Summary
Human metapneumovirus (HMPV) is a paramyxovirus that is a leading cause of acute respiratory disease. HMPV is difficult to cultivate and limited published data describe the in vitro growth characteristics of the virus and its ability to replicate in different cell lines. Stability of HMPV to different temperatures or environmental conditions has not been described. Nosocomial infections due to HMPV have been reported, and thus the survival of infectious particles on environmental surfaces is important. We tested multiple cell lines for the ability to support HMPV replication both in the presence and absence of exogenous trypsin. The most permissive monkey kidney epithelial cells were LLC-MK2 and Vero, while the most permissive human airway epithelial cell line was BEAS-2B. LLC-MK2 cells were tolerant of trypsin and thus remain an ideal cell line for HMPV cultivation. Spinoculation significantly increased the infectivity of HMPV for cells in monolayer culture. Infectious virus was very stable to repeat freeze-thaw cycles and ambient room temperature or 4°C, while incubation at 37 °C led to degradation of virus titer. Finally, nonporous materials such as metal or plastic retained infectious virus for prolonged periods, while virus deposited on tissue and fabric rapidly lost infectivity. These findings provide guidance for laboratories attempting to culture HMPV and relevant information for infection control policies.
Keywords: metapneumovirus, paramyxovirus, culture
1. Introduction
Human metapneumovirus (HMPV) is a major cause of upper and lower respiratory tract infections in children and adults [1–11]. HMPV is a member of the paramyxovirus family, along with the related respiratory syncytial virus (RSV) and parainfluenza virus (PIV). HMPV and RSV share a number of clinical and genetic similarities [2, 3, 6, 12, 13]. HMPV infection leads to significant morbidity in infants and other special populations, including immunocompromised, high-risk, and elderly patients [9, 14–21]. Nosocomial infections have been reported [22–27]. Thus, whether viable virus can survive in the environment and spread via fomites is important.
HMPV has been difficult for many groups to propagate readily in cell culture. It was first isolated in 2001in the Netherlands using tertiary monkey kidney cells [4]. There are several other reports of primary isolation in different cell lines [28–31]. However, there is no published systematic investigation of the replication of HMPV in continuous cell lines commonly used for research. Ongoing HMPV research would benefit from the ability to use cell lines from different tissues and species. Common classic virologic aspects of HMPV such as stability to temperature and freezing have not been reported. In this study, we investigated the capacity of multiple cell lines to support robust replication of HMPV. We determined the stability of the virus at different ambient temperatures and during freeze-thaw cycles. Finally, we studied the environmental persistence of infectious virus under various conditions.
2. Materials and methods
2.1. Cells and virus culture
BEAS-2B, BET2A, BBM, BZR, HEp2, LLC-MK2, MA-104, and Vero cell lines were obtained from ATCC. All cells were grown in OptiMEM reduced serum medium (Invitrogen) supplemented with 2% heat inactivated fetal bovine serum (FBS), 50ng/ml gentamicin, 2mM glutamine, and 2.5 µg/ml amphotericin (cell medium). All cells were passaged multiple times in this medium prior to performing virus experiments. Cells were incubated at 37°C in a humidified 5% CO2 incubator. Infected cells were cultured in the same medium without serum but with 100µg/ml CaCl2 and 5µg/ml trypsin (HMPV medium). In some experiments, the trypsin concentration varied. For virus infection, subconfluent cell monolayers in 6-well plates were washed twice with PBS and inoculated with 0.1ml of HMPV. The virus was allowed to adsorb for one hour at 37°C, with gentle rocking every 15 minutes. Wells were then overlaid with HMPV medium. The cells were checked daily for CPE using an inverted microscope.
2.2. Virus stocks and titration
The HMPV strain used in these experiments was TN/96-12, an A1 subgroup strain [32, 33]. This virus was passaged 5 times from the original clinical specimen in LLC-MK2 cells and then thrice plaque-purified. A single lot was titered and used for all experiments. Stock virus and experimental samples were titered as described [32]. Briefly, 0.1ml of serial 10-fold dilutions from 10−1 to 10−5 was inoculated into triplicate wells of a 24-well plate of confluent cells and adsorbed as described above. After adsorption, cells were overlaid with 0.75% methylcellulose in HMPV medium. After four days, the plates were fixed with 10% buffered formalin for one hour at room temperature, washed and blocked for 30 minutes at 37°C with 5% instant milk powder in PBS with 0.05% Tween. Plates were incubated with 200µl of a 1:1000 dilution of guinea pig polyclonal anti-HMPV serum [32] for two hours at 37°C. Plates were washed and incubated with HRP-labeled goat-anti-guinea pig IgG (Kirkegaard and Perry Labs, KPL) for two hours at 37°C. Plates were rinsed, TrueBlue peroxidase substrate (KPL) added and incubated for 10 minutes at room temperature. Plates were rinsed and allowed to dry before using a dissecting microscope to visualize and count plaques.
2.3. Spinoculation experiments
BEAS-2b cells grown in 12-well plates were infected with HMPV at a multiplicity of infection (MOI) of 0.31, 0.62, or 1.24 pfu/cell. Plates were either adsorbed as described (standard) or centrifuged at 700xg (spin) at RT for 60 min. After 24 h incubation in cell medium, cells were detached and assessed for surface expression of HMPV fusion (F) protein as a proxy for productive infection. Briefly, cells were incubated with hamster anti-HMPV F mAb 1017 (kindly provided by Nancy Ulbrandt, MedImmune), washed twice, stained with Cy5-conjugated goat anti-hamster IgG antibodies (Jackson ImmunoResearch), fixed with 2% paraformaldehyde and analyzed by flow cytometry using an LSRII (BD Biosciences). Percent of cells expressing surface HMPV F was calculated compared to uninfected control cells.
