Abstract
Purpose of review
Hypertension and edema are clinical manifestations of the extracellular volume expansion generated by abnormal renal sodium handling. Perturbations in epithelial sodium channel (ENaC) activity disrupt volume homeostasis. ENaC activity can be enhanced by proteases that cleave its long extracellular domains. Recent evidence suggests that this mechanism may be involved in individuals with volume overload and proteinuria.
Recent findings
Several observations indicate a link between proteinuria and hypertension, with proteinuria preceding and predicting the onset of incident hypertension in some individuals. Recently, enhanced cleavage of ENaC's extracellular loops was identified in kidney tissue of proteinuric mice. Plasmin, a serine protease known for its role in fibrinolysis, has been implicated as an activator of ENaC in proteinuric states as (i) nephrotic urine activates ENaC expressed in a mouse collecting duct cell line, (ii) aprotinin-affinity precipitation of nephrotic urine abolishes its ability to activate ENaC, (iii) plasmin is a major component within aprotinin-affinity purified nephrotic urine and is absent in non-proteinuric urine, and (iv) plasmin activates ENaC by cleaving the extracellular loop of its γ subunit.
Summary
Enhancement of ENaC activity by proteases represents a likely mechanism for extracellular volume overload relevant to some individuals with proteinuria. Proteases not normally found in the urine can enter the urinary space across damaged glomeruli and activate ENaC. Further understanding of this mechanism may guide targeted therapeutics in individuals with proteinuria, edema, and hypertension.
Keywords: epithelial sodium channel, proteinuria, plasmin, plasminogen, kidney tubules
Introduction
Expansion of extracellular volume develops in proteinuric diseases, such as the nephrotic syndrome, and can manifest clinically as edema and hypertension. In the aldosterone-sensitive distal nephron, the epithelial sodium channel (ENaC) provides fine control of salt and fluid reabsorption. Derangements in ENaC activity affect blood pressure and extracellular volume (1-4). It is now clear that extracellular proteases enhance ENaC activity (5), a mechanism that is likely relevant to proteinuric states where proteases not normally found in urine can enter the urinary space across damaged glomeruli. In this review, we examine how the protease plasmin contributes to volume expansion in proteinuric states through aberrant proteolytic activation of ENaC.
Proteinuria and volume expansion
Proteinuria appears to be more than just a marker of target end organ damage. Proteinuria is generally considered abnormal if urinary losses are greater than 150-200 mg of protein per day (6). Albuminuria, a surrogate marker for proteinuria, is considered abnormal in quantities >30mg per day (7). Proteinuria not only reflects glomerular damage, but also functions as a risk factor for cardiovascular disease, stroke, and end stage renal disease (8-10). Proteinuria has been associated with extracellular volume expansion and high blood pressure. Proteinuria correlates with elevation in blood pressure in multiple disparate populations such as male US army veterans with chronic kidney disease and pre-hypertensive men and women from Korea (11, 12). The relationship between proteinuria and blood pressure is complicated as hypertension can cause renal damage resulting in worsened proteinuria, and the development of essential hypertension does not require pre-existing proteinuria (13-15). However, multiple recent studies have examined the role of proteinuria as a risk factor for the development of elevated blood pressure.
A study involving normotensive adult men and women from Okinawa found the annual frequency for development of hypertension to be 2.4 fold higher if the patient had non-nephrotic proteinuria at baseline (16). Examination of nine potential biomarkers for hypertension risk in the normotensive, healthy male and female offspring of the Framingham Heart study participants found that urinary albumin-creatinine ratio, a marker of proteinuria, determined from a single void morning urine sample predicted the development of hypertension with an odds ratio of 1.21 (17). A type 1 diabetic population was also found to have increased risk of incident hypertension associated with albumin excretion rate (18). Another study found that higher levels of urinary albumin, despite being considered within the normal range, predicted incident hypertension in a population of healthy non-diabetic female nurses (7). Thus, proteinuria can predict the onset of incident hypertension in multiple different populations of normotensive individuals. How does proteinuria contribute to the pathogenesis of extracellular volume expansion and hypertension? Recent studies suggest that in some individuals with glomerular damage, proteases not normally found in urine enter the urinary space and aberrantly cleave ENaC (19-21). The proteolytic activation of ENaC would generate a primary defect in renal sodium handling, a mechanism that may be especially important in the development of the volume overload that accompanies the nephrotic syndrome.
Nephrotic syndrome is characterized by edema as well as hyperlipidemia, hypoalbuminemia, and massive proteinuria (22, 23). Hypertension often accompanies this disorder. The pathogenesis of volume expansion and edema formation in the setting of massive proteinuria remains undefined, but one leading theory often described as the “overfill hypothesis” suggests that volume overload is generated by a primary defect in renal sodium handling (3, 24). Evidence that supports this theory includes (i) the lack of plasma volume depletion in many nephrotic patients, (ii) suppression of components of the renin-angiotensin-aldosterone system during times of avid sodium retention in many nephrotic individuals, and (iii) the observed sodium retention only on the affected side of a unilateral nephrotic rat model (25-27). Enhanced ENaC activity by extracellular proteases may be a key event that creates the primary defect in sodium handling. However, not all patients with nephrotic syndrome fit this “overfill hypothesis” model. Up to 33% of nephrotic patients exhibit reduced plasma volume (28). Also, serum catecholamines, renin, and arginine vasopressin are increased in some nephrotic individuals (28). Enhanced ENaC activity by extracellular proteases may be one mechanism among many that generates extracellular volume overload. The ability to identify individuals with proteinuria, volume expansion, and enhanced ENaC activation would provide a population that might respond to targeted therapy with ENaC-specific inhibitors, like amiloride.
