Abstract
Layer-by-layer polyelectrolyte adsorption in porous polymeric membranes provides a simple way to create ion-exchange sites without greatly decreasing hydraulic permeability (<20% reduction in permeability). At 80% breakthrough, membranes coated with 3-bilayer poly(styrene sulfonate) (PSS)/polyethyleneimine (PEI) films bind 37±6 mg of negatively charged Au colloids per mL of membrane volume. The binding capacity of membranes coated with 1-bilayer films decreases in the order PSS/PEI>PSS/poly(diallyldimethyl ammonium chloride)>PSS/poly(allylamine hydrochloride). Films terminated with a polyanion present cation-exchange sites that bind lysozyme, and the lysozyme-binding capacities of (PSS/PEI)3/PSS films increase with the ionic strength of the solution from which the last PSS layer is deposited. Charge screening during deposition of the terminal PSS layer gives rise to a larger number of ion-exchange sites and lysozyme binding capacities as high as 16 mg per mL of membrane. At 10% breakthrough, a stack of 3 membranes binds 3 times as much lysozyme as a single membrane, showing that stacking is an effective way to increase capacity.
Keywords: membrane adsorbers, ion exchange, polyelectrolyte multilayers, lysozyme binding
1. Introduction
Packed columns have been the primary tools for protein separation and analysis for several decades. Nevertheless, separations with these columns have a number of drawbacks, such as high pressure drops, low throughput, mass transfer limitations, and large recovery volumes [1–5]. The recent use of monoliths as stationary phases can decrease mass transfer resistances and pressure drops, but such structures may be difficult to create on large scales [6]. Membrane adsorbers [7] provide another promising alternative to packed-bed columns because convective, rather than diffusive, transport of solutes to the binding sites in membranes accelerates the separation process [8–10]. The small thickness and short flow path through membranes also results in low pressure drops, and pore sizes as large as a few microns facilitate the access of large proteins or viruses to binding sites. In addition, the development of disposable membranes avoids the need for membrane cleaning and validation of the cleaning process [5]. Recent industrial applications of membrane adsorbers demonstrate their gradual acceptance as an alternative to columns [1, 11].
Ion-exchange-based protein separations and virus removal are among the most common applications of membrane adsorbers [1, 12], and approaches to prepare ion-exchange membranes include in-situ copolymerization [13, 14], radiation-induced grafting [2, 15, 16], and surface-initiated atom transfer radical polymerization techniques [3, 5, 17–19]. These methods modify the pores of the membrane with polymer films that can potentially provide a large number of binding sites. However, the synthesis of the polymers can be complicated, and more importantly, the polymer film may significantly decrease the membrane permeability.
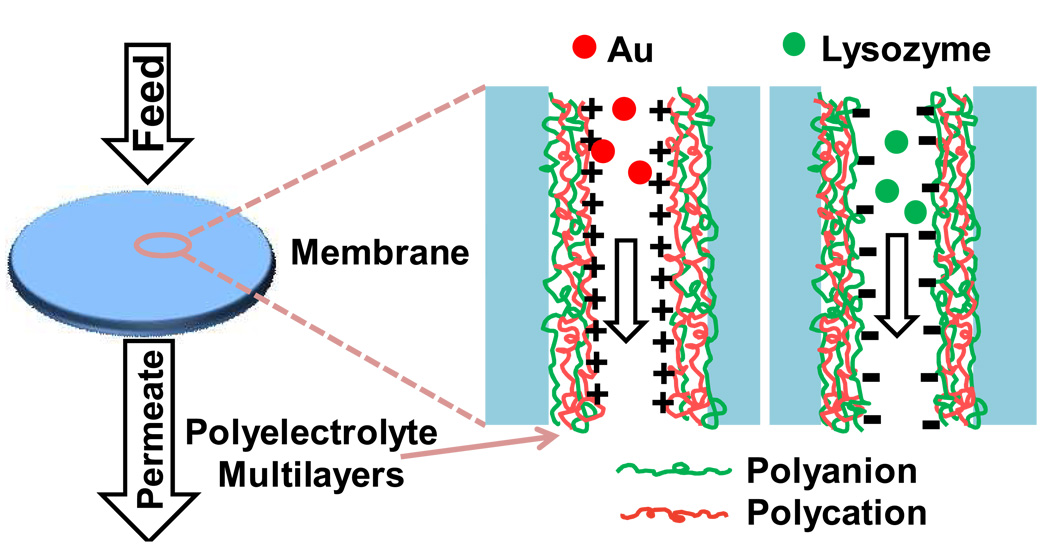
This paper reports the formation of ion-exchange membranes using layer-by-layer (LbL) deposition of polyelectrolyte multilayers (Figure 1) [20]. Several groups deposited LbL films on the top of porous ultrafiltration substrates to create composite membranes, but such systems are designed primarily for size-based or charge-based separation of small molecules [21–25]. Other studies demonstrated fabrication of polyelectrolyte nanotubes in the pores of alumina membranes [26, 27]. In this case, the porous membrane serves as a template for the preparation of multilayer films, and dissolution of the membrane yields the nanotubes. Recently, polyelectrolyte-modified membrane pores served as substrates for enzymes [28, 29], nanoparticle catalysts [30], and protein arrays [31].
Fig. 1.
Schematic diagram showing the binding of negatively charged Au nanoparticles (left) and positively charged lysozyme (right) to polyelectrolyte-multilayer-modified membranes terminated with a polycation (left) and a polyanion (right). Charges are only shown for the outer polyelectrolyte.
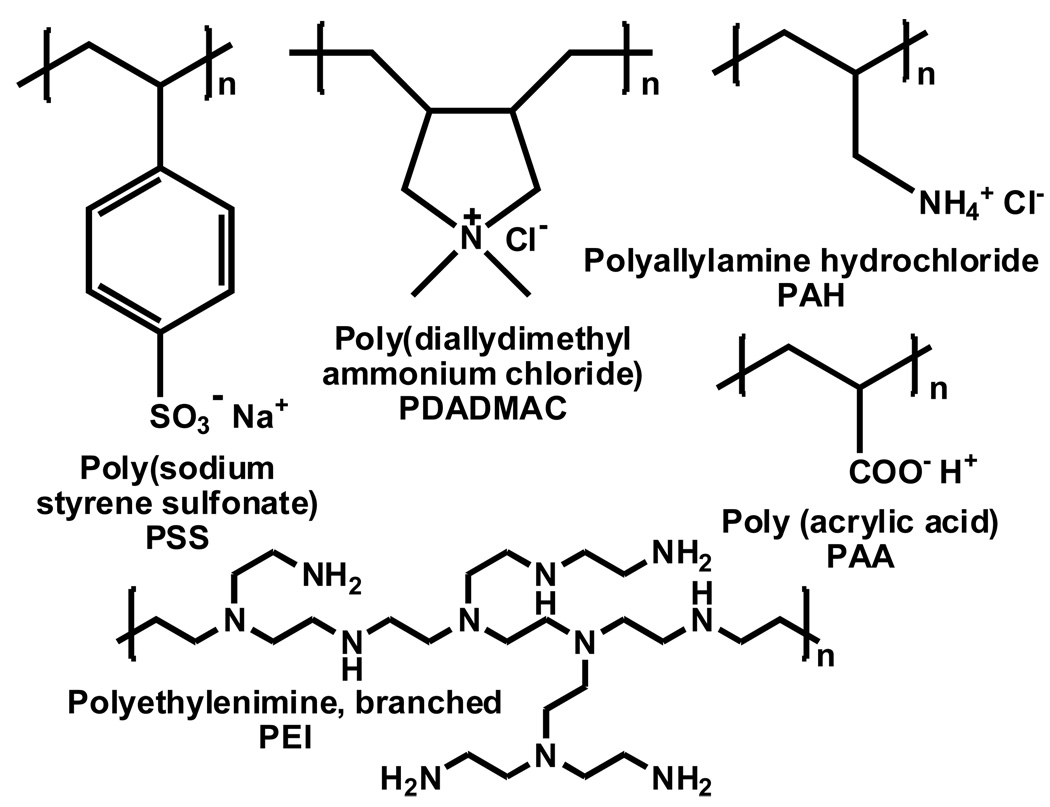
In this work, we show that substrates containing pores modified with LbL polyelectrolyte films are effective ion-exchange membranes for removal of charged Au nanoparticles and proteins from solution. The LbL technique is effective in a variety of membrane pores and does not greatly decrease permeability. Our research compares the binding capacities of membranes containing films prepared with two polyanions, poly(styrene sulfonate) (PSS) and poly(acrylic acid) (PAA), and three polycations, protonated polyethyleneimine (PEI), protonated poly(allylamine) (PAH), and poly(diallyldimethyl ammonium chloride) (PDADMAC). Scheme 1 shows the structures of these polyelectrolytes. Membranes containing films terminated with polycations remove negatively charged citrate-stabilized Au nanoparticles from solution, whereas films terminated with polyanions adsorb lysozyme. Importantly, the high permeabilities of the modified substrates allow the use of membrane stacks that exhibit low pressure drops, and the amount of protein adsorbed is proportional to the number of membranes in the stack.