2.4. Temperature and environmental stability experiments
HMPV stock was diluted 1:10 and 0.5ml aliquoted into each of 9 cryovials, snap frozen in an ethanol/dry ice bath, and placed at −80°C. At time -180 minutes, two vials were quickly thawed in a 37°C water bath, one was placed at room temperature, and one was placed on wet ice. This procedure was repeated at times -120, -60, -30, and -15 minutes. One vial was thawed at time 0 and all were titered as described. Virus aliquots were similarly incubated for determination of stability at 37°C. Stability to repeat freeze-thaw was performed similarly; virus was diluted and aliquoted into cryovials. One vial was placed on ice and remaining vials were snap frozen in a dry-ice/ ethanol bath. All frozen vials were quickly thawed in a 37°C water bath. One vial was placed on ice and the rest were snap frozen again. The freeze-thaw cycles were repeated until all tubes had been thawed and placed on ice; specimens were then titered as described.
To determine the stability of HMPV on various surfaces, 1-cm2 pieces of aluminum, plastic culture tube, latex glove, office paper, laboratory wipe and cotton were sterilized. 50 µl aliquots of virus stock were deposited onto the surfaces under sterile conditions. Specimens were maintained at room temperature in a sterile environment. For metal, plastic, and latex surfaces, aliquots were collected at the indicated time points, the surface was washed with an additional 50 µl of HMPV medium and both aliquots were combined into 400 µl of HMPV medium and titered as described. If the original aliquot had dried completely, the surface was washed with 100 µl of HMPV medium instead. To titer samples from paper, tissue and fabric, the material was placed into a tube containing 500 µl of HMPV medium, vortexed, and titered with the other samples.
2.5. Statistical analysis
Mean viral titers were compared between groups with a 2-tailed t test assuming unequal variance.
3. RESULTS
3.1. Ability of different cell lines to support HMPV replication
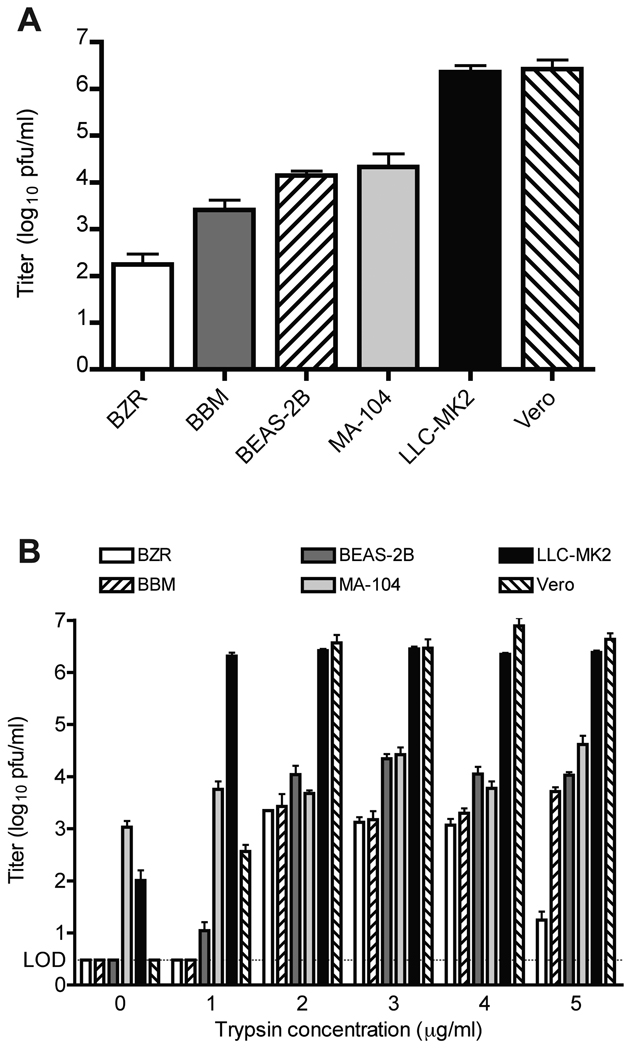
We tested the ability of multiple cell lines to support the growth of HMPV. Initially all cells were incubated with 5µg/ml trypsin in HMPV medium based on conditions that led to initial recovery of the virus in LLC-MK2 cells. Individual cell lines were inoculated with HMPV and incubated until extensive CPE was visible, then titered on the same cell lines. Productive replication with reasonable virus titers was observed in BZR, BBM, BEAS-2B, MA-104, LLC-MK2, and VERO cell lines (Figure 1A). BET-2A and HEp-2 cell lines did not tolerate the trypsin and most of the cells detached from the plate; these cell lines were not studied further. We observed that confluency of monolayers ranging from 40%–100% had minimal effect on the measured virus titer; however, plaques were visibly larger in sub-confluent monolayers (data not shown). The highest yield of virus in monkey kidney epithelial lines was in LLC-MK2 and Vero, while BEAS-2B was the most permissive human airway epithelial line tested.
Figure 1.
Replication of HMPV in different cell lines in the presence and absence of trypsin. A. Titer of HMPV grown in the indicated lines was calculated by plaque assay on the same cell lines, using medium supplemented with 5 µg/ml of trypsin. Data from three experiments performed in triplicate; error bars represent the SEM. B. Titer of HMPV grown in the indicated lines using medium supplemented with trypsin ranging from 0 to 5 µg/ml. Experiment performed in triplicate; error bars represent the SD. LOD = limit of detection.
