Abstract
Recent advances in defining the molecular signaling pathways that regulate the phagocytosis of apoptotic cells have improved our understanding of this complex and evolutionarily conserved process. Studies in mice and humans suggest that the prompt removal of dying cells is crucial for immune tolerance and tissue homeostasis. Failed or defective clearance has emerged as an important contributing factor to a range of disease processes. This review addresses how specific molecular alterations of engulfment pathways are linked to pathogenic states. A better understanding of the apoptotic cell clearance process in healthy and diseased states could offer new therapeutic strategies.
Introduction
Apoptosis plays an essential role in the development and maintenance of all mammalian tissues. The apoptotic program ensures that damaged, aged, or excess cells are deleted in a regulated manner that is not harmful to the host. Beyond the cell intrinsic apoptotic program initiated after a variety of insults, an integral second step in apoptosis is the removal of the cell corpse (Kerr et al., 1972). Indeed, the physical removal and subsequent degradation of the corpse via phagocytosis represents the final act necessary for the successful removal of a cell fated to die. Recent advances in our understanding of apoptotic cell clearance have led to the identification of molecules and signaling pathways that orchestrate this process (Lauber et al., 2004; Ravichandran and Lorenz, 2007; Erwig and Henson, 2008).
The efficiency of the phagocytic clearance of apoptotic cells appears enormous when one considers that despite the loss of >109 cells per day, the incidence of histologically detectable apoptotic cells is rare in normal tissues (Mochizuki et al., 1996; Scott et al., 2001; Schrijvers et al., 2005; Yang et al., 2006; Elliott et al., 2009). The engulfment of apoptotic cells is performed by both professional phagocytes (such as macrophages and dendritic cells) and by nonprofessional “neighboring” phagocytes (such as epithelial cells, endothelial cells, and fibroblasts). Current evidence suggests that the steps involved in the phagocytic clearance of apoptotic cells are similar between professional and nonprofessional phagocytes (Fig. 1), although the kinetics may differ, with professional phagocytes exhibiting higher rates and capacity for phagocytosis (Parnaik et al., 2000).
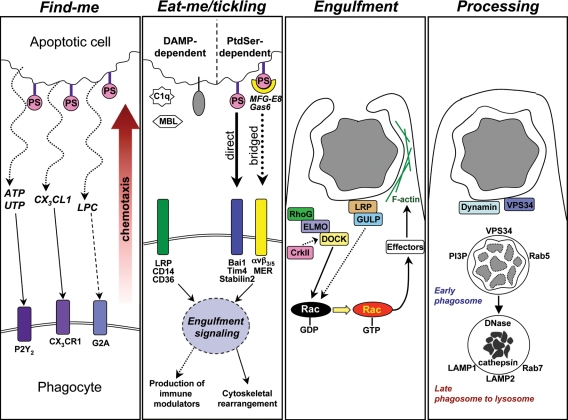
Figure 1.
Stages of apoptotic cell engulfment and associated cell signaling events that regulate each stage. The four stages of apoptotic cell clearance are shown, with some of the specific key signaling players identified. The “find-me” step occurs when apoptotic cells release soluble chemoattractants that promote chemotaxis of phagocytes via corresponding receptors on the phagocyte. The broken line from LPC to G2A indicates uncertainty of direct ligand–receptor interaction. The “eat-me” stage is characterized by the appearance of ligands on the surface of the dying cell that mark it as a target to be engulfed by phagocytes bearing appropriate DAMP or PtdSer recognition receptors. The “engulfment” stage occurs when signaling downstream of the apoptotic cell recognition receptors stimulates Rac-dependent cytoskeletal rearrangement and formation of the phagocytic cup around the target and subsequent internalization. Once fully internalized, the cell corpse undergoes “processing” through the phagolysosomal pathway that results in the degradation and reprocessing of the dead cell material. DAMP, damage-associated molecular patterns; LPC, lysophosphatidylcholine; MBL, mannose-binding lectin; PS, phosphatidylserine.
Based on work from many laboratories over the past decade, several broadly defined steps have been identified in the recognition and removal of apoptotic cells by phagocytes. Each step appears to be tightly regulated by signaling events to ensure swift and efficient clearance (Fig. 1). At the early stage of apoptosis, the dying cells release “find-me” signals that are sensed by motile phagocytes, which help attract these phagocytes to the proximity of the dying cell. Several soluble chemoattractant find-me signals released during apoptosis have been recently defined, including triphosphate nucleotides (ATP/UTP), lysophosphatidylchloline (lysoPC), and the chemokine CX3CL1 (Lauber et al., 2003; Truman et al., 2008; Elliott et al., 2009; Muñoz et al., 2010). Once in the proximity of the dying cell, the physical contact between the apoptotic cell and the phagocyte is mediated via ligands on apoptotic cells (referred to as “eat-me” signals) and engulfment receptors on phagocytes that can recognize these eat-me markers. Among the array of identified eat-me molecules (Ravichandran and Lorenz, 2007), the exposure of phosphatidylserine (PtdSer) on the outer leaflet of the apoptotic cell plasma membrane appears to be a key eat-me marker (Fadok et al., 1992; Vandivier et al., 2006). Phagocyte recognition of PtdSer is mediated directly via one or more PtdSer recognition receptors, including Bai1, Tim-4, and Stabilin-2 (Kobayashi et al., 2007; Park et al., 2007, 2008, 2009; Miyanishi et al., 2007; Nakayama et al., 2009), or by soluble bridging molecules that bind PtdSer on the apoptotic cell and a receptor on the phagocyte (MFG-E8/αvβ3/5, Gas6/MER; Savill et al., 1990; Scott et al., 2001; Hanayama et al., 2004). Engagement of the PtdSer receptors initiates signaling events within the phagocytes that lead to activation of the small GTPase Rac, and subsequent cytoskeletal reorganization of the phagocyte membrane to allow corpse internalization (Albert et al., 2000; Gumienny et al., 2001). From studies in Caenorhabditis elegans and Drosophila melanogaster, and in vitro mammalian cell experiments, two key evolutionarily conserved Rac-dependent apoptotic cell engulfment pathways have been identified (Fig. 1; Reddien and Horvitz, 2004; Kinchen, 2010). In addition to receptors that can directly signal after engaging eat-me signals, there are also contributions from other “tethering” receptors (e.g., CD14 and CD31) that help the binding/specific recognition between the apoptotic cell and the phagocyte (Brown et al., 2002; Devitt et al., 2003). Once inside the phagosome, the ingested apoptotic cargo is processed via a phagolysosomal pathway that shares both overlapping and unique features with the endocytic machinery (Erwig et al., 2006; Kinchen et al., 2008; Yu et al., 2008; Kinchen and Ravichandran, 2010; Bohdanowicz and Grinstein, 2010). Because of this overlap, it is difficult to distinguish disease states related specifically to aberrant signaling in the phagosomal pathways from those involving endocytosis dysfunction. Indeed, a role for endocytosis in human disease has been well established (Mosesson et al., 2008; Ballabio and Gieselmann, 2009). Thus, we will focus on diseases related to engulfment signaling upstream of corpse degradation.
Although the clearance of apoptotic cells occurs throughout the body, the specific molecular pathways can vary by tissue. For example the intracellular engulfment signaling molecules Rac, ELMO, and Dock180 appear to be widely expressed (Hasegawa et al., 1996; Gumienny et al., 2001), whereas the expression of many of the surface molecules responsible for recognition of apoptotic cells varies widely among different tissues and cell types (Ferrero et al., 1990; Graham et al., 1994; Falkowski et al., 2003; Miyanishi et al., 2007; Park et al., 2007). Thus, because of the redundancy in the engulfment machinery among cell types, it is critical to know the expression pattern of identified phagocytic receptors when considering apoptotic cell clearance in a specific tissue or by a particular cell type. Interestingly, many of the disease states linked to failed clearance have been associated with aberrations in the recognition or eat-me step of clearance (Table I). This observation might reflect an investigator-induced bias toward phagocyte–corpse interactions, or it may be the result of selective expression of phagocytic receptors that reduces the redundancy of uptake mechanisms, and thus is more likely to reveal failures in clearance.
Table I.
A survey of disease states associated with defects in engulfment-related genes
Genes are grouped by known roles in engulfment (find-me, eat-me, engulfment, and post-engulfment). AI, autoimmune phenotype; H, human; M, mouse.
There is evidence of genetic linkage but no direct causal relationship was established.
Regardless of the specific molecules mediating uptake, the ability to efficiently clear apoptotic cells is strongly linked to the homeostatic maintenance of healthy tissues in mammals. This is thought to be the result of two key features of the clearance process. The first is the obvious function of phagocytes as “garbage collectors,” mediating the physical removal of the dying cells. Such clearance sequesters the dying cell and prevents the release of potentially toxic or immunogenic intracellular contents from the dying cell into the local environment. This is a key distinction from necrotic cell death, where the unregulated release of dead cell material can cause very strong inflammatory responses (such as ischemic injury). The second homeostatic function of the clearance process is the production of anti-inflammatory mediators by phagocytes that suppress inflammation and facilitate the “immunologically silent” clearance of apoptotic cells.
The purpose of this review is to examine the current body of knowledge linking apoptotic cell clearance to disease pathogenesis. We will discuss several families of disease states that appear to have as a contributing factor some level of impaired cell clearance. We will also attempt to highlight how components of the engulfment signaling pathways may function in myriad disease processes.
Failed clearance, altered immune tolerance, and autoimmunity
Autoimmune disorders represent the best-characterized relationship between apoptotic cell clearance and disease pathogenesis (Table I; Savill et al., 2002; Gaipl et al., 2004; Erwig and Henson, 2007; Nagata et al., 2010). The self-contained, regulated nature of apoptotic cell death preserves membrane integrity and prevents the release of potentially inflammatory and immunogenic intracellular contents. However, if the apoptotic cells are not promptly cleared, the membrane integrity is lost over time, and apoptotic cells can progress to secondary necrosis. The release of intracellular contents from necrotic cells is thought to provoke an inflammatory response, particularly toward intracellular antigens and DNA released from the dying cells. This may provide the immunogenic impetus for the onset of some autoimmune disorders in humans, including systemic lupus erythematosus and rheumatoid arthritis (Gaipl et al., 2004). Early experiments in mice showed that the administration of excess syngeneic apoptotic cells or the masking of PtdSer on apoptotic cells via annexin V (to block PtdSer-mediated uptake) produces hallmarks of autoimmunity, such as autoantibody production and IgG deposition in the glomeruli (Mevorach et al., 1998; Asano et al., 2004). More recently, several genetic mouse models bearing defects in PtdSer-mediated recognition have further confirmed that the failure to efficiently clear apoptotic cells can result in autoimmunity (Botto et al., 1998; Scott et al., 2001; Cohen et al., 2002; Hanayama et al., 2004; Lacy-Hulbert et al., 2007; Rodriguez-Manzanet et al., 2010). Nuclear antigens, particularly DNA and DNA–protein complexes (e.g., high mobility group box 1–containing nucleosomes), appear especially crucial in human systemic lupus erythematosus and rheumatoid arthritis (Taniguchi et al., 2003). Studies in knockout mice demonstrated that to maintain self-tolerance, DNase-mediated degradation of apoptotic cell-derived DNA in the phagosome is necessary (Napirei et al., 2000; Krieser et al., 2002; Kawane et al., 2003). There is now a solid link between the inefficient engulfment of apoptotic cells and autoimmunity in humans (Ren et al., 2003; Gaipl et al., 2004).
