Abstract
Study Objective:
Incremental withdrawal of serotonin during wake to sleep transition is postulated as a key mechanism that renders the pharyngeal airway collapsible. While serotonin promotion with reuptake inhibitors have demonstrated modest beneficial effects during NREM sleep on obstructive sleep apnea (OSA), animal studies suggest a potential therapeutic role for selective serotonin receptor antagonists (5-HT3) in REM sleep. We aimed to test the hypothesis that a combination of ondansetron (Ond) and fluoxetine (Fl) may effectively reduce expression of disordered breathing during REM and NREM sleep in patients with OSA.
Design and Setting:
A prospective, parallel-groups, single-center trial in patients with OSA.
Participants:
35 adults with apnea hypopnea index (AHI) > 10; range 10-98.
Intervention:
Subjects were randomized to placebo, n = 7; Ond (24 mg QD), n = 9; Fl (5 mg QD) + Ond (12 mg QD), n = 9; and Fl (10 mg QD) + Ond (24 mg QD), n = 10.
Measurements and Results:
AHI was measured by in-lab polysomnography after a 7-day no-treatment period (Baseline) and on days 14 and 28 of treatment. The primary endpoint was AHI reduction at days 14 and 28. OND+FL resulted in approximately 40% reduction of baseline AHI at days 14 and 28 (unadjusted P < 0.03 for each) and improved oximetry trends. This treatment-associated relative reduction in AHI was also observed in REM and supine sleep.
Conclusions:
Combined treatment with OND+FL is well-tolerated and reduces AHI, yielding a potentially therapeutic response in some subjects with OSA.
Citation:
Prasad B; Radulovacki M; Olopade C; Herdegen JJ; Logan T; Carley DW. Prospective trial of efficacy and safety of ondansetron and fluoxetine in patients with obstructive sleep apnea syndrome. SLEEP 2010;33(7):982-989.
Keywords: Apnea, drug treatment, clinical trial, randomized, placebo-controlled, OSA, ondansetron, fluoxetine
THE HIGH PREVALENCE OF OSA1 AND PLETHORA OF ASSOCIATED COMORBIDITIES AND CONSEQUENCES OF OSA HAVE BEEN EXTENSIVELY INVESTIGATED and elucidated over the past few decades.2,3 Despite the accrual of a large body of scientific evidence, the underlying neural mechanisms and pathogenesis of upper airway closure in OSA is not fully understood, limiting the development of effective pharmacologic interventions. Although the current standard of therapy, continuous positive airway pressure (CPAP) is effective in controlling the expression of sleep disordered breathing and in ameliorating many of the associated comorbidities,4–6 these salutary effects of CPAP are highly dependent on adherence,7 which remains a challenge.8 Putative pharmacologic interventions have attempted neuromodulation to increase upper airway muscle tone, to accentuate central respiratory drive, or to suppress REM sleep. Currently, there is no generally effective pharmacologic treatment for OSA, with only a minority of the interventions tested showing beneficial effects on short-term outcomes.9
Decreased serotonergic facilitation of upper-airway motor neurons during sleep may be an important mechanism rendering the upper airway vulnerable to collapse in OSA.10 The effect of serotonergic activation on the expression of sleep apnea is determined by the site of activation (central vs. peripheral). This is further complicated by the multitude of serotonin receptor subtypes in the central and peripheral nervous system,11 as well as their differential effects on phrenic nerve12 and upper airway motor outputs,13 and alterations of chemical and mechanical ventilatory reflexes.14 Furthermore, evidence from animal studies suggest that apnea-induced long-term facilitation (LTF) or augmentation of respiratory activity of the phrenic and hypoglossal motor neurons is serotonin dependent.15
In the brainstem, endogenous serotonin release promotes drive to the upper airway dilators in the waking state, primarily via postsynaptic 5-HT2 receptors,13 while peripherally, serotonin release at 5-HT3 receptors in the nodose ganglion promotes the expression of REM-related apnea.16 These observations provide a theoretical framework that may reconcile some of the inconsistent therapeutic effects associated with the use of systemic serotonergic neuromodulation on sleep disordered breathing (SDB) attempted with buspar,17 paroxetine,18 and fluoxetine.19 Collectively, these studies suggest only limited therapeutic effects of selective serotonin reuptake inhibitors (SSRI) on SDB, and only during NREM sleep.
We hypothesized that combined stimulation of central 5-HT2 and inhibition of peripheral 5-HT3 receptors may result in more robust and consistent inhibition of apnea-genesis, independent of sleep stage. Two distinct compounds with 5-HT3 receptor antagonistic properties, administered intraperitoneally to a rat model of sleep apnea suppressed the expression of spontaneous central apnea. These salutary effects were remarkably state dependent, being most significant in REM sleep.20,21 Ondansetron, a selective 5-HT3 receptor antagonist administered orally, has also been shown to be effective in reduction of SDB expression in the English bulldog, an animal model of OSA.22 However, the same drug showed no therapeutic potential in a single-dose placebo controlled human study of moderate to severe OSA.23 While efficacy of mirtazapine, an antidepressant with 5-HT1 agonist as well as 5-HT2 and 5-HT3 antagonist effects in sleep apnea expression has been reported in animal studies,24 the results are mixed for human trials.25,26
We conducted a proof of concept, double-blind, placebo-controlled, parallel-groups clinical trial aimed to test the hypothesis that a combination of ondansetron and fluoxetine (Ond + Fl) can reduce the expression of sleep disordered breathing in patients with obstructive sleep apnea syndrome. The primary objective was to demonstrate the efficacy of this drug combination on the AHI; the most widely accepted objective measure of disease severity. Secondary objectives included examination of the dose-effect on AHI, and evaluation of dose effects on objective measures of sleep quality and other polysomnography based variables indicative of disease severity.