Scheme 1.
Structures of the polyelectrolytes used in this study. (The protonation sites for PEI have not been determined.)
2. Experimental
2.1 Materials
Hydrophilic nylon and polyethersulfone (PES) membrane filters (25 mm disks) with 5 µm nominal pore sizes and thicknesses of ~80 and ~135 µm, respectively, were purchased from GE Osmonics. Regenerated cellulose (RC) membranes (Whatman, 47 mm discs) with a thickness of 75 µm and a 1 µm nominal pore size were cut to a diameter of 25 mm before use. Hydrophilic polyvinylidene difluoride (PVDF) membranes (25 mm discs) with a thickness of 125 µm and nominal pore size of 5 µm were obtained from Millipore. Finally, hydrophilic nylon sheets with a 1.2 µm nominal pore size and a thickness of ~110 µm were kindly provided by Pall Corporation and were cut into 25 mm discs before use.
Poly(sodium 4-styrene-sulfonate) (Mw = 70,000), poly(allylamine hydrochloride) (Mw = 15,000), polyethyleneimine (branched, Mw = 25,000), poly(diallyldimethylammonium chloride) (20 wt % in water, Mw =100,000 – 200,000), poly(acrylic acid) (Mw = 70,000, 25% aqueous solution), gold (III) chloride trihydrate (≥99.9% trace metal basis), lysozyme (from chicken egg white), sodium citrate, BSA, and TWEEN-20 surfactant were purchased from Sigma-Aldrich. Coomassie protein assay reagent was obtained from Thermo Scientific, and buffers were prepared using analytical grade chemicals and deionized (Milli-Q, 18.2 MΩcm) water.
2.2 Membrane Modification
For modification of nylon, PES, RC, and PVDF membranes by LbL adsorption, the membrane was placed in an Amicon 8010 membrane cell (Millipore), and a solution containing 0.02 M PSS and 0.5 M NaCl was first circulated through the membrane for 15 minutes (flux of 80 cm/h, the exposed area of the top of the membranes was 3.1 cm2) using a peristaltic pump. After passing 10 mL of H2O through the membrane, a polycation (PAH, PEI, or PDADMAC) was deposited in the same manner prior to rinsing with 10 mL of H2O. Subsequent polyanion and polycation layers were deposited similarly. The pH values of PSS, PAA, PAH, PEI, and PDADMAC deposition solutions were adjusted to 4, 4, 4, 9, and 6, respectively, with 0.1 M NaOH or HCl, and all deposition solutions contained 0.02 M polymer and 0.5 M NaCl, unless otherwise noted. (Polymer concentrations are always given with respect to the repeating unit.) Membranes were dried thoroughly with N2 after deposition and rinsing of the final layer. Rinsing should remove any loosely bound polyelectrolytes.
2.3 ATR-FTIR Spectroscopy
ATR-FTIR spectra (Perkin Elmer Spectrum One) of bare and modified membranes were obtained using 16 scans at 4 cm−1 resolution, and the spectrum of the bare diamond crystal (45 degrees) in air served as a background. Membranes were placed on a flat stage and pressed against the crystal with a lever arm.
2.4 Determination of Hydraulic Permeability
Using a pressurized feed tank connected to an Amicon cell, the permeabilities of the membranes to pure water were determined after deposition of each polyelectrolyte layer. The feed tank was filled with deionized water, the system was pressured with N2 to 0.69 bar, and the permeate was collected over specific time intervals to determine the pure water flux. Three measurements of permeate flux were recorded and averaged after each deposition step.
2.5 Zeta Potential Studies and Adsorption Isotherms
Streaming potential measurements were conducted using a streaming potential analyzer (BI-EKA, Brookhaven Instruments, Holtsville, NY) with an asymmetric clamping cell (Anton Paar, Graz, Austria) [32, 33]. The cell contains a 10 × 20 mm grooved poly(methyl methacrylate) (PMMA) spacer, and the 50 × 50 mm membrane substrate (1.2 µm nylon) was placed against the PMMA spacer and sandwiched by another larger PMMA block. The potential across the cell was measured using Ag/AgCl electrodes while flowing a 1 mM KCl solution through the channels of the PMMA spacer, and the streaming potential of a PMMA sheet was determined prior to measuring the streaming potential of the membrane [32, 33]. The internal surface area of the membranes was determined using N2 adsorption measurements (Micromeritics ASAP 2010 Sorptometer) and analysis of the data using a BET model.
2.6 Preparation of Gold Colloids
The synthesis of ~12 nm-diameter gold nanoparticles followed a modified literature procedure [34]. In this method, 150 mL of 1mM HAuCl4·3 H2O was heated to boiling with vigorous stirring in a 500 mL round-bottom flask, and 15 mL of 38.8 mM boiling sodium citrate dihydrate was added rapidly to the gold solution. After ~30 seconds, the mixture turned from pale yellow to dark black and then burgundy, and was subsequently heated with stirring for 10 minutes followed by an additional 15-minute stirring without heating. The resulting solution showed a maximum absorbance at 519 nm. The aqueous colloids were stored in a dark bottle and diluted before use.
2.7 Binding of Gold Nanoparticles and Proteins to Modified Membranes
With the polyelectrolyte-modified membrane in the Amicon cell (exposed membrane area of 3.1 cm2), a 0.05 mM Au-colloid solution (concentration is defined as the molarity of Au atoms) was passed through the membrane using a peristaltic pump [35]. The flux was adjusted by varying the spinning rate of the pump and determined by measuring the permeate volume over a period of time. To determine the concentration of Au colloids in the feed or permeate, the absorbance of the solution at 519 nm (λmax) was converted to concentration with a linear calibration curve. (Figure S1a of the supplementary material shows the calibration curve. All figure numbers beginning with S refer to the supplementary material).
For lysozyme binding, a solution containing 0.1 mg/mL lysozyme in 20 mM phosphate buffer (pH 7.2) was forced through the modified membrane at a constant flux using a peristaltic pump. Thirty µL of each permeate aliquot was mixed with 1.5 mL of Coomassie protein assay reagent (1:50 v/v), and the absorbance of this solution at 585 nm was obtained relative to phosphate buffer mixed with Coomassie reagent. Absorbances were obtained using a Perkin-Elmer Lambda 40 UV-Vis spectrophotometer. The concentration of lysozyme in the permeate was then determined using a calibration curve (Figure S1b). Unless specified otherwise, all breakthrough curves are representative of experiments with at least two membranes.
2.8 Scanning Electron Microscopy
Membrane samples were characterized by scanning electron microscopy (SEM) using a Hitachi S-4700 II field-emission scanning electron microscope. Prior to imaging, samples were fractured with tweezers in liquid nitrogen, and the cross section was coated with 10 nm of Au using a Pelco SC-7 sputter coater. Cross-sectional images were used to determine the thicknesses of nylon and PES membranes (Figure S2), and images of cross sections and the top and bottom of membranes were taken to examine adsorption of Au nanoparticles.
3 Results and Discussion
3.1 Membrane Modification and Characterization
Alternating adsorption of polycations and polyanions allows modification of nylon, PES, PVDF, and RC membranes. For each membrane, PSS served as the initially adsorbed polyanion to facilitate comparisons between membrane types. The PSS deposition pH of 4 is within about one unit of the isoelectric point for all of the different membrane materials in this study [36–39], so electrostatic interactions may not dominate the adsorption process. Nonionic interactions (e.g., hydrophobic, π-π, dipolar, and van der Waals forces) should, however, facilitate PSS adsorption on nylon, PES, and RC membranes. Due to its high chemical resistance and low surface charge, PVDF may exhibit lower levels of polyelectrolyte adsorption than other membranes, regardless of which polyelectrolyte is used. Prior studies showed that adsorption of PSS/polycation/nanoparticle films occurs throughout the pores of nylon, track-etched polycarbonate, and alumina membranes [30, 35].