3.2. Effect of varying trypsin concentrations
Cell lines were tested for HMPV replication with differing concentrations of trypsin (Figure 1B). Only MA-104 and LLC-MK2 cells allowed HMPV replication in the absence of trypsin, but the titers were lower than in the presence of trypsin. LLC-MK2 cells supported high levels of replication of HMPV at all concentrations ≥ 1µg/ml. Vero cells required ≥ 2µg/ml to achieve maximum growth, with undetectable replication in the absence of trypsin. MA-104 cells supported replication in the absence of trypsin, and titers were similar regardless of trypsin concentration. Titers in BZR cells decreased at higher trypsin concentrations, likely because cells were detaching. We observed signs of excess trypsin effects (cell rounding and detachment) in all cell lines except LLC-MK2 cells, especially at concentrations ≥ 3µg/ml. We have observed previously that LLC-MK2 cells are tolerant of trypsin at 5µg/ml for 14 to 21 days when used for primary isolation [2, 32] and thus this concentration was used for all subsequent LLC-MK2 experiments.
3.3. HMPV replication is not dependent on MOI
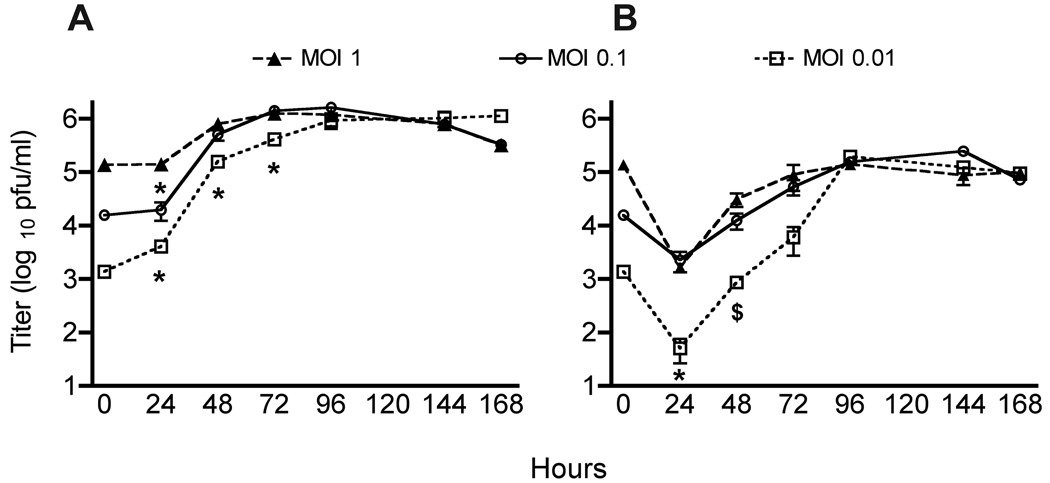
In order to determine whether inoculum size affects viral growth, we performed kinetic curve experiments in LLC-MK2 cells. Cell monolayers were adsorbed with HMPV at MOI of 1, 0.1, and 0.01, overlaid with medium without removal of inoculum, and infected monolayers harvested for viral titration at different time points. Inoculation with a lower MOI led to delayed growth kinetics, with significantly reduced titers at 24 hours for MOI 0.1, and at 24, 48 and 72-hour time points for MOI 0.01 (Figure 2A). However, the titers in all three conditions became equivalent at 96 and 144 hours. Titers in all three groups declined slightly at 168 hours, likely due to deterioration of cells. Supernatant and cell monolayers were both collected and titered at the indicated time points, and the titer in supernatant was one to two log10 lower than the cell fraction at each point (Figure 2B), showing that HMPV is highly cell-associated. Furthermore, the titer in either supernatant or cells was not significantly affected by washing off the inoculum after the one-hour adsorption (data not shown), suggesting that virus binding (if not fusion and entry) is complete within one hour. Therefore, for early time point experiments, inoculation MOI has a substantial effect, while for maximal yield of virus stock production the initial MOI is relatively unimportant.
Figure 2.
Kinetics of HMPV replication at different MOI. LLC-MK2 cell monolayers were inoculated with the indicated MOI and harvested for plaque titration at the indicated time points. A. Titer of HMPV in the cell fraction. B. Titer of HMPV in the supernatant. $ = p<0.05, * = p<0.005 compared to MOI = 1, Student’s t test. Experiment performed in triplicate; error bars represent the SD.