METHODS
Subjects
Subjects were selected from a clinical population referred to a single tertiary-care center Sleep laboratory with a known or suspected diagnosis of OSAS. After providing written informed consent at the initial screening visit, all subjects underwent history, physical, and laboratory examination. After a 7-day no-treatment period, during which subjects discontinued any current therapy for their OSAS, subjects underwent baseline overnight polysomnography. Subjects aged 21 to 65 years with AHI ≥ 10 were then randomized as to treatment group subject to the following exclusion criteria: arterial oxygen saturation < 75% for more than 5% of total sleep time on screening PSG; severe OSA that precluded withdrawal of PAP treatment; history of shift work or rotating shifts within preceding 1 month; history of any surgical treatment for OSA at any time or other major surgery within 6 months; participation in any form of medically managed weight loss program within 6 months, any clinically significant cardiopulmonary, gastrointestinal, pancreatic, hepatic, renal, hematologic, endocrine (including type 1 diabetes), neurological, urogenital, psychiatric, or sleep disorder other than OSA. Additional exclusion criteria included use of any central nervous system-active drug or serotonergic drug, pregnancy, alcohol or recreational drug use, positive plasma drug screen, or clinically significant abnormality on complete blood count. This study was approved by the Institutional Review Board of the University of Illinois at Chicago.
Study Protocol
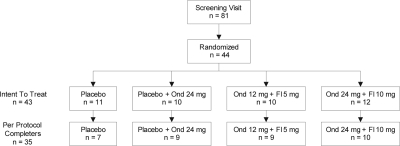
Figure 1 depicts the disposition of all subjects enrolled in the study. Eighty-one subjects provided initial informed consent to participate and were enrolled. Of these, 44 subjects met all inclusion/exclusion criteria and were randomized to specific treatment groups. Although the randomization schedule was balanced, the number of subjects to complete the study per-protocol ranged from 7 (placebo) to 10 (high-dose combination).
Figure 1.
Randomization flow chart. Ond, Ondansetron; Fl, Fluoxetine.
Randomized subjects initiated treatment the day immediately following their first overnight PSG performed 7 to 10 days after discontinuation of CPAP treatment, which served as their baseline PSG as well as the final screening step to verify inclusion/exclusion criteria. Randomized subjects were provided blinded study agent in white capsules on coded blister cards and instructed to take one labeled pill with breakfast (placebo or fluoxetine) and one labeled pill 30 minutes before bed (placebo or ondansetron) each night, and to complete a sleep/activity/drug-administration log daily. Randomization of subjects was to one of 4 treatment groups: placebo AM/placebo PM, placebo AM + Ond (24 mg QD PM), Fl (5 mg QD AM) + Ond (12 mg QD PM) or Fl (10 mg QD AM) + Ond (24 mg QD PM). Subjects continued daily AM/PM dosing of study medications for 28 days with subsequent overnight PSG studies on treatment days 14 and 28. The dose of fluoxetine and ondansetron were chosen to minimize adverse effects and in consideration of the results of our previous experiments where we found a ratio of approximately 1:1 by weight was best in the rat-model of sleep disordered breathing.34 Because the bioavailability of fluoxetine is higher in humans than in rats, we estimated that a ratio of 1:2.4 (Fl: Ond) by mouth would be likely to yield a plasma ratio similar to that which pertained in the animal studies. Treatment adherence was assessed by self-report, accounting of all returned treatment units as well as venous blood pharmacokinetic (pK) analysis of study drugs on days 14 and 28 of the study period. Five to ten days after completing the treatment period (day 28), subjects returned for a final post-study visit comprising a brief physical examination, adverse event monitoring, and where appropriate, compliance monitoring for re-institution of CPAP treatment. There was a single subject from the placebo group who was discontinued after randomization due to adverse events.
After randomization and throughout the duration of the protocol each subject was instructed to maintain a regular schedule and sleep/wake/activity pattern, was given sleep/activity/drug-administration logs, instructed in their use, and was asked to bring completed logs to each subsequent visit. Subjects that required discontinuation of their continuous positive airway pressure (CPAP) treatment were instructed to expect recurrence of the symptoms of their sleep apnea, including daytime sleepiness. They were also instructed in how to chart these symptoms and received follow-up from the study coordinator on days 3 and 5 after treatment suspension to assess the occurrence and severity of such symptoms. In the final 2 days of the CPAP washout period as well as each of the two 2-week treatment periods, the subjects completed a Stanford Sleepiness Scale every 2 h while awake for a total 2 days each time.
Each subject underwent overnight polysomnography in the Center for Sleep and Ventilatory Disorders at the University of Illinois Hospital on days 0 (screening), 14, and 28. Subjects reported to the Center at 21:00 and were instrumented for electroencephalogram (C3/A2, O2/A1), bilateral electroculogram, electromyogram (submental, bilateral anterior tibial), electrocardiogram, oronasal airflow (thermistor and nasal pressure transducer), thoracoabdominal motion (piezocrystal, EPM Systems), arterial oxygen saturation by pulse oximetry, and body position. All signals, including digital infrared video, were acquired, processed, and stored using the Alice 3 digital system (Respironics). Subjects were administered the PM dose of their study medication on days 14 and 28, 30 minutes before lights out. All subjects were given a minimum of 8 hours time in bed opportunity.