The kidney plays an important role in the generation of extracellular volume expansion and hypertension as sodium balance has a profound impact on extracellular fluid volume and blood pressure. Several hypertensive disease states involving ENaC implicate this channel as a vital part of renal sodium handling. Liddle's syndrome, characterized by hypertension, hypokalemia, and metabolic alkalosis, involves a mutation in ENaC that prevents retrieval of the channel from the apical membrane (29-32). In hyperaldosteronism, overexpression of the volume regulatory hormone aldosterone leads to increased ENaC activity in principal cells and subsequently hypertension (33-35). ENaC activity can also be enhanced by extracellular proteases that cleave its large extracellular loops (5). Aberrant proteolytic activation of ENaC is a likely mechanism responsible for the volume expansion seen in some proteinuric disease states.
Proteolytic activation of ENaC
ENaC is composed of three structurally related subunits (α, β, and γ) that contain intracellular N- and C- termini, two transmembrane spanning domains, and a large extracellular loop (36, 37). Enhanced ENaC activation by proteases was first observed by Chraibi et al. when they applied the serine proteases trypsin and chymotrypsin to solutions bathing cells expressing ENaC (38). Vuagniaux et al. subsequently identified a family of serine proteases that can stimulate ENaC currents when co-expressed in Xenopus oocytes, and designated them channel activating proteases (CAPs) (39). More recently, several proteases, including furin, prostasin (CAP1), TMPRSS4 (CAP2), plasmin, neutrophil elastase, pancreatic elastase, and kallikrein, have been implicated in cleavage and activation of ENaC (20, 39-45).
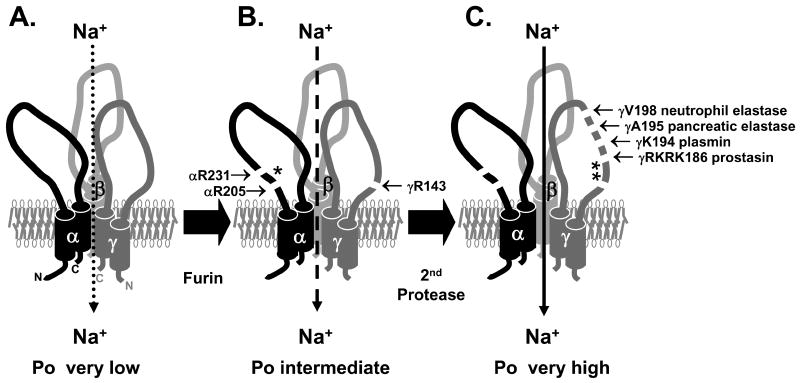
Proteolytic activation of ENaC involves double cleavage events in the long extracellular loops of the channel's α and γ subunits to liberate inhibitory domains (Figure 1). Furin, a proprotein convertase residing primarily in the trans-Golgi network, cleaves the channel during channel maturation in the biosynthetic pathway (51). Furin cleaves the α subunit twice flanking a 26 residue inhibitory region (44, 52). Electrophysiologic experiments using the Xenopus oocyte expression system confirmed this phenomenon. Channels with α subunits that contained mutated furin-consensus cleavage sites were not cleaved by furin and had significantly decreased activity compared to wild type channels (44, 52). Furthermore, channels with α subunits containing furin-consensus cleavage site mutations and a simultaneous deletion of the intervening 26 amino acid tract were not cleaved, but exhibited activity similar to processed/cleaved wild type channels (52). A synthetic peptide corresponding to the 26-mer inhibitory domain of α, when applied externally to wild type channels in mouse cortical collecting ducts and human airway epithelial cells, inhibited these channels (52).
Figure 1. ENaC is activated by proteolytic cleavage and release of inhibitory peptides.
(A) Non-cleaved trimers of αβγENaC likely represent near-silent channels found on the cell surface with Po<0.1 (46-49). (B) αβγENaC is normally cleaved by furin as it transits the biosynthetic pathway and exhibits an intermediate Po (50). Furin cleaves α twice to release a 26-mer inhibitory peptide (*) and γ is cleaved once (51, 52). (C) Subsequent cleavage of the γ subunit at a site distal to the furin cleavage site, releases a second inhibitory peptide (**) and increases the Po to near 1 (46, 50, 53). Several proteases cleave γ at this distal site (20, 40, 50). For review, see (1, 5).
