Abstract
Since adolescence is a critical period for the initiation of tobacco use, we have systematically compared behavioral and endocrine responses to nicotine in Sprague-Dawley rats of both sexes at early adolescence (postnatal day (P) 28), mid- adolescence (P38) and adulthood (P90). Locomotion and center time in a novel open field were evaluated for 30 min following intravenous injection of saline or nicotine (60 µg/kg), followed by measurement of plasma corticosterone. Complex age and sex differences in behavioral and endocrine response were observed, which were dependent on the functional endpoint examined. Whereas there were age differences in nicotine effects on all functional measures, sex differences were largely restricted to adult stress-related corticosterone and center-time responses. Although significant drug effects were detected at P28 and P90, there was no effect of nicotine at P38 on any measure examined. In saline-treated males, but not females, there were significant positive correlations across age between initial ambulatory counts and both initial vertical counts and total center time. Nicotine treatment increased correlations in both sexes, and yielded a significant negative interaction between initial ambulatory counts and plasma corticosterone. The unique responses of adolescents to nicotine are consistent with an immature function of nicotinic acetylcholine receptors at this age.
Keywords: adolescence, anxiety, corticosterone, locomotion, stress, sex differences
INTRODUCTION
Adolescence is a critical period of vulnerability for the onset of smoking (Kandel and Logan, 1984). Approximately 80% of smokers initiate tobacco use during adolescence, with those who begin smoking in early adolescence being more likely to experience difficulty quitting than those who start at later ages (Chen and Millar, 1998). Compared to adult smokers, adolescents more readily develop symptoms of tobacco dependence (DiFranza et al. , 2006), showing withdrawal symptoms, craving for cigarettes and failed attempts at quitting in the first weeks of smoking (DiFranza, 2008). Although there are similarities in initiation of smoking between sexes (Marshall et al. , 2006), the prevalence of tobacco use in girls is even higher than that in boys in some countries (Stanton et al. , 1996). Women are also more likely to experience mood changes during abstinence, resulting in shorter or less frequent abstinent periods compared to their male counterparts (Perkins et al. , 1999). Furthermore, there are sex differences in the association of tobacco withdrawal and stress hormone response (al’Absi, 2006). Given these clinical findings, it is important to understand the factors that underlie sex and age differences in smoking behaviors.
Animal studies have shown that adolescents differ in their response to nicotine, as compared to adults. Adolescence in rodents, a stage when risk-taking and novelty seeking behaviors emerge, has been conservatively defined as the fifth and sixth postnatal weeks (Spear, 2000). Adolescent rodents are more sensitive than adults to the rewarding effects of nicotine as shown in both conditioned place preference (CPP; Belluzzi et al. , 2004, Kota et al. , 2008, Kota et al. , 2007, Torres et al. , 2009, Torres et al. , 2008) and self-administration models (Belluzzi et al. , 2004, Chen et al. , 2007, Kota et al. , 2008, Kota et al. , 2007, Levin et al. , 2007, Levin et al. , 2003, Torres et al. , 2009, Torres et al. , 2008), whereas they are less sensitive to the aversive effects (Wilmouth and Spear, 2004). Sex differences, which are well characterized in adults, also begin to emerge during adolescence. The developmental differences in males are more pronounced than those in females (Park et al., 2007, Torres et al., 2009, Torres et al., 2008). Specifically, males but not females show a substantial age-related decline in nicotine self-administration (Levin, et al, 2007, Levin, et al, 2003, Park et al., 2007). Adolescent females show greater motivation to self-administer nicotine (Lynch, 2009), higher sensitivity to nicotine conditioning (Isiegas et al. , 2009), and increased withdrawal signs (Kota, et al., 2008, Kota, et al. , 2007) while the developmental differences in nicotine-induced CPP is smaller in females as compared to males (Torres et al. , 2009, Torres et al. , 2008).
Other behavioral effects of nicotine also exhibit substantial age and sex differences. Whereas locomotor depressant effects of nicotine are frequently observed in adult rats, nicotine stimulates locomotor activity in adolescents (Belluzzi et al., 2004, Elliott et al. , 2004, Vastola et al. , 2002). Furthermore, females have been found to be more sensitive than males to the locomotor effects of nicotine in both adolescent and adult rats (Elliott et al. 2004, Kanyt et al. , 1999). Nicotine is also reported to have both anxiolytic and anxiogenic effects. However, these effects are complex and vary depending on age, sex and experimental condition (Cheeta et al. , 2001, Elliott et al., 2004, Picciotto et al. , 2002).