Sleep was scored according to standard criteria on 30-sec epochs, and respiratory events were scored according to previously published American Academy of Sleep Medicine guidelines.27 Specifically, hypopneas were scored when a reduction in airflow of > 50% occurred and was associated with either an oxygen desaturation > 3% or an arousal. Electroencephalogram arousals were scored according to American Academy of Sleep Medicine guidelines.28 Time in bed was computed as the number of minutes in bed between lights out and lights on; total sleep time as the number of minutes of sleep between lights out and lights on; and sleep efficiency as the ratio of the two: total sleep time divided by time in bed. The distribution of sleep stages was separately determined as percentages of total sleep time scored as: (1) stage N1 or stage N2, (2) stage N3 or N4, and (3) REM sleep. In view of the above technologic limitations, and because AHI was the primary outcome variable, we sought to minimize variability of respiratory-event scoring by using a single polysomnographer blinded to subject and treatment information.
Statistical Analysis
The primary efficacy analysis was performed on the per-protocol (PP) population. The PP population included all randomized subjects who: (1) received at least one dose of study medication (the intent to treat [ITT] population); (2) missed no more than 4 PM doses in treatment period 2 (days 15 to 28); and (3) received both doses of study medication (AM and PM) on study day 28. Safety analyses were performed on the ITT population, comprising all subjects who received at least one dose of study medication.
The primary efficacy measurement was the change from baseline in the AHI at treatment days 14 and 28. The changes from baseline in the active treatment groups were compared to the placebo group using an ANCOVA design with treatment group as the main effect and body mass index (BMI) and baseline AHI as covariates. Dunnett test was used to adjust the post hoc pairwise comparisons between each active treatment group and placebo. All analyses were performed using SAS 8.2, and a P-value < 0.05 was considered significant.
RESULTS
There were no significant differences in the incidence of adverse events in subjects between the active treatment groups and placebo, with most of the reported adverse events being gastrointestinal symptoms, including constipation. Eight randomized subjects failed to complete the trial per protocol (attrition from ITT to PP population):1 due to AE in the placebo group, and two in the placebo and Ond 24 + Fl 10 treatment groups each, and 1 each from the other groups due to noncompliance. As described in Table 1, baseline AHI, mean oxygen saturation nadir following respiratory events, and sleep efficiency did not differ significantly among treatment groups. Mean arousal index was higher in the placebo group than the active treatment groups (Table 1).
Table 1.
Screening demographic characteristics of all subjects completing per protocol
| Parameter Mean (SD) | Placebo (N = 7) |
Ondasetron (N = 9) |
Fluoxetine (5 mg) + Ondasetron (12 mg) (N = 9) |
Fluoxetine (10 mg) + Ondasetron (24 mg) (N = 10) |
P-value |
|---|---|---|---|---|---|
| Gender [n (%)] | |||||
| Male | 6 (85.7) | 5 (55.6) | 5 (55.6) | 7 (70) | 0.554 |
| Female | 1 (14.3) | 4 (44.4) | 4 (44.4) | 3 (30) | |
| Race [n (%)] | |||||
| White | 3 (42.9) | 2 (22.2) | 6 (66.7) | 4 (40) | 0.626 |
| African American | 4 (57.1) | 5 (55.6) | 3 (33.3) | 5 (50) | |
| Hispanic | 1 (14.3) | 2 (22.2) | 1 (11.1) | 0 (0.0) | |
| Other | 0 (0.0) | 2 (22.2) | 0 (0.0) | 1 (10) | |
| Age (years) | 46 (10.57) | 46.1 (12.26) | 50.9 (8.07) | 49.4 (10.35) | 0.706 |
| BMI (kg/m2) | 35.2 (3.63) | 30.9 (5.21) | 37.5 (6.76) | 35.2 (7.13) | 0.149 |
| Polysomnographic Variables | |||||
| Sleep efficiency (%) | 91.8 (3.55) | 91.2 (6.73) | 76.8 (21.14) | 87.5 (12.66) | 0.098 |
| Arousal Index (/hour) | 43.6 (13.67) | 20.1 (14.93) | 32.3 (21.54) | 24.2 (11.99) | 0.022 |
| AHI (/h) | 54.2 (29.75) | 33.6 (19.82) | 44.0 (30.79) | 37.0 (23.28) | 0.422 |
| Minimum SpO2 | 87.0 (3.83) | 91.4 (2.30) | 89.2 (2.68) | 89.0 (3.86) | 0.112 |
| Stanford Sleepiness Scale | 4.25 (0.56) | 4.72 (0.74) | 4.31 (0.84) | 3.70 (0.39) | 0.076 |
SD, Standard deviation; SpO2, oxygen saturation by pulse oximetry; AHI, apnea hypopnea index;
The P-values are for ANOVA.
Primary Endpoint: Efficacy
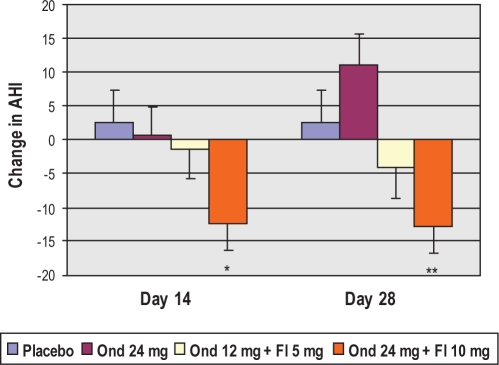
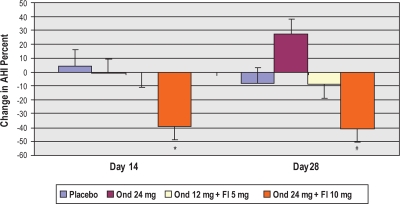
Change in AHI from baseline (no treatment) to day 28 of treatment within the per protocol groups was the primary efficacy endpoint. As shown in Figures 2 and 3, the high dose combination treatment showed a trend toward improvement with a mean of 12.9 events/h reduction in AHI, or 40.5% decrease at treatment day 28 in comparison to baseline (unadjusted P = 0.005, adjusted P = 0.19). Conversely, there was a lack of change with treatment in the placebo group. Numerically, the lower dose combination treatment exhibited a more modest reduction in AHI of 4.2 events per hour, or 8.8%. While comparison of the high- and low-dose combination effects on AHI is consistent with a dose-response relationship, the lower dose effects did not achieve statistical significance and will require further clinical testing to firmly establish a threshold dose for efficacy. In the ondansetron alone group, there was a trend toward increased AHI post-treatment, which was not statistically significant.