3.4. Spinoculation increases the efficiency of HMPV infection
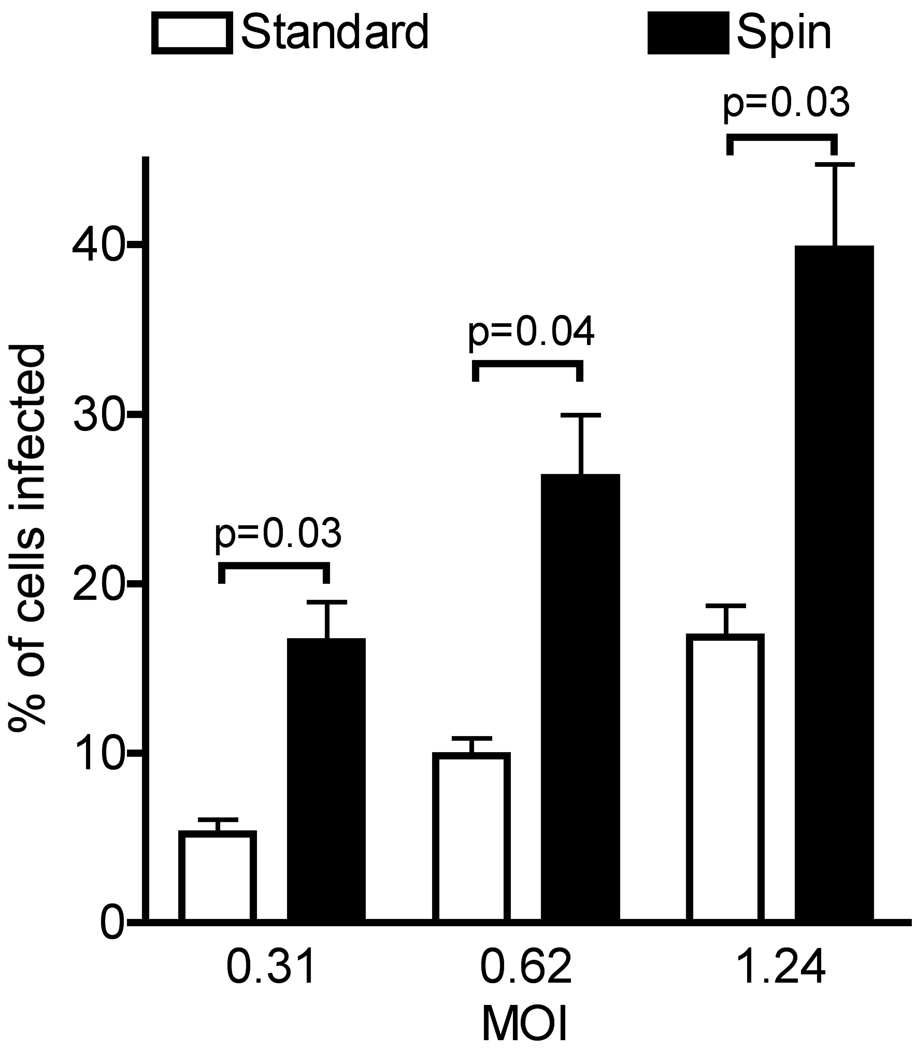
We sought to determine whether enhancing virus binding to cells could increase the effective MOI and achieve a greater number of infected cells. We infected BEAS-2b cells with HMPV using either the standard method of gently rocking virus inoculum on cells, or spinoculation of cells with virus at ~700xg. Twenty-four hours post infection, infected cells were distinguished from uninfected cells by staining for surface expressed HMPV fusion (F) protein, and the percentage of infected cells was quantified using flow cytometry. Spinoculation resulted in a significant 2- to 3-fold increase in the percentage of HMPV-infected cells compared to standard inoculation (Figure 3). We found at other MOI below and above this range (0.1 to 4.5 pfu/cell) that the increased efficiency of spinoculation was consistent (data not shown). Standard adsorption was slightly more reproducible in that variation observed between different experiments was lower than for spinoculation, as evidenced by the error bars in Figure 3. The variation associated with spinoculation was greater with subconfluent target cells and repeat experiments at higher cell confluency were less variable (data not shown). Reflecting the findings in the MOI experiments, spinoculation did not increase the yield at later time points (data not shown). Thus, spinoculation increases the efficiency of HMPV infection and may be the method of choice for some experiments, as low-to-moderate HMPV titers often limit the highest achievable effective MOI.
Figure 3.
Effects of different virus adsorption methods on the efficiency of HMPV infection. BEAS-2B cells were infected with HMPV at a MOI of 0.31, 0.62, or 1.24 pfu/cell by standard or spin inoculation. At 24 h post-infection, infected cells were identified by indirect immunostaining using flow cytometry. Values represent the mean percentage of infected cells for three independent experiments performed in triplicate; error bars represent the SEM.
3.5. Stability to temperature variability and repeated freeze/thaw cycles
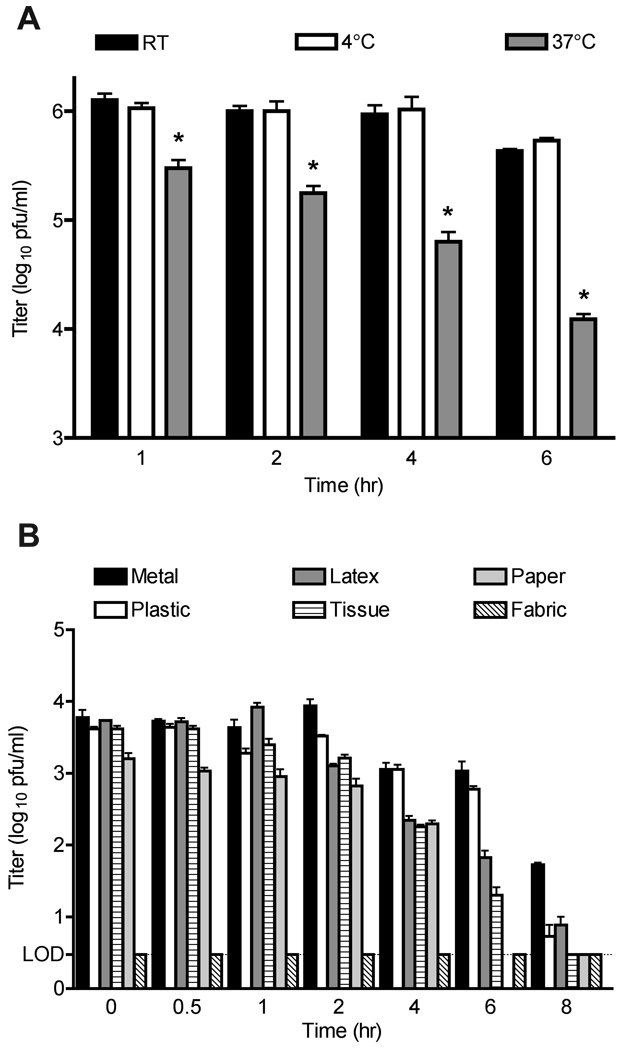
We tested the ability of HMPV incubated at various temperatures to retain infectivity. HMPV showed little loss of titer when allowed to sit at room temperature or at 4°C for up to six hours, losing only about 0.6 log10 after three hours at room temperature and slightly less at 4°C (Figure 4A). In contrast, significant infectious viral titer was lost during incubation at 37°C, with ~99% loss of titer after 6 hours. The results of multiple freeze thaw cycles on the titer of HMPV are shown in Table 1. Repeated freeze thaw cycles resulted in loss of less than one log10 of infectious virus with each cycle.
Figure 4.