An additional means for controlling the immune response to apoptotic cells is through the active production of anti-inflammatory mediators by phagocytes. The PtdSer-dependent recognition of apoptotic cells by a phagocyte elicits the release of anti-inflammatory mediators such as IL-10, TGFβ, and prostaglandins in vitro (Voll et al., 1997; Fadok et al., 1998; McDonald et al., 1999; Ogden et al., 2005). Moreover, this recognition actively suppresses inflammatory cytokine release in vitro, particularly those elicited via Toll-like receptors (TLRs; Voll et al., 1997; Fadok et al., 1998). This immunosuppressive response extends in vivo, as studies in mice have shown that the systemic administration of apoptotic cells induces a tolerizing effect on the immune response in rodent allograft models (Sun et al., 2004; Wang et al., 2009). Recently, key insights into the signaling events that regulate the release of these immune modulators have been gained. PtdSer-dependent engagement of apoptotic cells induces in phagocytes the p38 MAPK-dependent transcriptional regulation of IL-10, as well as translational control of TGFβ in the phagocyte (Chung et al., 2007; Xiao et al., 2008). The ability of apoptotic cells to suppress TLR-dependent release of IL-6, IL-8, and TNF has also been shown to be regulated at the transcript level (Cvetanovic and Ucker, 2004). Thus, in addition to the physical removal of dying cells, the “tickling” of phagocytic receptors generates signals that lead to regulation of anti-inflammatory mediators and in turn, the elicitation of an immunosuppressive environment during removal of apoptotic cells. Even under normal healthy conditions, there is a turnover of >200 billion cells per day in many tissues throughout our body, and therefore interruptions to the finely tuned clearance system can lead to inflammation, tissue destruction, and the onset of disease.
Respiratory diseases and impaired cell clearance
Intriguingly, increased levels of apoptotic cells are seen in the sputum and lung tissue of several serious respiratory diseases, including chronic obstructive pulmonary disease (COPD), cystic fibrosis (CF), and asthma (Henson and Tuder, 2008). Because aberrant lung inflammation is a common feature of these diseases, one possibility is that uncleared apoptotic cells progressing to secondary necrosis may contribute to lung inflammation. But a common underlying question is whether or not these “uncleared” apoptotic cells represent increased rates of apoptosis or defects in apoptotic cell clearance. In the past few years, several studies have established considerable links between respiratory disease and inefficient apoptotic cell clearance in the lung (Vandivier et al., 2002; Hodge et al., 2003; Huynh et al., 2005). Although the focus of these studies has primarily been on the phagocytic activity of lung resident macrophages (alveolar macrophages), it will be interesting to determine the relative contribution of healthy lung epithelial cells in the clearance of neighboring apoptotic cells.
The environment of the diseased lung contributes to poor apoptotic cell clearance. Cigarette smoking, the leading cause of COPD, is correlated with increased apoptotic cell debris in the lung (Hodge et al., 2005), and cigarette smoke impairs the uptake of apoptotic cells by alveolar macrophages in vitro (Kirkham et al., 2004; Hodge et al., 2007). Sputum from CF patients, when added to normal alveolar macrophages, inhibits their ability to engulf apoptotic targets in vitro (Vandivier et al., 2002). At least two factors in CF sputum have been shown to disrupt apoptotic cell engulfment, including elevated levels of neutrophil-derived elastase, which may cleave eat-me signals (Vandivier et al., 2002), and pyocyanin, a toxic by-product of Pseudomonas aeruginosa, a common infectious pathogen found in the lungs of about half of all CF patients (Bianchi et al., 2008). Finally, the inflammation associated with lung disease appears to create a cytokine milieu (notably increased TNF) that may suppress apoptotic cell engulfment (Borges et al., 2009), perhaps by hindering the differentiation of monocytes to macrophages, thus exacerbating these clearance defects.
Intrinsic defects in macrophages in the context of the diseased lung also appear to contribute to the reduced clearance seen in these respiratory diseases. Alveolar macrophages from COPD, CF, and asthma patients show a decreased ability to engulf apoptotic cells in vitro (Hodge et al., 2003, 2007; Huynh et al., 2005; Vandivier et al., 2009). To date, there are no reported links to specific engulfment pathways that are defective in these lung diseases, although decreased expression of at least two collectins (mannose-binding lectin and surfactant protein-D) in COPD patients suggests a possible role for decreased pattern recognition receptor (PRR)/C1q receptor–mediated uptake (Hodge et al., 2008). Intriguingly, Vandivier et al. (2009) recently found that cystic fibrosis transmembrane conductance regulator (CFTR)-deficient epithelial cells are defective in the phagocytosis of apoptotic cells, whereas CFTR-deficient alveolar macrophages show no engulfment defect. These findings suggest that a persistent disease state in the lung (i.e., COPD) and/or genetic anomalies may drive engulfment defects, and thus point to a prominent role for engulfment in the establishment and progression of disease. Moreover, the relative contributions of macrophages and the epithelial cells for apoptotic cell clearance, as well as the anti-inflammatory cytokines generated (or lack thereof), need to be determined in the context of lung inflammation. Future genetic studies that target engulfment genes in particular phagocyte populations may reveal some important information on the onset and progression of lung inflammation.
An interesting feature of defective apoptotic cell clearance in the diseased lung is the potential role of the small GTPase RhoA. During engulfment, activation of the small GTPase Rac in the phagocyte is crucial for actin rearrangement during corpse internalization (Fig. 1). In contrast, RhoA antagonizes Rac in this process, and increased levels of RhoA-GTP potently impair engulfment (Leverrier and Ridley, 2001; Tosello-Trampont et al., 2003; Nakaya et al., 2006). Independent studies have shown that CFTR deficiency in lung epithelial cells results in higher basal levels of activated RhoA (Kreiselmeier et al., 2003; Vandivier et al., 2009). Studies using in vitro treated lung epithelial cells similarly show increased basal levels of RhoA-GTP in response to cigarette smoke (Richens et al., 2009). Pharmacological inhibitors of RhoA activity, particularly statins, enhance apoptotic cell engulfment in vitro and in vivo, and thus suggest that elevated RhoA-GTP levels may play a significant role in the impaired clearance observed in diseased lungs (Morimoto et al., 2006). Although the molecular events leading to increased levels of RhoA-GTP levels are poorly understood, cigarette smoke exerts a similar effect (activation of RhoA) and may in part explain the defective engulfment seen in COPD (Richens et al., 2009). There is currently no definite linkage between lung disease and specific engulfment receptors, and the high rate of cell death in the lung due to inhaled toxins could provide valuable insights into clearance mechanisms through the use of genetically modified mice.
Atherosclerosis and engulfment-related consequences
Macrophages play a prominent role in the development of atherosclerotic plaques, and their function in clearing apoptotic cells appears to be a key to the pathogenesis of this widespread and life-threatening disease. At the onset of plaque formation, monocytes in the blood adhere to intimal smooth muscle cells and differentiate almost exclusively to macrophages. These macrophages then take up low-density lipoprotein (LDL) via scavenger receptors and, once they are cholesterol-laden, are known as “foam cells.” These foam cells eventually undergo apoptosis, yet early atherosclerotic lesions display few uncleared apoptotic cells, which suggests efficient clearance (Tabas, 2005). As leukocytes continue to infiltrate the lesion and release inflammatory mediators, cell death increases (Schrijvers et al., 2005). Indeed, late plaques feature much higher levels of free, uncleared apoptotic cells, and eventually a necrotic core forms and becomes unstable, leading to possible lesions that can cause thrombosis (Tabas, 2005).
In recent years, the role of apoptotic cell clearance has begun to be appreciated in atherogenesis. Through the use of atherosclerosis mouse models—ApoE−/− and Ldlr−/−—genetic studies of engulfment molecules have demonstrated the role of cell clearance in atherosclerosis (Table I). Mice deficient in the apoptotic cell-bridging molecules MFG-E8 (Ait-Oufella et al., 2007) and C1q (Bhatia et al., 2007) develop accelerated atherogenesis and display increased plaque-bound apoptotic cells on ApoE−/− and Ldlr−/− genetic backgrounds, respectively. Likewise, mice deficient in transglutamase 2 (TG2), a cross-linking enzyme that promotes engulfment via αvβ3/5 (Lorand and Graham, 2003; Szondy et al., 2003), also enhances atherosclerotic plaque formation in Ldlr−/−-deficient mice (Boisvert et al., 2006), but not in ApoE-deficient mice (Williams et al., 2010). In addition, the receptor tyrosine kinase MER, which recognizes apoptotic cells via the PtdSer-binding Gas6 bridging molecule, functions in vivo to inhibit plaque formation and can promote apoptotic cell clearance in atherosclerosis models (Ait-Oufella et al., 2008; Thorp et al., 2008). Paradoxically, Gas6 deficiency on the ApoE−/− background leads to the formation of more stable plaques with smaller necrotic cores, fewer macrophages, and increased TGFβ levels (Lutgens et al., 2008), which suggests possible additional nonengulfment related antiatherogenic roles for MER. These studies suggest divergent roles for the receptor–ligand interactions in atherogenesis, which may be due to nonengulfment functions of both proteins or the lack of our full understanding of cell death/cell clearance in an atherosclerotic plaque.
Lipid handling by macrophages plays an important role in atherosclerosis, and so it is interesting that there is considerable overlap in the cellular mechanisms that regulate lipid metabolism and apoptotic cell engulfment. We and others have found that macrophages engulfing apoptotic cells up-regulate the key lipid transporter ABCA1, and this leads to enhanced cholesterol efflux from the phagocytes (Gerbod-Giannone et al., 2006; Kiss et al., 2006a). This cholesterol efflux requires PtdSer-dependent recognition and signaling within the phagocytes (Kiss et al., 2006a). These findings reveal that a phagocyte taking up an apoptotic cell has the ability to regulate and normalize the level of cellular material. Another intracellular engulfment signaling protein, GULP1, has been shown to promote cholesterol efflux, and GULP1 functions downstream of the LDL-receptor related protein 1 (LRP1), which is also linked to engulfment of apoptotic cells (Su et al., 2002; Gardai et al., 2005; Kiss et al., 2006b). Nuclear receptors, a family of transcriptional regulators that control the response to cellular lipids (Hong and Tontonoz, 2008), have been implicated in this response, as antagonists blocked this efflux (Gerbod-Giannone et al., 2006; Kiss et al., 2006a). As further evidence of the interplay between engulfment and lipid metabolism, mice deficient in the LXRα/β or PPARδ nuclear receptors showed decreased expression of engulfment genes, with impaired engulfment of apoptotic cells by macrophages in vitro and in vivo (A-Gonzalez et al., 2009; Mukundan et al., 2009). These mice also showed aberrant expression of inflammatory mediators and eventually develop hallmarks of autoimmunity. Because uncleared dead cells are a fundamental issue in atherogenesis, it would seem that the ability to modulate apoptotic cell clearance in this environment could serve as a useful and novel tool to prevent or treat disease.