The processing of the γ subunit involves two important differences compared to α processing: (i) the γ subunit is only cleaved once by furin and thus requires a second protease to cleave and release an inhibitory peptide and (ii) cleavage of the γ subunit is dominant over α cleavage in activating the channels such that channels that have only γ doubly cleaved are nearly fully active (51, 54). Prostasin, designated by Vuagniaux et al. as CAP1, a GPI anchored serine protease found in renal epithelia, can provide the second cleavage event distal to the furin site in the γ subunit (39, 50). Wild type channels co-expressed with prostasin in Xenopus oocytes were fully activated. Prostasin was found to cleave at a site 43 residues distal to the furin site in γ, and mutation of this site prevented channels from being cleaved and activated by prostasin (50). A synthetic peptide corresponding to the 43-mer inhibitory domain, when applied externally to wild type channels in mouse cortical collecting ducts and human airway epithelial cells, inhibits these channels with an IC50 of 2-3 μM (50). Channels that possess a mutation at the γ furin consensus cleavage site and deletion of the intervening amino acid tract including the prostasin-dependent cleavage site, were not cleaved, but were fully active (50). When these mutant γ subunits were expressed along with the mutant α subunits that cannot be cleaved by furin, they were still near fully active showing the dominance of γ processing (54). Other proteases besides prostasin that have been shown to cleave the γ subunit near the prostasin-dependent cleavage site, liberate the intervening inhibitory domain, and activate the channel, include pancreatic elastase. neutrophil elastase and plasmin (20, 40, 41, 43).
Proteolytic processing of ENaC enhances activity by increasing the channel's open probability (Po) (Figure 1) (46, 49, 50). ENaCs expressed in Xenopus oocytes undergo furin cleavage of the α subunit, liberating the α inhibitory domain, but retain their γ inhibitory domain and have a Po of ∼0.3-0.4 (although ENaC Po is known to be highly variable) (50, 55). Near silent channels have a Po of less than 0.1, similar to ENaCs, expressed in oocytes, that contain furin-dependent cleavage site mutations in the α subunit (i.e., these channels retain their inhibitory domains) (47, 49). Application of extracellular trypsin has been shown to enhance the Po of near silent channels expressed in fibroblasts to about 0.6-0.7 (46). Co-expression of prostasin and ENaC in Xenopus oocytes results in channels with a Po of 0.8-0.9 (50). ENaCs that have the γ inhibitory region deleted and furin processed α subunits have a Po >0.9 (50).
Not all ENaCs undergo furin-dependent maturation. There exists a pool of ENaC that reaches the cell surface that has escaped proteolytic processing (48). This pool of channels may also be the target of extracellular proteases in disease states. Single channel recordings of near silent channels exposed to trypsin showed a significant increase in activity and Po demonstrating that near silent channels can be activated by extracellular proteases (46, 53). This trypsin-dependent activation was not observed when α and β subunits were expressed without γ, whereas trypsin-dependent activation was observed in αγ channels, suggesting that the γ subunit plays a key role in channel activation by extracellular proteases (53). ENaCs in the aldosterone-sensitive distal nephron that have not been fully processed may be substrates for plasmin in proteinuric states.
Plasmin, proteinuria and processing of ENaC
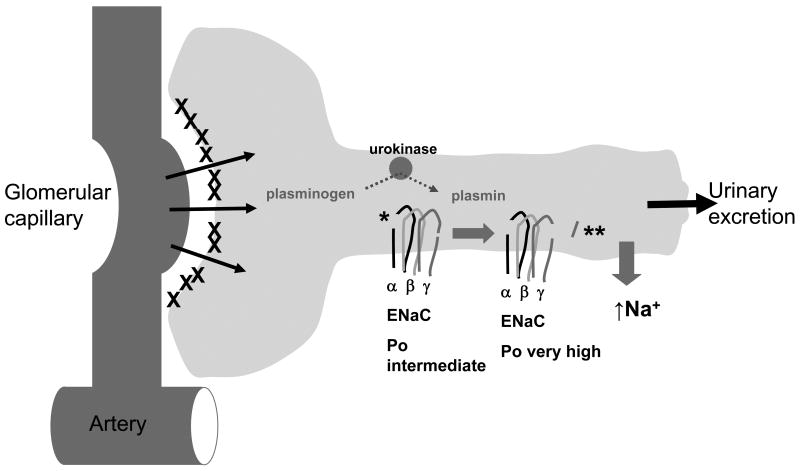
In proteinuric states, proteases not normally found in the urinary space cross the glomerular barrier and have the potential to cleave ENaC (Figure 2) (61). Recently, enhanced cleavage of the γ subunit of ENaC was demonstrated by Kastner et al. in kidney tissue of proteinuric mice (injected with an anti-glomerular basement membrane (GBM) antibody) (19). Kidney homogenates blotted with antibody prepared against the C-terminus of the γ subunit showed an approximate two-fold increase in a 70kDa fragment consistent with cleavage near the known prostasin-dependent cleavage site (i.e., a cleavage event that liberates the γ inhibitory domain) (19). The enzyme responsible for ENaC activation is plasmin, a serine protease known for its role in fibrinolysis, derived from processing of its inactive precursor plasminogen by the activating enzymes tissue-type (tpa) and urokinase-type plasminogen activator (urokinase). Plasminogen is not normally found in the urine of humans that are nonproteinuric, but has been shown to be present under nephrotic conditions (56, 57). Cells lining the proximal and distal nephron release urokinase to convert plasminogen, which has crossed damaged glomeruli, to plasmin in the urinary space (58-60). Furthermore, immunohistochemical staining for urokinase in human and rat nephrectomy specimens demonstrated the presence of urokinase on the apical surface of cortical collecting ducts (21).