Stress is an important factor in smoking behaviors, with a bi-directional interaction between nicotine and the hypothalamus-pituitary-adrenal (HPA) axis. Nicotine administration activates the HPA axis and increases corticosteroid release in adults (Balfour et al. , 1975, Cam and Bassett, 1983, Matta et al. , 1998). Glucocorticoids, in turn, modulate responsiveness to nicotine (Caggiula et al. , 1998, Pauly et al. , 1988). Both clinical and animal studies have shown that control of the HPA axis, and resulting stress responses, continue to mature throughout adolescence (reviewed by Leslie and Park, 2009). Differences between adolescents and adults have also been observed in the responses of the HPA axis to nicotine, which vary depending on the experimental condition used (Cruz et al. , 2008, Matta et al. , 1987, McCormick and Ibrahim, 2007). We have previously shown that male adolescents are less sensitive to nicotine activation of the HPA axis as compared to adults (Cao et al. , 2007a). Although sex differences during adolescence have not been studied, adult females have been reported to be more sensitive than males to the HPA activating effects of nicotine (Rhodes et al. , 2001).
Given the complexity of nicotine-induced behavioral and endocrine effects, in which comparisons are often confounded by differences in experimental conditions across studies, the aim of the present study was to systematically compare age and sex differences in parallel within the same animals. The nicotine dose chosen in this study has been used routinely in our previous studies to examine adolescent responses to tobacco components (Cao, et al., 2007a, McQuown et al. , 2007, Park et al. , 2006). We have examined the effects of acute intravenous drug administration at a dose comparable to human blood levels of nicotine after inhalation of 3–4 cigarettes (Benowitz et al. , 1990). Intravenous administration reduces the stress of drug administration and better models the pharmacokinetics of smoking compared to other modes of drug administration. Behaviorally, we evaluated the locomotor effects of nicotine in a novel open field apparatus and its anxiolytic or anxiogenic effects, as determined by time spent in the center of the field. Plasma corticosterone levels were further assessed in the same animals after behavioral testing. This behavioral paradigm was developed to model the initial phase of self-administration testing and to examine behavioral and endocrine responses to nicotine which underlie responding. Our data indicate that the effects of nicotine are highly dependent on both age and sex, and provide strong evidence for unique adolescent behavioral and endocrine responses to nicotine in both sexes.
MATERIALS AND METHODS
Animals
Both male and female Sprague-Dawley rats were maintained in a temperature (21°C) and humidity (50%) controlled room on a 12-h light-dark cycle (lights on 0700–1900) with unlimited access to food and water. All experiments were carried out in accordance with the Institutional Animal Care and Use Committee at the University of California, Irvine, and were consistent with Federal guidelines. To minimize prenatal stress effects on the offspring, early adolescents, aged postnatal day (P) 28, and older adolescents, aged P38, were delivered with dams at P16 and habituated in the vivarium for five days prior to weaning at P21. Male and female pups were housed separately in group cages after weaning. Adults, aged P80, were delivered a minimum of one week before use. All animals were group housed with 5 animals per cage until implantation of intravenous catheters, after which they were singly housed.
Surgical implantation of intravenous catheters
Surgery was conducted on animals at the age of P24, P38 and P86 respectively. Catheter construction and implantation were as described previously (Belluzzi et al. , 2005). Animals were anesthetized with Equithesin (2.5 ml/kg adolescents, 3 ml/kg adults, i.p.) and a chronic catheter was surgically implanted into the right external jugular vein. The catheter was passed subcutaneously from the animals’ back to the jugular vein where the tubing was inserted. The cannula assembly was mounted on the animal's back and was sealed to prevent clogging and to keep a closed system. The wounds were closed with wound clips; antiseptic ointment was applied to the wounds. The animals were kept in a warm cage for postsurgical observation until they emerged from anesthesia. The animals were allowed to recover from surgery for 3 days.
Drug treatment and sample collection
For three days prior to the experiment, catheters were flushed with 0.2 ml of a heparinized saline solution (600U or 300U of heparin in 30 ml saline for adult and young animals, respectively) to prevent clogging. Propofol (10mg/ml), a fast-acting, short-lived intravenous anesthetic, was administered (0.1 ml for adults and 0.05 ml for adolescents) to test catheter patency one day before the experiment. Only rats that were immediately anesthetized by propofol and recovered within 5 min were used. On the test day, male and female animals, aged P28, P38 and P90 (n=5–8 per group), were habituated to the lab for 30 min prior to drug administration. Experiments were carried out, and samples collected, from 0800–1100 AM when diurnal levels of plasma corticosterone are lowest (Critchlow et al. , 1963). Animals were given two intravenous injections, spaced 1 min apart, of saline or nicotine tartrate solution (30 µg/kg/100µl calculated as nicotine free base), a drug administration paradigm that was chosen to model the first two injections in self-administration tests (Belluzzi et al., 2005). Animals were placed into locomotion boxes immediately after drug treatment and monitored behaviorally for 30 min, and were then immediately sacrificed by decapitation. Trunk blood (2 ml) was collected, EDTA (1.48 mg/ml) was added, and samples centrifuged at 4 °C for 30 min at 2200 rpm. Aliquots of plasma supernatant were stored at –20 °C before analysis of corticosterone levels. In another group of animals, the time course of nicotine-induced corticosterone release was evaluated in males aged P28 and P90 (n=5–6 per group). Under the same handling procedure and experimental environment, animals were sacrificed and trunk blood collected immediately prior to, or 15, 30 and 60 min after, injection with nicotine (30 µg/kg/100 µl × 2). Separate groups of control animals were injected with saline and placed into the locomotor apparatus for 15 and 30 min prior to blood collection.