Cell clearance defects in neurological diseases
Over a decade ago, several studies identified excess apoptotic cells associated with chronic neurodegenerative diseases, including in patients with Parkinson’s, Alzheimer’s, and Huntington’s disease, and in aging brains (Su et al., 1994; Thomas et al., 1995; Zhang et al., 1995; Mochizuki et al., 1996). Microglia are one of the primary phagocytes for apoptotic cells and debris in the brain (Witting et al., 2000; Magnus et al., 2002; Stolzing and Grune, 2004; Garden and Möller, 2006). Considered to be of myeloid lineage, these highly motile cells provide necessary surveillance to respond to cell death associated with acute injury and stroke (Davalos et al., 2005; Garden and Möller, 2006). Upon the initiation of neuronal cell death, microglia migrate to the site of injury and mediate the inflammatory response (Davalos et al., 2005; Koizumi et al., 2007). Recently, engulfment signaling pathways have been implicated in glial function during chronic neurological diseases. Although the discussion in the following paragraph focuses on microglial cells, it is important to keep in mind that other cell types in the brain such as astrocytes can also engulf apoptotic cells (Chang et al., 2000; Magnus et al., 2002; Park et al., 2007) and thus may play a role in clearance and disease in the brain.
To date, MFG-E8 is the engulfment-related molecule best linked to clearance of apoptotic cells in the brain. Cultured astrocytes and microglia produce MFG-E8, and MFG-E8 can promote the phagocytosis of apoptotic neurons by microglia in vitro (Boddaert et al., 2007; Fuller and Van Eldik, 2008). There is also a correlative relationship between MFG-E8 and Alzheimer’s disease, as suppressed levels of MFG-E8 are associated with the disease in humans and mice (Boddaert et al., 2007; Fuller and Van Eldik, 2008). Additional evidence of engulfment signaling in the brain comes from studies of microglial chemoattractants. Dying neurons release find-me cues, namely extracellular nucleotides as well as CX3CL1 (fractalkine or neurotactin) that promote chemotaxis of microglia via the P2Y and CX3CR1 receptors, respectively (Harrison et al., 1998; Koizumi et al., 2007). Interestingly, both fractalkine and UDP appear to enhance glial cell engulfment: fractalkine by enhancing microglial secretion of MFG-E8, and UDP through an as yet unknown mechanism (Koizumi et al., 2007; Fuller and Van Eldik, 2008). The role of fractalkine signaling has been studied in the context of amyotrophic lateral sclerosis and Parkinson’s disease using CX3CR1-deficient mice. In these disease models, loss of fractalkine signaling resulted in increased numbers of dying neurons, which suggests a potential role for fractalkine as an important find-me signal in the maintenance of brain homeostasis (Cardona et al., 2006). A key unexplored area of clearance in the central nervous system is the immune response generated by microglial cells or astrocytes during engulfment (i.e., the release of anti-inflammatory mediators) and how that impacts homeostasis and disease. Finally, in the developed brain, cell turnover is thought to be quite low with the exception of restricted regions where adult neurogenesis takes place (Kempermann et al., 2004; Zhao et al., 2008; Taupin, 2009). Defining how apoptotic cell clearance impacts other developmental processes in the brain related to cell turnover, including adult neurogenesis, will require additional studies with appropriate neurological models.
Tumorigenesis and cell clearance
Because apoptotic cell clearance typically generates an immunosuppressive environment, its role in the development and progression of cancer is enigmatic. As has been reviewed elsewhere (Coussens and Werb, 2002; Condeelis and Pollard, 2006; Solinas et al., 2009), chronic inflammation is a key factor in tumorigenesis. Thus, the efficient clearance of dying cells, and the associated production of anti-inflammatory mediators, would be predicted to be beneficial in limiting tumorigenesis. However, within a tumor environment where rapid cell proliferation and apoptosis are ongoing, phagocyte-mediated clearance can exert an unwanted immunosuppressive effect. This is particularly the case upon the administration of antitumor chemotherapeutics, most of which act by inducing apoptosis of tumor cells. In this setting, efficient engulfment and the characteristic release of anti-inflammatory mediators, particularly TGFβ, upon encounter with eat-me signals during this process appear to suppress the antitumor immune response. Indeed, in several rodent tumor models, treatment with monoclonal antibodies to block PtdSer-mediated uptake retards the growth of tumors (Huang et al., 2005; Ran et al., 2005; He et al., 2009). Similarly, vaccination of mice with UV-irradiated lymphoma cells coated with annexin V to mask PtdSer provides significant tumor protection against subsequent challenge with living tumor cells, presumably by initiating an antitumor inflammatory response (Bondanza et al., 2004). Antibody depletion of MFG-E8 in mouse models of solid tumors also enhances antitumor activity (Jinushi et al., 2008; Jinushi et al., 2009). These findings suggest that interfering with PtdSer uptake promotes dendritic cell-mediated antitumor activity by favoring inflammatory uptake mechanisms. Still, despite what appears to be a plausible scenario wherein apoptotic cell clearance could have a profound impact on carcinogenesis, there is only limited genetic evidence to implicate specific engulfment signaling pathways in this process. Indeed, the expression of several key engulfment players, including MER (Linger et al., 2008) and αvβ5 (Burvenich et al., 2008), is up-regulated in neoplastic cells, but the importance of this observation is unclear.
With the recent discovery of several “find-me” factors released by apoptotic cells that act to promote recruitment of phagocytes to apoptotic cells, new insights have been gained in our understanding of connections between cell clearance and tumorigenesis. Several insightful studies from the laboratory of C.D. Gregory (Ogden et al., 2005; Truman et al., 2008) have focused on how macrophages sense and subsequently engulf apoptotic Burkitt lymphoma cells and how these signaling events may impact disease progression. These neoplastic B cells express high levels of fractalkine on their surface that is cleaved during apoptosis and subsequently functions as a potent chemoattractant for macrophages (Truman et al., 2008). Recruitment of macrophages to splenic follicles is impaired in fractalkine receptor-deficient mice, an observation consistent with a role for fractalkine as a key mediator of macrophage recruitment to germinal centers (Truman et al., 2008). Within the germinal center environment, high levels of IL-10 (likely produced by the engulfing macrophages) appear to suppress tumor immunity, whereas the release of B cell survival factors by engulfing macrophages is thought to promote tumor growth (Ogden et al., 2005).
Additionally, we have recently found that apoptotic cells release nucleotide triphosphates (ATP/UTP) early during the apoptotic process (within 2–4 h), and that these nucleotides act as chemoattractants for monocytes and macrophages in vitro and in vivo (Elliott et al., 2009). The amount of ATP released by apoptotic cells under these conditions, which promotes silent clearance, represents a very small percentage of the total intracellular pool of nucleotides (<2%; Elliott et al., 2009). In contrast, a few other recent studies have demonstrated that ATP is released by tumor cells undergoing apoptosis in response to chemotherapeutics, with considerably higher amounts of ATP release (10–100 fold greater) seen at later times after induction (12–24 h; Ghiringhelli et al., 2009; Martins et al., 2009; Aymeric et al., 2010). This apoptotic cell-derived ATP stimulates activation of the NLRP3 inflammasome in dendritic cells via the P2X7 receptor (Ghiringhelli et al., 2009). This heightened activation state appears necessary to drive IL-1β secretion and subsequent priming of CD8+ T cells for IFNγ production and antitumor responses. These studies highlight an emerging role for factors released by apoptotic cells in shaping the immune response in normal and tumor environments. This has led to the concept of “immunogenic” versus “nonimmunogenic” cell death, and the idea that immunogenic cell death may be beneficial in antitumor therapies (Green et al., 2009; Locher et al., 2009). Thus, whether apoptotic cell clearance has a beneficial or detrimental effect in the context of tumor progression or anticancer therapies will depend on gaining a better understanding of the role of factors released by apoptotic tumor cells.
Engulfment molecules in microbial pathogenesis
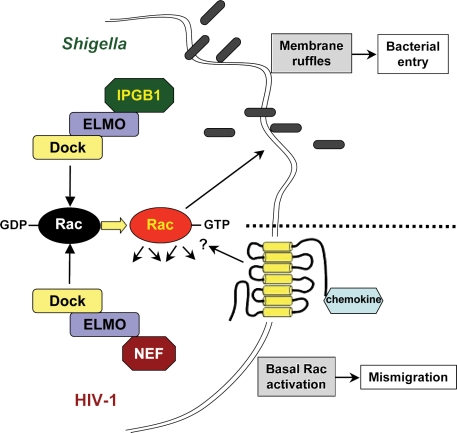
An emerging facet of engulfment signaling is how these pathways can be usurped by microbial pathogens. It has been known for some time that bacteria can hijack or mimic host signaling pathways to aid in pathogenic steps, including cell entry and immune evasion (Stebbins and Galán, 2001). This is achieved by delivery of bacterial effector proteins into the host cell that mimic a range of cellular activities. As key regulators of the cytoskeleton and numerous other cellular processes, small G proteins, particularly the Rho family (e.g., RhoA, Rac, and Cdc42), are frequent targets for these clever effector mechanisms (Mattoo et al., 2007). The signaling machinery that controls phagocyte morphology during apoptotic cell engulfment relies on these GTPases as well, and thus it is not surprising that several bacteria target these pathways. In particular, the RhoG–ELMO–Dock–Rac pathway has been found to be such a target (Fig. 2). The invasive pathogen Shigella flexneri utilizes a type III secretion system to inject effectors to promote entry into epithelial cells, including IPGB1 (Handa et al., 2007). IPGB1 promotes membrane ruffling via Rac activation in a mechanism that requires binding to ELMO1. The small GTPase RhoG acts upstream of ELMO1, and active RhoG-GTP interacts with ELMO1, and thereby recruits the ELMO–Dock180 complex to the membrane to promote Rac activation, membrane ruffling, and engulfment (Katoh and Negishi, 2003; deBakker et al., 2004). IPGB1 mimics the activity of RhoG-GTP, and the Rac-generated ruffles serve as a site of entry for S. flexneri (Handa et al., 2007). Similarly, Yersinia enterocolitica virulence factors Invasin and YopE also modulate Rac1 activity at the level of RhoG, and appear to do so in an ELMO–Dock180-dependent manner in cultured cells (Roppenser et al., 2009). However, neither of these Y. enterocolitica virulence factors have been reported to directly interact with ELMO–Dock180, and the role of this module was inferred by expression of a dominant-negative mutant of ELMO1 that did not further alter Rac activation in the presence of YopE (Roppenser et al., 2009).