Stability of HMPV under varying environmental conditions. A. Viral aliquots were incubated at room temperature, 4°C, or 37°C and harvested for plaque titration at the indicated time points. * = p<0.05 compared to 4°C, student’s t test. Data from three experiments performed in triplicate; error bars represent the SEM. B. Aliquots of virus were adsorbed to material surfaces, incubated and recovered for plaque titration at the indicated time points. Experiment performed in triplicate; error bars represent the SD. LOD = limit of detection.
Table 1.
Stability of HMPV to repeated freeze-thaw cycles.
| No. of freeze- thaw cycles |
Titer (log10 pfu/ml) ± SD |
|---|---|
| 1 | 5.83 ± 0.24 |
| 2 | 5.89 ± 0.11 |
| 3 | 5.90 ± 0.05 |
| 4 | 5.85 ± 0.10 |
| 5 | 5.86 ± 0.07 |
| 6 | 5.66 ± 0.03 |
| 7 | 5.63 ± 0.03 |
| 8 | 5.60 ± 0.03 |
| 9 | 5.56 ± 0.05 |
| 10 | 5.52 ± 0.05 |
3.6. Stability on environmental surfaces
We determined the capacity of HMPV to retain infectivity when incubated on environmental surfaces at room temperature. For these experiments, a lower inoculating titer of 104 pfu/ml was used to approximate the expected titer shed in secretions from infected patients. HMPV genome copy numbers in nasal secretions between 105–107 copies/ml have been reported [1, 34, 35]. We determined that a genome copy number of 106 correlated with an infectious titer of 104 pfu/ml (data not shown). In general, nonporous surfaces protected HMPV from degradation for two hours, after which infectivity was lost slowly (Figure 4B). The metal surface alone retained a small amount of infectious virus after 8 hours, but this still represented ~99% loss of titer. Virus deposited on tissue and paper was less stable, with only 4% and 12% viable at 2 and 4 hours, respectively. No infectious virus could be recovered from the fabric sample at any time point.
4. Discussion
We sought to take a classic virological approach to HMPV and determine several important characteristics of this recently identified virus. We found that HMPV replicates to high titers in two monkey kidney epithelial cells lines, LLK-MK2 and Vero, as well as the human bronchial epithelial cell line BEAS-2B. This provides an opportunity to confirm in vitro biology of HMPV in cells derived from two different tissues and two primate species. While many cell lines tested supported the replication of HMPV, most were poorly tolerant of trypsin and thus probably not suitable for routine laboratory work. We found LLC-MK2 cells to be resistant to trypsin and thus more amenable for most viral cultivation. We have previously found that the trypsin tolerance of LLC-MK2 cells makes them more sensitive than other cell types for primary recovery of HMPV from clinical specimens [2]. However, Vero cells have been used for production of several live attenuated vaccines approved for humans and thus might be preferable for vaccine-oriented research [36]. Notably, Vero cells did not support detectable HMPV replication in the absence of trypsin. This may explain the failure to identify HMPV in routine diagnostic virology, where Vero cells are frequently used. Other groups investigated the capacity of HMPV to infect different cell types, but most of these used measurement of viral RNA or fluorescent-focus assays to enumerate viral protein-expressing cells after 24 hours [28, 37]; these techniques measure entry, RNA replication, and protein translation. We used methods that determine productive virus infection in order to determine the generation of fully infectious particles and viable multicycle growth.
The original isolate was passed five times in LLC-MK2 cells prior to use in these experiments. We think it unlikely that significant cell adaptation occurred in such few passages. However, we cannot exclude this possibility; mutations in RSV G protein that affect replication in a cell-line specific manner have been detected after a single passage in Vero cells [38]. Thus, our findings are most relevant for viruses propagated in vitro and it is possible that other cell lines may also be suitable for recovery of primary isolates.
These experiments confirmed that once an HMPV strain has been isolated, and with proper detection reagents, HMPV is not difficult to grow and plaque. However, cell culture isolation is neither a sensitive nor simple method for primary identification of HMPV. Clinical specimens usually require prolonged culture and/or blind passage before CPE appears and the presence of the virus can be visually identified [2, 28, 39]. Antibody-based immunofluorescent staining methods performed directly on clinical specimens have reasonable sensitivity and specificity [29, 40]. The titer of virus shed in patients would be expected to affect the number of infected cells and thus, the sensitivity of this technique.
Similarly, we found that early replication kinetics of HMPV were inoculum-dependent; this has implications for culture-based detection methods with or without antibody mediated fluorescent detection. Infecting cell monolayers with a low MOI results in the maximal number of infected cells appearing at >72 hours. Conversely, the MOI of the input virus had very little effect on the titer of the virus at 96 hours in the range of 0.01 to 1.0 MOI. However, the increase in infectious virus titer was modest when inoculated with an MOI of 1.0. We speculate that this apparent lack of robust replication was due to persistence of the input virus masking an eclipse phase of viral replication. Several of the experiments presented here demonstrate the stability of the HMPV virion, supporting this interpretation. Thus, for production of high titered working virus stock, initial MOI is relatively unimportant. However, this data informs experiments of cellular biology of HMPV. Experiments designed to study early cellular immune and other signaling responses to HMPV are likely to have greater discrimination between virus-induced phenomena and background with a high MOI and a greater number of infected cells.
We confirmed that a greater number of cells also could be infected using the spinoculation technique, though spinoculation did not increase the final virus yield. This was not entirely unexpected, since several reports have used spinoculation and shell-vial culture techniques to detect HMPV in clinical specimens [41–43]. The centrifugation shell vial technique has been used to enhance the detection of many viruses [44–48]. However, the quantitation of this method provides useful data for HMPV research. One challenge in studying cellular responses to HMPV is the fact that HMPV grows to modest titers, usually in the 106 – 107 pfu/ml range. This limits the achievable MOI in an experiment; spinoculation provides a reproducible means to double the effective MOI. Thus, combining spinoculation with the highest achievable MOI is a useful approach to study early events in HMPV entry and replication.