Behavioral test: locomotion and center time
Locomotor activity was measured for 30 min using an open-field activity system measuring 43.2 × 43.2 × 30.5 cm (MED Associates, Inc., St. Albans, VT). Horizontal movement was monitored by 16 evenly spaced infrared beams located along two adjacent sides of the chamber. Vertical movement was monitored by vertical infrared beams, the height of which was adjusted to be proportionate to the average body size at each age. Repetitive activity was automatically monitored as repeated interruption of the same infrared beams by the system. The time spent in the center area of the apparatus was monitored simultaneously. The center area was defined based on the average body size at each age.
Corticosterone radioimmunoassay
Plasma corticosterone concentrations were determined in duplicate samples using a commercial RIA kit (ICN, Costa Mesa, CA, USA) as instructed. Briefly, diluted rat plasma samples were incubated with rabbit anti-corticosterone serum in the presence of synthetic [125I]-corticosterone at room temperature for 2 h. The complex was precipitated by centrifugation at 2400 r.p.m. for 15 min after adding goat anti-rabbit gamma globulin, and the radioactivity in the precipitate was determined by gamma scintillation counter. A standard curve was generated with a series of known corticosterone samples using commercial software (Prism, GraphPad Software, San Diego, CA, USA) and the plasma hormonal concentrations in the unknown samples were interpolated from the curve.
Statistics
Locomotor activity and center time were analyzed separately using a 4-way ANOVA for Sex × Age × Drug × Time, with repeated measures on Time. Significant main effects or interactions were further tested separately by ANOVAs and two-sided Bonferroni corrected post-hoc multiple comparison tests. Differences were considered statistically significant at p<0.05. Data were analyzed using SYSTAT 10.01 statistical software.
Plasma corticosterone levels at 30 min were analyzed using a 3-way ANOVA for Age × Drug × Sex. The time course of change in plasma corticosterone levels was analyzed using a 3-way ANOVA for Age × Drug × Time. Significant main effects or interactions were further tested separately by ANOVAs with two-sided Bonferroni- or Dunnett’s-corrected post-hoc multiple comparison tests. Differences were considered statistically significant at p<0.05. Data were analyzed using SYSTAT 10.01 statistical software.
For correlation analyses, data from three ages were combined in each treatment and each sex, and correlations between each measurement were evaluated using Pearson correlation analysis. Differences were considered statistically significant at p<0.05. Data were analyzed using Prism software.
RESULTS
Age, but not sex, differences in nicotine-induced locomotor activity
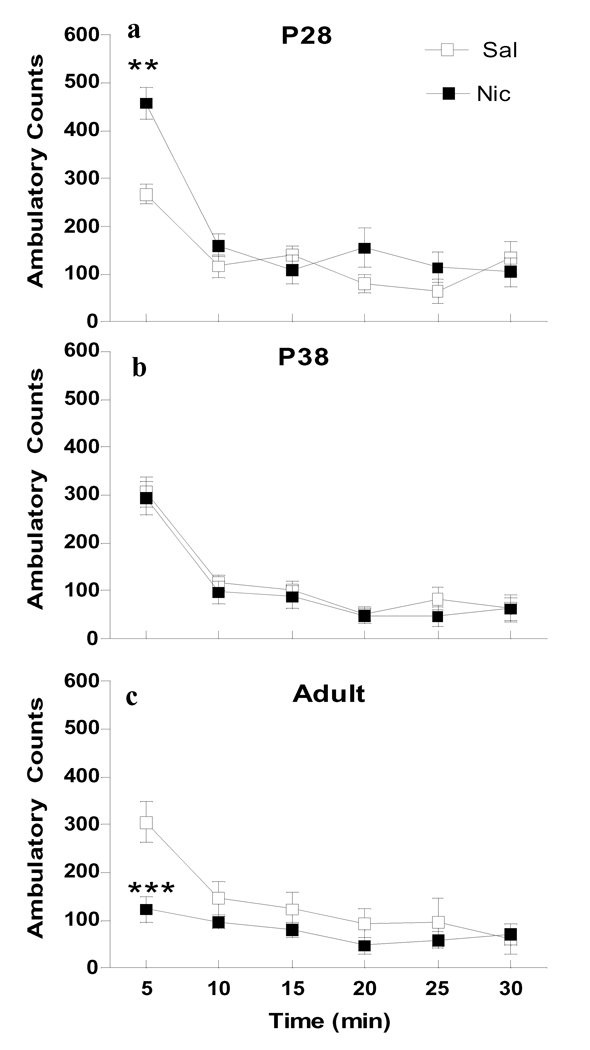
There was a significant effect of age, but not sex, on nicotine-induced changes in horizontal (Fig. 1), vertical (Fig. 2) and repetitive (Fig. 3) locomotor activity. For horizontal activity, overall 4-way ANOVA for Age × Sex × Drug × Time showed significant effects of age (F2, 61=5.554, p<0.01), time (F5, 305=95.144, p<0.001) and an interaction of Age × Time (F10, 290=3.782, p<0.001). Since there was no significant sex effect (F1, 61=0.193, p=0.662), data from males and females were combined. Further 2-way ANOVA for Age × Drug was conducted at each time point. The analysis showed a significant effect of age (F2, 67=12.311, p<0.001) and an interaction of Age × Drug (F2, 67=13.118, p<0.001), which was limited to the first 5 min after drug administration. Nicotine’s effects transitioned from locomotor stimulant to suppressant effects as age increased with no age differences following saline treatment (Fig. 1). Specifically, nicotine significantly increased horizontal activity at 5 min at P28 (p<0.001), and decreased it in adults (p<0.001). No drug effect was observed at P38.