Figure 2.
Adjusted mean change from baseline in AHI within groups at treatment day 14 and treatment day 28. *P = 0.069; **P = 0.057.
Figure 3.
Adjusted mean percent change from baseline in AHI within groups at treatment day 14 and treatment day 28.*P= 0.02; #P = 0.19.
Primary Endpoint: Stratification
Beyond the biometric characteristics and upper airway structural parameters of individual subjects, several other factors are well-recognized to influence the expression of disordered breathing events. Most important among these additional factors are sleep stage and body position. Therefore, the treatment effects on AHI were stratified according to REM sleep versus NREM sleep; and according supine versus non-supine sleep. Among these, REM sleep and supine sleep are the most provocative states for apnea/hypopnea expression. In addition, because the kinetics of ondansetron may not have been optimal to achieving all-night treatment effects (mean elimination half-life of 5.7 h), the primary endpoint also was stratified according to time of night: first half (4 hours) versus second half (4 h).
Table 2 summarizes the stratified primary endpoint analysis as described above, for the high dose combination treatment and placebo groups. The AHI exhibited similar (approximately 40%) reductions for all strata: REM, NREM, supine, non-supine, first half and second half of the night. In addition, similar reductions were observed in both apnea index and hypopnea index. These reductions achieved statistical significance for REM AHI and AHI during non-supine sleep. Notably, the most sustained effect on AHI within the high dose combination treatment group occurred in the second half of the night and was significant at both days 14 and day 28. Because combination treatments may affect individual drug levels, and their relative plasma levels may also affect treatment-related effects. Thus, we examined the effect of the morning serum samples for Ond to Fl drug levels ratios after overnight polysomnography performed at the end of each treatment period. Within the Ond 24 + Fl 10 mg group, the Ond to Fl ratio appeared to affect AHI reduction at day 14, when an increase in ratio from 0-5 correlated with reduction in AHI significantly (r = 0.76, P = 0.009). No significant correlations were observed within this group at day 28 or in the combined low-dose and high-dose combination treatment groups at either day 14 or day 28.
Table 2.
Summary of stratified findings for primary endpoint analysis in the per-protocol population
| PSG | Placebo/Placebo (N = 7) |
Fl 10 mg/Ond 24 mg (N = 10) |
||||
|---|---|---|---|---|---|---|
| Baseline | Day 14 | Day 28 | Baseline | Day 14 | Day 28 | |
| REM AHI | 55.2 | 58.6 | 62.4 | 45.5 | 27.3P = 0.018 | 36.8 |
| NREM AHI | 53.8 | 55.4 | 55.2 | 33.8 | 23.4 | 20.5 |
| Supine AHI | 84.4 | 64.9 | 92.6 | 48.4 | 40.9 | 33.4 |
| Non-Supine AHI | 50.3 | 56.4 | 53.6 | 22.7 | 16.1P = 0.044 | 14.8 |
| AHI 1st Half | 63.5 | 59.8 | 61.7 | 37.9 | 29.7 | 23.3 |
| AHI 2nd Half | 45.5 | 52.5 | 52.8 | 36.5 | 20.4P = 0.004 | 25.0P = 0.035 |
| Apnea Index | 24.4 | 27.5 | 25.2 | 13.8 | 7.1 | 8.0 |
| Hypopnea Index | 29.8 | 28.7 | 31.8 | 23.2 | 17.8 | 16.2 |
| Arousal Index | 43.6 | 46.3 | 40.7 | 24.2 | 18.9 | 21.4 |
Fl, Fluoxetine; Ond, Ondansetron; PSG, polysomnogram; AHI, apnea hyponea index;
1s and 2nd half of the entire night of PSG recording. The polysomnographic parameters with statistically significant change at days 14 and 28 of treatment compared to baseline are indicated within the table with numerical P values specified.
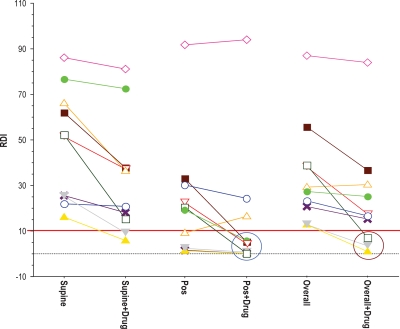
It is crucial that an effective therapy control apnea/hypopnea expression independent of the sleep position of the patient. The impact of the high-dose combination treatment on AHI (overall and according to sleep position) is presented for each of the 10 individual subjects of this group in Figure 4. As expected, AHI was higher during supine than during non-supine sleep in 9 of the 10 subjects. The most important finding illustrated by Figure 4 is the general consistency of the therapeutic effect for all subjects. It does appear that there may be a loss of effect at very high baseline AHI (AHI > 70), but verifying this possibility will require significant additional data from future study phases. In addition, the three responders (defined by a > 50% reduction and a post-treatment AHI at day 28 < 10) are highlighted by the brown circle at the lower right of Figure 4. The responder group increased to 4 of 6 possible responders when only non-supine sleep was considered.