Figure 2. Plasmin in the renal tubule can aberrantly cleave and activate ENaC.
Plasminogen is absent in the urine of normal rats or humans, but is present in the setting of nephrotic syndrome (20, 21, 56, 57). It is likely that secreted tubular urokinase generates active plasmin from plasminogen, and that plasmin cleaves the γ subunit distal to the furin cleavage site to release the γ inhibitory peptide (**) (20, 21, 58-60). The α inhibitory peptide (*) is already released by furin cleavage at two sites (44, 51). This figure is adapted from Eaton, DC and Pooler, JP (61).
Plasmin is identifiable in the urine of animals and humans with proteinuria. We examined urine from male obese ZSF-1 rats (diabetic and hypertensive) and their lean littermates for the presence of plasminogen and plasmin (20). Obese ZSF-1 rats were proteinuric compared to lean littermate controls and Western blotting with an anti-plasminogen antibody revealed plasminogen and plasmin to be readily detectable in the urine of obese ZSF1 rats and largely absent in urine from lean littermate controls (20). Svenningsen et al. found that urine from nephrotic humans and rats can activate ENaC expressed in mouse collecting duct cells, an observation that was abolished by aprotinin, a serine protease inhibitor, or heat inactivation of the urine (21). Control urine had no effect. Aprotinin-affinity precipitation of the nephrotic urine abolished the ability of the urine to activate ENaC despite not changing its total protein content, but the precipitate retained the ability to activate ENaC in collecting duct cells (21). By subjecting the precipitate to several purification steps followed by matrix-assisted laser desorption/ionization time-of flight mass spectrometry (21), plasmin was identified as the channel activating protease. Western blotting of urine from nephrotic and control humans with an antibody against plasminogen revealed plasminogen and plasmin in only the nephrotic urine and not in controls (21).
Plasmin has the ability to activate ENaC by cleaving its γ subunit (20, 21). We showed that plasmin (at 10μM) enhanced ENaC currents ∼2 fold when applied externally to Xenopus laevis oocytes expressing wild type ENaC after four minutes of exposure to the enzyme (20). Plasmin exhibited a time dependent increase in whole cell amiloride-sensitive currents, similar to the time course for activation of ENaC by extracellular trypsin, and reached a maximal effect by 10 minutes (20, 38, 62). Western blotting of surface channels expressed in oocytes exposed to 10 μM plasmin for four minutes, identified a new C-terminal 70kDa cleavage product from the γ subunit that was absent in controls not exposed to plasmin (20). This 70 kDa fragment is similar in length to the fragment generated from prostasin cleavage of ENaC as well as the fragment identified in blots of kidney lysates from proteinuric mice (exposed to anti-GBM antibody) (19, 50). A similar cleavage product was also demonstrated in western blots, probed for the C-terminus of γ, of surface channels exposed to extracellular trypsin, chymotrypsin, or a combination of plasminogen and urokinase (21). Plasmin's ability to cleave and activate ENaC was abolished by a specific mutation of a residue within a predicted plasmin cleavage site that is near the prostasin-dependent cleavage site, suggesting the enzyme activates the channel by liberating the intervening inhibitory tract (similar to other enzymes like prostasin and elastase that activate the channel) (20). Plasmin can cleave and activate furin processed channels. However, the ability of plasmin to activate fully non-cleaved channels, by cleaving near the furin cleavage site in the γ subunit, was not examined. Svenningsen et al. introduced a hexahistadine tag between the furin and prostasin cleavage sites in γ. Mouse collecting duct cells that expressed this tagged ENaC in the presence of NTA-Atto550 fluorophore exhibited a fluorescent signal. The signal is abolished when the cells were preincubated with plasmin or urine from nephrotic patients suggesting that plasmin cleavage liberates the inhibitory tract (21). Presumably the mouse collecting duct cells produced channels that were not fully processed. Plasmin, therefore, not only activates ENaC by cleaving the γ subunit, but appears to be the predominant aprotinin-sensitive serine protease identified in nephrotic urine from rats and humans.
Conclusions
Activation of ENaC by the serine protease plasmin appears to play a role in the development of extracellular volume expansion seen in some proteinuric states. Enhanced proteolytic cleavage of ENaC has been identified in a proteinuric mouse model. Plasminogen can cross damaged glomeruli and is activated by urokinase in the urinary space as evidenced by the detection of plasminogen and plasmin in proteinuric urine of animals and humans and their absence in control urine. Plasmin possesses the ability to activate ENaC by cleaving its γ subunit and liberates the intervening inhibitory tract when channels have also been processed by furin. Further understanding of this mechanism may help elucidate the pathogenesis of extracellular volume overload and guide therapeutics in individuals with proteinuria, edema, and hypertension.
Acknowledgments
Sponsorship: this work was supported by a grant from the National Institutes of Health (DK065161 and F32-DK080574)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bhalla V, Hallows KR. Mechanisms of ENaC regulation and clinical implications. J Am Soc Nephrol. 2008;19:1845–54. doi: 10.1681/ASN.2008020225. [DOI] [PubMed] [Google Scholar]; * This article reviews proteolytic processing, ubiquitination, and recycling of ENaC with implications for diseases related to the kidney and lung.