Figure 1.
Age differences in nicotine-induced horizontal activity. Data are collapsed across males and females for ages P28 (a), P38 (b) and adult (c) during the 30 min following i.v. injection of nicotine (30 µg/kg/injection × 2, filled symbols) or saline (open symbols), n=9–14 per group. * p<0.05, ** p<0.01(Bonferroni) significantly different from saline treatment at the same age.
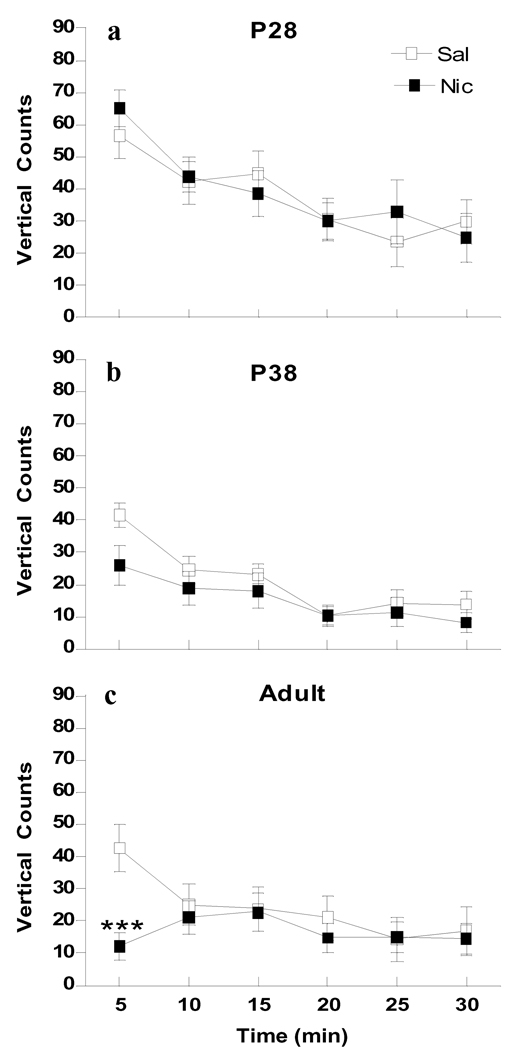
Figure 2.
Age differences in nicotine-induced vertical activity. Data are collapsed across males and females for ages P28 (a), P38 (b) and adult (c) during the 30 min following i.v. injection of nicotine (30 µg/kg/injection × 2, filled symbols) or saline (open symbols), n=5–8 per group. * p<0.05 (Bonferroni) significantly different from saline treatment at the same age.