We examined the effect of temperature on the stability of HMPV. HMPV particles remained infectious for hours at room temperature and 4°C, while significant degradation of infectivity occurred at 37°C. These data suggest that HMPV can be maintained at 4°C for hours during experimental procedures or processing of clinical specimens. Consistent with these findings, we have not observed a decrease in HMPV detection in clinical specimens maintained at 4°C for up to 24 hours (data not shown). Surprisingly, HMPV was quite stable to repeated freeze-thaw cycles, losing <1 log10 pfu/ml over ten cycles. These experiments were performed on virus stock without the inclusion of additives shown to stabilize RSV, such as sucrose, MgSO4, or 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) [49, 50]. Thus, the results are in sharp contrast to RSV, where in the absence of stabilizers, RSV infectivity was reduced by >2 log10 TCID50/ml after five freeze-thaw cycles [50]. In keeping with these data, we have found that HMPV stocks retain infectivity for months at −80°C storage without stabilizers (data not shown), in contrast to RSV losing 2–3 log10 TCID50/ml within three months at −80°C [50].
HMPV was stable on nonporous surfaces for hours, retaining most of its infectivity. In contrast, we recovered diminishing amounts of infectious HMPV from paper and tissue over time, and were unable to recover infectious virus from fabric. These data have important implications for transmission of HMPV and infection control. One brief report described limited data on HMPV persistence on environmental surfaces, but the data were based on qualitative RT-PCR and observation for HMPV CPE in culture for five days [51]; data from many groups including ours has shown that CPE in primary culture is insensitive for HMPV detection. We used an inoculum of 104 pfu/ml to simulate the expected titer in nasal secretions for these experiments. It is important to note that biological substances present in human respiratory secretions also could affect the stability of HMPV, but we did not test for this. Our data show that substantial amounts of infectious HMPV can persist on nonporous surfaces for hours. This environmental stability and resulting nosocomial transmission has been well described for RSV [52–55]. Thus, infection control measures for children with suspected or proven HMPV infection should follow recommendations for RSV infection control [56]. Since RSV and HMPV seasonality overlap considerably in most temperate regions [1–3, 5, 10, 57], specific viral diagnostic tests would avoid nosocomial transmission of these two viruses between children due to the common practice of cohorting children with shared diagnoses.
The studies presented in this manuscript have some limitations. We performed these experiments using an A1 subgroup virus TN/96-12, but the A2, B1, and B2 subgroup viruses perform similarly in our laboratory (data not shown). We did not test the effects of various viral stabilizers such as sugars, amino acids and salts [50, 58]; those experiments are ongoing. Some variability in the cell culture characteristics for different HMPV subgroups have been suggested [37], but the biologic significance of this is unknown, since all four subgroups infect humans readily.
In summary, we have shown that HMPV exhibits productive high titer replication in several primate cell lines from different tissues. HMPV replication is MOI dependent during early growth cycles, but achieves similar maximum virus titers after several days in culture. Spinoculation increases the effective MOI of HMPV. HMPV is stable to repeat freeze-thaw cycles and can persist on nonporous environmental surfaces for many hours. These data can guide both basic HMPV laboratory research and clinical care for patients with HMPV infection.
Acknowledgements
Financial support: Supported by grants from the National Institute of Allergy and Infectious Diseases, National Institutes of Health AI-56170, AI-073697 and AI-082417 (JVW); and 5T32AI-007611-08 (RGC). Flow Cytometry experiments were performed in the VMC Flow Cytometry Shared Resource. The VMC Flow Cytometry Shared Resource is supported by the Vanderbilt Ingram Cancer Center (P30 CA68485) and the Vanderbilt Digestive Disease Research Center (DK058404).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Williams JV, et al. The role of human metapneumovirus in upper respiratory tract infections in children: a 20-year experience. Infect Dis. 2006;193(3):387–395. doi: 10.1086/499274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams JV, et al. Human metapneumovirus and lower respiratory tract disease in otherwise healthy infants and children. N Engl J Med. 2004;350(5):443–450. doi: 10.1056/NEJMoa025472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van den Hoogen BG, et al. Prevalence and clinical symptoms of human metapneumovirus infection in hospitalized patients. J Infect Dis. 2003;188(10):1571–1577. doi: 10.1086/379200. [DOI] [PubMed] [Google Scholar]
- 4.van den Hoogen BG, et al. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat Med. 2001;7(6):719–724. doi: 10.1038/89098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sloots TP, et al. Human metapneumovirus, Australia, 2001–2004. Emerg Infect Dis. 2006;12(8):1263–1266. doi: 10.3201/eid1208.051239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peiris JS, et al. Children with respiratory disease associated with metapneumovirus in Hong Kong. Emerg Infect Dis. 2003;9(6):628–633. doi: 10.3201/eid0906.030009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mullins JA, et al. Human metapneumovirus infection among children hospitalized with acute respiratory illness. Emerg Infect Dis. 2004;10(4):700–705. doi: 10.3201/eid1004.030555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martinello RA, et al. Human metapneumovirus and exacerbations of chronic obstructive pulmonary disease. J Infect. 2006;53(4):248–254. doi: 10.1016/j.jinf.2005.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Falsey AR, et al. Human metapneumovirus infections in young and elderly adults. J Infect Dis. 2003;187(5):785–790. doi: 10.1086/367901. [DOI] [PubMed] [Google Scholar]