Figure 4.
Individual treatment responses for the high dose combination group at day 28 (n = 10). The solid line marker is for an AHI of 10; the dashed line an AHI of 0. The circles identify the clinical responders defined a priori: Post-treatment AHI of less than 10 and reduction in AHI of > 50% from baseline. RDI (Respiratory Disturbance Index) reflects the apnea hypopnea index (AHI). Each subject is represented by a different color and individual line segments connect the observed values for AHI in that subject at baseline (left side of each segment) and at day 28 of high dose combination treatment (right side of each segment). The left column presents observations from supine sleep, the center column (Pos) from non-supine sleep, and the right column from overall sleep (same values reflected in the aggregate in Figures 2 and 3).
Secondary Endpoints
Oximetry
Transient oxygen desaturation of arterial hemoglobin is commonly associated with individual apnea/hypopnea events, and these desaturations are believed to contribute to both cardiovascular and cognitive impairment. Secondary endpoints to assess treatment-related improvement in oxygen delivery included change from baseline to day 28 of treatment in 2 measures: (1) time (minutes) spent with oxygen saturation < 90% and (2) percent of total sleep time at oxygen saturation below 90%. As summarized in Table 3, even the high-dose combination treatment exerted only a modest trend toward improvement in either of these measures.
Table 3.
Summary of secondary oximetry endpoint analysis in the per-protocol population
| Treatment group | Time (min) at SpO2 < 90% Mean (SD) |
Percent of TST at SpO2 < 90% Mean (SD) |
||||
|---|---|---|---|---|---|---|
| Baseline | Day 14 | Day 28 | Baseline | Day 14 | Day 28 | |
| Placebo/Placebo (N = 7) | 85.9 (85.67) | 104.2 (100.33) | 96.4 (101.85) | 19.2 (18.80) | 25.1 (22.82) | 23.4 (24.10) |
| Placebo / Ond 24 mg (N = 9) | 13.2 (17.44) | 20.0 (26.66) | 51.8 (69.61) | 2.9 (3.63) | 4.4 (5.76) | 11.2 (14.47) |
| Fl 5 mg / Ond 24 mg (N = 9) | 38.5 (41.34) | 66.5 (84.43) | 77.6 (91.29) | 10.1 (9.26) | 18.7 (22.29) | 18.7 (22.03) |
| Fl 10 mg / Ond 24 mg (N = 10) | 53.6 (71.18) | 36.6 (45.74) | 46.3 (58.49) | 12.9 (16.78) | 9.6 (13.15) | 11.7 (15.11) |
TST, total sleep time; SpO2, oxygen saturation by pulse oximetry; Ond, ondansetron; Fl, fluoxetine.
Dunnett adjusted P-values were not significant for all group and time comparisons.
Sleep Architecture
Arousal index, a measure of the frequency of brief (3-15 s) arousals observable in the cortical EEG is a commonly employed metric of sleep continuity. In the present POC study, there were no statistically significant treatment effects observed on arousal index. As delineated in Table 2, the high dose combination treatment was associated with a numerical decrease in arousal index ranging from 10% to 20% with respect to baseline.
Finally, consideration was given to measures of REM sleep expression and integrity because fluoxetine is known to suppress REM sleep. No significant treatment-related changes in REM expression were demonstrated, and baseline measures of REM sleep (latency, duration, percentage of TST) were within normal limits in all 4 treatment groups. There was a trend toward degraded REM sleep in the group receiving high dose combination treatment: REM latency increased by 10%, and REM duration and REM percent of TST decreased by approximately 5%; none of these were statistically significant.
DISCUSSION
This randomized, double-blind, placebo-controlled study demonstrated that AHI was reduced by combination treatment with fluoxetine and ondansetron in patients with OSA. This effect was consistent across sleep stages, body position, and throughout the night.
Serotonergically active drugs have been previously examined as putative OSA therapeutics. In an uncontrolled human trial,19 fluoxetine was reported to reduce AHI during NREM sleep. In a placebo-controlled randomized trial on men with OSA, paroxetine was found to significantly reduce apnea index by approximately 35% but only during NREM sleep, while having no effect on hypopnea event density in any stage of sleep.18
Animal data regarding the impact of serotonin on respiratory control during sleep demonstrate considerable complexity, with a multitude of site- and receptor-specific effects. State-dependent withdrawal of serotonin release in the brainstem (nadir in REM sleep) has been reported during natural sleep,29,30 and an excitatory effect of serotonin on hypoglossal motor neurons via 5HT2A receptors has been demonstrated.13 Notwithstanding the above, the role of endogenous serotonin in the facilitation of genioglossus muscle tone during sleep has been questioned.31,32
Other data also are not fully consistent. While application of a serotonergic neurotoxin in the brainstem inhibits respiratory chemoreflexes,14 intravenous injection of 5-HT induces a dose-dependent apnea.33 Intraperitoneal administration of 5-HT in freely behaving rats augments the expression of apnea during REM sleep and is blocked by pretreatment with ondansetron.16 The likely site for these observed effects is the 5HT3 receptors on the nodose ganglion, as neither ondansetron nor serotonin effectively crosses the blood-brain barrier. Indeed, ondansetron has been shown to reduce apnea expression during REM sleep in two different animal models.21,22 These results however, were not replicated in a 10-subject, single-dose human study of moderate OSA.23 The authors postulated that the low-dose, single-night administration, and species-specific effects of ondansetron as potential explanations for the contradictory results observed.
