeks to understand the underlying relationship processes and physiological and psychological mechanisms that can account for the links between stable marriage and sleep. Indeed, there may be direct physiological effects of sleeping with a partner on sleep, stemming from close physical contact and intimacy within couples. A particularly compelling area of future research is to examine the degree to which oxytocin, a neurohormone that is critically involved in intimacy and pair-bonding,49 and has been shown to have sedating properties,50 serves as a neurobiological substrate linking close relationships with sleep.42 Reconceptualizing sleep as a social behavior that is intimately tied with the social environment represents a shift in traditional sleep research, and one that may ultimately elucidate a critical pathway linking social relationships with health and well-being.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
Funding for the SWAN Sleep Study is from the National Institute on Aging (Grants AG019360, AG019361, AG019362, AG019363). The Study of Women's Health Across the Nation (SWAN) has grant support from the National Institutes of Health, DHHS, through the National Institute on Aging, the National Institute of Nursing Research and the NIH Office of Research on Women's Health (Grants NR004061, AG012505, AG012535, AG012531, AG012539, AG012546, AG012553, AG012554, AG012495). Sleep data were processed with the support of RR024153. Funding for the first author was provided by K23Hl093220 from the National Heart Lung Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Aging, National Heart Lung Blood Institute, National Institute of Nursing Research, Office of Research on Women's Health, or the National Institutes of Health.
Clinical Centers: University of Michigan, Ann Arbor–MaryFran Sowers, PI; Massachusetts General Hospital, Boston, MA–Robert Neer, PI 1994–1999; Joel Finkelstein, PI 1999–present; Rush University, Rush University Medical Center, Chicago, IL–Lynda Powell, PI 1994–2009; Howard Kravitz, PI 2009–present; University of California, Davis/Kaiser–Ellen Gold, PI; University of California, Los Angeles–Gail Greendale, PI; University of Medicine and Dentistry–New Jersey Medical School, Newark–Gerson Weiss, PI 1994–2004; Nanette Santoro, PI 2004–present; and the University of Pittsburgh, Pittsburgh, PA–Karen Matthews, PI.
NIH Program Office: National Institute on Aging, Bethesda, MD–Marcia Ory 1994–2001; Sherry Sherman 1994–present; National Institute of Nursing Research, Bethesda, MD–Program Officers.
Central Laboratory: University of Michigan, Ann Arbor–Daniel McConnell (Central Ligand Assay Satellite Services).
Coordinating Center: New England Research Institutes, Watertown, MA–Sonja McKinlay, PI 1995–2001; University of Pittsburgh, Pittsburgh, PA–Kim Sutton–Tyrrell, PI 2001–present.
Steering Committee: Chris Gallagher, Chair; Susan Johnson, Chair.
We thank the study staff at each site and all the women who participated in SWAN.
Institutions where the study was performed: Rush University Medical Center, Chicago, IL; University of California-Davis, Davis, CA; University of Michigan, Ann Arbor, MI; University of Pittsburgh, Pittsburgh, PA. Portions of this data were presented at the meeting of the Associated Professional Sleep Societies, June 10, 2009, Seattle, WA.
Footnotes
A commentary on this article appears in this issue on page862.
REFERENCES
- 1.Chen Y, Kawachi I, Subramanian SV, Acevedo-Garcia D, Lee Y-J. Can social factors explain sex differences in insomnia? Findings from a national survey in Taiwan. J Epidemiol Community Health. 2005;59:488–94. doi: 10.1136/jech.2004.020511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doi Y, Minowa M, Okawa M, Uchiyama M. Prevalence of sleep disturbance and hypnotic medication use in relation to sociodemographic factors in the general Japanese adult population. J Epidemiol. 2000;10:79–86. doi: 10.2188/jea.10.79. [DOI] [PubMed] [Google Scholar]
- 3.Hajak G Sine Study Group. Epidemiology of severe insomnia and its consequences in Germany. Eur Arch Psychiatry Clin Neurosci. 2001;251:49–56. doi: 10.1007/s004060170052. [DOI] [PubMed] [Google Scholar]
- 4.Hale L. Who has time to sleep? J Public Health. 2005;27:205–11. doi: 10.1093/pubmed/fdi004. [DOI] [PubMed] [Google Scholar]
- 5.Brown LF, Reynolds CF, 3rd, Monk TH, et al. Social rhythm stability following late-life spousal bereavement: associations with depression and sleep impairment. Psychiatry Res. 1996;62:161–9. doi: 10.1016/0165-1781(96)02914-9. [DOI] [PubMed] [Google Scholar]
