Abstract
Cell–cell adhesions are sites where cells experience and resist tugging forces. It has long been postulated, but not directly tested, that cadherin adhesion molecules may serve in mechanotransduction at cell–cell contacts. In this issue, Le Duc et al. (2010. J. Cell Biol. doi: 10.1083/jcb.201001149) provide direct evidence that E-cadherin participates in a mechanosensing pathway that regulates the actomyosin cytoskeleton to modulate cell stiffness in response to pulling force.
All of the cells in our body experience force: from the shear stress of blood flow experienced by the vascular endothelium to the tugging of other cells in skeletal muscle. Accordingly, cellular mechanisms exist to preserve tissue integrity by resisting this play of forces. Characteristically, these mechanisms involve adhesion receptors that are mechanically coupled to the cytoskeleton. However, these apparatuses do not simply support passive resistance. Instead, there has been great recent interest in the concept that adhesion receptors contribute to cell signaling pathways, which sense the magnitude of force exerted on cells and trigger cellular responses to those forces (Vogel and Sheetz, 2006). This notion is best established for integrin cell–matrix adhesion molecules in which well-characterized signaling pathways are clearly involved in mechanotransduction, which modifies focal adhesion size in response to force (Balaban et al., 2001) and may ultimately affect processes that range from stem cell differentiation (Engler et al., 2006) to tumor cell progression (Levental et al., 2009).
At cell–cell contacts, classical cadherin adhesion molecules play major roles in morphogenesis and in the maintenance of tissue integrity. A role for cadherins in mechanotransduction has often been suspected (Schwartz and DeSimone, 2008) but not directly tested. One challenge in dissecting this problem is to distinguish responses principally elicited by the cadherin from juxtacrine events that occur when adhesion systems bring native cell surfaces into contact with one another. In this issue, Le Duc et al. circumvent this problem by using recombinant cadherin ligands, which contain the entire adhesive ectodomain, to test the capacity for a classical cadherin to participate in mechanosensing. The authors allowed magnetic beads coated with recombinant E-cadherin ectodomains to adhere to the dorsal surfaces of cultured cells. Classical cadherins engage in homophilic interactions via their ectodomains, and ligation of cellular cadherins by these immobilized ligands is a commonly used approach to generate adhesive contacts through E-cadherin alone. They used an oscillating magnetic field to twist the beads, thereby applying shear forces onto the sites of adhesion. By measuring the displacement of the beads in response to twisting stimuli, they could calculate changes in the local stiffness of the adhesive contact of each bead.
Strikingly, they found that these adhesive contacts between the cadherin-coated beads and the cells stiffened in response to repetitive twisting force. The magnitude of stiffening increased with the magnitude of the applied force, which is evidence for the existence of a mechanism that could apparently measure the applied force and calibrate a proportionate cellular response. The use of E-cadherin as the ligand for homophilic engagement implied that the cellular cadherin was key to the force-sensing apparatus. This was further substantiated by the demonstration that the stiffening response did not occur when cadherin function was disrupted by removing extracellular calcium or adding a function-blocking antibody. Moreover, stiffening could not be elicited by beads coated with cadherin antibodies, suggesting that a native ligand was required rather than simple binding to the cellular cadherin ectodomain. Moreover, cell stiffening required an intact actomyosin cytoskeleton, implying that it reflected a cellular mechanical response to applied force. Overall, these findings indicate that E-cadherin engaged in homophilic interactions can serve to sense force and trigger a cellular response that involves the actin cytoskeleton, classical hallmarks of a mechanotransduction pathway (Fig. 1).
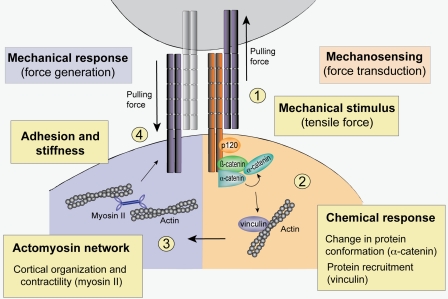
Figure 1.
E-cadherin mechanotransduction. Forces acting on surface E-cadherin molecules activate mechanosensing processes that lead to proportionate mechanical responses from cells. (1) In this model, E-cadherin engaged in homophilic adhesive interactions acts as a surface receptor for forces that tug on cells. (2) This induces an intracellular signaling cascade, which includes events such as alterations in protein conformation (notably α-catenin) and recruitment of proteins such as vinculin. (3 and 4) The subsequent mechanical response involves the actomyosin cytoskeleton (3), which can alter adhesion stiffness (4) by diverse processes such as changes in cortical organization and contractility. One potential outcome is that this cellular response will be felt as a pulling force by the neighboring cell that initiated the cascade, leading to cooperative interactions between the cells.