Based on the collective evidence presented above, it may be hypothesized that an OSA pharmacologic intervention targeting the central 5HT2A and the peripheral 5HT3 receptors may prove to be efficacious across the sleep stages and may be expected to result in a meaningful overall clinical response. In fact, our experiments on a rat model of SDB, showed a significant reduction in apnea index with administration of ondansetron (at 1 mg/kg dose) alone and a significant potentiation of this observed therapeutic effect by combining ondansetron with fluoxetine (at 1 mg/kg each).34
To our knowledge, this randomized, double-blind, placebo-controlled study is the first to examine a combined pharmacologic approach targeting the previously observed site and receptor specific serotonin-mediated effects in humans with OSAS. The observed high-dose combination treatment-related mean reduction in AHI of 40% is clinically meaningful, given this small POC trial, in which the baseline AHI of subjects ranged from mild (AHI = 10) to severe (AHI = 98). Although, we did not observe a concomitant improvement in daytime sleepiness assessed by the Stanford Sleepiness Scale, the present study was not adequately powered to examine subjective responses. A reduction in AHI on the order of 40% to 50% may be expected to result in reduced daytime symptoms in some patients and potentially reduce risks for incidence or progression of cardiovascular or metabolic disease in most patients with OSAS.35,36 Nevertheless, a reduction in AHI from 80 to 40 still leaves clinically significant untreated disease (and its attendant morbidity) in place; whereas a reduction from 15 to 7.5, for example, may be sufficient to control both the risks and symptoms associated with OSAS. Both the practical constraints of pharmacotherapeutics in OSAS, and the specific findings of the present POC study, suggest that the high-dose combination treatment may yield a fully therapeutic response in only a subset of patients with mild-moderate OSA.
With respect to respiratory event density index (AHI), the operational definition for a “clinical responder” was a subject who exhibited: an AHI reduction from baseline of at least 50% and a final on-treatment (day 28) AHI of less than 10 events per hour. Using this definition, a responder analysis revealed that 3 of 10 (30%) of subjects receiving the high dose combination treatment were clinical “responders” in contrast to 0 of 7 receiving placebo, 0 of 9 receiving low dose combination treatment, and 1of 9 receiving ondansetron alone. Ondansetron alone also exhibited a trend toward increased AHI at day 28. These findings collectively support the view that fluoxetine is necessary to the efficacy of the combination treatment, but leaves the necessity of ondansetron in question. A previous study with fluoxetine showed similar numerical reduction in AHI of approximately 40% at 4 weeks during NREM sleep only.19 However, another study with paroxetine did not demonstrate any treatment effect on hypopneic events in NREM and the AHI overall in REM sleep at 6 weeks.18 In consideration of above, our observation that the percent reduction in AHI achieved statistical significance only at day 14 suggests an initial therapeutic response in OSAS to serotonin neuromodulation in the central nervous system that wanes at day 28, likely as a result of neuroplasticity, potentially limiting the role of this therapeutic approach.
Notwithstanding the improvement in the respiratory event density measures of OSA disease severity in the high dose combination treatment group, this group failed to demonstrate a significant treatment response assessed by the oximetry-based indices. The nonlinear and dynamic characteristics of oxygen binding to hemoglobin and the further degradation of ventilation/perfusion matching within the lungs during REM sleep provide a physiologic mechanism and in our speculation a rationale for this observation.
The study population is too small to attempt any firm characterization of the responder group. However, it may be noteworthy that the 3 responders among those receiving the high dose combination treatment were also among the least obese of the study population, with BMI < 32 for each. This suggests that these subjects may have had a higher “effective” dose exposure than other subjects in the same treatment group. If true, this may imply that the 24 mg/ 10 mg combination was very near or even just below the threshold therapeutic dose and that greater efficacy might be observed at even higher doses. This possibility provides a rationale to examine higher combination doses during future clinical trials.
None of the above findings changes appreciably when the intent to treat (ITT) population is assessed rather than the per protocol (PP) population. In addition, similar proportionate reductions in the two major disordered breathing event types, apneas and hypopneas, were observed in the high dose combination treatment group. Both of these facts suggest the robustness of the findings regarding the high dose combination treatment, despite the small study population. The stratified analyses suggest that the high dose combination treatment may simply work better on mild-to-moderate OSA (AHI < 40) than it does on severe OSA. The improved treatment response (e.g., more responders) with change from supine to non-supine sleep may potentially be attributed to a more mild expression of OSA in the non-supine posture.
The minimal changes of sleep architecture with treatment merit some discussion, given the concomitant 40% decrease in AHI. While it is true that most disordered breathing events are terminated with EEG-observable arousal, many are not. Another factor confounding the relationship between AHI and arousal index arises from scoring conventions that place time-duration constraints on the formal scoring of arousals with regards to duration of preceding sleep as well as EEG frequency shifts.28,37 Collectively, these considerations may explain the observation that the frequency of arousals was reduced less than disordered breathing events during all studies in all groups.
There were no statistically significant treatment-related changes in any of the secondary endpoints with regards to sleep architecture: sleep efficiency, sleep latency, REM sleep latency or the duration (minutes) and percent of TST, NREM, or REM sleep. This is unexpected, as poor sleep is a common feature of OSA. If sleep disturbance is secondary to disordered breathing events, then reducing the AHI should be expected to improve sleep. In this regard it is important to note that the referenced measures demonstrated remarkably little gross sleep disturbance even at baseline. For example, sleep efficiency above 90% is broadly considered to be normal. From this viewpoint, the placebo and ondansetron alone subject groups exhibited normal sleep efficiency even at baseline, despite having a mean AHI in the moderate to severe range. The high-dose combination treatment group showed a mean sleep efficiency of 87.5% at baseline, leaving little potential for improvement, despite the 40% reduction in AHI. The small changes in REM sleep most probably reflected the low doses of fluoxetine utilized in both groups in this study. It is also possible that, by unknown mechanisms, the co-administration of ondansetron with fluoxetine may have blunted or eliminated the REM suppressing effect of fluoxetine. Insights into this possibility may be gained from future studies incorporating both ondansetron and fluoxetine alone arms, as well as combination treatment arms.