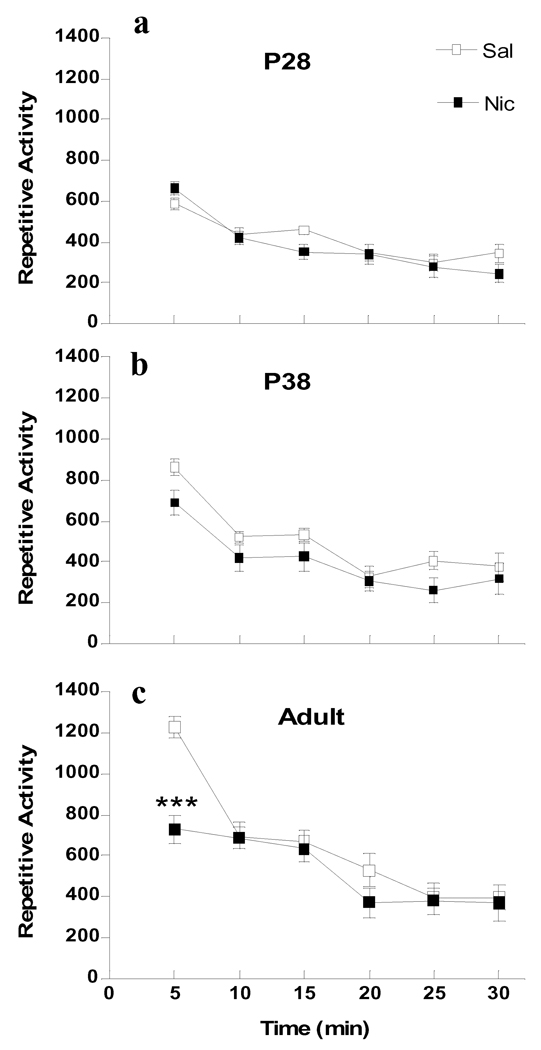
Figure 3.
Age differences in nicotine-induced repetitive activity. Data are collapsed across males and females for ages P28 (a), P38 (b) and adult (c) during the 30 min following i.v. injection of nicotine (30 µg/kg/injection × 2, filled symbols) or saline (open symbols), n=5–8 per group. * p<0.05 (Bonferroni) significantly different from saline treatment at the same age.
For vertical activity, overall 4-way ANOVA for Age × Sex × Drug × Time showed significant effects of age (F2, 61=11.949, p<0.0001), time (F5, 305=41.062, p<0.0001) and interactions of Age × Time (F10, 305=3.660, p<0.0001) and Time × Drug (F5, 305=2.804, p<0.05). Since there was no significant sex effect (F1, 61=0.559, p=0.457), data from males and females were combined. Further 2-way ANOVA for Age × Drug was conducted at each time point and the analysis showed significant effects of age (F2, 67=25.170, p<0.001), drug (F2, 67=25.170, p<0.001) and an interaction of Age × Drug (F2, 67=13.118, p<0.001), which was limited to the first 5 min after injection. Saline treated P28 animals showed significantly higher activity in the initial 5 min as compared to adults (p<0.05). Nicotine had no effects on vertical activity at either P28 or P38, whereas it significantly decreased vertical counts in adults (p<0.001) for the first 5 min after nicotine injection (Fig. 2).
For repetitive activity, overall 4-way ANOVA for Age × Sex × Drug × Time showed significant effects of age (F2, 61=11.306, p<0.0001), drug (F1, 61=6.6368, p<0.05) and time (F5, 305=91.157, p<0.0001), and interactions of Time × Age (F5, 305=10.307, p<0.0001) and Time × Age × Drug (F5, 305=5.223, p<0.001). Since there was no significant effect of sex (F1, 61=0.0021, p=0.9633), data from males and females were combined. Further 2-way ANOVA for Age × Drug was conducted at each time point. The analysis showed significant effects of age (F2, 67=24.185, p<0.001), drug (F1, 67=21.737, p<0.001) and an interaction of Age × Drug (F2, 67=16.071, p<0.001), which was limited to the first 5 min after injection. P28 and P38 animals showed significantly lower basal activity in the initial 5 min as compared to adults. Consistent with vertical activity, nicotine had no effect on repetitive activity at either P28 or P38, whereas it significantly decreased repetitive activity in adults (p<0.001) for the first 5 min after drug injection (Fig. 3).