- 2.Rossier BC, Pradervand S, Schild L, Hummler E. Epithelial sodium channel and the control of sodium balance: interaction between genetic and environmental factors. Annu Rev Physiol. 2002;64:877–97. doi: 10.1146/annurev.physiol.64.082101.143243. [DOI] [PubMed] [Google Scholar]
- 3.Deschenes G, Feraille E, Doucet A. Mechanisms of oedema in nephrotic syndrome: old theories and new ideas. Nephrol Dial Transplant. 2003;18:454–6. doi: 10.1093/ndt/18.3.454. [DOI] [PubMed] [Google Scholar]
- 4.Strazzullo P, Galletti F, Barba G. Altered renal handling of sodium in human hypertension: short review of the evidence. Hypertension. 2003;41:1000–5. doi: 10.1161/01.HYP.0000066844.63035.3A. [DOI] [PubMed] [Google Scholar]
- 5.Kleyman TR, Carattino MD, Hughey RP. ENaC at the Cutting Edge: Regulation of Epithelial Sodium Channels by Proteases. J Biol Chem. 2009;284:20447–51. doi: 10.1074/jbc.R800083200. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This review article provides an overview of the regulation of ENaC activity by specific proteases.
- 6.Lemann J, Jr, Doumas BT. Proteinuria in health and disease assessed by measuring the urinary protein/creatinine ratio. Clin Chem. 1987;33:297–9. [PubMed] [Google Scholar]
- 7.Forman JP, Fisher ND, Schopick EL, Curhan GC. Higher levels of albuminuria within the normal range predict incident hypertension. J Am Soc Nephrol. 2008;19:1983–8. doi: 10.1681/ASN.2008010038. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This cohort study of non-diabetic nurses showed that higher albumin to creatinine ratios within the normal range, are associated with a risk of incident hypertension.
- 8.Hsu CY, Iribarren C, McCulloch CE, Darbinian J, Go AS. Risk factors for end-stage renal disease: 25-year follow-up. Arch Intern Med. 2009;169:342–50. doi: 10.1001/archinternmed.2008.605. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This large cohort study examining ESRD risk factors found that proteinuria and excess weight were the two strongest predictors of ESRD.
- 9.Lambers Heerspink HJ, Brantsma AH, de Zeeuw D, Bakker SJ, de Jong PE, Gansevoort RT. Albuminuria assessed from first-morning-void urine samples versus 24-hour urine collections as a predictor of cardiovascular morbidity and mortality. Am J Epidemiol. 2008;168:897–905. doi: 10.1093/aje/kwn209. [DOI] [PubMed] [Google Scholar]; * This prospective cohort study showed that a first morning void urinary albumin to creatinine ratio predicted cardiovascular mortality similarly to 24-hour urine albumin excretion rate.
- 10.Ninomiya T, Perkovic V, Verdon C, Barzi F, Cass A, Gallagher M, et al. Proteinuria and stroke: a meta-analysis of cohort studies. Am J Kidney Dis. 2009;53:417–25. doi: 10.1053/j.ajkd.2008.08.032. [DOI] [PubMed] [Google Scholar]; * This meta-analysis of observational cohort studies in diabetics demonstrated a relationship between proteinuria and stroke.
- 11.Agarwal R, Andersen MJ. Correlates of systolic hypertension in patients with chronic kidney disease. Hypertension. 2005;46:514–20. doi: 10.1161/01.HYP.0000178102.85718.66. [DOI] [PubMed] [Google Scholar]
- 12.Kim BJ, Lee HJ, Sung KC, Kim BS, Kang JH, Lee MH, et al. Comparison of microalbuminuria in 2 blood pressure categories of prehypertensive subjects. Circ J. 2007;71:1283–7. doi: 10.1253/circj.71.1283. [DOI] [PubMed] [Google Scholar]
- 13.Feld LG, Brentjens JR, Van Liew JB. Renal injury and proteinuria in female spontaneously hypertensive rats. Ren Physiol. 1981;4:46–56. doi: 10.1159/000172803. [DOI] [PubMed] [Google Scholar]
- 14.Rossi GP, Bernini G, Desideri G, Fabris B, Ferri C, Giacchetti G, et al. Renal damage in primary aldosteronism: results of the PAPY Study. Hypertension. 2006;48:232–8. doi: 10.1161/01.HYP.0000230444.01215.6a. [DOI] [PubMed] [Google Scholar]
- 15.Wang G, Lai FM, Kwan BC, Lai KB, Chow KM, Li PK, et al. Podocyte loss in human hypertensive nephrosclerosis. Am J Hypertens. 2009;22:300–6. doi: 10.1038/ajh.2008.360. [DOI] [PubMed] [Google Scholar]; * A decrease in podocyte number and podocyte related molecules in kidney biopsy specimens from hypertensive individuals was observed when compared to non-hypertensive individuals.