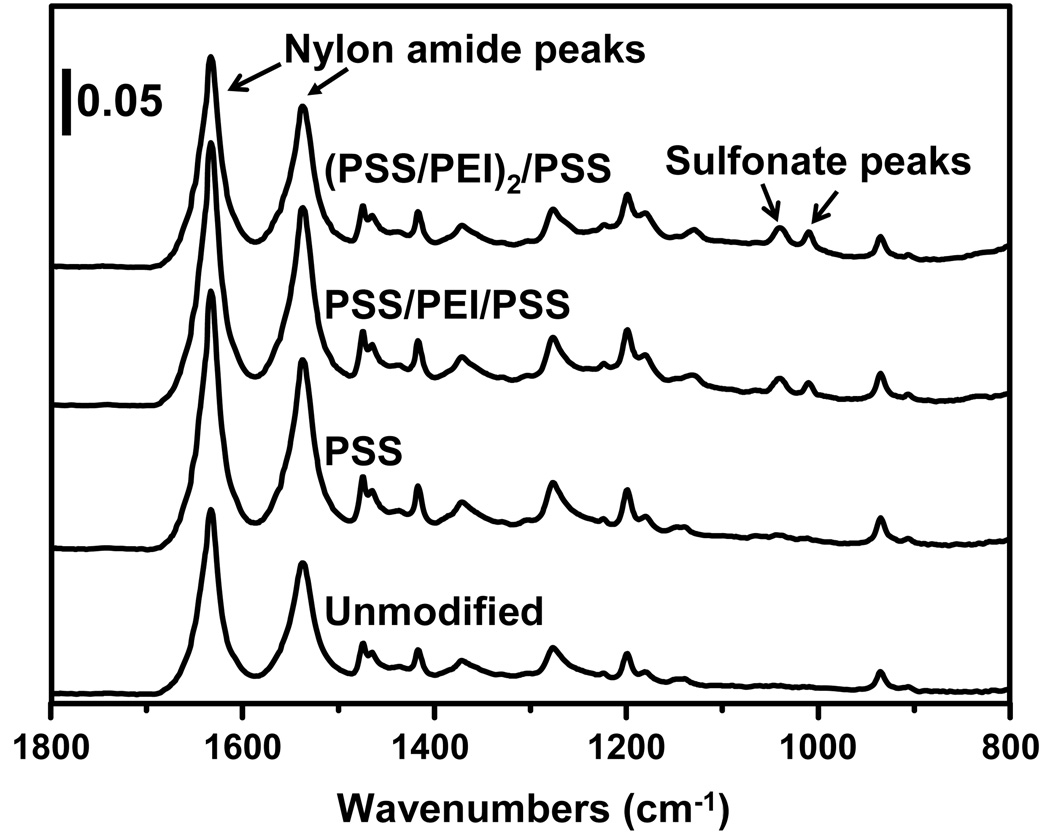
Figure 2 shows the ATR-FTIR spectra of nylon membranes before and after deposition of PSS-terminated polyelectrolyte films. Although most of the peaks in the spectra stem from the nylon membrane, peaks due to the sulfonate moieties of PSS (1010 cm−1 and 1040 cm−1) provide evidence for successful film formation. The sulfonate bands are very small after deposition of the initial layer, but the ratios of the sulfonate absorbances to the amide I absorbance (1633 cm−1) of the nylon substrate increase with the number of adsorbed layers, as would be expected with LbL growth. Because the intensities of peaks in ATR-IR spectra vary with the quality of the contact with the ATR crystal, absorbance ratios are a better indicator of polyelectrolyte adsorption than absolute absorbances. Signals due to PEI stretches are not visible because they have lower extinction coefficients than PSS bands and overlap with nylon absorbances.
Fig. 2.
ATR-FTIR spectra of 5-µm nylon membranes before and after modification with PSS/PEI films. The ratios of the sulfonate absorbances to the amide I absorbance increase with deposition of more PEI/PSS layers.
A small peak at 1040 cm−1 also appears in the ATR-FTIR spectra of PES membranes after the deposition of PSS-terminated polyelectrolyte films (Figure S3). Unfortunately, peaks due to sulfonate moieties were not clearly visible in the ATR-FTIR spectra of RC (Figure S4) and PVDF (Figure S5) membranes after deposition of PSS-terminated films. This might indicate less polyelectrolyte adsorption on these materials than on nylon and PES, but overlap of sulfonate peaks with absorbances from the base membrane may also obscure PSS signals on RC and PVDF.
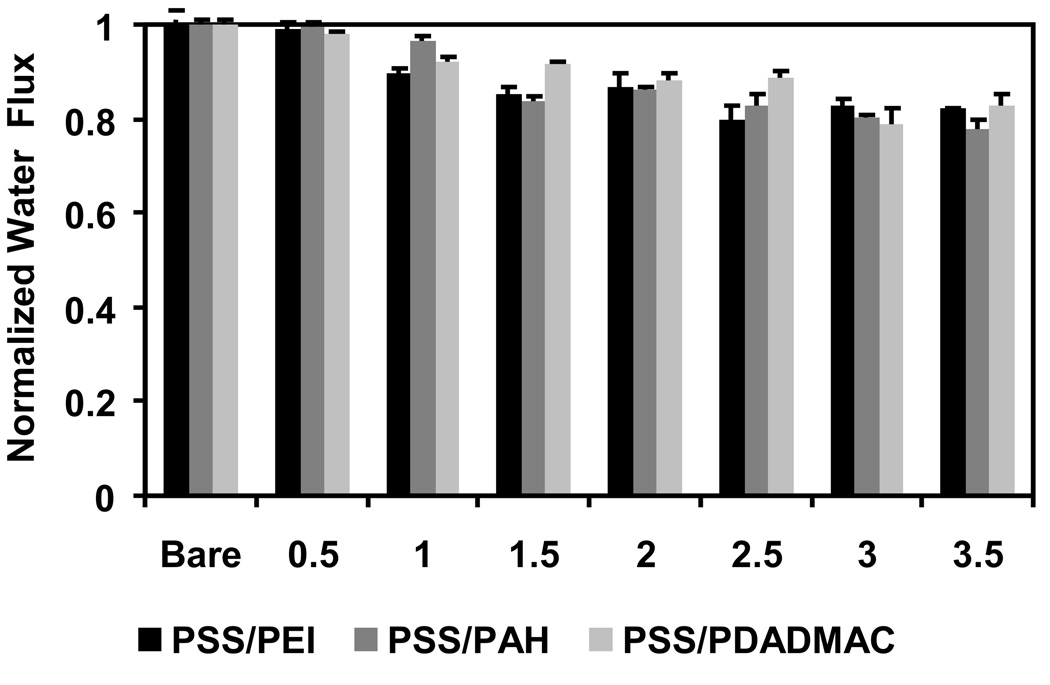
One concern in modifying membranes using the LbL method is that the polyelectrolyte film may plug membrane pores, and the spongy structure of many polymeric membranes may be particularly prone to pore blocking. To avoid plugging, we use membranes with pore sizes > 1 µm. Figure 3 shows the declines in pressure-driven (0.69 bar) pure water flux through nylon membranes (5 µm nominal pores) after each step in the deposition of (PSS/PEI)3/PSS, (PSS/PAH)3/PSS and (PSS/PDADMAC)3/PSS films. The fluxes through the membranes decrease only slightly after each modification step, and the final flux through membranes modified with 3.5-bilayer films is still ~80% of that through the bare membrane. The polyelectrolytes apparently occlude only a small fraction of the pore volume. However, it is possible that blocking or constriction of especially small pores could lead to increased dispersion in breakthrough curves with increasing flow rate (see below).
Fig. 3.
Normalized pure water flux through 5-µm nylon membranes before and after modification with PSS/PEI, PSS/PAH, and PSS/PDADMAC films. Films containing integer numbers of bilayers terminate with polycations, whereas films containing N+0.5 bilayers terminate with PSS. The flux was measured at 0.69 bar and normalized to the flux through the bare membrane, which was 2.0±0.3 cm·s−1·bar−1.
The hydraulic permeability of nylon membranes modified with 3.5-bilayer films is 1.7 ± 0.2 cm·s−1·bar−1, which is about the same as the permeability of commercial Sartobind™ ion-exchange membranes, taking into account the differences in membrane thicknesses [40]. Pall Mustang™ ion-exchange membranes have a smaller pore size (0.8 µm) [41] than Sartobind membranes and likely a lower permeability. The high permeabilities of polyelectrolyte-modified porous substrates will allow the use of thick membranes or membrane stacks to increase capacity without creating an unmanageable pressure drop.
Modification of other types of membranes, i.e., 5-µm PVDF and 1-µm RC with PSS/PEI films also results in a modest decline in permeability as more layers are added, confirming that at least some layer-by-layer adsorption occurs in these materials. The permeabilities of all substrates modified with 3 polyelectrolyte bilayers are ~80% of the permeabilities of the corresponding bare membranes. Interestingly, for 5-µm PES membranes, the flux after the deposition of PEI is always higher than that after deposition of the previous PSS layer (Figure S6). Greater aqueous swelling of PSS-terminated films relative to PEI-terminated coatings likely causes more occlusion of membrane pores and, hence, lower flux when films end in PSS. This phenomenon only occurred when using PES as a support. Previous studies show that the support can affect the structure of the first few layers in polyelectrolyte films [42] and that swelling also depends on whether films end in a polycation or a polyanion [43]. Changes in swelling likely result from variations in the degree of ionic cross-linking within the film.
3.2 Polycation-terminated Films for Binding of Au Colloids
The deposition of polycation-terminated films in membranes creates anion-exchange materials because of the positively charged film surface. Similarly, films terminated with a polyanion function as cation-exchangers. In initial investigations of anion-exchange, we examined breakthrough curves for binding of citrate-stabilized Au colloids to polycation-terminated films in membranes. The Au colloids have a strong optical absorbance for their analysis, and previous studies show that binding of gold colloids to polyelectrolyte-coated membranes yields catalytic reactors [23, 30, 35].