Age and sex differences in nicotine-induced anxiolytic and anxiogenic effects
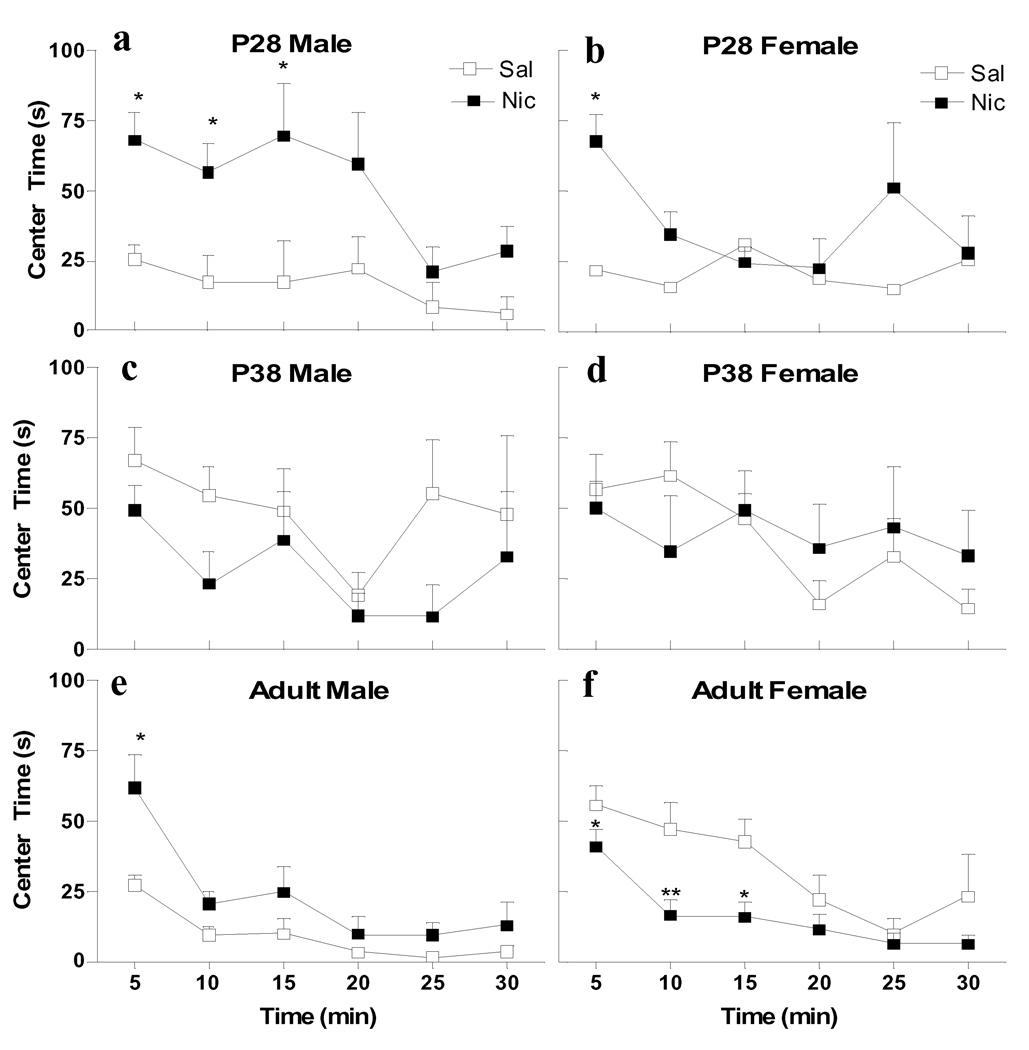
In the same groups of animals, anxiolytic and anxiogenic effects of nicotine were evaluated by measuring time spent in the center area of the novel apparatus during locomotor monitoring (Fig. 4). Unlike locomotor activity, there were both age and sex differences in this measure. Overall 4-way ANOVA for Age × Sex × Drug × Time showed significant effects of age (F2, 57=8.704, p<0.005) and time (F5, 285=13.937, p<0.0001), and interactions of Drug × Sex (F2, 57=6.582, p<0.005), and Drug × Age × Sex (F2, 57=4.971, p<0.05). There was a significant age effect (F2, 24=12.90, p<0.001) and a significant interaction of Age × Sex (F2, 24=6.675, p<0.001) in the basal center time during 30 min after saline injection. P38 males showed significantly increased basal center times as compared to P28 (p<0.05) and adult males (p<0.01). In contrast, P38 females showed significantly increased basal levels as compared to P28 females (p<0.05) but not adults.
Figure 4.
Age and sex differences in the anxiolytic and anxiogenic effects of nicotine. Center time (s) from male (a, c, e) and female (b, d, f) animals aged P28 (a, b), P38 (c, d) and adult (e, f) were collected during the 30 min following i.v. injection of saline (open symbols) or nicotine (30 µg/kg/injection × 2; filled symbols). n=5–8 per group. * p<0.05, ** p<0.01 (Bonferroni) significantly different from saline treatment at the same age and sex.
At P28, nicotine increased center time, which indicates an anxiolytic effect, in both sexes with a more prolonged effect in males (p<0.05 at 5, 10 and 15 min in males, p<0.05 at 5 min in females, Fig. 4a, b). As with locomotor activity, there were no drug effects on center time in males or females aged P38 (Fig. 4c, d). In adults, there was a significant sex difference in basal anxiety levels, with saline-treated females spending significantly more time in the center of the locomotor apparatus than males within 30 min after injections (p<0.01). Nicotine significantly increased center time in adult males (p<0.05 at 5 min), but decreased it in adult females (p<0.05 at 5 and 15 min and p<0.01 at 10 min) (Fig. 4e, f). Thus nicotine induced anxiolytic effects in adult males, but anxiogenic effects in adult females.