- 16.Inoue T, Iseki K, Higashiuesato Y, Nagahama K, Matsuoka M, Iseki C, et al. Proteinuria as a significant determinant of hypertension in a normotensive screened cohort in Okinawa, Japan. Hypertens Res. 2006;29:687–93. doi: 10.1291/hypres.29.687. [DOI] [PubMed] [Google Scholar]
- 17.Wang TJ, Gona P, Larson MG, Levy D, Benjamin EJ, Tofler GH, et al. Multiple biomarkers and the risk of incident hypertension. Hypertension. 2007;49:432–8. doi: 10.1161/01.HYP.0000256956.61872.aa. [DOI] [PubMed] [Google Scholar]
- 18.de Boer IH, Kestenbaum B, Rue TC, Steffes MW, Cleary PA, Molitch ME, et al. Insulin therapy, hyperglycemia, and hypertension in type 1 diabetes mellitus. Arch Intern Med. 2008;168:1867–73. doi: 10.1001/archinternmed.2008.2. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This cohort study in type 1 diabetics demonstrated that albumin excretion rate, body mass index, age, hemoglobin A1C, and glycemic control were associated with increased risk of incident hypertension.
- 19.Kastner C, Pohl M, Sendeski M, Stange G, Wagner CA, Jensen B, et al. Effects of receptor-mediated endocytosis and tubular protein composition on volume retention in experimental glomerulonephritis. Am Physiol Renal Physiol. 2009;296:F902–11. doi: 10.1152/ajprenal.90451.2008. [DOI] [PubMed] [Google Scholar]; ** Kidney tissue homogenates from a proteinuric mouse model demonstrated an increase in proteolytically processed α and γ subunits as compared to controls. This observation fits the proposed mechanism of an extracellular protease entering the urinary space across damaged glomeruli (in this case anti-GBM mediated injury) to aberrantly cleave ENaC.
- 20.Passero CJ, Mueller GM, Rondon-Berrios H, Tofovic SP, Hughey RP, Kleyman TR. Plasmin activates epithelial Na+ channels by cleaving the gamma subunit. J Biol Chem. 2008;283:36586–91. doi: 10.1074/jbc.M805676200. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** The serine protease plasmin activated ENaC by cleaving a specific residue in the extracellular loop of the γ subunit. Plasmin and its precursor plasminogen were readily identified in the urine of obese proteinuric, hypertensive rats and largely absent in the urine of their lean, nonproteinuric littermates.
- 21.Svenningsen P, Bistrup C, Friis UG, Bertog M, Haerteis S, Krueger B, et al. Plasmin in nephrotic urine activates the epithelial sodium channel. Am Soc Nephrol. 2009;20:299–310. doi: 10.1681/ASN.2008040364. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This study demonstrated that nephrotic urine from humans and rats can activate ENaC currents in mouse collecting duct cells. Aprotinin-affinity precipitation of the urine abolished this effect. Plamin was identified as the serine protease responsible for ENaC activation by mass spectrometry of precipitated fragments. External purified plasmin application to channels with an epitope tag within the γ inhibitory domain demonstrated that plasmin removed the inhibitory tract of the γ subunit of ENaC, activating the channel.
- 22.Crew RJ, Radhakrishnan J, Appel G. Complications of the nephrotic syndrome and their treatment. Clin Nephrol. 2004;62:245–59. doi: 10.5414/cnp62245. [DOI] [PubMed] [Google Scholar]
- 23.Rasool A, Palevsky PM. Treatment of edematous disorders with diuretics. Am J Med Sci. 2000;319:25–37. doi: 10.1097/00000441-200001000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Doucet A, Favre G, Deschenes G. Molecular mechanism of edema formation in nephrotic syndrome: therapeutic implications. Pediatr Nephrol. 2007;22:1983–90. doi: 10.1007/s00467-007-0521-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Usberti M, Gazzotti RM. Hyporeninemic hypoaldosteronism in patients with nephrotic syndrome. Am J Nephrol. 1998;18:251–5. doi: 10.1159/000013347. [DOI] [PubMed] [Google Scholar]
- 26.Vande Walle JG, Donckerwolcke RA, van Isselt JW, Derkx FH, Joles JA, Koomans HA. Volume regulation in children with early relapse of minimal-change nephrosis with or without hypovolaemic symptoms. Lancet. 1995;346:148–52. doi: 10.1016/s0140-6736(95)91210-x. [DOI] [PubMed] [Google Scholar]
- 27.Ichikawa I, Rennke HG, Hoyer JR, Badr KF, Schor N, Troy JL, et al. Role for intrarenal mechanisms in the impaired salt excretion of experimental nephrotic syndrome. J Clin Invest. 1983;71:91–103. doi: 10.1172/JCI110756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schrier RW, Fassett RG. A critique of the overfill hypothesis of sodium and water retention in the nephrotic syndrome. Kidney Int. 1998;53:1111–7. doi: 10.1046/j.1523-1755.1998.00864.x. [DOI] [PubMed] [Google Scholar]