Figure 2.
Pathogens usurp the ELMO–Dock–Rac engulfment module. Examples of mechanisms whereby microbial pathogens use the ELMO–Dock–Rac module to alter the host cellular response. The area above the broken line shows mechanism of enhanced S. flexneri invasion via IPGB1 interaction with ELMO, leading to enhanced Rac activation and membrane ruffles that serve as entry points for the bacteria. The area below the broken line shows that HIV-1 uses Nef interaction with the ELMO–Dock2 complex to disrupt CXCR4-dependent chemotaxis in CD4+ T cells.
Usurping the engulfment machinery is not exclusive to bacteria, and in fact can be used by viruses to promote pathogenesis. Janardhan et al. (2004) found that the Nef gene product of HIV-1 is able to complex with the ELMO2–Dock2 module in T cells to promote Rac activation. Further, we have found that Nef interacts with Dock2 in Jurkat T cells and promotes the activation of a key cytoskeletal Rac effector, p21-activated kinase (PAK; unpublished data). The outcome of this interaction appears to be dysregulated Rac activation, which is associated with enhanced activation through the T cell receptor and improper CXCR4-dependent chemotaxis. However, the hijacking of the engulfment signaling machinery has only been shown using cultured cells, and it will be important to determine if in vivo pathogenesis is dependent on these activities as well.
Engulfment genes and other types of disease associations
Several recent studies have discovered associations with human disease and genetic mutations of components of the engulfment signaling machinery. For example, several point mutations in the intronic regions of Elmo1 have been linked to diabetic nephropathy and diabetes (Shimazaki et al., 2005; Pezzolesi et al., 2009a,b). ELMO has also been shown to promote the invasive phenotype of glioblastoma cells in concert with Dock180 and Rac (Jarzynka et al., 2007). Although the role of MFG-E8 in cell clearance and self-tolerance in mice is well-established, there is now evidence that improper splicing of this gene in humans, which results in the production of a PtdSer-binding mutant protein, can be seen in some systemic lupus erythematosus patients (Yamaguchi et al., 2010). Finally, several mutations in engulfment-related genes have been seen in human diseases, including Alzheimer’s disease, schizophrenia, and multiple types of cancer (Table I). It will be important to determine the contribution of these genes in these diseases and understand how they relate to engulfment- and nonengulfment-related cell signaling. As such, these studies point to the importance of the signaling molecules relevant for apoptotic cell engulfment, or the respective signaling pathways in disease, and may help unravel a few of the complex disease pathologies.
Conclusions
The past decade has seen an impressive expansion of our knowledge regarding the fundamentals of apoptotic cell clearance. Despite the complexity and what appears to be redundancy of this process, several key themes emerge, with relevance for disease onset and progression. First, the presence of excess apoptotic cells, particularly in a disease state, is not simply a sign of disease but is likely to have a role in pathogenesis. With the tools currently available, it is difficult to distinguish whether excess apoptotic cells observed in vivo are the result of normal cell death with failed clearance, or an increase in the rate of cell death. However, most tissues appear to have mechanisms in place to support very efficient clearance of apoptotic cells, and uncleared apoptotic cells thus likely represent, at least to some extent, a failure of clearance. A key question related to this idea is the relative contribution of the actual physical removal of the dying cell versus the anti-inflammatory signaling generated by this event in homeostasis and disease. This question will require a more thorough understanding of the signaling events that regulate these two closely related, but experimentally distinguishable, steps of engulfment. Finally, it is also important to carefully consider the impact of apoptotic cell clearance on progression of particular disease states, as in most cases apoptotic cell clearance is a beneficial event. Yet, as in the case of tumorigenesis, it may be that certain disease conditions are exacerbated by the clearance of apoptotic cells. The widespread role of apoptotic cell clearance in many tissues and the recent flood of information on this topic (using in vivo models) portend potentially therapeutic benefits by targeting the components of the engulfment machinery.
Acknowledgments
We thank members of the Ravichandran laboratory for helpful comments during preparation of this manuscript.
This work was supported by a post-doctoral fellowship from the American Cancer Society (to M.R. Elliott), and grants from the National Institute of General Medical Sciences/National Institutes of Health (to K.S. Ravichandran). K.S. Ravichandran is a William Benter Senior Fellow of the American Asthma Foundation.
Footnotes
Abbreviations used in this paper:
- CF
- cystic fibrosis
- CFTR
- cystic fibrosis transmembrane conductance regulator
- COPD
- chronic obstructive pulmonary disease
- LDL
- low-density lipoprotein
- PtdSer
- phosphatidylserine
References
- A-Gonzalez N., Bensinger S.J., Hong C., Beceiro S., Bradley M.N., Zelcer N., Deniz J., Ramirez C., Díaz M., Gallardo G., et al. 2009. Apoptotic cells promote their own clearance and immune tolerance through activation of the nuclear receptor LXR. Immunity. 31:245–258 10.1016/j.immuni.2009.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ait-Oufella H., Kinugawa K., Zoll J., Simon T., Boddaert J., Heeneman S., Blanc-Brude O., Barateau V., Potteaux S., Merval R., et al. 2007. Lactadherin deficiency leads to apoptotic cell accumulation and accelerated atherosclerosis in mice. Circulation. 115:2168–2177 10.1161/CIRCULATIONAHA.106.662080 [DOI] [PubMed] [Google Scholar]
- Ait-Oufella H., Pouresmail V., Simon T., Blanc-Brude O., Kinugawa K., Merval R., Offenstadt G., Lesèche G., Cohen P.L., Tedgui A., Mallat Z. 2008. Defective mer receptor tyrosine kinase signaling in bone marrow cells promotes apoptotic cell accumulation and accelerates atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 28:1429–1431 10.1161/ATVBAHA.108.169078 [DOI] [PubMed] [Google Scholar]
- Albert M.L., Kim J.I., Birge R.B. 2000. alphavbeta5 integrin recruits the CrkII-Dock180-rac1 complex for phagocytosis of apoptotic cells. Nat. Cell Biol. 2:899–905 10.1038/35046549 [DOI] [PubMed] [Google Scholar]
- Asano K., Miwa M., Miwa K., Hanayama R., Nagase H., Nagata S., Tanaka M. 2004. Masking of phosphatidylserine inhibits apoptotic cell engulfment and induces autoantibody production in mice. J. Exp. Med. 200:459–467 10.1084/jem.20040342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aymeric L., Apetoh L., Ghiringhelli F., Tesniere A., Martins I., Kroemer G., Smyth M.J., Zitvogel L. 2010. Tumor cell death and ATP release prime dendritic cells and efficient anticancer immunity. Cancer Res. 70:855–858 10.1158/0008-5472.CAN-09-3566 [DOI] [PubMed] [Google Scholar]
- Ballabio A., Gieselmann V. 2009. Lysosomal disorders: from storage to cellular damage. Biochim. Biophys. Acta. 1793:684–696 10.1016/j.bbamcr.2008.12.001 [DOI] [PubMed] [Google Scholar]
- Bhatia V.K., Yun S., Leung V., Grimsditch D.C., Benson G.M., Botto M.B., Boyle J.J., Haskard D.O. 2007. Complement C1q reduces early atherosclerosis in low-density lipoprotein receptor-deficient mice. Am. J. Pathol. 170:416–426 10.2353/ajpath.2007.060406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi S.M., Prince L.R., McPhillips K., Allen L., Marriott H.M., Taylor G.W., Hellewell P.G., Sabroe I., Dockrell D.H., Henson P.W., Whyte M.K. 2008. Impairment of apoptotic cell engulfment by pyocyanin, a toxic metabolite of Pseudomonas aeruginosa. Am. J. Respir. Crit. Care Med. 177:35–43 10.1164/rccm.200612-1804OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boddaert J., Kinugawa K., Lambert J.C., Boukhtouche F., Zoll J., Merval R., Blanc-Brude O., Mann D., Berr C., Vilar J., et al. 2007. Evidence of a role for lactadherin in Alzheimer’s disease. Am. J. Pathol. 170:921–929 10.2353/ajpath.2007.060664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohdanowicz M., Grinstein S. 2010. Vesicular traffic: a Rab SANDwich. Curr. Biol. 20:R311–R314 10.1016/j.cub.2010.02.030 [DOI] [PubMed] [Google Scholar]
- Boisvert W.A., Rose D.M., Boullier A., Quehenberger O., Sydlaske A., Johnson K.A., Curtiss L.K., Terkeltaub R. 2006. Leukocyte transglutaminase 2 expression limits atherosclerotic lesion size. Arterioscler. Thromb. Vasc. Biol. 26:563–569 10.1161/01.ATV.0000203503.82693.c1 [DOI] [PubMed] [Google Scholar]
- Bondanza A., Zimmermann V.S., Rovere-Querini P., Turnay J., Dumitriu I.E., Stach C.M., Voll R.E., Gaipl U.S., Bertling W., Pöschl E., et al. 2004. Inhibition of phosphatidylserine recognition heightens the immunogenicity of irradiated lymphoma cells in vivo. J. Exp. Med. 200:1157–1165 10.1084/jem.20040327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges V.M., Vandivier R.W., McPhillips K.A., Kench J.A., Morimoto K., Groshong S.D., Richens T.R., Graham B.B., Muldrow A.M., Van Heule L., et al. 2009. TNFalpha inhibits apoptotic cell clearance in the lung, exacerbating acute inflammation. Am. J. Physiol. Lung Cell. Mol. Physiol. 297:L586–L595 10.1152/ajplung.90569.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botto M., Dell’Agnola C., Bygrave A.E., Thompson E.M., Cook H.T., Petry F., Loos M., Pandolfi P.P., Walport M.J. 1998. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat. Genet. 19:56–59 10.1038/ng0598-56 [DOI] [PubMed] [Google Scholar]