Age and sex differences in nicotine-induced corticosterone release
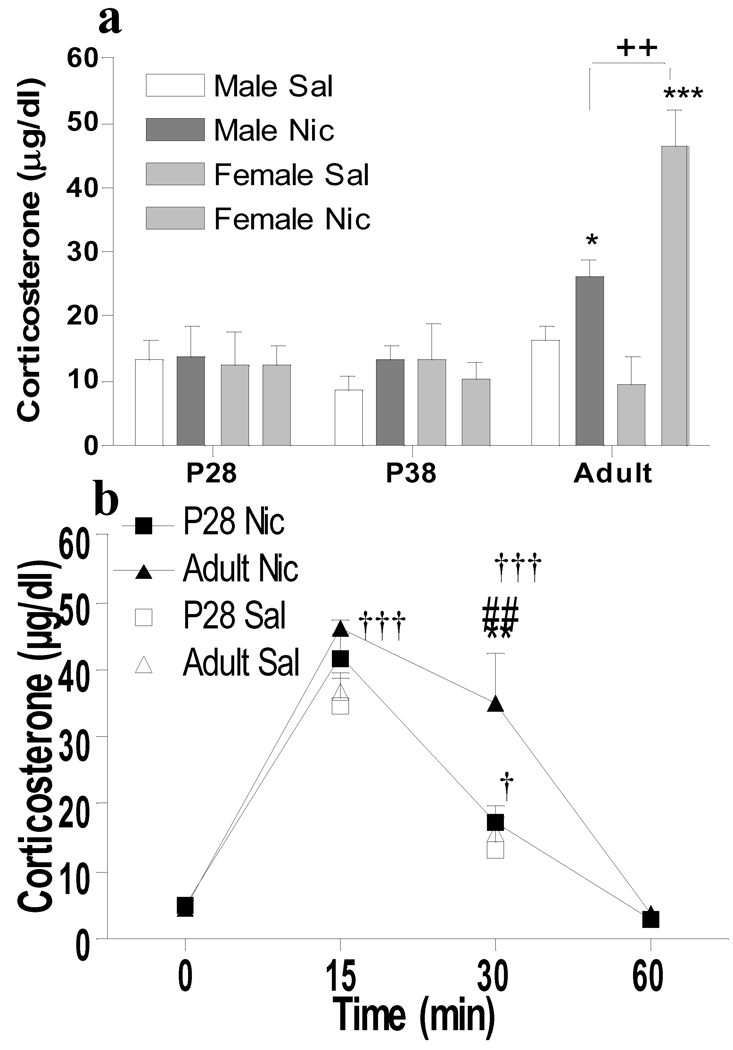
Plasma corticosterone levels in the behaviorally tested animals were evaluated 30 min after drug injections (Fig. 5a). Overall 3-way ANOVA for Age × Sex × Drug showed significant effects of age (F2, 62=11.9955, p<0.0001), drug (F1, 62=10.4744, p<0.005) and an interaction of age by drug (F2, 62=6.9233, p<0.005). Baseline corticosterone levels following saline injection did not show age (F2, 30=0.021, p=0.979) or sex differences (F1, 30=0.048, p=0.828). There was no significant nicotine-induced corticosterone release in either sex at P28 or P38. In contrast, nicotine significantly increased corticosterone release in both adult males (p<0.05) and females (p<0.001), with greater effects in females as compared to males (p<0.01).
Figure 5.
Age and sex differences in nicotine-induced corticosterone release. a) Plasma corticosterone levels in males and females, aged P28, P38 and adult, 30 min following i.v. injection of nicotine (30 µg/kg/injection × 2, male: closed bars; female: vertical bars) or saline (male: open bars; female: horizontal bars), n=5–8 per group. b) Time course of plasma corticosterone levels in P28 (squares) and adult (triangles) males, prior to or 15, 30 or 60 min after injection of nicotine (30 µg/kg/injection × 2, closed symbols) or saline (open symbols), n=5–6 per group. *p<0.01, **p<0.05, ***p<0.001 (Bonferroni) significantly different from saline treatment in the same sex. ++p<0.01(Bonferroni) significant sex differences. ##p<0.01 (Bonferroni) significant age differences. †p<0.05, †††p<0.001 (Bonferroni) significantly different from time point 0 at which no injections were conducted.
To further clarify age differences in nicotine-induced HPA activation, a time course of changes in plasma corticosterone levels was evaluated (Fig. 5b). Since there were no sex differences in HPA response to nicotine during adolescence, and P28 and P38 animals showed similar responses, the time course was evaluated only in P28 and adult males. Both adolescent and adult saline-injected controls showed significant elevations of plasma corticosterone 15 min after placement in the novel locomotor apparatus (p<0.01), which then declined substantially towards baseline levels by the 30 min timepoint. Nicotine had no effect as compared to saline-treated controls at 15 min in either age group. However, corticosterone levels were significantly increased 30 min after nicotine injection as compared to saline-treated controls (p<0.01) in adults, but not at P28.
Correlations
Correlational analysis revealed significant relationships between some effects of nicotine treatment (Table 1). There were no significant correlations between any measurements in saline treated females. In contrast, in nicotine-treated females, plasma corticosterone levels were negatively correlated with initial vertical and ambulatory counts (p<0.05), whereas positive correlations were observed between center time during the first 30 min after nicotine treatment and initial ambulatory counts (p<0.01) and vertical counts (p<0.001). In addition, initial vertical and ambulatory counts in nicotine treated females were positively correlated with each other (p<0.001).