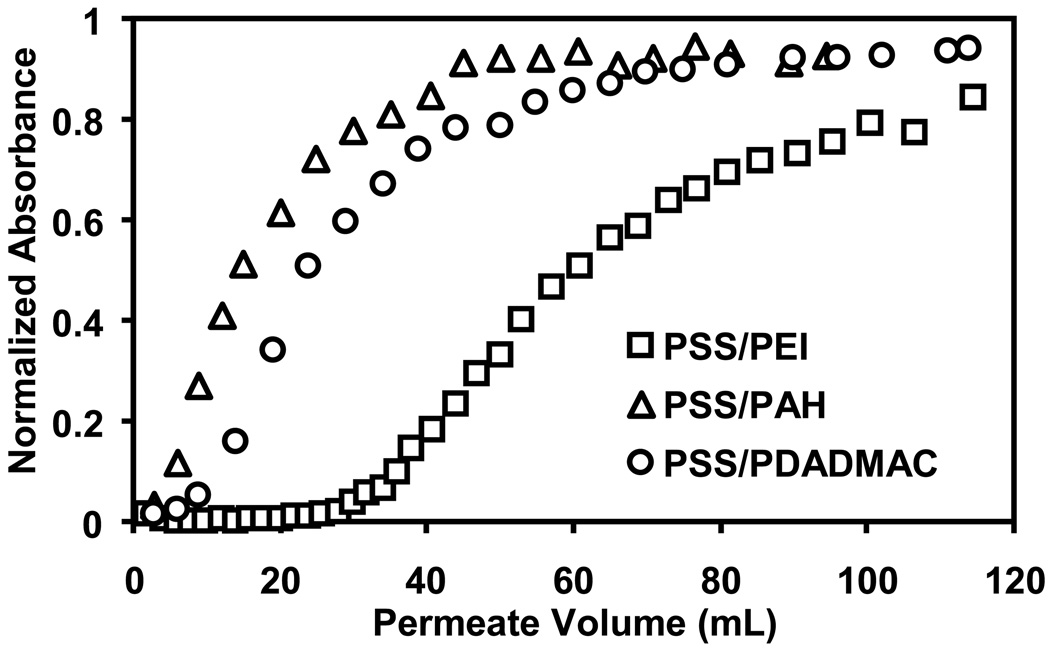
Figure 4 shows the breakthrough curves for passage of Au-colloid solutions through 5-µm nylon membranes coated with 1-bilayer PSS/PEI, PSS/PAH, and PSS/PDADMAC films. The PEI-containing film binds the most Au, and breakthrough is less than 10% for about 36 mL of suspension, or over 1400 membrane volumes. The binding capacity of these PEI-terminated films is 27 mg Au/mL of membrane at 80% breakthrough (defined as the capacity when the permeate concentration is 80% of the feed value) and 14 mg/mL for 10% breakthrough. The PSS/PAH and PSS/PDADMAC films give binding capacities of 8 and 12 mg/mL, respectively, for 80% breakthrough. The branched structure of the PEI may increase film thickness and binding capacity relative to coatings containing PAH or PDADMAC. Additionally, when fully protonated, PEI is also the polycation with the highest charge density. Deposition of PEI at pH 9 rather than 4, the pH used for the other polycations, could enhance the charge density in PSS/PEI films because these coatings will be more protonated at neutral pH than during PEI deposition.
Fig. 4.
Breakthrough curves for passage of Au-colloid solutions through 5-µm nylon membranes modified with 1-bilayer PSS/PEI, PSS/PAH, and PSS/PDADMAC films. Absorbance, which is normalized to the absorbance of the feed solution, is proportional to colloid concentration. The colloid feed solution contained 0.05 mM Au and was pumped through the membrane at a flux of 19 cm/h.
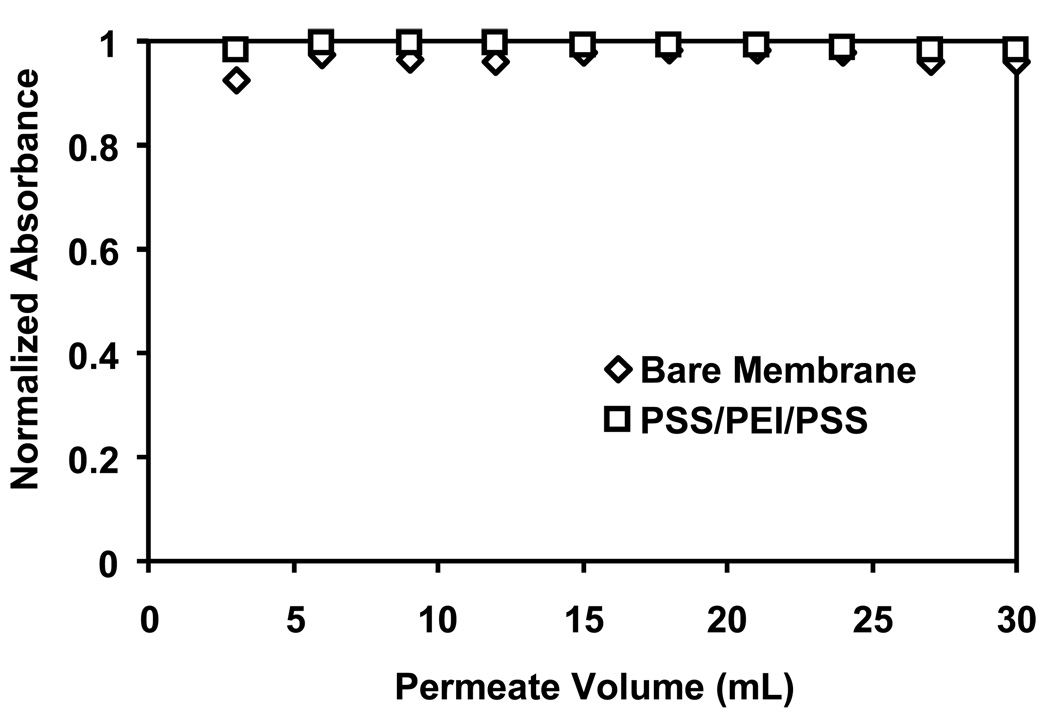
Figure 5 presents breakthrough curves for an unmodified nylon membrane and a membrane modified with a PSS/PEI/PSS film. These control experiments show almost no adsorption and demonstrate that Au-colloid binding to polycation-terminated films occurs via anion-exchange. Moreover, the minimal adsorption to the PSS/PEI/PSS film suggests that the cation-exchange occurs at the surface of the polyelectrolyte film and not in its interior.
Fig. 5.
Breakthrough curves for passage of Au-colloid solutions through an unmodified 5-µm nylon membrane and a membrane modified with a PSS/PEI/PSS film. The feed solution contained 0.05 mM Au and was pumped through the membranes at a flux of 19 cm/h.
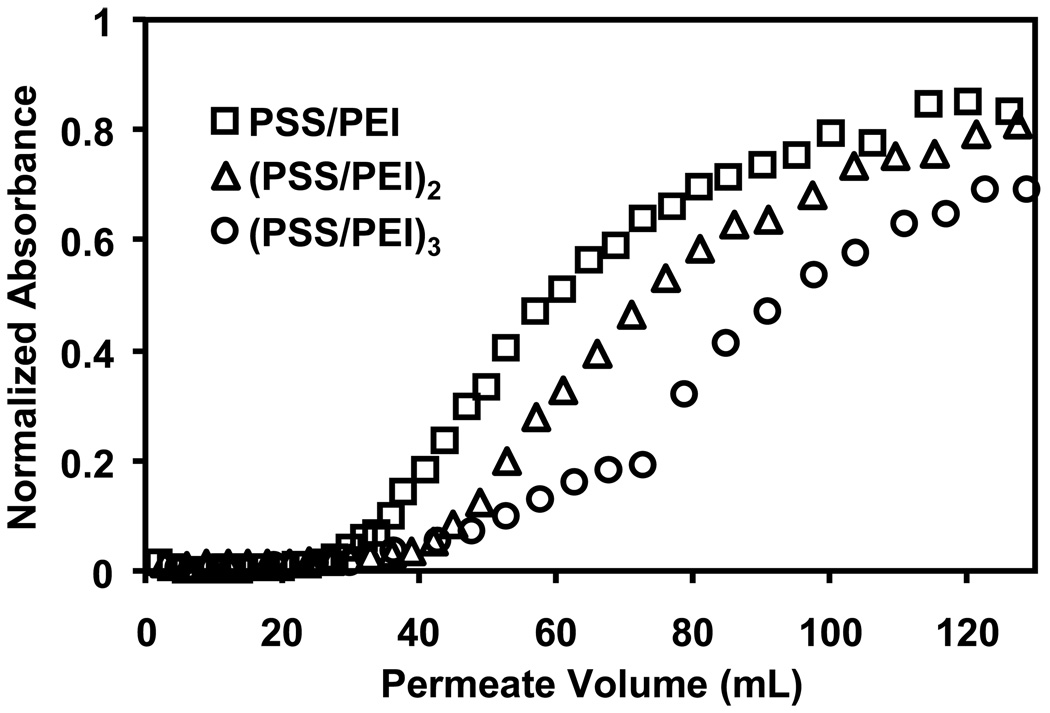
We also examined Au-colloid breakthrough curves for membranes modified with 2- and 3- bilayer films. Figure 6 shows a gradual increase in colloid binding with the addition of more PSS/PEI layers. Adsorption of a second PSS/PEI bilayer increases binding capacities by about 15%, whereas a third PSS/PEI bilayer improves capacity by an additional 20%. Deposition of the extra polyelectrolyte layers likely yields a more continuous film with increased surface charge and more ion-exchange sites. Nevertheless, although the 1-bilayer film has fewer ion-exchange sites than 2- and 3-bilayer films, the zeta potentials of PEI-terminated membranes do not vary significantly with the number of PSS/PEI bilayers (Figure S7). The majority of the anion-exchange sites may be compensated by counterions inside the plane of shear that defines the zeta potential.