This study, despite its promising preliminary results, has at least two apparent limitations.
The assessment of all efficacy measures was limited to 28 days. Longer-term sustained therapeutic responses remain to be demonstrated. Any clinical implications must be drawn with caution, given small sample size of the present POC study. A definitive evaluation of the therapeutic response with a high dose combination treatment with fluoxetine and ondansetron will require additional data from future studies.
DISCLOSURE STATEMENT
This study was supported by a grant from BTG International, which holds a license from University of Illinois at Chicago to issued and pending patents relevant to the combined use of ondansetron and fluoxetine to treat sleep apnea. This study involved off-label investigational use of ondansetron and fluoxetine. Investigational drug supply was purchased by the University of Illinois at Chicago Investigational Drug Service from a pharmacy. Investigational ondansetron was manufactured by GlaxoSmithKline and fluoxetine was manufactured by Eli Lily. None of the authors have a financial relationship with the manufacturers of ondansetron or fluoxetine. BTG neither manufactures nor markets ondansetron or fluoxetine. No University of Illinois at Chicago or BTG personnel were involved in the statistical analysis of study results, which was contracted to a third-party service provider: ProSoft Inc. Dr. Logan is a full time Vice President of BTG International, the study sponsor. Dr. Carley serves as an unpaid Director for SteadySleep Rx Co., and holds stock in this company. Drs. Carley and Radulovacki are inventors on patents and patent applications that disclose the use of serotonin antagonists and reuptake inhibitors to treat sleep apnea. Dr. Carley and Dr. Radulovacki have received research support from SteadySleep Rx Co. Dr. Radulovacki holds stock in SteadySleep Rx Co. Dr. Prasad, Dr. Olpade, and Dr. Herdegen have indicated no conflicts of interest.
REFERENCES
- 1.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. New Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 2.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–39. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 3.Marshall NS, Wong KK, Liu PY, Cullen SR, Knuiman MW, Grunstein RR. Sleep apnea as an independent risk factor for all-cause mortality: the Busselton Health Study. Sleep. 2008;31:1079–85. [PMC free article] [PubMed] [Google Scholar]
- 4.Harsch IA, Schahin SP, Radespiel-Troger M, et al. Continuous positive airway pressure treatment rapidly improves insulin sensitivity in patients with obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2004;169:156–62. doi: 10.1164/rccm.200302-206OC. [DOI] [PubMed] [Google Scholar]
- 5.Faccenda JF, Mackay TW, Boon NA, Douglas NJ. Randomized placebo-controlled trial of continuous positive airway pressure on blood pressure in the sleep apnea-hypopnea syndrome. Am J Respir Crit Care Med. 2001;163:344–8. doi: 10.1164/ajrccm.163.2.2005037. [DOI] [PubMed] [Google Scholar]
- 6.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–53. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 7.Dorkova Z, Petrasova D, Molcanyiova A, Popovnakova M, Tkacova R. Effects of continuous positive airway pressure on cardiovascular risk profile in patients with severe obstructive sleep apnea and metabolic syndrome. Chest. 2008;134:686–92. doi: 10.1378/chest.08-0556. [DOI] [PubMed] [Google Scholar]
- 8.Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thoracic Soc. 2008;5:173–8. doi: 10.1513/pats.200708-119MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith I, Lasserson TJ, Wright J. Drug therapy for obstructive sleep apnoea in adults. Cochrane Database Syst Rev. 2006:CD003002. doi: 10.1002/14651858.CD003002.pub2. [DOI] [PubMed] [Google Scholar]
- 10.Heym J, Steinfels GF, Jacobs BL. Activity of serotonin-containing neurons in the nucleus raphe pallidus of freely moving cats. Brain Res. 1982;251:259–76. doi: 10.1016/0006-8993(82)90743-0. [DOI] [PubMed] [Google Scholar]
- 11.Ling L. Serotonin and NMDA receptors in respiratory long-term facilitation. Respir Physiol Neurobiol. 2008;164:233–41. doi: 10.1016/j.resp.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Valic M, Pecotic R, Dogas Z. Phrenic nerve activity is enhanced by 5-HT1A receptor agonist 8-OH-DPAT in spontaneously breathing anesthetized rats. J Physiol Pharmacol. 2008;59:17–25. [PubMed] [Google Scholar]
- 13.Fenik P, Veasey SC. Pharmacological characterization of serotonergic receptor activity in the hypoglossal nucleus. Am J Respir Crit Care Med. 2003;167:563–9. doi: 10.1164/rccm.200202-107OC. [DOI] [PubMed] [Google Scholar]
- 14.Martin-Body RL, Grundy HR. Effects of neurotoxin-induced brainstem lesions on the respiratory responses of conscious rats. Clin Exp Pharmacol Physiol. 1985;12:427–37. doi: 10.1111/j.1440-1681.1985.tb00890.x. [DOI] [PubMed] [Google Scholar]