What do we know of the molecular players in this E-cadherin–activated mechanotransduction pathway? A comprehensive answer to this question must ultimately encompass the signal transduction pathways that are activated by mechanical stimulation of E-cadherin and the elicited downstream cytoskeletal responses. Many different kinds of signaling events are implicated in other forms of mechanosensing, including the Src tyrosine kinase and ion channels (Vogel and Sheetz, 2006), which can be found at cell–cell contacts (Wang et al., 2006). Another molecular paradigm involves alterations in protein conformation in response to applied force, which thereby reveals novel sites for posttranslational modification or protein binding (del Rio et al., 2009). In this regard, a recent study identifies an apparently cryptic site in α-catenin that is sensitive to the cellular force generator myosin II (Yonemura et al., 2010). The study found that junctional staining with a monoclonal antibody directed to the central region of α-catenin was abolished in cells treated with the myosin II inhibitor blebbistatin, although α-catenin protein remained at cell–cell contacts. Notably, the epitope for this monoclonal antibody resides close to the region of α-catenin that can directly bind the actin regulator vinculin. Both Le Duc et al. (2010) and Yonemura et al. (2010) show that the recruitment of vinculin to cell–cell junctions is blebbistatin sensitive. Moreover, Le Duc et al. (2010) demonstrate that cellular stiffening in response to twisting force is reduced in vinculin-deficient cells. This suggests the attractive hypothesis that transmission of force to α-catenin that is incorporated into the E-cadherin complex may alter its conformation and capacity to interact with binding partners such as vinculin. This notion warrants more detailed analysis; however, if experience with integrin mechanotransduction is any guide (Vogel and Sheetz, 2006; Schwartz and DeSimone, 2008), force-induced conformational change in proteins such as α-catenin are likely to be but one part of a more complex network of signal transduction mechanisms.
The force-dependent recruitment of vinculin also provides a potential mechanism to coordinate a cytoskeletal response to E-cadherin mechanosensing. Although long known to concentrate at the zonula adherens (as well for its better-known localization in focal adhesions), the precise role that vinculin plays in cell–cell interactions remains enigmatic. Nonetheless, depletion of vinculin reduces cell–cell adhesion and disrupts the integrity of epithelial cell–cell junctions (Peng et al., 2010). Vinculin depletion also perturbs the junctional actin cytoskeleton (Maddugoda et al., 2007), and vinculin has the capacity to bind actin filaments and diverse actin regulators, thereby influencing both filament bundling and dynamics (Le Clainche et al., 2010). However, vinculin is unlikely to be the sole mediator of the cytoskeletal response. Many cytoskeletal regulators act at E-cadherin cell–cell junctions to control actin filament dynamics and organization. A particularly interesting case is nonmuscle myosin II, which was implicated as the dominant cellular force generator in recent studies (Le Duc et al., 2010; Yonemura et al., 2010). However, the contribution of myosin II is likely to be complex, as myosin II is also necessary for the cytoskeletal response to force (Le Duc et al., 2010). Moreover, there is emerging evidence for both contractile and noncontractile functions for myosin II (Choi et al., 2008). Also, the myosin II A and B isoforms can have distinct contributions to E-cadherin clustering and apical actin regulation (Smutny et al., 2010). Thus, myosin II may have several contributions to cadherin mechanotransduction.
Finally, what functions might be served by cadherin-based mechanosensing? One possibility is that local stiffening, and perhaps the cytoskeletal response more broadly, might provide a mechanism to strengthen adhesions against potentially disruptive forces. This notion is supported by a recent study by Liu et al. (2010), who analyzed forces at the contacts between pairs of cells grown on micropatterned substrata. Force vectors oriented approximately perpendicular to the cell–cell contacts could be extracted from their data, and strikingly, the authors identified a linear relationship between the magnitude of the forces and the size of the contacts. This appeared to reflect the coordinated action of Rho- and Rac-based signaling pathways. They proposed that force-dependent growth of adhesions may be a mechanism to reduce stress at the contacts and thus preserve their integrity. In addition, it is interesting to consider the possibility that mechanosensing through E-cadherin could provide a mechanism for cells to assess the mechanical properties of their neighboring cells. Cells appear to use integrin-based mechanosensing to assess the stiffness of their surrounding matrix (Schwartz and DeSimone, 2008; Levental et al., 2009). Their ability to use myosin II–based contractility to pull on adhesion sites is likely critical for cells to assess stiffness of their surroundings. However, an important difference between cell–matrix and cell–cell mechanosensing is that although in the former case the environment is passive, in the latter case, it is active (i.e., neighboring cells can pull back). Is the mechanical response of a neighboring cell an important parameter in cadherin-based cell–cell recognition? Clearly, then, the new work of Le Duc et al. (2010) opens many new avenues for understanding the role of mechanosensing in cadherin biology and tissue organization.