- Brown S., Heinisch I., Ross E., Shaw K., Buckley C.D., Savill J. 2002. Apoptosis disables CD31-mediated cell detachment from phagocytes promoting binding and engulfment. Nature. 418:200–203 10.1038/nature00811 [DOI] [PubMed] [Google Scholar]
- Burvenich I., Schoonooghe S., Vervoort L., Dumolyn C., Coene E., Vanwalleghem L., Van Huysse J., Praet M., Cuvelier C., Mertens N., et al. 2008. Monoclonal antibody 14C5 targets integrin alphavbeta5. Mol. Cancer Ther. 7:3771–3779 10.1158/1535-7163.MCT-08-0600 [DOI] [PubMed] [Google Scholar]
- Cardona A.E., Pioro E.P., Sasse M.E., Kostenko V., Cardona S.M., Dijkstra I.M., Huang D., Kidd G., Dombrowski S., Dutta R., et al. 2006. Control of microglial neurotoxicity by the fractalkine receptor. Nat. Neurosci. 9:917–924 10.1038/nn1715 [DOI] [PubMed] [Google Scholar]
- Chang G.H., Barbaro N.M., Pieper R.O. 2000. Phosphatidylserine-dependent phagocytosis of apoptotic glioma cells by normal human microglia, astrocytes, and glioma cells. Neuro-oncol. 2:174–183 10.1215/15228517-2-3-174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Sun C., Chen Q., O’Neill F.A., Walsh D., Fanous A.H., Chowdari K.V., Nimgaonkar V.L., Scott A., Schwab S.G., et al. 2009. Apoptotic engulfment pathway and schizophrenia. PLoS One. 4:e6875 10.1371/journal.pone.0006875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung E.Y., Liu J., Homma Y., Zhang Y., Brendolan A., Saggese M., Han J., Silverstein R., Selleri L., Ma X. 2007. Interleukin-10 expression in macrophages during phagocytosis of apoptotic cells is mediated by homeodomain proteins Pbx1 and Prep-1. Immunity. 27:952–964 10.1016/j.immuni.2007.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P.L., Caricchio R., Abraham V., Camenisch T.D., Jennette J.C., Roubey R.A., Earp H.S., Matsushima G., Reap E.A. 2002. Delayed apoptotic cell clearance and lupus-like autoimmunity in mice lacking the c-mer membrane tyrosine kinase. J. Exp. Med. 196:135–140 10.1084/jem.20012094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combadière C., Potteaux S., Gao J.L., Esposito B., Casanova S., Lee E.J., Debré P., Tedgui A., Murphy P.M., Mallat Z. 2003. Decreased atherosclerotic lesion formation in CX3CR1/apolipoprotein E double knockout mice. Circulation. 107:1009–1016 10.1161/01.CIR.0000057548.68243.42 [DOI] [PubMed] [Google Scholar]
- Condeelis J., Pollard J.W. 2006. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 124:263–266 10.1016/j.cell.2006.01.007 [DOI] [PubMed] [Google Scholar]
- Coussens L.M., Werb Z. 2002. Inflammation and cancer. Nature. 420:860–867 10.1038/nature01322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cvetanovic M., Ucker D.S. 2004. Innate immune discrimination of apoptotic cells: repression of proinflammatory macrophage transcription is coupled directly to specific recognition. J. Immunol. 172:880–889 [DOI] [PubMed] [Google Scholar]
- Davalos D., Grutzendler J., Yang G., Kim J.V., Zuo Y., Jung S., Littman D.R., Dustin M.L., Gan W.B. 2005. ATP mediates rapid microglial response to local brain injury in vivo. Nat. Neurosci. 8:752–758 10.1038/nn1472 [DOI] [PubMed] [Google Scholar]
- deBakker C.D., Haney L.B., Kinchen J.M., Grimsley C., Lu M., Klingele D., Hsu P.K., Chou B.K., Cheng L.C., Blangy A., et al. 2004. Phagocytosis of apoptotic cells is regulated by a UNC-73/TRIO-MIG-2/RhoG signaling module and armadillo repeats of CED-12/ELMO. Curr. Biol. 14:2208–2216 10.1016/j.cub.2004.12.029 [DOI] [PubMed] [Google Scholar]
- Devitt A., Pierce S., Oldreive C., Shingler W.H., Gregory C.D. 2003. CD14-dependent clearance of apoptotic cells by human macrophages: the role of phosphatidylserine. Cell Death Differ. 10:371–382 10.1038/sj.cdd.4401168 [DOI] [PubMed] [Google Scholar]
- Elliott M.R., Chekeni F.B., Trampont P.C., Lazarowski E.R., Kadl A., Walk S.F., Park D., Woodson R.I., Ostankovich M., Sharma P., et al. 2009. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature. 461:282–286 10.1038/nature08296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erwig L.P., Henson P.M. 2007. Immunological consequences of apoptotic cell phagocytosis. Am. J. Pathol. 171:2–8 10.2353/ajpath.2007.070135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erwig L.P., Henson P.M. 2008. Clearance of apoptotic cells by phagocytes. Cell Death Differ. 15:243–250 10.1038/sj.cdd.4402184 [DOI] [PubMed] [Google Scholar]
- Erwig L.P., McPhilips K.A., Wynes M.W., Ivetic A., Ridley A.J., Henson P.M. 2006. Differential regulation of phagosome maturation in macrophages and dendritic cells mediated by Rho GTPases and ezrin-radixin-moesin (ERM) proteins. Proc. Natl. Acad. Sci. USA. 103:12825–12830 10.1073/pnas.0605331103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadok V.A., Voelker D.R., Campbell P.A., Cohen J.J., Bratton D.L., Henson P.M. 1992. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J. Immunol. 148:2207–2216 [PubMed] [Google Scholar]
- Fadok V.A., Bratton D.L., Konowal A., Freed P.W., Westcott J.Y., Henson P.M. 1998. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J. Clin. Invest. 101:890–898 10.1172/JCI1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkowski M., Schledzewski K., Hansen B., Goerdt S. 2003. Expression of stabilin-2, a novel fasciclin-like hyaluronan receptor protein, in murine sinusoidal endothelia, avascular tissues, and at solid/liquid interfaces. Histochem. Cell Biol. 120:361–369 10.1007/s00418-003-0585-5 [DOI] [PubMed] [Google Scholar]
- Ferrero E., Hsieh C.L., Francke U., Goyert S.M. 1990. CD14 is a member of the family of leucine-rich proteins and is encoded by a gene syntenic with multiple receptor genes. J. Immunol. 145:331–336 [PubMed] [Google Scholar]
- Fonseca M.I., Zhou J., Botto M., Tenner A.J. 2004. Absence of C1q leads to less neuropathology in transgenic mouse models of Alzheimer’s disease. J. Neurosci. 24:6457–6465 10.1523/JNEUROSCI.0901-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller A.D., Van Eldik L.J. 2008. MFG-E8 regulates microglial phagocytosis of apoptotic neurons. J. Neuroimmune Pharmacol. 3:246–256 10.1007/s11481-008-9118-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaipl U.S., Franz S., Voll R.E., Sheriff A., Kalden J.R., Herrmann M. 2004. Defects in the disposal of dying cells lead to autoimmunity. Curr. Rheumatol. Rep. 6:401–407 10.1007/s11926-004-0016-1 [DOI] [PubMed] [Google Scholar]
- Gal A., Li Y., Thompson D.A., Weir J., Orth U., Jacobson S.G., Apfelstedt-Sylla E., Vollrath D. 2000. Mutations in MERTK, the human orthologue of the RCS rat retinal dystrophy gene, cause retinitis pigmentosa. Nat. Genet. 26:270–271 10.1038/80002 [DOI] [PubMed] [Google Scholar]
- Gardai S.J., McPhillips K.A., Frasch S.C., Janssen W.J., Starefeldt A., Murphy-Ullrich J.E., Bratton D.L., Oldenborg P.A., Michalak M., Henson P.M. 2005. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell. 123:321–334 10.1016/j.cell.2005.08.032 [DOI] [PubMed] [Google Scholar]
- Garden G.A., Möller T. 2006. Microglia biology in health and disease. J. Neuroimmune Pharmacol. 1:127–137 10.1007/s11481-006-9015-5 [DOI] [PubMed] [Google Scholar]
- Gerbod-Giannone M.C., Li Y., Holleboom A., Han S., Hsu L.C., Tabas I., Tall A.R. 2006. TNFalpha induces ABCA1 through NF-kappaB in macrophages and in phagocytes ingesting apoptotic cells. Proc. Natl. Acad. Sci. USA. 103:3112–3117 10.1073/pnas.0510345103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiringhelli F., Apetoh L., Tesniere A., Aymeric L., Ma Y., Ortiz C., Vermaelen K., Panaretakis T., Mignot G., Ullrich E., et al. 2009. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat. Med. 15:1170–1178 10.1038/nm.2028 [DOI] [PubMed] [Google Scholar]
- Graham D.K., Dawson T.L., Mullaney D.L., Snodgrass H.R., Earp H.S. 1994. Cloning and mRNA expression analysis of a novel human protooncogene, c-mer. Cell Growth Differ. 5:647–657 [PubMed] [Google Scholar]
- Green D.R., Ferguson T., Zitvogel L., Kroemer G. 2009. Immunogenic and tolerogenic cell death. Nat. Rev. Immunol. 9:353–363 10.1038/nri2545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumienny T.L., Brugnera E., Tosello-Trampont A.C., Kinchen J.M., Haney L.B., Nishiwaki K., Walk S.F., Nemergut M.E., Macara I.G., Francis R., et al. 2001. CED-12/ELMO, a novel member of the CrkII/Dock180/Rac pathway, is required for phagocytosis and cell migration. Cell. 107:27–41 10.1016/S0092-8674(01)00520-7 [DOI] [PubMed] [Google Scholar]
- Hanayama R., Tanaka M., Miyasaka K., Aozasa K., Koike M., Uchiyama Y., Nagata S. 2004. Autoimmune disease and impaired uptake of apoptotic cells in MFG-E8-deficient mice. Science. 304:1147–1150 10.1126/science.1094359 [DOI] [PubMed] [Google Scholar]
- Handa Y., Suzuki M., Ohya K., Iwai H., Ishijima N., Koleske A.J., Fukui Y., Sasakawa C. 2007. Shigella IpgB1 promotes bacterial entry through the ELMO-Dock180 machinery. Nat. Cell Biol. 9:121–128 10.1038/ncb1526 [DOI] [PubMed] [Google Scholar]