Fig. 6.
Breakthrough curves for passage of Au-colloid solutions through 5-µm nylon membranes modified with 1, 2, and 3 bilayers of PSS/PEI. The feed solution contained 0.05 mM Au and was pumped through the membrane at a flux of 19 cm/h.
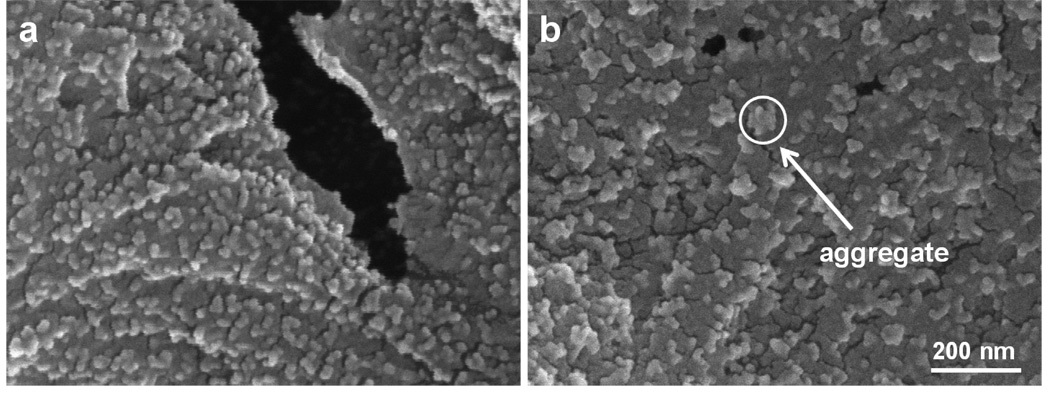
Nylon membranes modified with 1, 2, and 3 bilayers of PSS/PAH and PSS/PDADMAC also show increases in binding capacity with the addition of more bilayers (Figure S8). Table 1 shows gradual increases in binding capacities for all three types of films on going from 1 to 3 bilayers, although the binding capacity with PSS/PAH is not significantly different for 2-bilayer and 3-bilayer films. Interestingly, membranes coated with 3 bilayers of PSS/PDADMAC have the largest binding capacity, but the permeability of these membranes dramatically declines during binding of Au colloids, presumably because of pore clogging. (The peristaltic pump cannot maintain a 19 cm/h flux with these membranes even with an increase in rotation rate.) The clogging may result from aggregated Au nanoparticles that plug pores. SEM images show a uniform dispersion of Au nanoparticles on (PSS/PEI)3-coated membranes (Figure 7a) and significant aggregation of Au nanoparticles on (PSS/PDADMAC)3-coated membranes (Figure 7b). Compared with the other polyelectrolytes in this study, PSS/PDADMAC films are unique in that the film thickness increases nonlinearly with the number of adsorbed layers when deposition occurs at high ionic strength [44]. This phenomenon, which is sometimes termed exponential growth [45], occurs because newly deposited PDADMAC penetrates the entire film to create a highly swellable coating with a high density of anion-exchange sites [32, 46]. The swelling and high positive charge of PSS/PDADMAC films may allow the adsorption and aggregation of relatively large amounts of Au nanoparticles.
Table 1.
Au-colloid binding capacities of 5-µm nylon membranes modified with multilayer polyelectrolyte films. The Au-colloid feed solution contained 0.05 mM Au and was passed through the membrane at a flux of 19 cm/h. (Multiple numbers represent experiments with different membranes.)
| Polyelectrolyte Film |
aBinding Capacity at 80% Breakthrough (mg/mL) | ||
|---|---|---|---|
| 1 bilayer | 2 bilayers | 3 bilayers | |
| PSS/PEI | 26,28 | 31,31 | 30,39,40,42 |
| PSS/PAH | 9,6 | 14,23 | 14,25 |
| PSS/PDADMAC | 10,17 | 37,31 | 37,41,73a |
For these membranes, the capacity was determined at <80% breakthrough in two of three cases because these particular systems sometimes plug, or flux greatly decreases.
Fig. 7.
SEM images of Au nanoparticles adsorbed on 5-µm nylon membranes modified with 3 bilayers of (a) PSS/PEI and (b) PSS/PDADMAC. Both images were taken at 100,000 × magnification. The circle in the right image shows a nanoparticle aggregate formed on the PDADMAC-terminated film.
The presence of supporting electrolyte during the deposition of multilayer polyelectrolyte films significantly alters the structures of these films because the salt screens charge on the polyelectrolytes to create more loops and tails in the film [47]. Typically, higher ionic strengths during film deposition give rise to a higher surface charge [44], which might increase binding capacity. To examine the effect of deposition ionic strength on the binding of Au colloids, we modified several 5-µm nylon membranes with 3 PSS/PEI bilayers and varied the NaCl concentrations in the solutions used to deposit the last layer of PEI. Breakthrough curves for Au-colloid binding are essentially independent of the salt concentration (0.5 to 2 M) used in the deposition of the outer PEI layer (Figure S9). Thus, at least for this polyelectrolyte system, deposition of the terminal layer from a solution of high ionic strength does not increase the number of binding sites for gold colloids.
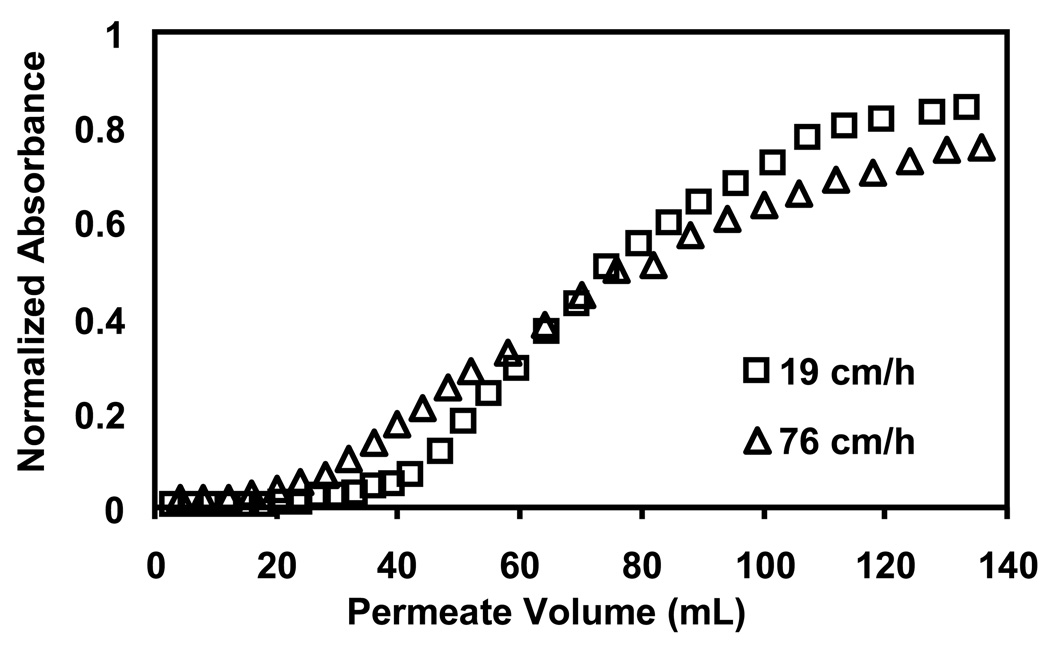
To briefly examine binding as a function of residence time in the membrane, the Au-colloid suspension was passed through modified membranes at fluxes of 19 cm/h and 76 cm/h. As Figure 8 shows, the higher flux gives rise to an earlier breakthrough in 5-µm nylon membranes modified with (PSS/PEI)3 films. Similar trends occur with membranes coated with 1 and 2 bilayers of PSS/PEI (Figure S10). Thus, the binding in these systems is not simply limited by mass flow into the membrane. However, modeling of colloid binding in this complicated membrane internal structure is beyond the scope of this work.
Fig. 8.