- 29.Botero-Velez M, Curtis JJ, Warnock DG. Brief report: Liddle's syndrome revisited--a disorder of sodium reabsorption in the distal tubule. N Engl J Med. 1994;330:178–81. doi: 10.1056/NEJM199401203300305. [DOI] [PubMed] [Google Scholar]
- 30.Rotin D, Schild L. ENaC and its regulatory proteins as drug targets for blood pressure control. Curr Drug Targets. 2008;9:709–16. doi: 10.2174/138945008785132367. [DOI] [PubMed] [Google Scholar]
- 31.Staub O, Verrey F. Impact of Nedd4 proteins and serum and glucocorticoid-induced kinases on epithelial Na+ transport in the distal nephron. J Am Soc Nephrol. 2005;16:3167–74. doi: 10.1681/ASN.2005050454. [DOI] [PubMed] [Google Scholar]
- 32.Vehaskari VM. Heritable forms of hypertension. Pediatr Nephrol. 2007;24 doi: 10.1007/s00467-007-0537-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eaton DC, Malik B, Saxena NC, Al-Khalili OK, Yue G. Mechanisms of aldosterone's action on epithelial Na + transport. J Membr Biol. 2001;184:313–9. doi: 10.1007/s00232-001-0098-x. [DOI] [PubMed] [Google Scholar]
- 34.Griffing GT, Cole AG, Aurecchia SA, Sindler BH, Komanicky P, Melby JC. Amiloride in primary hyperaldosteronism. Clin Pharmacol Ther. 1982;31:56–61. doi: 10.1038/clpt.1982.9. [DOI] [PubMed] [Google Scholar]
- 35.Verrey F. Early aldosterone action: toward filling the gap between transcription and transport. Am J Physiol. 1999;277:F319–27. doi: 10.1152/ajprenal.1999.277.3.F319. [DOI] [PubMed] [Google Scholar]
- 36.Canessa CM, Merillat AM, Rossier BC. Membrane topology of the epithelial sodium channel in intact cells. Am J Physiol. 1994;267:C1682–90. doi: 10.1152/ajpcell.1994.267.6.C1682. [DOI] [PubMed] [Google Scholar]
- 37.Snyder PM, McDonald FJ, Stokes JB, Welsh MJ. Membrane topology of the amiloride-sensitive epithelial sodium channel. J Biol Chem. 1994;269:24379–83. [PubMed] [Google Scholar]
- 38.Chraibi A, Vallet V, Firsov D, Hess SK, Horisberger JD. Protease modulation of the activity of the epithelial sodium channel expressed in Xenopus oocytes. J Gen Physiol. 1998;111:127–38. doi: 10.1085/jgp.111.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vuagniaux G, Vallet V, Jaeger NF, Hummler E, Rossier BC. Synergistic activation of ENaC by three membrane-bound channel-activating serine proteases (mCAP1, mCAP2, and mCAP3) and serum- and glucocorticoid-regulated kinase (Sgk1) in Xenopus Oocytes. J Gen Physiol. 2002;120:191–201. doi: 10.1085/jgp.20028598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adebamiro A, Cheng Y, Rao US, Danahay H, Bridges RJ. A segment of gamma ENaC mediates elastase activation of Na+ transport. J Gen Physiol. 2007;130:611–29. doi: 10.1085/jgp.200709781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Caldwell RA, Boucher RC, Stutts MJ. Neutrophil elastase activates near-silent epithelial Na+ channels and increases airway epithelial Na+ transport. Am J Physiol Lung Cell Mol Physiol. 2005;288:L813–9. doi: 10.1152/ajplung.00435.2004. [DOI] [PubMed] [Google Scholar]
- 42.Garcia-Caballero A, Dang Y, He H, Stutts MJ. ENaC proteolytic regulation by channel-activating protease 2. J Gen Physiol. 2008;132:521–35. doi: 10.1085/jgp.200810030. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This article demonstrated that the type II transmembrane serine protease TMPRSS4 (channel-activating protease 2) cleaves the γ subunit and activates ENaC.
- 43.Harris M, Firsov D, Vuagniaux G, Stutts MJ, Rossier BC. A novel neutrophil elastase inhibitor prevents elastase activation and surface cleavage of the epithelial sodium channel expressed in Xenopus laevis oocytes. J Biol Chem. 2007;282:58–64. doi: 10.1074/jbc.M605125200. [DOI] [PubMed] [Google Scholar]
- 44.Hughey RP, Bruns JB, Kinlough CL, Harkleroad KL, Tong Q, Carattino MD, et al. Epithelial sodium channels are activated by furin-dependent proteolysis. J Biol Chem. 2004;279:18111–4. doi: 10.1074/jbc.C400080200. [DOI] [PubMed] [Google Scholar]
- 45.Picard N, Eladari D, El Moghrabi S, Planes C, Bourgeois S, Houillier P, et al. Defective ENaC processing and function in tissue kallikrein-deficient mice. J Biol Chem. 2008;283:4602–11. doi: 10.1074/jbc.M705664200. [DOI] [PubMed] [Google Scholar]
- 46.Caldwell RA, Boucher RC, Stutts MJ. Serine protease activation of near-silent epithelial Na+ channels. Am J Physiol Cell Physiol. 2004;286:C190–4. doi: 10.1152/ajpcell.00342.2003. [DOI] [PubMed] [Google Scholar]