- Harrison J.K., Jiang Y., Chen S., Xia Y., Maciejewski D., McNamara R.K., Streit W.J., Salafranca M.N., Adhikari S., Thompson D.A., et al. 1998. Role for neuronally derived fractalkine in mediating interactions between neurons and CX3CR1-expressing microglia. Proc. Natl. Acad. Sci. USA. 95:10896–10901 10.1073/pnas.95.18.10896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa H., Kiyokawa E., Tanaka S., Nagashima K., Gotoh N., Shibuya M., Kurata T., Matsuda M. 1996. DOCK180, a major CRK-binding protein, alters cell morphology upon translocation to the cell membrane. Mol. Cell. Biol. 16:1770–1776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J., Yin Y., Luster T.A., Watkins L., Thorpe P.E. 2009. Antiphosphatidylserine antibody combined with irradiation damages tumor blood vessels and induces tumor immunity in a rat model of glioblastoma. Clin. Cancer Res. 15:6871–6880 10.1158/1078-0432.CCR-09-1499 [DOI] [PubMed] [Google Scholar]
- Henson P.M., Tuder R.M. 2008. Apoptosis in the lung: induction, clearance and detection. Am. J. Physiol. Lung Cell. Mol. Physiol. 294:L601–L611 10.1152/ajplung.00320.2007 [DOI] [PubMed] [Google Scholar]
- Hodge S., Hodge G., Scicchitano R., Reynolds P.N., Holmes M. 2003. Alveolar macrophages from subjects with chronic obstructive pulmonary disease are deficient in their ability to phagocytose apoptotic airway epithelial cells. Immunol. Cell Biol. 81:289–296 10.1046/j.1440-1711.2003.t01-1-01170.x [DOI] [PubMed] [Google Scholar]
- Hodge S., Hodge G., Holmes M., Reynolds P.N. 2005. Increased airway epithelial and T-cell apoptosis in COPD remains despite smoking cessation. Eur. Respir. J. 25:447–454 10.1183/09031936.05.00077604 [DOI] [PubMed] [Google Scholar]
- Hodge S., Hodge G., Ahern J., Jersmann H., Holmes M., Reynolds P.N. 2007. Smoking alters alveolar macrophage recognition and phagocytic ability: implications in chronic obstructive pulmonary disease. Am. J. Respir. Cell Mol. Biol. 37:748–755 10.1165/rcmb.2007-0025OC [DOI] [PubMed] [Google Scholar]
- Hodge S., Hodge G., Jersmann H., Matthews G., Ahern J., Holmes M., Reynolds P.N. 2008. Azithromycin improves macrophage phagocytic function and expression of mannose receptor in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 178:139–148 10.1164/rccm.200711-1666OC [DOI] [PubMed] [Google Scholar]
- Hong C., Tontonoz P. 2008. Coordination of inflammation and metabolism by PPAR and LXR nuclear receptors. Curr. Opin. Genet. Dev. 18:461–467 10.1016/j.gde.2008.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X., Bennett M., Thorpe P.E. 2005. A monoclonal antibody that binds anionic phospholipids on tumor blood vessels enhances the antitumor effect of docetaxel on human breast tumors in mice. Cancer Res. 65:4408–4416 10.1158/0008-5472.CAN-05-0031 [DOI] [PubMed] [Google Scholar]
- Huynh M.L., Malcolm K.C., Kotaru C., Tilstra J.A., Westcott J.Y., Fadok V.A., Wenzel S.E. 2005. Defective apoptotic cell phagocytosis attenuates prostaglandin E2 and 15-hydroxyeicosatetraenoic acid in severe asthma alveolar macrophages. Am. J. Respir. Crit. Care Med. 172:972–979 10.1164/rccm.200501-035OC [DOI] [PubMed] [Google Scholar]
- Janardhan A., Swigut T., Hill B., Myers M.P., Skowronski J. 2004. HIV-1 Nef binds the DOCK2-ELMO1 complex to activate rac and inhibit lymphocyte chemotaxis. PLoS Biol. 2:E6 10.1371/journal.pbio.0020006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarzynka M.J., Hu B., Hui K.M., Bar-Joseph I., Gu W., Hirose T., Haney L.B., Ravichandran K.S., Nishikawa R., Cheng S.Y. 2007. ELMO1 and Dock180, a bipartite Rac1 guanine nucleotide exchange factor, promote human glioma cell invasion. Cancer Res. 67:7203–7211 10.1158/0008-5472.CAN-07-0473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinushi M., Nakazaki Y., Carrasco D.R., Draganov D., Souders N., Johnson M., Mihm M.C., Dranoff G. 2008. Milk fat globule EGF-8 promotes melanoma progression through coordinated Akt and twist signaling in the tumor microenvironment. Cancer Res. 68:8889–8898 10.1158/0008-5472.CAN-08-2147 [DOI] [PubMed] [Google Scholar]
- Jinushi M., Sato M., Kanamoto A., Itoh A., Nagai S., Koyasu S., Dranoff G., Tahara H. 2009. Milk fat globule epidermal growth factor-8 blockade triggers tumor destruction through coordinated cell-autonomous and immune-mediated mechanisms. J. Exp. Med. 206:1317–1326 10.1084/jem.20082614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh H., Negishi M. 2003. RhoG activates Rac1 by direct interaction with the Dock180-binding protein Elmo. Nature. 424:461–464 10.1038/nature01817 [DOI] [PubMed] [Google Scholar]
- Kawane K., Fukuyama H., Yoshida H., Nagase H., Ohsawa Y., Uchiyama Y., Okada K., Iida T., Nagata S. 2003. Impaired thymic development in mouse embryos deficient in apoptotic DNA degradation. Nat. Immunol. 4:138–144 10.1038/ni881 [DOI] [PubMed] [Google Scholar]
- Keating A.K., Salzberg D.B., Sather S., Liang X., Nickoloff S., Anwar A., Deryckere D., Hill K., Joung D., Sawczyn K.K., et al. 2006. Lymphoblastic leukemia/lymphoma in mice overexpressing the Mer (MerTK) receptor tyrosine kinase. Oncogene. 25:6092–6100 10.1038/sj.onc.1209633 [DOI] [PubMed] [Google Scholar]
- Kempermann G., Jessberger S., Steiner B., Kronenberg G. 2004. Milestones of neuronal development in the adult hippocampus. Trends Neurosci. 27:447–452 10.1016/j.tins.2004.05.013 [DOI] [PubMed] [Google Scholar]
- Kerr J.F., Wyllie A.H., Currie A.R. 1972. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer. 26:239–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinchen J.M. 2010. A model to die for: signaling to apoptotic cell removal in worm, fly and mouse. Apoptosis. In press [DOI] [PubMed] [Google Scholar]
- Kinchen J.M., Ravichandran K.S. 2010. Identification of two evolutionarily conserved genes regulating processing of engulfed apoptotic cells. Nature. 464:778–782 10.1038/nature08853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinchen J.M., Doukoumetzidis K., Almendinger J., Stergiou L., Tosello-Trampont A., Sifri C.D., Hengartner M.O., Ravichandran K.S. 2008. A pathway for phagosome maturation during engulfment of apoptotic cells. Nat. Cell Biol. 10:556–566 10.1038/ncb1718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkham P.A., Spooner G., Rahman I., Rossi A.G. 2004. Macrophage phagocytosis of apoptotic neutrophils is compromised by matrix proteins modified by cigarette smoke and lipid peroxidation products. Biochem. Biophys. Res. Commun. 318:32–37 10.1016/j.bbrc.2004.04.003 [DOI] [PubMed] [Google Scholar]
- Kiss R.S., Elliott M.R., Ma Z., Marcel Y.L., Ravichandran K.S. 2006a. Apoptotic cells induce a phosphatidylserine-dependent homeostatic response from phagocytes. Curr. Biol. 16:2252–2258 10.1016/j.cub.2006.09.043 [DOI] [PubMed] [Google Scholar]
- Kiss R.S., Ma Z., Nakada-Tsukui K., Brugnera E., Vassiliou G., McBride H.M., Ravichandran K.S., Marcel Y.L. 2006b. The lipoprotein receptor-related protein-1 (LRP) adapter protein GULP mediates trafficking of the LRP ligand prosaposin, leading to sphingolipid and free cholesterol accumulation in late endosomes and impaired efflux. J. Biol. Chem. 281:12081–12092 10.1074/jbc.M600621200 [DOI] [PubMed] [Google Scholar]
- Kobayashi N., Karisola P., Peña-Cruz V., Dorfman D.M., Jinushi M., Umetsu S.E., Butte M.J., Nagumo H., Chernova I., Zhu B., et al. 2007. TIM-1 and TIM-4 glycoproteins bind phosphatidylserine and mediate uptake of apoptotic cells. Immunity. 27:927–940 10.1016/j.immuni.2007.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi S., Shigemoto-Mogami Y., Nasu-Tada K., Shinozaki Y., Ohsawa K., Tsuda M., Joshi B.V., Jacobson K.A., Kohsaka S., Inoue K. 2007. UDP acting at P2Y6 receptors is a mediator of microglial phagocytosis. Nature. 446:1091–1095 10.1038/nature05704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreiselmeier N.E., Kraynack N.C., Corey D.A., Kelley T.J. 2003. Statin-mediated correction of STAT1 signaling and inducible nitric oxide synthase expression in cystic fibrosis epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 285:L1286–L1295 [DOI] [PubMed] [Google Scholar]
- Krieser R.J., MacLea K.S., Longnecker D.S., Fields J.L., Fiering S., Eastman A. 2002. Deoxyribonuclease IIalpha is required during the phagocytic phase of apoptosis and its loss causes perinatal lethality. Cell Death Differ. 9:956–962 10.1038/sj.cdd.4401056 [DOI] [PubMed] [Google Scholar]
- Lacy-Hulbert A., Smith A.M., Tissire H., Barry M., Crowley D., Bronson R.T., Roes J.T., Savill J.S., Hynes R.O. 2007. Ulcerative colitis and autoimmunity induced by loss of myeloid alphav integrins. Proc. Natl. Acad. Sci. USA. 104:15823–15828 10.1073/pnas.0707421104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauber K., Bohn E., Kröber S.M., Xiao Y.J., Blumenthal S.G., Lindemann R.K., Marini P., Wiedig C., Zobywalski A., Baksh S., et al. 2003. Apoptotic cells induce migration of phagocytes via caspase-3-mediated release of a lipid attraction signal. Cell. 113:717–730 10.1016/S0092-8674(03)00422-7 [DOI] [PubMed] [Google Scholar]
- Lauber K., Blumenthal S.G., Waibel M., Wesselborg S. 2004. Clearance of apoptotic cells: getting rid of the corpses. Mol. Cell. 14:277–287 10.1016/S1097-2765(04)00237-0 [DOI] [PubMed] [Google Scholar]
- Le L.Q., Kabarowski J.H., Weng Z., Satterthwaite A.B., Harvill E.T., Jensen E.R., Miller J.F., Witte O.N. 2001. Mice lacking the orphan G protein-coupled receptor G2A develop a late-onset autoimmune syndrome. Immunity. 14:561–571 10.1016/S1074-7613(01)00145-5 [DOI] [PubMed] [Google Scholar]