Breakthrough curves for passage of a Au-colloid solution through 5-µm nylon membranes modified with (PSS/PEI)3 films. The solution, which contained 0.05 mM Au, was passed through the membranes at fluxes of 19 cm/h and 76 cm/h.
Even for the same polyelectrolyte film, both the composition and internal surface area of the substrate membrane should influence binding capacity. The membrane chemistry may affect the structure and amount of polyelectrolyte deposited, whereas increased pore surface areas should lead to enhanced binding. Table 2 presents Aunanoparticle binding capacities for (PSS/PEI)3 films in 5-µm nylon, 5-µm PES, 5-µm PVDF, and 1-µm regenerated cellulose membranes. Although the modified 5-µm PES and nylon membranes bind similar amounts of Au, the nylon membrane shows slightly higher binding capacities at 80% breakthrough because its thickness (80 µm, as determined from SEM cross-sectional images) is less than that of PES membranes (135 µm). The PVDF and RC membranes exhibit much lower binding capacities than the other membranes, perhaps in part because the chemical structures of the PVDF and RC membranes resist polyelectrolyte adsorption to give thinner or less uniform films that bind fewer gold particles. More importantly, the low internal surface areas of PVDF and RC membranes probably also decrease binding capacity, The surface areas per membrane volume calculated from BET isotherms are 3.3, 2.8, 1.9, and 1.6 m2/mL for nylon, PES, PVDF, and RC membranes, respectively. SEM images of the different membranes (Figure S11) suggest that the PVDF and RC membranes are much more porous than the nylon and PES substrates, which likely explains the low surface areas.
Table 2.
Au-colloid binding capacities of 5-µm nylon, PES, and PVDF, and 1 µm RC membranes modified with (PSS/PEI)3 films. The binding occurred at a flux of 19 cm/h for a colloid solution containing 0.05 mM Au atoms.
| Breakthrough Level |
Breakthrough Binding Capacity (mg/mL) | |||
|---|---|---|---|---|
| Nylon | PES | PVDF | RC | |
| 80% | 37±6 | 29±4 | 5±4 | 8±2 |
| 10% | 20±3 | 22±5 | ≤1 | ≤2 |
3.3 Cation-Exchange Membranes for Lysozyme Binding
3.3.1 Lysozyme Binding Properties
Deposition of polyanion-terminated films in membrane pores should create cation-exchange sites that bind positively charged proteins. Lysozyme has an isoelectric point of 10.7, and in a 20 mM phosphate buffer at pH 7.2, this protein is highly positively charged (+8) [48] and should bind strongly to polyanions via ionic interactions. In initial experiments, we compared the lysozyme binding capacities of membranes terminated with PSS and PAA. Membranes modified with PSS/PEI/PSS and PSS/PEI/PAA films show distinct binding profiles (Figure S12). For the PSS-terminated membranes, breakthrough occurs quickly, whereas PAA-capped films show a more gradual saturation. The binding capacity of PSS/PEI/PAA-modified membranes at 80% breakthrough is 14 mg/mL, whereas that for PSS/PEI/PSS films is 11 mg/mL.
The charge density of the polycation in the film might also affect the surface charge of polyanion-terminated films and, hence, the binding capacity of membranes modified with such films. However, the lysozyme breakthrough curves for (PSS/PEI)3/PSS, (PSS/PAH)3/PSS, and (PSS/PDADMAC)3/PSS films in nylon membranes are all similar (Figure S13). Thus, the polycation has little effect on binding to these polyanion-terminated films.
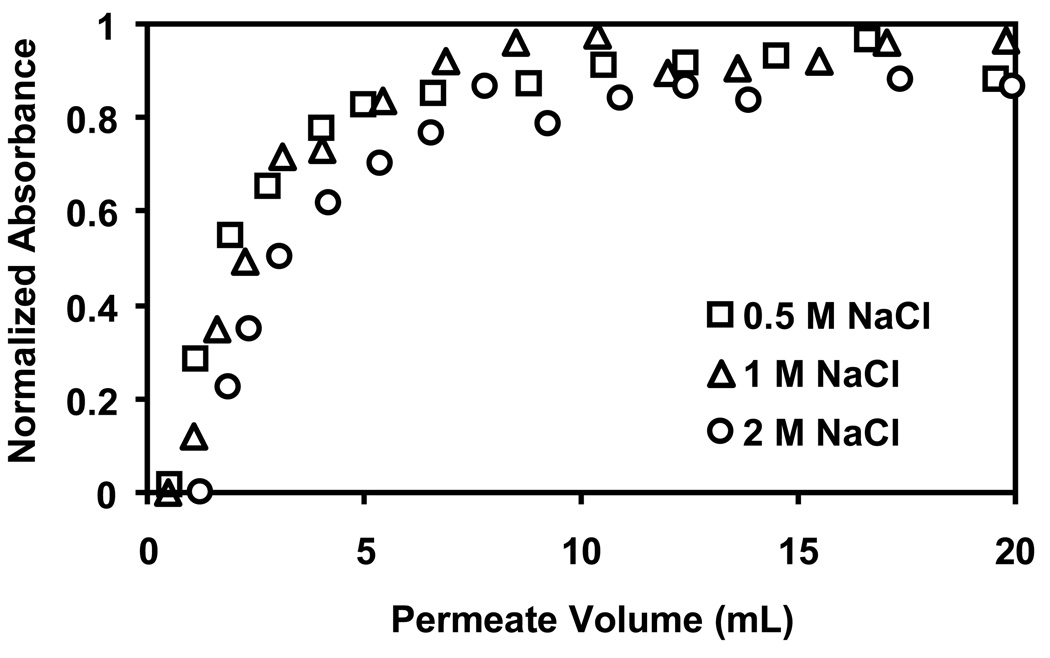
In contrast, Figure 9 shows that the ionic strength of the solution used to deposit the terminating PSS layer significantly affects lysozyme binding. PSS-terminated membranes prepared using 2 M NaCl in the last PSS deposition solution exhibit capacities that are about twice those for films deposited from solutions containing only 0.5 M NaCl. The salt concentration evidently affects the number of ion-exchanges sites more when depositing PSS than PEI, as binding of gold colloids to PEI-terminated films shows little dependence on the ionic strength of the deposition solution for the last layer (see Figure S9).
Fig. 9.
Breakthrough curves for binding of lysozyme to 5-µm nylon membranes modified with (PSS/PEI)3/PSS films. The solutions employed for deposition of the last layer of PSS contained 0.5 M, 1 M, or 2 M NaCl, and the lysozyme feed solution (0.1 mg/mL, pH 7.2) was passed through the membrane at 19 cm/h.
As with binding of Au colloids to PSS/PEI films, lysozyme binding increases modestly upon going from (PSS/PEI)/PSS to (PSS/PEI)2/PSS and (PSS/PEI)3/PSS films in membranes (Figure S14). The binding capacities at 80% breakthrough are 11, 13, and 16 mg/mL for membranes containing (PSS/PEI)/PSS, (PSS/PEI)2/PSS, and (PSS/PEI)3/PSS films, respectively. This lysozyme-binding capacity of membranes containing (PSS/PEI)3PSS is 34–73% of the reported static lysozyme binding capacities of commercial ion-exchange membranes [49, 50]. Although the static binding capacities of the polyelectrolyte-modified membranes may be somewhat higher than those at 80% breakthrough, they will probably still be less than those of optimized commercial membranes. Nevertheless, the high permeabilities of polyelectrolyte-modified membranes, especially relative to commercial membranes with the highest binding capacities, and the simplicity of polyelectrolyte adsorption may make this modification technique attractive for creating disposable filters.
Notably, breakthrough curves for a bare nylon membrane and a nylon membrane modified with a PSS/PEI film show very low lysozyme binding (Figure S15). More importantly, nylon membranes modified with (PSS/PEI)3/PSS films bind very little bovine serum albumin (BSA) when passing a 0.1 mg/mL solution of BSA through the membrane (Figure S16), presumably because BSA is negatively charged at neutral pH. The low BSA binding of these membranes and the lack of lysozyme binding to polycation-terminated films are consistent with lysozyme adsorption to the membrane through a cation-exchange mechanism. Gel electrophoresis shows that passage of a solution containing both BSA and lysozyme through a membrane modified with (PSS/PEI)3/PSS selectively removes lysozyme from the solution (Figure S17).
After protein binding, we made several attempts to elute the bound lysozyme by passing buffers such as 1 M KSCN in 50 mM phosphate at pH 8 and 2 M NaCl in 50 mM phosphate at pH 10 through the membrane. Unfortunately, these attempts recovered less than 2% of the bound protein. The multiple electrostatic interactions between lysozyme and the polyelectrolyte and perhaps other non-electrostatic interactions will likely require stronger eluents such as higher pH (>10.5) solutions for recovery of the lysozyme. Elution of gold nanoparticles from the anion-exchange membranes was similarly unsuccessful. Thus, these membranes may be most suitable for removing contaminant proteins, viruses, and DNA from solutions, because in those cases elution is not needed for single-use membranes.