- 6.Reynolds CF, 3rd, Hoch CC, Buysse DJ, et al. Sleep after spousal bereavement: a study of recovery from stress. Biol Psychiatry. 1993;34:791–7. doi: 10.1016/0006-3223(93)90068-o. [DOI] [PubMed] [Google Scholar]
- 7.Cartwright RD, Wood E. Adjustment disorders of sleep: the sleep effects of a major stressful event and its resolution. Psychiatry Res. 1991;39:199–209. doi: 10.1016/0165-1781(91)90088-7. [DOI] [PubMed] [Google Scholar]
- 8.Prigerson HG, Maciejewski PK, Rosenheck RA. The effects of marital dissolution and marital quality on health and health service use among women. Med Care. 1999;37:858–73. doi: 10.1097/00005650-199909000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Troxel WM, Buysse DJ, Hall M, Matthews KA. Marital happiness and sleep disturbances in a multi-ethnic sample of middle-aged women. [see comment] Beh Sleep Med. 2009;7:2–19. doi: 10.1080/15402000802577736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carmichael CL, Reis HT. Attachment, sleep quality, and depressed affect. Health Psychol. 2005;24:526–31. doi: 10.1037/0278-6133.24.5.526. [DOI] [PubMed] [Google Scholar]
- 11.Scharfe E, Eldredge D. Associations between attachment representations and health behaviors in late adolescence. J Health Psychol. 2001;6:295–307. doi: 10.1177/135910530100600303. [DOI] [PubMed] [Google Scholar]
- 12.Troxel WM, Cyranowski JM, Hall M, Frank E, Buysse DJ. Attachment anxiety, relationship context, and sleep in women with recurrent major depression. Psychosom Med. 2007;69:692–9. doi: 10.1097/PSY.0b013e3180cc2ec7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buysse DJ, Germain A, Moul DE. Diagnosis, epidemiology, and consequences of insomnia. Prim Psychiatry. 2005;12:37–44. [Google Scholar]
- 14.Freedman RR, Roehrs TA. Sleep disturbance in menopause. Menopause. 2007;14:826–9. doi: 10.1097/GME.0b013e3180321a22. [DOI] [PubMed] [Google Scholar]
- 15.Kuh DL, Wadsworth M, Hardy R. Women's health in midlife: the influence of the menopause, social factors and health in earlier life. Br J Obstetr Gynaecol. 1997;104:923–33. doi: 10.1111/j.1471-0528.1997.tb14352.x. [DOI] [PubMed] [Google Scholar]
- 16.Kiecolt-Glaser J, K , Newton TL. Marriage and health: his and hers. Psychol Bull. 2001;127:472–503. doi: 10.1037/0033-2909.127.4.472. [DOI] [PubMed] [Google Scholar]
- 17.Morin CM, Rodrigue S, Ivers H. Role of stress, arousal, and coping skills in primary insomnia. Psychosom Med. 2003;65:259–67. doi: 10.1097/01.psy.0000030391.09558.a3. [DOI] [PubMed] [Google Scholar]
- 18.Nofzinger EA, Price JC, Meltzer CC, et al. Towards a neurobiology of dysfunctional arousal in depression: The relationship between beta EEG power and regional cerebral glucose metabolism during NREM sleep. Psychiatry Res: Neuroimaging. 2000;98:71–91. doi: 10.1016/s0925-4927(00)00045-7. [DOI] [PubMed] [Google Scholar]
- 19.Roth T, Roehrs T, Pies R. Insomnia: pathophysiology and implications for treatment. Sleep Med Rev. 2007;11:71–9. doi: 10.1016/j.smrv.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 20.Hall M, Thayer JF, Germain A, et al. psychological stress is associated with heightened physiological arousal during NREM sleep in primary insomnia. Beh Sleep Med. 2007;5:178–93. doi: 10.1080/15402000701263221. [DOI] [PubMed] [Google Scholar]
- 21.Hall M, Buysse DJ, Nofzinger EA, et al. Financial strain is a significant correlate of sleep continuity disturbances in late-life. Biol Psychol. 2008;77:217–22. doi: 10.1016/j.biopsycho.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hall MH, Matthews KA, Kravitz HM, et al. Race and financial strain are independent correlates of sleep in midlife women: the SWAN sleep study. Sleep. 2009;32:73–82. [PMC free article] [PubMed] [Google Scholar]
- 23.Mezick EJ, Matthews KA, Hall M, al E. Influence of race and socioeconomic status on sleep: Pittsburgh SleepSCORE project. Psychosom Med. 2008;70:410–16. doi: 10.1097/PSY.0b013e31816fdf21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson N, Backlund E, Sorlie PD, Loveless CA. Marital status and mortality: the national longitudinal mortality study. Ann Epidemiol. 2000;10:224–38. doi: 10.1016/s1047-2797(99)00052-6. [DOI] [PubMed] [Google Scholar]
- 25.McLanahan S, Percheski C. Family structure and the reproduction of inequalities. Annu Rev Sociol. 2008;34:257–76. [Google Scholar]
- 26.Bramlett MD, Mosher WD. Cohabitation, marriage, divorce, and remarriage in the United States: National Center for Health Statistics. Vital Health Stat. 2002 [PubMed] [Google Scholar]
- 27.Ohayon MM, Shapiro CM. Tenses of insomnia epidemiology. J Psychosom Res. 2002;53:525–7. doi: 10.1016/s0022-3999(02)00442-7. [DOI] [PubMed] [Google Scholar]