- 47.Firsov D, Schild L, Gautschi I, Merillat AM, Schneeberger E, Rossier BC. Cell surface expression of the epithelial Na channel and a mutant causing Liddle syndrome: a quantitative approach. Proc Natl Acad Sci U S A. 1996;93:15370–5. doi: 10.1073/pnas.93.26.15370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hughey RP, Bruns JB, Kinlough CL, Kleyman TR. Distinct pools of epithelial sodium channels are expressed at the plasma membrane. J Biol Chem. 2004;279:48491–4. doi: 10.1074/jbc.C400460200. [DOI] [PubMed] [Google Scholar]
- 49.Sheng S, Carattino MD, Bruns JB, Hughey RP, Kleyman TR. Furin cleavage activates the epithelial Na+ channel by relieving Na+ self-inhibition. Am J Physiol Renal Physiol. 2006;290:F1488–96. doi: 10.1152/ajprenal.00439.2005. [DOI] [PubMed] [Google Scholar]
- 50.Bruns JB, Carattino MD, Sheng S, Maarouf AB, Weisz OA, Pilewski JM, et al. Epithelial Na+ channels are fully activated by furin- and prostasin-dependent release of an inhibitory peptide from the gamma-subunit. J Biol Chem. 2007;282:6153–60. doi: 10.1074/jbc.M610636200. [DOI] [PubMed] [Google Scholar]
- 51.Hughey RP, Mueller GM, Bruns JB, Kinlough CL, Poland PA, Harkleroad KL, et al. Maturation of the epithelial Na+ channel involves proteolytic processing of the alpha- and gamma-subunits. J Biol Chem. 2003;278:37073–82. doi: 10.1074/jbc.M307003200. [DOI] [PubMed] [Google Scholar]
- 52.Carattino MD, Sheng S, Bruns JB, Pilewski JM, Hughey RP, Kleyman TR. The epithelial Na+ channel is inhibited by a peptide derived from proteolytic processing of its alpha subunit. J Biol Chem. 2006;281:18901–7. doi: 10.1074/jbc.M604109200. [DOI] [PubMed] [Google Scholar]
- 53.Diakov A, Bera K, Mokrushina M, Krueger B, Korbmacher C. Cleavage in the {gamma}-subunit of the epithelial sodium channel (ENaC) plays an important role in the proteolytic activation of near-silent channels. J Physiol. 2008;586:4587–608. doi: 10.1113/jphysiol.2008.154435. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Near-silent ENaCs can be activated by extracellular proteases, with processing of the γ subunit playing a key role.
- 54.Carattino MD, Hughey RP, Kleyman TR. Proteolytic processing of the epithelial sodium channel gamma subunit has a dominant role in channel activation. J Biol Chem. 2008;283:25290–5. doi: 10.1074/jbc.M803931200. [DOI] [PMC free article] [PubMed] [Google Scholar]; * ENaCs with mutations making them resistant to processing of the α subunit by proteases are nearly fully active when the γ inhibitory domain is removed showing the dominance of proteolytic processing of γ over α.
- 55.Maarouf AB, Sheng N, Chen J, Winarski KL, Okumura S, Carattino MD, et al. Novel determinants of epithelial sodium channel gating within extracellular thumb domains. J Biol Chem. 2009;284:7756–65. doi: 10.1074/jbc.M807060200. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Specific sites within the extracellular “thumb” domain of ENaC affect sodium self inhibition and channel gating. Substitutions at these sites within the α and γ subunits alter the open probability of the channel.
- 56.Lau SO, Tkachuck JY, Hasegawa DK, Edson JR. Plasminogen and antithrombin III deficiencies in the childhood nephrotic syndrome associated with plasminogenuria and antithrombinuria. J Pediatr. 1980;96:390–2. doi: 10.1016/s0022-3476(80)80678-0. [DOI] [PubMed] [Google Scholar]
- 57.Vaziri ND, Gonzales EC, Shayestehfar B, Barton CH. Plasma levels and urinary excretion of fibrinolytic and protease inhibitory proteins in nephrotic syndrome. J Lab Clin Med. 1994;124:118–24. [PubMed] [Google Scholar]
- 58.Kristensen P, Eriksen J, Dano K. Localization of urokinase-type plasminogen activator messenger RNA in the normal mouse by in situ hybridization. J Histochem Cytochem. 1991;39:341–9. doi: 10.1177/39.3.1899685. [DOI] [PubMed] [Google Scholar]
- 59.Muellbacher W, Maier M, Binder BR. Regulation of plasminogen activation in isolated perfused rat kidney. Am J Physiol. 1989;256:F787–93. doi: 10.1152/ajprenal.1989.256.5.F787. [DOI] [PubMed] [Google Scholar]
- 60.Piedagnel R, Tiger Y, Lelongt B, Ronco PM. Urokinase (u-PA) is produced by collecting duct principal cells and is post-transcriptionally regulated by SV40 large-T, arginine vasopressin, and epidermal growth factor. J Cell Physiol. 2006;206:394–401. doi: 10.1002/jcp.20485. [DOI] [PubMed] [Google Scholar]
- 61.Eaton DC, Pooler JP. Vander's Renal Physiology. 6th. New York: McGraw-Hill; 2004. Chapter1 Renal Functions, Anatomy, and Basic Processes; p. 17. Figure 1-7. [Google Scholar]
- 62.Vallet V, Chraibi A, Gaeggeler HP, Horisberger JD, Rossier BC. An epithelial serine protease activates the amiloride-sensitive sodium channel. Nature. 1997;389:607–10. doi: 10.1038/39329. [DOI] [PubMed] [Google Scholar]