- 10.Esper F, et al. A 1-year experience with human metapneumovirus in children aged <5 years. J Infect Dis. 2004;189(8):1388–1396. doi: 10.1086/382482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boivin G, et al. Human metapneumovirus infections in hospitalized children. Emerg Infect Dis. 2003;9(6):634–640. doi: 10.3201/eid0906.030017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McAdam AJ, et al. Human metapneumovirus in children tested at a tertiary-care hospital. J Infect Dis. 2004;190(1):20–26. doi: 10.1086/421120. [DOI] [PubMed] [Google Scholar]
- 13.van den Hoogen BG, et al. Analysis of the genomic sequence of a human metapneumovirus. Virology. 2002;295(1):119–132. doi: 10.1006/viro.2001.1355. [DOI] [PubMed] [Google Scholar]
- 14.Englund JA, et al. Brief communication: fatal human metapneumovirus infection in stem-cell transplant recipients. Ann Intern Med. 2006;144(5):344–349. doi: 10.7326/0003-4819-144-5-200603070-00010. [DOI] [PubMed] [Google Scholar]
- 15.Larcher C, et al. Human metapneumovirus infection in lung transplant recipients: clinical presentation and epidemiology. J Heart Lung Transplant. 2005;24(11):1891–1901. doi: 10.1016/j.healun.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 16.Boivin G, et al. An outbreak of severe respiratory tract infection due to human metapneumovirus in a long-term care facility. Clin Infect Dis. 2007;44(9):1152–1158. doi: 10.1086/513204. [DOI] [PubMed] [Google Scholar]
- 17.Hamelin ME, et al. Human metapneumovirus infection in adults with community-acquired pneumonia and exacerbation of chronic obstructive pulmonary disease. Clin Infect Dis. 2005;41(4):498–502. doi: 10.1086/431981. [DOI] [PubMed] [Google Scholar]
- 18.van den Hoogen BG. Respiratory tract infection due to human metapneumovirus among elderly patients. Clin Infect Dis. 2007;44(9):1159–1160. doi: 10.1086/513295. [DOI] [PubMed] [Google Scholar]
- 19.Vicente D, et al. Human metapneumovirus and chronic obstructive pulmonary disease. Emerg Infect Dis. 2004;10(7):1338–1339. doi: 10.3201/eid1007.030633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams JV, et al. Human metapneumovirus infection plays an etiologic role in acute asthma exacerbations requiring hospitalization in adults. J Infect Dis. 2005;192(7):1149–1153. doi: 10.1086/444392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williams JV, et al. A prospective study comparing human metapneumovirus with other respiratory viruses in adults with hematologic malignancies and respiratory tract infections. J Infect Dis. 2005;192(6):1061–1065. doi: 10.1086/432732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chano F, et al. Epidemiological survey of human metapneumovirus infection in a large pediatric tertiary care center. J Clin Microbiol. 2005;43(11):5520–5525. doi: 10.1128/JCM.43.11.5520-5525.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng VC, et al. Outbreak of human metapneumovirus infection in psychiatric inpatients: implications for directly observed use of alcohol hand rub in prevention of nosocomial outbreaks. J Hosp Infect. 2007;67(4):336–343. doi: 10.1016/j.jhin.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 24.Esper F, et al. Human metapneumovirus infection in the United States: clinical manifestations associated with a newly emerging respiratory infection in children. Pediatrics. 2003;111(6 Pt 1):1407–1410. doi: 10.1542/peds.111.6.1407. [DOI] [PubMed] [Google Scholar]
- 25.Evashuk KM, et al. Respiratory failure associated with human metapneumovirus infection in an infant posthepatic transplant. Am J Transplant. 2008;8(7):1567–1569. doi: 10.1111/j.1600-6143.2008.02278.x. [DOI] [PubMed] [Google Scholar]
- 26.Kim S, et al. Molecular epidemiological investigation of a nosocomial outbreak of human metapneumovirus infection in a pediatric hemato-oncology patient population. J Clin Microbiol. 2009;47(4):1221–1224. doi: 10.1128/JCM.01959-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee N, et al. Co-circulation of human metapneumovirus and SARS-associated coronavirus during a major nosocomial SARS outbreak in Hong Kong. J Clin Virol. 2007;40(4):333–337. doi: 10.1016/j.jcv.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deffrasnes C, Cote S, Boivin G. Analysis of replication kinetics of the human metapneumovirus in different cell lines by real-time PCR. J Clin Microbiol. 2005;43(1):488–490. doi: 10.1128/JCM.43.1.488-490.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ingram RE, et al. Detection of human metapneumovirus in respiratory secretions by reverse-transcriptase polymerase chain reaction, indirect immunofluorescence, and virus isolation in human bronchial epithelial cells. J Med Virol. 2006;78(9):1223–1231. doi: 10.1002/jmv.20685. [DOI] [PubMed] [Google Scholar]
- 30.Schildgen V, et al. Human HepG2 cells support respiratory syncytial virus and human metapneumovirus replication. J Virol Methods. 2009 doi: 10.1016/j.jviromet.2009.08.024. [DOI] [PubMed] [Google Scholar]
- 31.Shirogane Y, et al. Efficient multiplication of human metapneumovirus in Vero cells expressing the transmembrane serine protease TMPRSS2. J Virol. 2008;82(17):8942–8946. doi: 10.1128/JVI.00676-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Williams JV, et al. The cotton rat (Sigmodon hispidus) is a permissive small animal model of human metapneumovirus infection, pathogenesis, and protective immunity. J Virol. 2005;79(17):10944–10951. doi: 10.1128/JVI.79.17.10944-10951.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van den Hoogen BG, et al. Antigenic and genetic variability of human metapneumoviruses. Emerg Infect Dis. 2004;10(4):658–666. doi: 10.3201/eid1004.030393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kunz AN, et al. Detection of multiple respiratory viruses by real-time polymerase chain reaction in infants attending an outpatient clinic. Eur J Clin Microbiol Infect Dis. 2008;27(12):1245–1248. doi: 10.1007/s10096-008-0558-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuypers J, et al. Detection and quantification of human metapneumovirus in pediatric specimens by real-time RT-PCR. J Clin Virol. 2005;33(4):299–305. doi: 10.1016/j.jcv.2004.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barrett PN, et al. Vero cell platform in vaccine production: moving towards cell culture-based viral vaccines. Expert Rev Vaccines. 2009;8(5):607–618. doi: 10.1586/erv.09.19. [DOI] [PubMed] [Google Scholar]