3.3.2 Membrane Stacking
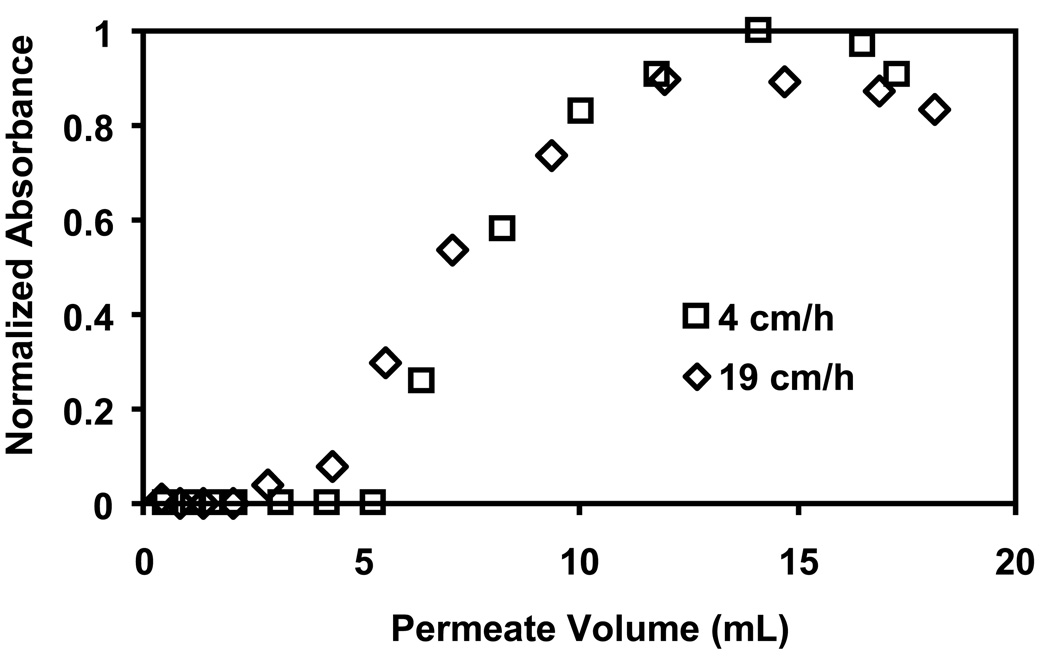
The above results show that the greatest amount of lysozyme binding occurs with 5-µm nylon membranes coated with (PSS/PEI)3/PSS films prepared with 2 M NaCl present during deposition of the last layer of PSS. However, a single membrane gives a 10% breakthrough capacity of only 6 mg/mL of membrane. Nevertheless, because these membranes are highly permeable, stacking of membranes can increase the capacity while still maintaining low pressure drops [51]. In initial studies, we modified three stacked membranes at once and examined the immobilization of lysozyme in these systems. The 3-membrane stack binds 0.43 mg lysozyme (5.7 mg/mL of total membrane) at 10% breakthrough, which is essentially 3 times the amount of lysozyme that binds to a single membrane (0.15 mg). Thus, stacking has no deleterious effect on the performance of individual membranes. Figure 10 shows breakthrough curves for passage of a lysozyme solution through a stack of 3 membranes at fluxes of 19 and 4 cm/h. The dynamic capacity (10% breakthrough) increases from 5.7 to 7.5 mg/mL on decreasing the flux. Thus, lowering the flux 5-fold improves the binding only to a small extent. Diffusion and kinetic limitations to binding are present, but they appear to be relatively small.
Fig. 10.
Breakthrough curves for binding of lysozyme to membrane stacks. A stack of three 5-µm nylon membranes was modified with 3.5 bilayers of PSS/PEI, and the solution employed for deposition of the last layer of PSS contained 2 M NaCl. The 0.1 mg/mL lysozyme feed solution (pH 7.2) was passed through the membrane stacks at 4 and 19 cm/h.
4. Conclusions
LbL polyelectrolyte deposition in porous substrates provides a simple method to prepare ion-exchange membranes. Increases in the intensity of sulfonate peaks in ATR-FTIR spectra and small declines in pure water flux after deposition of each layer provide evidence for the immobilization of polyelectrolytes, but even after adsorption of 3 bilayers, the membrane permeability is 80% of that through a bare membrane. Thus, separations with these materials can occur at low pressures. In the case of anion-exchange, PSS/PEI films typically bind more Au colloids than either PSS/PAH or PSS/PDADMAC coatings. For 5-µm nylon membranes modified with (PSS/PEI)3 films, the Au-colloid binding capacity at 80% breakthrough is 37±6 mg/mL. Among the polymer membranes examined, 5-µm nylon substrates give the highest binding capacities due to their large internal surface area. In the case of PSS-terminated cation-exchange membranes, increasing the supporting electrolyte concentration in the terminal PSS deposition solution from 0.5 to 2 M increases lysozyme binding capacity by 100%, and the high permeability of polyelectrolyte-modified membranes permits stacking of membranes to improve binding capacity. A 3-membrane stack provides the expected 3-fold increase in binding capacity.
Supplementary Material
Acknowledgement
We thank the US National Institutes of Health (GM080511) and the National Science Foundation (OIS 0530174) for funding this work, and Sebastian Grajales for helping with adsorption isotherms.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Appendix A. Supplementary data
Supplementary data associated with this article can be found in the online version at …
References
- 1.Ghosh R. Protein separation using membrane chromatography: Opportunities and challenges. J. Chromatogr. A. 2002;952:13–27. doi: 10.1016/s0021-9673(02)00057-2. [DOI] [PubMed] [Google Scholar]
- 2.Kawai T, Saito K, Lee W. Protein binding to polymer brush, based on ion-exchange, hydrophobic, and affinity interactions. J. Chromatogr. B. 2003;790:131–142. doi: 10.1016/s1570-0232(03)00090-4. [DOI] [PubMed] [Google Scholar]
- 3.Bhut BV, Husson SM. Dramatic performance improvement of weak anion-exchange membranes for chromatographic bioseparations. J. Membr. Sci. 2009;337:215–223. [Google Scholar]
- 4.Ghosh R. Separation of proteins using hydrophobic interaction membrane chromatography. J. Chromatogr. A. 2001;923:59–64. doi: 10.1016/s0021-9673(01)00956-6. [DOI] [PubMed] [Google Scholar]
- 5.Singh N, Wang J, Ulbricht M, Wickramasinghe SR, Husson SM. Surface-initiated atom transfer radical polymerization: A new method for preparation of polymeric membrane adsorbers. J. Membr. Sci. 2008;309:64–72. [Google Scholar]
- 6.Jungbauer A, Hahn R. Monoliths for fast bioseparation and bioconversion and their applications in biotechnology. J. Sep. Sci. 2004;27:767–778. doi: 10.1002/jssc.200401812. [DOI] [PubMed] [Google Scholar]
- 7.Saxena A, Tripathi BP, Kumar M, Shahi VK. Membrane-based techniques for the separation and purification of proteins: An overview. Adv. Colloid Interface Sci. 2009;145:1–22. doi: 10.1016/j.cis.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 8.Knudsen HL, Fahrner RL, Xu Y, Norling LA, Blank GS. Membrane ion-exchange chromatography for process-scale antibody purification. J. Chromatogr. A. 2001;907:145–154. doi: 10.1016/s0021-9673(00)01041-4. [DOI] [PubMed] [Google Scholar]
- 9.Lightfoot EN, Moscariello JS. Bioseparations. Biotechnol. Bioeng. 2004;87:259–273. doi: 10.1002/bit.20111. [DOI] [PubMed] [Google Scholar]
- 10.Thommes J, Etzel M. Alternatives to chromatographic separations. Biotechnol. Progr. 2007;23:42–45. doi: 10.1021/bp0603661. [DOI] [PubMed] [Google Scholar]
- 11.Zhou JX, Tressel T, Gottschalk U, Solamo F, Pastor A, Dermawan S, Hong T, Reif O, Mora J, Hutchison F, Murphy M. New Q membrane scale-down model for process-scale antibody purification. J. Chromatogr. A. 2006;1134:66–73. doi: 10.1016/j.chroma.2006.08.064. [DOI] [PubMed] [Google Scholar]
- 12.Specht R, Han B, Wickramasinghe SR, Carlson JO, Czermak P, Wolf A, Reif O-W. Densonucleosis virus purification by ion exchange membranes. Biotechnol. Bioeng. 2004;88:465–473. doi: 10.1002/bit.20270. [DOI] [PubMed] [Google Scholar]
- 13.Xianfang Z, Ruckenstein E. Membrane chromatography: Preparation and applications to protein separation. Biotechnol. Progr. 1999;15:1003–1019. doi: 10.1021/bp990120e. [DOI] [PubMed] [Google Scholar]
- 14.Ulbricht M. Advanced functional polymer membranes. Polymer. 2006;47:2217–2262. [Google Scholar]
- 15.Mika AM, Childs RF, Dickson JM, McCarry BE, Gagnon DR. Porous, polyelectrolyte-filled membranes: Effect of cross-linking on flux and separation. J. Membr. Sci. 1997;135:81–92. [Google Scholar]
- 16.Ulbricht M, Yang H. Porous polypropylene membranes with different carboxyl polymer brush layers for reversible protein binding via surface-initiated graft copolymerization. Chem. Mater. 2005;17:2622–2631. [Google Scholar]
- 17.Singh N, Husson SM, Zdyrko B, Luzinov I. Surface modification of microporous PVDF membranes by ATRP. J. Membr. Sci. 2005;262:81–90. [Google Scholar]
- 18.Sun L, Dai J, Baker GL, Bruening ML. High-capacity, protein-binding membranes based on polymer brushes grown in porous substrates. Chem. Mater. 2006;18:4033–4039. [Google Scholar]