- Leak T.S., Perlegas P.S., Smith S.G., Keene K.L., Hicks P.J., Langefeld C.D., Mychaleckyj J.C., Rich S.S., Kirk J.K., Freedman B.I., et al. 2009. Variants in intron 13 of the ELMO1 gene are associated with diabetic nephropathy in African Americans. Ann. Hum. Genet. 73:152–159 10.1111/j.1469-1809.2008.00498.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leverrier Y., Ridley A.J. 2001. Requirement for Rho GTPases and PI 3-kinases during apoptotic cell phagocytosis by macrophages. Curr. Biol. 11:195–199 10.1016/S0960-9822(01)00047-1 [DOI] [PubMed] [Google Scholar]
- Linger R.M., Keating A.K., Earp H.S., Graham D.K. 2008. TAM receptor tyrosine kinases: biologic functions, signaling, and potential therapeutic targeting in human cancer. Adv. Cancer Res. 100:35–83 10.1016/S0065-230X(08)00002-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locher C., Rusakiewicz S., Tesnière A., Ghiringhelli F., Apetoh L., Kroemer G., Zitvogel L. 2009. Witch hunt against tumor cells enhanced by dendritic cells. Ann. N. Y. Acad. Sci. 1174:51–60 10.1111/j.1749-6632.2009.04940.x [DOI] [PubMed] [Google Scholar]
- Lorand L., Graham R.M. 2003. Transglutaminases: crosslinking enzymes with pleiotropic functions. Nat. Rev. Mol. Cell Biol. 4:140–156 10.1038/nrm1014 [DOI] [PubMed] [Google Scholar]
- Lutgens E., Tjwa M., Garcia de Frutos P., Wijnands E., Beckers L., Dahlbäck B., Daemen M.J., Carmeliet P., Moons L. 2008. Genetic loss of Gas6 induces plaque stability in experimental atherosclerosis. J. Pathol. 216:55–63 10.1002/path.2381 [DOI] [PubMed] [Google Scholar]
- Magnus T., Chan A., Linker R.A., Toyka K.V., Gold R. 2002. Astrocytes are less efficient in the removal of apoptotic lymphocytes than microglia cells: implications for the role of glial cells in the inflamed central nervous system. J. Neuropathol. Exp. Neurol. 61:760–766 [DOI] [PubMed] [Google Scholar]
- Martins I., Tesniere A., Kepp O., Michaud M., Schlemmer F., Senovilla L., Séror C., Métivier D., Perfettini J.L., Zitvogel L., Kroemer G. 2009. Chemotherapy induces ATP release from tumor cells. Cell Cycle. 8:3723–3728 10.4161/cc.8.22.10026 [DOI] [PubMed] [Google Scholar]
- Mattoo S., Lee Y.M., Dixon J.E. 2007. Interactions of bacterial effector proteins with host proteins. Curr. Opin. Immunol. 19:392–401 10.1016/j.coi.2007.06.005 [DOI] [PubMed] [Google Scholar]
- McDonald P.P., Fadok V.A., Bratton D., Henson P.M. 1999. Transcriptional and translational regulation of inflammatory mediator production by endogenous TGF-beta in macrophages that have ingested apoptotic cells. J. Immunol. 163:6164–6172 [PubMed] [Google Scholar]
- Mevorach D., Zhou J.L., Song X., Elkon K.B. 1998. Systemic exposure to irradiated apoptotic cells induces autoantibody production. J. Exp. Med. 188:387–392 10.1084/jem.188.2.387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyanishi M., Tada K., Koike M., Uchiyama Y., Kitamura T., Nagata S. 2007. Identification of Tim4 as a phosphatidylserine receptor. Nature. 450:435–439 10.1038/nature06307 [DOI] [PubMed] [Google Scholar]
- Mochizuki H., Goto K., Mori H., Mizuno Y. 1996. Histochemical detection of apoptosis in Parkinson’s disease. J. Neurol. Sci. 137:120–123 10.1016/0022-510X(95)00336-Z [DOI] [PubMed] [Google Scholar]
- Morimoto K., Janssen W.J., Fessler M.B., McPhillips K.A., Borges V.M., Bowler R.P., Xiao Y.Q., Kench J.A., Henson P.M., Vandivier R.W. 2006. Lovastatin enhances clearance of apoptotic cells (efferocytosis) with implications for chronic obstructive pulmonary disease. J. Immunol. 176:7657–7665 [DOI] [PubMed] [Google Scholar]
- Mosesson Y., Mills G.B., Yarden Y. 2008. Derailed endocytosis: an emerging feature of cancer. Nat. Rev. Cancer. 8:835–850 10.1038/nrc2521 [DOI] [PubMed] [Google Scholar]
- Mukundan L., Odegaard J.I., Morel C.R., Heredia J.E., Mwangi J.W., Ricardo-Gonzalez R.R., Goh Y.P., Eagle A.R., Dunn S.E., Awakuni J.U., et al. 2009. PPAR-delta senses and orchestrates clearance of apoptotic cells to promote tolerance. Nat. Med. 15:1266–1272 10.1038/nm.2048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz L.E., Peter C., Herrmann M., Wesselborg S., Lauber K. 2010. Scent of dying cells: the role of attraction signals in the clearance of apoptotic cells and its immunological consequences. Autoimmun. Rev. 9:425–430 10.1016/j.autrev.2009.11.016 [DOI] [PubMed] [Google Scholar]
- Nagata S., Hanayama R., Kawane K. 2010. Autoimmunity and the clearance of dead cells. Cell. 140:619–630 10.1016/j.cell.2010.02.014 [DOI] [PubMed] [Google Scholar]
- Nakaya M., Tanaka M., Okabe Y., Hanayama R., Nagata S. 2006. Opposite effects of rho family GTPases on engulfment of apoptotic cells by macrophages. J. Biol. Chem. 281:8836–8842 10.1074/jbc.M510972200 [DOI] [PubMed] [Google Scholar]
- Nakayama M., Akiba H., Takeda K., Kojima Y., Hashiguchi M., Azuma M., Yagita H., Okumura K. 2009. Tim-3 mediates phagocytosis of apoptotic cells and cross-presentation. Blood. 113:3821–3830 10.1182/blood-2008-10-185884 [DOI] [PubMed] [Google Scholar]
- Nandrot E.F., Anand M., Almeida D., Atabai K., Sheppard D., Finnemann S.C. 2007. Essential role for MFG-E8 as ligand for alphavbeta5 integrin in diurnal retinal phagocytosis. Proc. Natl. Acad. Sci. USA. 104:12005–12010 10.1073/pnas.0704756104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napirei M., Karsunky H., Zevnik B., Stephan H., Mannherz H.G., Möröy T. 2000. Features of systemic lupus erythematosus in Dnase1-deficient mice. Nat. Genet. 25:177–181 10.1038/76032 [DOI] [PubMed] [Google Scholar]
- Ogden C.A., Pound J.D., Batth B.K., Owens S., Johannessen I., Wood K., Gregory C.D. 2005. Enhanced apoptotic cell clearance capacity and B cell survival factor production by IL-10-activated macrophages: implications for Burkitt’s lymphoma. J. Immunol. 174:3015–3023 [DOI] [PubMed] [Google Scholar]
- Park D., Tosello-Trampont A.C., Elliott M.R., Lu M., Haney L.B., Ma Z., Klibanov A.L., Mandell J.W., Ravichandran K.S. 2007. BAI1 is an engulfment receptor for apoptotic cells upstream of the ELMO/Dock180/Rac module. Nature. 450:430–434 10.1038/nature06329 [DOI] [PubMed] [Google Scholar]
- Park S.Y., Jung M.Y., Kim H.J., Lee S.J., Kim S.Y., Lee B.H., Kwon T.H., Park R.W., Kim I.S. 2008. Rapid cell corpse clearance by stabilin-2, a membrane phosphatidylserine receptor. Cell Death Differ. 15:192–201 10.1038/sj.cdd.4402242 [DOI] [PubMed] [Google Scholar]
- Park D., Hochreiter-Hufford A., Ravichandran K.S. 2009. The phosphatidylserine receptor TIM-4 does not mediate direct signaling. Curr. Biol. 19:346–351 10.1016/j.cub.2009.01.042 [DOI] [PubMed] [Google Scholar]
- Parnaik R., Raff M.C., Scholes J. 2000. Differences between the clearance of apoptotic cells by professional and non-professional phagocytes. Curr. Biol. 10:857–860 10.1016/S0960-9822(00)00598-4 [DOI] [PubMed] [Google Scholar]
- Pezzolesi M.G., Katavetin P., Kure M., Poznik G.D., Skupien J., Mychaleckyj J.C., Rich S.S., Warram J.H., Krolewski A.S. 2009a. Confirmation of genetic associations at ELMO1 in the GoKinD collection supports its role as a susceptibility gene in diabetic nephropathy. Diabetes. 58:2698–2702 10.2337/db09-0641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezzolesi M.G., Poznik G.D., Mychaleckyj J.C., Paterson A.D., Barati M.T., Klein J.B., Ng D.P., Placha G., Canani L.H., Bochenski J., et al. 2009b. Genome-wide association scan for diabetic nephropathy susceptibility genes in type 1 diabetes. Diabetes. 58:1403–1410 10.2337/db08-1514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qingchun H., Runyue H., LiGang J., Yongliang C., Song W., Shujing Z. 2008. Comparison of the expression profile of apoptosis-associated genes in rheumatoid arthritis and osteoarthritis. Rheumatol. Int. 28:697–701 10.1007/s00296-008-0534-7 [DOI] [PubMed] [Google Scholar]
- Ran S., He J., Huang X., Soares M., Scothorn D., Thorpe P.E. 2005. Antitumor effects of a monoclonal antibody that binds anionic phospholipids on the surface of tumor blood vessels in mice. Clin. Cancer Res. 11:1551–1562 10.1158/1078-0432.CCR-04-1645 [DOI] [PubMed] [Google Scholar]
- Ravichandran K.S., Lorenz U. 2007. Engulfment of apoptotic cells: signals for a good meal. Nat. Rev. Immunol. 7:964–974 10.1038/nri2214 [DOI] [PubMed] [Google Scholar]
- Reddien P.W., Horvitz H.R. 2004. The engulfment process of programmed cell death in caenorhabditis elegans. Annu. Rev. Cell Dev. Biol. 20:193–221 10.1146/annurev.cellbio.20.022003.114619 [DOI] [PubMed] [Google Scholar]
- Ren Y., Tang J., Mok M.Y., Chan A.W., Wu A., Lau C.S. 2003. Increased apoptotic neutrophils and macrophages and impaired macrophage phagocytic clearance of apoptotic neutrophils in systemic lupus erythematosus. Arthritis Rheum. 48:2888–2897 10.1002/art.11237 [DOI] [PubMed] [Google Scholar]
- Richens T.R., Linderman D.J., Horstmann S.A., Lambert C., Xiao Y.Q., Keith R.L., Boé D.M., Morimoto K., Bowler R.P., Day B.J., et al. 2009. Cigarette smoke impairs clearance of apoptotic cells through oxidant-dependent activation of RhoA. Am. J. Respir. Crit. Care Med. 179:1011–1021 10.1164/rccm.200807-1148OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Manzanet R., Sanjuan M.A., Wu H.Y., Quintana F.J., Xiao S., Anderson A.C., Weiner H.L., Green D.R., Kuchroo V.K. 2010. T and B cell hyperactivity and autoimmunity associated with niche-specific defects in apoptotic body clearance in TIM-4-deficient mice. Proc. Natl. Acad. Sci. USA. 107:8706–8711 10.1073/pnas.0910359107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roppenser B., Röder A., Hentschke M., Ruckdeschel K., Aepfelbacher M. 2009. Yersinia enterocolitica differentially modulates RhoG activity in host cells. J. Cell Sci. 122:696–705 10.1242/jcs.040345 [DOI] [PubMed] [Google Scholar]