- 28.Simon RW. Revisiting the relationships among gender, marital status, and mental health. AJS. 2002;107:1065–96. doi: 10.1086/339225. [DOI] [PubMed] [Google Scholar]
- 29.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 30.Reynolds CF, Hoch CC, Buysse DJ, Houck PR, et al. Electroencephalographic sleep in spousal bereavement and bereavement-related depression of late life. Biol Psychiatry. 1992;31:69–82. doi: 10.1016/0006-3223(92)90007-m. [DOI] [PubMed] [Google Scholar]
- 31.Marks NF, Lambert JD. Marital status continuity and change among young and midlife adults: longitudinal effects on psychological well-being. J Fam Issues. 1998;19:652–86. [Google Scholar]
- 32.Sowers MF, Crawford SL, Sternfeld B, Morganstein D, Gold EB, Greendale GA. SWAN: A multicenter, multiethnic, community-based cohort study of women and the menopausal transition. In: Lobo R, Marcus R, Kelsey J, editors. Menopause: biology and pathobiology. San Diego: Academic Press; 2000. pp. 175–88. [Google Scholar]
- 33.Rechtschaffen A, Kales A. Washington, DC: Department of Health Education and Welfare; 1968. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects NIH Publication 204. [Google Scholar]
- 34.The American Academy of Sleep Medicine Task Force. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 35.Edinger JD, Marsh GR, McCall WV, Erwin CW, Lininger AW. Sleep variability across consecutive nights of home monitoring in older mixed DIMS patients. Sleep. 1991;14:13–7. [PubMed] [Google Scholar]
- 36.Matthews KA, Kiefe CI, Lewis CE, Liu K, Sidney S, Yunis C. Socioeconomic trajectories and incident hypertension in a biracial cohort of young adults. Hypertension. 2002;39:772–6. doi: 10.1161/hy0302.105682. [DOI] [PubMed] [Google Scholar]
- 37.Rush A, Trivedi MH, Ibrahim HM, et al. Biol Psychiatry. 2003. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression; pp. 573–83. [DOI] [PubMed] [Google Scholar]
- 38.National Institutes of Health, National Heart Lung Blood Institute, and National Institute of Diabetes Digestive and Kidney Diseases. Washington, DC: NIH Publication No 98-4083; 1998. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults. [Google Scholar]
- 39.Akerstedt T. Psychosocial stress and impaired sleep. Scand J Work Environ Health. 2006;32:493–501. [PubMed] [Google Scholar]
- 40.Hall M, Buysse DJ, Nowell PD, et al. Symptoms of stress and depression as correlates of sleep in primary insomnia. Psychosom Med. 2000;62:227–30. doi: 10.1097/00006842-200003000-00014. [DOI] [PubMed] [Google Scholar]
- 41.Ross CE. Reconceptualizing marital status as a continuum of social attachment. J Marriage Fam. 1995;57:129. [Google Scholar]
- 42.Troxel WM, Robles TF, Hall M, Buysse DJ. Marital quality and the marital bed: examining the covariation between relationship quality and sleep. Sleep Med Rev. 2007;11:389–404. doi: 10.1016/j.smrv.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Waterman AL, Kerkhof G. Sleep-wake patterns of partners. Percept Mot Skills. 1998;86:1141–2. doi: 10.2466/pms.1998.86.3c.1141. [DOI] [PubMed] [Google Scholar]
- 44.Monroe LJ. Transient changes in EEG sleep patterns of married good sleepers: The effects of altering sleeping arrangement. Psychophysiology. 1969;6:330–7. doi: 10.1111/j.1469-8986.1969.tb02910.x. [DOI] [PubMed] [Google Scholar]
- 45.Pankhurst FP, Horne JA. The influence of bed partners on movement during sleep. Sleep. 1994;17:308–15. doi: 10.1093/sleep/17.4.308. [DOI] [PubMed] [Google Scholar]
- 46.Call V, Sprecher S, Schwartz P. The incidence and frequency of marital sex in a national sample. J Marriage Fam. 1995;57:639. [Google Scholar]
- 47.Laditka JN, Laditka SB. Increased hospitalization risk for recently widowed older women and the protective effects of social contacts. J Women Aging. 2003;15:7–28. doi: 10.1300/J074v15n02_02. [DOI] [PubMed] [Google Scholar]
- 48.Overbeek G, Vollebergh W, de Graaf R, Scholte R, de Kemp R, Engels R. Longitudinal associations of marital quality and marital dissolution with the incidence of DSM-III-R disorders. J Fam Psychol. 2006;20:284–91. doi: 10.1037/0893-3200.20.2.284. [DOI] [PubMed] [Google Scholar]
- 49.Neumann I. Brain oxytocin: A key regulator of emotional and social behaviours in both females and males. J Neuroendocrinol. 2008;20:858–65. doi: 10.1111/j.1365-2826.2008.01726.x. [DOI] [PubMed] [Google Scholar]
- 50.Lancel M, Kromer SA, Neumann ID. Intracerebral oxytocin modulates sleep-wake behavior in male rats. Regul Pept. 2003;114:145–52. doi: 10.1016/s0167-0115(03)00118-6. [DOI] [PubMed] [Google Scholar]