- 19.Bhut BV, Wickramasinghe SR, Husson SM. Preparation of high-capacity, weak anion-exchange membranes for protein separations using surface-initiated atom transfer radical polymerization. J. Membr. Sci. 2008;325:176–183. [Google Scholar]
- 20.Decher G. Fuzzy nanoassemblies: Toward layered polymeric multicomposites. Science. 1997;277:1232–1237. [Google Scholar]
- 21.Krasemann L, Tieke B. Selective ion transport across self-assembled alternating multilayers of cationic and anionic polyelectrolytes. Langmuir. 2000;16:287–290. [Google Scholar]
- 22.Jin W, Toutianoush A, Tieke B. Use of polyelectrolyte layer-by-layer assemblies as nanofiltration and reverse osmosis membranes. Langmuir. 2003;19:2550–2553. [Google Scholar]
- 23.Bruening ML, Dotzauer DM, Jain P, Ouyang L, Baker GL. Creation of functional membranes using polyelectrolyte multilayers and polymer brushes. Langmuir. 2008;24:7663–7673. doi: 10.1021/la800179z. [DOI] [PubMed] [Google Scholar]
- 24.Hong SU, Malaisamy R, Bruening ML. Separation of fluoride from other monovalent anions using multilayer polyelectrolyte nanofiltration membranes. Langmuir. 2007;23:1716–1722. doi: 10.1021/la061701y. [DOI] [PubMed] [Google Scholar]
- 25.Malaisamy R, Bruening ML. High-flux nanofiltration membranes prepared by adsorption of multilayer polyelectrolyte membranes on polymeric supports. Langmuir. 2005;21:10587–10592. doi: 10.1021/la051669s. [DOI] [PubMed] [Google Scholar]
- 26.Liang Z, Susha AS, Yu A, Caruso F. Nanotubes prepared by layer-by-layer coating of porous membrane templates. Adv. Mater. 2003;15:1849–1853. [Google Scholar]
- 27.Ai S, Lu G, He Q, Li J. Highly flexible polyelectrolyte nanotubes. J. Am. Chem. Soc. 2003;125:11140–11141. doi: 10.1021/ja0356378. [DOI] [PubMed] [Google Scholar]
- 28.Datta S, Cecil C, Bhattacharyya D. Functionalized membranes by layer-by-layer assembly of polyelectrolytes and in situ polymerization of acrylic acid for applications in enzymatic catalysis. Ind. Eng. Chem. Res. 2008;47:4586–4597. doi: 10.1021/ie800142d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smuleac V, Butterfield DA, Bhattacharyya D. Layer-by-layer-assembled microfiltration membranes for biomolecule immobilization and enzymatic catalysis. Langmuir. 2006;22:10118–10124. doi: 10.1021/la061124d. [DOI] [PubMed] [Google Scholar]
- 30.Dotzauer DM, Dai J, Sun L, Bruening ML. Catalytic membranes prepared using layer-by-layer adsorption of polyelectrolyte/metal nanoparticle films in porous supports. Nano Lett. 2006;6:2268–2272. doi: 10.1021/nl061700q. [DOI] [PubMed] [Google Scholar]
- 31.Dai J, Baker GL, Bruening ML. Use of porous membranes modified with polyelectrolyte multilayers as substrates for protein arrays with low nonspecific adsorption. Anal. Chem. 2005;78:135–140. doi: 10.1021/ac0513966. [DOI] [PubMed] [Google Scholar]
- 32.Adusumilli M, Bruening ML. Variation of ion-exchange capacity, ζ potential, and ion-transport selectivities with the number of layers in a multilayer polyelectrolyte film. Langmuir. 2009;25:7478–7485. doi: 10.1021/la900391q. [DOI] [PubMed] [Google Scholar]
- 33.Ouyang L, Malaisamy R, Bruening ML. Multilayer polyelectrolyte films as nanofiltration membranes for separating monovalent and divalent cations. J. Membr. Sci. 2008;310:76–84. [Google Scholar]
- 34.Grabar KC, Freeman RG, Hommer MB, Natan MJ. Preparation and characterization of Au colloid monolayers. Anal. Chem. 1995;67:735–743. [Google Scholar]
- 35.Dotzauer DM, Bhattacharjee S, Wen Y, Bruening ML. Nanoparticle-containing membranes for the catalytic reduction of nitroaromatic compounds. Langmuir. 2009;25:1865–1871. doi: 10.1021/la803220z. [DOI] [PubMed] [Google Scholar]
- 36.Kim KJ, Fane AG, Nystrom M, Pihlajamaki A, Bowen WR, Mukhtar H. Evaluation of electroosmosis and streaming potential for measurement of electric charges of polymeric membranes. J. Membr. Sci. 1996;116:149–159. [Google Scholar]
- 37.Okamura Y, Gotoh K, Kosaka M, Tagawa M. Capillary wetting rates in nylon fibrous assemblies. J. Adhesion Sci. Technol. 1998;12:639–654. [Google Scholar]
- 38.Werner C, König U, Augsburg A, Arnhold C, Körber H, Zimmerman R, Jacobasch H-J. Electrokinetic surface characterization of biomedical polymers - a survey. Colloids & Surfaces A. 1999;159:519–529. [Google Scholar]
- 39.Pontié M, Chasseray X, Lemordant D, Lainé JM. The streaming potential method for the characterization of ultrafiltration organic membranes and the control of cleaning treatments. J. Membr. Sci. 1997;129:125–133. [Google Scholar]
- 40.Sartobind® membrane adsorbers for rapid purification of proteins. [accessed January 18, 2010]; http://www.sartorius.hr/pdf/bio/kromatografija/Ionski_izmjenjivaci.pdf.
- 41.Acrodisc® units with mustang® Q membranes. [accessed January 18, 2010]; http://labfilters.pall.com/catalog/laboratory_19993.asp.
- 42.Kolasinska M, Warszynski P. The effect of support material and conditioning on wettability of PAH/PSS multilayer films. Bioelectrochemistry. 2005;66:65–70. doi: 10.1016/j.bioelechem.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 43.Miller MD, Bruening ML. Correlation of the swelling and permeability of polyelectrolyte multilayer films. Chem. Mater. 2005;17:5375–5381. [Google Scholar]
- 44.Schlenoff JB, Dubas ST. Mechanism of polyelectrolyte multilayer growth: Charge overcompensation and distribution. Macromolecules. 2001;34:592–598. [Google Scholar]
- 45.Lavalle P, Gergely C, Cuisinier FJG, Decher G, Schaaf P, Voegel JC, Picart C. Comparison of the structure of polyelectrolyte multilayer films exhibiting a linear and an exponential growth regime: An in situ atomic force microscopy study. Macromolecules. 2002;35:4458–4465. [Google Scholar]
- 46.Miller MD, Bruening ML. Controlling the nanofiltration properties of multilayer polyelectrolyte membranes through variation of film composition. Langmuir. 2004;20:11545–11551. doi: 10.1021/la0479859. [DOI] [PubMed] [Google Scholar]
- 47.McAloney RA, Sinyor M, Dudnik V, Goh MC. Atomic force microscopy studies of salt effects on polyelectrolyte multilayer film morphology. Langmuir. 2001;17:6655–6663. [Google Scholar]
- 48.Bergers JJ, Vingerhoeds MH, van Bloois L, Herron JN, Janssen LHM, Fischer MJE, Crommelin DJA. The role of protein charge in protein-lipid pH-dependent changes of the electrophoretic mobility of liposomes through adsorption of water-soluble interactions globular proteins. Biochemistry. 2002;32:4641–4649. doi: 10.1021/bi00068a023. [DOI] [PubMed] [Google Scholar]
- 49.Wang J, Dismer F, Hubbuch J, Ulbricht M. Detailed analysis of membrane adsorber pore structure and protein binding by advanced microscopy. J. Membr. Sci. 2008;320:456–467. [Google Scholar]
- 50.Purification on an acrodisc® unit with mustang® S membrane 4.1.3. [accessed January 18, 2010]; http://www.pall.com/laboratory_49524.asp.
- 51.Lin S-Y, Suen S-Y. Protein separation using plate-and-frame modules with ion-exchange membranes. J. Membr. Sci. 2002;204:37–51. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.