References
- Balaban N.Q., Schwarz U.S., Riveline D., Goichberg P., Tzur G., Sabanay I., Mahalu D., Safran S., Bershadsky A., Addadi L., Geiger B. 2001. Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nat. Cell Biol. 3:466–472 10.1038/35074532 [DOI] [PubMed] [Google Scholar]
- Choi C.K., Vicente-Manzanares M., Zareno J., Whitmore L.A., Mogilner A., Horwitz A.R. 2008. Actin and alpha-actinin orchestrate the assembly and maturation of nascent adhesions in a myosin II motor-independent manner. Nat. Cell Biol. 10:1039–1050 10.1038/ncb1763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Rio A., Perez-Jimenez R., Liu R., Roca-Cusachs P., Fernandez J.M., Sheetz M.P. 2009. Stretching single talin rod molecules activates vinculin binding. Science. 323:638–641 10.1126/science.1162912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler A.J., Sen S., Sweeney H.L., Discher D.E. 2006. Matrix elasticity directs stem cell lineage specification. Cell. 126:677–689 10.1016/j.cell.2006.06.044 [DOI] [PubMed] [Google Scholar]
- Le Clainche C., Dwivedi S.P., Didry D., Carlier M.F. 2010. Vinculin is a dually regulated actin filament barbed-end capping and side-binding protein. J. Biol. Chem. 10.1074/jbc.M110.102830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Duc Q., Shi Q., Blonk I., Sonnenberg A., Wang N., Leckband D., de Rooij J. 2010. Vinculin potentiates E-cadherin mechanosensing and is recruited to actin-anchored sites within adherens junctions in a myosin II–dependent manner. J. Cell Biol. 189:1107–1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levental K.R., Yu H., Kass L., Lakins J.N., Egeblad M., Erler J.T., Fong S.F., Csiszar K., Giaccia A., Weninger W., et al. 2009. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 139:891–906 10.1016/j.cell.2009.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Tan J.L., Cohen D.M., Yang M.T., Sniadecki N.J., Ruiz S.A., Nelson C.M., Chen C.S. 2010. Mechanical tugging force regulates the size of cell-cell junctions. Proc. Natl. Acad. Sci. USA. 10.1073/pnas.0914547107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddugoda M.P., Crampton M.S., Shewan A.M., Yap A.S. 2007. Myosin VI and vinculin cooperate during the morphogenesis of cadherin cell–cell contacts in mammalian epithelial cells. J. Cell Biol. 178:529–540 10.1083/jcb.200612042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X., Cuff L.E., Lawton C.D., DeMali K.A. 2010. Vinculin regulates cell-surface E-cadherin expression by binding to beta-catenin. J. Cell Sci. 123:567–577 10.1242/jcs.056432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M.A., DeSimone D.W. 2008. Cell adhesion receptors in mechanotransduction. Curr. Opin. Cell Biol. 20:551–556 10.1016/j.ceb.2008.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smutny M., Cox H.L., Joanne M., Leerberg E.M., Kovacs M.A., Conti C., Ferguson N.A., Hamilton R.G., Adelstein Parton R.S., Yap A.S. 2010. Myosin II isoforms identify distinct functional modules that support integrity of the epithelial zonula adherens. Nat. Cell Biol. 10.1038/ncb2072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel V., Sheetz M. 2006. Local force and geometry sensing regulate cell functions. Nat. Rev. Mol. Cell Biol. 7:265–275 10.1038/nrm1890 [DOI] [PubMed] [Google Scholar]
- Wang Y., Jin G., Miao H., Li J.Y., Usami S., Chien S. 2006. Integrins regulate VE-cadherin and catenins: dependence of this regulation on Src, but not on Ras. Proc. Natl. Acad. Sci. USA. 103:1774–1779 10.1073/pnas.0510774103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonemura S., Wada Y., Watanabe T., Nagafuchi A., Shibata M. 2010. alpha-catenin as a tension transducer that induces adherens junction development. Nat. Cell Biol. 12:533–542 10.1038/ncb2055 [DOI] [PubMed] [Google Scholar]