- 15.Mahamed S, Mitchell GS. Simulated apnoeas induce serotonin-dependent respiratory long-term facilitation in rats. J Physiol. 2008;586:2171–81. doi: 10.1113/jphysiol.2007.149047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carley DW, Radulovacki M. Role of peripheral serotonin in the regulation of central sleep apneas in rats. Chest. 1999;11:1397–401. doi: 10.1378/chest.115.5.1397. [DOI] [PubMed] [Google Scholar]
- 17.Mendelson WB, Maczaj M, Holt J. Buspirone administration to sleep apnea patients. J Clin Psychopharmacol. 1991;11:71–2. doi: 10.1097/00004714-199102000-00019. [DOI] [PubMed] [Google Scholar]
- 18.Kraiczi H, Hedner J, Dahlof P, Ejnell H, Carlson J. Effect of serotonin uptake inhibition on breathing during sleep and daytime symptoms in obstructive sleep apnea. Sleep. 1999;22:61–7. [PubMed] [Google Scholar]
- 19.Hanzel DA, Proia NG, Hudgel DW. Response of obstructive sleep apnea to fluoxetine and protriptyline. Chest. 1991;100:416–21. doi: 10.1378/chest.100.2.416. [DOI] [PubMed] [Google Scholar]
- 20.Carley DW, Depoortere H, Radulovacki M. R-zacopride, a 5-HT3 antagonist/5-HT4 agonist, reduces sleep apneas in rats. Pharmacol Biochem Behav. 2001;69:283–9. doi: 10.1016/s0091-3057(01)00535-4. [DOI] [PubMed] [Google Scholar]
- 21.Radulovacki M, Trbovic SM, Carley DW. Serotonin 5-HT3-receptor antagonist GR 38032F suppresses sleep apneas in rats. Sleep. 1998;21:131–6. doi: 10.1093/sleep/21.2.131. [DOI] [PubMed] [Google Scholar]
- 22.Veasey SC, Chachkes J, Fenik P, Hendricks JC. The effects of ondansetron on sleep-disordered breathing in the English bulldog. Sleep. 2001;24:155–60. doi: 10.1093/sleep/24.2.155. [DOI] [PubMed] [Google Scholar]
- 23.Stradling J, Smith D, Radulovacki M, Carley D. Effect of ondansetron on moderate obstructive sleep apnoea, a single night, placebo-controlled trial. J Sleep Res. 2003;12:169–70. doi: 10.1046/j.1365-2869.2003.00342.x. [DOI] [PubMed] [Google Scholar]
- 24.Carley DW, Radulovacki M. Mirtazapine, a mixed-profile serotonin agonist/antagonist, suppresses sleep apnea in the rat. Am J Respir Crit Care Med. 1999;160:1824–9. doi: 10.1164/ajrccm.160.6.9902090. [DOI] [PubMed] [Google Scholar]
- 25.Carley DW, Olopade C, Ruigt GS, Radulovacki M. Efficacy of mirtazapine in obstructive sleep apnea syndrome. Sleep. 2007;30:35–41. doi: 10.1093/sleep/30.1.35. [DOI] [PubMed] [Google Scholar]
- 26.Marshall NS, Yee BJ, Desai AV, et al. Two randomized placebo-controlled trials to evaluate the efficacy and tolerability of mirtazapine for the treatment of obstructive sleep apnea. Sleep. 2008;31:824–31. doi: 10.1093/sleep/31.6.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 28.EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15:173–84. [PubMed] [Google Scholar]
- 29.Blanco-Centurion CA, Salin-Pascual RJ. Extracellular serotonin levels in the medullary reticular formation during normal sleep and after REM sleep deprivation. Brain Res. 2001;923:128–36. doi: 10.1016/s0006-8993(01)03209-7. [DOI] [PubMed] [Google Scholar]
- 30.Strecker RE, Thakkar MM, Porkka-Heiskanen T, Dauphin LJ, Bjorkum AA, McCarley RW. Behavioral state-related changes of extracellular serotonin concentration in the pedunculopontine tegmental nucleus: a microdialysis study in freely moving animals. Sleep Res Online. 1999;2:21–7. [PubMed] [Google Scholar]
- 31.Sood S, Raddatz E, Liu X, Liu H, Horner RL. Inhibition of serotonergic medullary raphe obscurus neurons suppresses genioglossus and diaphragm activities in anesthetized but not conscious rats. J Appl Physiol. 2006;100:1807–21. doi: 10.1152/japplphysiol.01508.2005. [DOI] [PubMed] [Google Scholar]
- 32.Sood S, Morrison JL, Liu H, Horner RL. Role of endogenous serotonin in modulating genioglossus muscle activity in awake and sleeping rats. Am J Respir Crit Care Med. 2005;172:1338–47. doi: 10.1164/rccm.200502-258OC. [DOI] [PubMed] [Google Scholar]
- 33.Matsumoto S. Effects of carotid Body chemoreceptor stimulation by 5-HT on phrenic nerve activity and ventilation in the rabbit. Arch Int Pharmacodyn Ther. 1981;254:282–92. [PubMed] [Google Scholar]
- 34.Carley DW, Radulovacki M. Apnea suppression by ondansetron and fluoxetine alone and combined. Sleep. 2005;28(Abstract Supplement):A12. [Google Scholar]
- 35.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–84. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 36.Punjabi NM, Shahar E, Redline S, Gottlieb DJ, Givelber R, Resnick HE. Sleep-disordered breathing, glucose intolerance, and insulin resistance: the Sleep Heart Health Study. Am J Epidemiol. 2004;160:521–30. doi: 10.1093/aje/kwh261. [DOI] [PubMed] [Google Scholar]
- 37.Iber C, A-I S, Chesson A, Quan SF. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]