- Savill J., Dransfield I., Hogg N., Haslett C. 1990. Vitronectin receptor-mediated phagocytosis of cells undergoing apoptosis. Nature. 343:170–173 10.1038/343170a0 [DOI] [PubMed] [Google Scholar]
- Savill J., Dransfield I., Gregory C., Haslett C. 2002. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat. Rev. Immunol. 2:965–975 10.1038/nri957 [DOI] [PubMed] [Google Scholar]
- Schrijvers D.M., De Meyer G.R., Kockx M.M., Herman A.G., Martinet W. 2005. Phagocytosis of apoptotic cells by macrophages is impaired in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 25:1256–1261 10.1161/01.ATV.0000166517.18801.a7 [DOI] [PubMed] [Google Scholar]
- Scott R.S., McMahon E.J., Pop S.M., Reap E.A., Caricchio R., Cohen P.L., Earp H.S., Matsushima G.K. 2001. Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature. 411:207–211 10.1038/35075603 [DOI] [PubMed] [Google Scholar]
- Shimazaki A., Kawamura Y., Kanazawa A., Sekine A., Saito S., Tsunoda T., Koya D., Babazono T., Tanaka Y., Matsuda M., et al. 2005. Genetic variations in the gene encoding ELMO1 are associated with susceptibility to diabetic nephropathy. Diabetes. 54:1171–1178 10.2337/diabetes.54.4.1171 [DOI] [PubMed] [Google Scholar]
- Solinas G., Germano G., Mantovani A., Allavena P. 2009. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J. Leukoc. Biol. 86:1065–1073 10.1189/jlb.0609385 [DOI] [PubMed] [Google Scholar]
- Stebbins C.E., Galán J.E. 2001. Structural mimicry in bacterial virulence. Nature. 412:701–705 10.1038/35089000 [DOI] [PubMed] [Google Scholar]
- Stolzing A., Grune T. 2004. Neuronal apoptotic bodies: phagocytosis and degradation by primary microglial cells. FASEB J. 18:743–745 [DOI] [PubMed] [Google Scholar]
- Su J.H., Anderson A.J., Cummings B.J., Cotman C.W. 1994. Immunohistochemical evidence for apoptosis in Alzheimer’s disease. Neuroreport. 5:2529–2533 10.1097/00001756-199412000-00031 [DOI] [PubMed] [Google Scholar]
- Su H.P., Nakada-Tsukui K., Tosello-Trampont A.C., Li Y., Bu G., Henson P.M., Ravichandran K.S. 2002. Interaction of CED-6/GULP, an adapter protein involved in engulfment of apoptotic cells with CED-1 and CD91/low density lipoprotein receptor-related protein (LRP). J. Biol. Chem. 277:11772–11779 10.1074/jbc.M109336200 [DOI] [PubMed] [Google Scholar]
- Sun E., Gao Y., Chen J., Roberts A.I., Wang X., Chen Z., Shi Y. 2004. Allograft tolerance induced by donor apoptotic lymphocytes requires phagocytosis in the recipient. Cell Death Differ. 11:1258–1264 10.1038/sj.cdd.4401500 [DOI] [PubMed] [Google Scholar]
- Szondy Z., Sarang Z., Molnar P., Nemeth T., Piacentini M., Mastroberardino P.G., Falasca L., Aeschlimann D., Kovacs J., Kiss I., et al. 2003. Transglutaminase 2-/- mice reveal a phagocytosis-associated crosstalk between macrophages and apoptotic cells. Proc. Natl. Acad. Sci. USA. 100:7812–7817 10.1073/pnas.0832466100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabas I. 2005. Consequences and therapeutic implications of macrophage apoptosis in atherosclerosis: the importance of lesion stage and phagocytic efficiency. Arterioscler. Thromb. Vasc. Biol. 25:2255–2264 10.1161/01.ATV.0000184783.04864.9f [DOI] [PubMed] [Google Scholar]
- Taniguchi N., Kawahara K., Yone K., Hashiguchi T., Yamakuchi M., Goto M., Inoue K., Yamada S., Ijiri K., Matsunaga S., et al. 2003. High mobility group box chromosomal protein 1 plays a role in the pathogenesis of rheumatoid arthritis as a novel cytokine. Arthritis Rheum. 48:971–981 10.1002/art.10859 [DOI] [PubMed] [Google Scholar]
- Taupin P. 2009. Adult neurogenesis, neural stem cells and Alzheimer’s disease: developments, limitations, problems and promises. Curr. Alzheimer Res. 6:461–470 10.2174/156720509790147151 [DOI] [PubMed] [Google Scholar]
- Thomas L.B., Gates D.J., Richfield E.K., O’Brien T.F., Schweitzer J.B., Steindler D.A. 1995. DNA end labeling (TUNEL) in Huntington’s disease and other neuropathological conditions. Exp. Neurol. 133:265–272 10.1006/exnr.1995.1029 [DOI] [PubMed] [Google Scholar]
- Thorp E., Cui D., Schrijvers D.M., Kuriakose G., Tabas I. 2008. Mertk receptor mutation reduces efferocytosis efficiency and promotes apoptotic cell accumulation and plaque necrosis in atherosclerotic lesions of apoe-/- mice. Arterioscler. Thromb. Vasc. Biol. 28:1421–1428 10.1161/ATVBAHA.108.167197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosello-Trampont A.C., Nakada-Tsukui K., Ravichandran K.S. 2003. Engulfment of apoptotic cells is negatively regulated by Rho-mediated signaling. J. Biol. Chem. 278:49911–49919 10.1074/jbc.M306079200 [DOI] [PubMed] [Google Scholar]
- Truman L.A., Ford C.A., Pasikowska M., Pound J.D., Wilkinson S.J., Dumitriu I.E., Melville L., Melrose L.A., Ogden C.A., Nibbs R., et al. 2008. CX3CL1/fractalkine is released from apoptotic lymphocytes to stimulate macrophage chemotaxis. Blood. 112:5026–5036 10.1182/blood-2008-06-162404 [DOI] [PubMed] [Google Scholar]
- Vandivier R.W., Fadok V.A., Hoffmann P.R., Bratton D.L., Penvari C., Brown K.K., Brain J.D., Accurso F.J., Henson P.M. 2002. Elastase-mediated phosphatidylserine receptor cleavage impairs apoptotic cell clearance in cystic fibrosis and bronchiectasis. J. Clin. Invest. 109:661–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandivier R.W., Henson P.M., Douglas I.S. 2006. Burying the dead: the impact of failed apoptotic cell removal (efferocytosis) on chronic inflammatory lung disease. Chest. 129:1673–1682 10.1378/chest.129.6.1673 [DOI] [PubMed] [Google Scholar]
- Vandivier R.W., Richens T.R., Horstmann S.A., deCathelineau A.M., Ghosh M., Reynolds S.D., Xiao Y.Q., Riches D.W., Plumb J., Vachon E., et al. 2009. Dysfunctional cystic fibrosis transmembrane conductance regulator inhibits phagocytosis of apoptotic cells with proinflammatory consequences. Am. J. Physiol. Lung Cell. Mol. Physiol. 297:L677–L686 10.1152/ajplung.00030.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voll R.E., Herrmann M., Roth E.A., Stach C., Kalden J.R., Girkontaite I. 1997. Immunosuppressive effects of apoptotic cells. Nature. 390:350–351 10.1038/37022 [DOI] [PubMed] [Google Scholar]
- Wang Z., Shufesky W.J., Montecalvo A., Divito S.J., Larregina A.T., Morelli A.E. 2009. In situ-targeting of dendritic cells with donor-derived apoptotic cells restrains indirect allorecognition and ameliorates allograft vasculopathy. PLoS One. 4:e4940 10.1371/journal.pone.0004940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng S., Zemany L., Standley K.N., Novack D.V., La Regina M., Bernal-Mizrachi C., Coleman T., Semenkovich C.F. 2003. Beta3 integrin deficiency promotes atherosclerosis and pulmonary inflammation in high-fat-fed, hyperlipidemic mice. Proc. Natl. Acad. Sci. USA. 100:6730–6735 10.1073/pnas.1137612100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams H., Pease R.J., Newell L.M., Cordell P.A., Graham R.M., Kearney M.T., Jackson C.L., Grant P.J. 2010. Effect of transglutaminase 2 (TG2) deficiency on atherosclerotic plaque stability in the apolipoprotein E deficient mouse. Atherosclerosis. 210:94–99 10.1016/j.atherosclerosis.2009.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witting A., Müller P., Herrmann A., Kettenmann H., Nolte C. 2000. Phagocytic clearance of apoptotic neurons by Microglia/Brain macrophages in vitro: involvement of lectin-, integrin-, and phosphatidylserine-mediated recognition. J. Neurochem. 75:1060–1070 10.1046/j.1471-4159.2000.0751060.x [DOI] [PubMed] [Google Scholar]
- Xiao Y.Q., Freire-de-Lima C.G., Schiemann W.P., Bratton D.L., Vandivier R.W., Henson P.M. 2008. Transcriptional and translational regulation of TGF-beta production in response to apoptotic cells. J. Immunol. 181:3575–3585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi H., Fujimoto T., Nakamura S., Ohmura K., Mimori T., Matsuda F., Nagata S. 2010. Aberrant splicing of the milk fat globule-EGF factor 8 (MFG-E8) gene in human systemic lupus erythematosus. Eur. J. Immunol. 40:1778–1785 [DOI] [PubMed] [Google Scholar]
- Yang S.K., Attipoe S., Klausner A.P., Tian R., Pan D., Rich T.A., Turner T.T., Steers W.D., Lysiak J.J. 2006. In vivo detection of apoptotic cells in the testis using fluorescence labeled annexin V in a mouse model of testicular torsion. J. Urol. 176:830–835 10.1016/j.juro.2006.03.073 [DOI] [PubMed] [Google Scholar]
- Yu X., Lu N., Zhou Z. 2008. Phagocytic receptor CED-1 initiates a signaling pathway for degrading engulfed apoptotic cells. PLoS Biol. 6:e61 10.1371/journal.pbio.0060061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Kokkonen G., Roth G.S. 1995. Identification of neuronal programmed cell death in situ in the striatum of normal adult rat brain and its relationship to neuronal death during aging. Brain Res. 677:177–179 10.1016/0006-8993(95)00197-X [DOI] [PubMed] [Google Scholar]
- Zhao C., Deng W., Gage F.H. 2008. Mechanisms and functional implications of adult neurogenesis. Cell. 132:645–660 10.1016/j.cell.2008.01.033 [DOI] [PubMed] [Google Scholar]