- 37.Abiko C, et al. Outbreak of human metapneumovirus detected by Vero E6 cell line in Yamagata, Japan between 2004 and 2005. J Clin Microbiol. 2007 doi: 10.1128/JCM.01251-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kwilas S, et al. Respiratory syncytial virus grown in Vero cells contains a truncated attachment protein that alters its infectivity and dependence on glycosaminoglycans. J Virol. 2009;83(20):10710–10718. doi: 10.1128/JVI.00986-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boivin G, et al. Virological features and clinical manifestations associated with human metapneumovirus: a new paramyxovirus responsible for acute respiratory-tract infections in all age groups. J Infect Dis. 2002;186(9):1330–1334. doi: 10.1086/344319. [DOI] [PubMed] [Google Scholar]
- 40.Ebihara T, et al. Detection of human metapneumovirus antigens in nasopharyngeal secretions by an immunofluorescent-antibody test. J Clin Microbiol. 2005;43(3):1138–1141. doi: 10.1128/JCM.43.3.1138-1141.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jun KR, et al. Detection of human metapneumovirus by direct antigen test and shell vial cultures using immunofluorescent antibody staining. J Virol Methods. 2008;152(1–2):109–111. doi: 10.1016/j.jviromet.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Landry ML, et al. Detection of human metapneumovirus in clinical samples by immunofluorescence staining of shell vial centrifugation cultures prepared from three different cell lines. J Clin Microbiol. 2005;43(4):1950–1952. doi: 10.1128/JCM.43.4.1950-1952.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reina J, et al. Comparison of different cell lines and incubation times in the isolation by the shell vial culture of human metapneumovirus from pediatric respiratory samples. J Clin Virol. 2007;40(1):46–49. doi: 10.1016/j.jcv.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 44.Hughes JH. Physical and chemical methods for enhancing rapid detection of viruses and other agents. Clin Microbiol Rev. 1993;6(2):150–175. doi: 10.1128/cmr.6.2.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matthey S, et al. Rapid detection of respiratory viruses by shell vial culture and direct staining by using pooled and individual monoclonal antibodies. J Clin Microbiol. 1992;30(3):540–544. doi: 10.1128/jcm.30.3.540-544.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O'Doherty U, Swiggard WJ, Malim MH. Human immunodeficiency virus type 1 spinoculation enhances infection through virus binding. J Virol. 2000;74(21):10074–10080. doi: 10.1128/jvi.74.21.10074-10080.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rabalais GP, et al. Rapid diagnosis of respiratory viral infections by using a shell vial assay and monoclonal antibody pool. J Clin Microbiol. 1992;30(6):1505–1508. doi: 10.1128/jcm.30.6.1505-1508.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scanlan PM, et al. Spinoculation of heparan sulfate deficient cells enhances HSV-1 entry, but does not abolish the need for essential glycoproteins in viral fusion. J Virol Methods. 2005;128(1–2):104–112. doi: 10.1016/j.jviromet.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 49.Fernie BF, Gerin JL. The stabilization and purification of respiratory syncytial virus using MgSO4. Virology. 1980;106(1):141–144. doi: 10.1016/0042-6822(80)90229-9. [DOI] [PubMed] [Google Scholar]
- 50.Gupta CK, et al. Stabilization of respiratory syncytial virus (RSV) against thermal inactivation and freeze-thaw cycles for development and control of RSV vaccines and immune globulin. Vaccine. 1996;14(15):1417–1420. doi: 10.1016/s0264-410x(96)00096-5. [DOI] [PubMed] [Google Scholar]
- 51.Muller A, et al. Stability of human metapneumovirus and human coronavirus NL63 on medical instruments and in the patient environment. J Hosp Infect. 2008;69(4):406–408. doi: 10.1016/j.jhin.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hall CB, Douglas RG, Jr, Geiman JM. Possible transmission by fomites of respiratory syncytial virus. J Infect Dis. 1980;141(1):98–102. doi: 10.1093/infdis/141.1.98. [DOI] [PubMed] [Google Scholar]
- 53.Hall CB. Respiratory syncytial virus: its transmission in the hospital environment. Yale J Biol Med. 1982;55(3–4):219–223. [PMC free article] [PubMed] [Google Scholar]
- 54.Hall CB. Nosocomial respiratory syncytial virus infections: the "Cold War" has not ended. Clin Infect Dis. 2000;31(2):590–596. doi: 10.1086/313960. [DOI] [PubMed] [Google Scholar]
- 55.Halasa NB, et al. Medical and economic impact of a respiratory syncytial virus outbreak in a neonatal intensive care unit. Pediatr Infect Dis J. 2005;24(12):1040–1044. doi: 10.1097/01.inf.0000190027.59795.ac. [DOI] [PubMed] [Google Scholar]
- 56.Diagnosis and management of bronchiolitis. Pediatrics. 2006;118(4):1774–1793. doi: 10.1542/peds.2006-2223. [DOI] [PubMed] [Google Scholar]
- 57.Hamelin ME, Abed Y, Boivin G. Human metapneumovirus: a new player among respiratory viruses. Clin Infect Dis. 2004;38(7):983–990. doi: 10.1086/382536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ausar SF, et al. High-throughput screening of stabilizers for respiratory syncytial virus: identification of stabilizers and their effects on the conformational thermostability of viral particles. Hum Vaccin. 2007;3(3):94–103. doi: 10.4161/hv.3.3.4149. [DOI] [PubMed] [Google Scholar]