Table 1.
Correlations between responses to nicotine.
| Female Saline |
Female Nicotine |
Male Saline |
Male Nicotine |
||
|---|---|---|---|---|---|
| Corticosterone Levels vs. Ambulatory Counts |
r | 0.2341 | −0.5152 | −0.1415 | −0.5162 |
| p | 0.3829 | 0.0343* | 0.5879 | 0.0283* | |
| Corticosterone Levels vs. Vertical Counts |
r | 0.4829 | −0.5268 | −0.03380 | −0.2658 |
| p | 0.0581 | 0.0247* | 0.8975 | 0.2863 | |
| Corticosterone Levels vs. Center time |
r | 0.0239 | −0.4480 | −0.2993 | −0.1002 |
| p | 0.9353 | 0.0622 | 0.3205 | 0.7224 | |
| Center time vs. Ambulatory Counts |
r | 0.4349 | 0.5540 | 0.7291 | 0.6911 |
| p | 0.1201 | 0.0210* | 0.0047** | 0.0043** | |
| Center Time vs. Vertical Counts |
r | 0.1652 | 0.5511 | 0.2061 | 0.8562 |
| p | 0.5726 | 0.0178* | 0.4992 | <0.0001*** | |
| Vertical Counts Vs. Ambulatory Counts |
r | 0.4339 | 0.6865 | 0.7476 | 0.8060 |
| p | 0.0931 | 0.0023** | 0.0006*** | <0.0001*** |
Pearson r and P value in correlation analysis of the responses to nicotine. Correlation analysis is applied to the combined data from three ages (P28, P38 and adult) of the same sex with the same drug treatment. n=13–18 pairs in each analysis.
p<0.05
p<0.01 and
p<0.001 significant correlations.
In contrast to females, significant positive correlations were observed in saline treated males between center time and ambulatory counts (p<0.01), as well as between vertical and ambulatory counts (p<0.001). In nicotine treated males, there was a significant negative correlation between corticosterone levels and ambulatory counts (p<0.05), but not between corticosterone levels and vertical counts. In contrast, center time positively correlated with both ambulatory and vertical counts (p<0.05), and there was a significant correlation between initial ambulatory and vertical counts in nicotine treated males (p<0.01).
DISCUSSION
Whereas prior studies have also shown age and/or sex differences in response to acute and chronic nicotine, comparisons are often confounded by differences in experimental conditions across studies. In the present analysis, we have therefore systematically evaluated behavioral and endocrine responses to nicotine in both sexes during three developmental stages - early adolescence, mid- adolescence and adulthood. This experimental design allowed us to evaluate all measures in parallel and undertake a correlation analyses to assess relationships between these drug responses. Understanding the mechanisms underlying such complex interactions may provide new insight into normative responses to nicotine during adolescence and permit development of novel strategies for the prevention and treatment of adolescent smoking.
We have found that there are complex age and sex differences in responses to acute nicotine treatment, which are dependent on the functional endpoint examined. Our data also show that adolescence is not a homogeneous period, but that there are significant differences in response to nicotine between early and mid-adolescence. This is consistent with the concept that adolescence consists of different stages (McCormick and Mathews, 2007), during which there may be large differences in drug response. Given that nicotine exerts its functions by acting on nicotinic acetylcholine receptors (nAChRs), the observed age differences suggest immature functions of nAChRs and their cellular substrates in adolescents, as has been reported previously (Leslie et al. , 2004, Placzek et al. , 2009).
Locomotor activity
The present findings are consistent with prior studies that have shown a transition from ambulatory stimulant effects of nicotine in early adolescence to depressant effects in adulthood (Cao et al., 2007a). We now also show that the peripubertal period, P38, is a transition point in which there is no ambulatory response to nicotine. The fact that locomotor responses to nicotine continue to change throughout adolescence may underlie the variability in the existing literature in which animals of different adolescent stages have been used (Belluzzi et al., 2004, Schochet et al. , 2004, Vastola et al., 2002).
Locomotor effects were observed only in the first five minutes after nicotine injection. Although a more detailed study with a one-minute interval would help us to determine when the drug effects exactly occurred, our data suggests an interaction between the drug and the novel environment, as has been shown previously in adults (O'Neill et al. , 1991). Locomotor activity in a novel environment is closely associated with self-administration of many addictive substances (Hooks et al. , 1991, Piazza et al. , 1989, Piazza et al. , 1998, Suto et al. , 2001) and this association is specifically observed with inescapable novelty (Klebaur et al. , 2001, Zheng et al. , 2004), as was used in the present study. Specifically, high responders (HRs) in a novel environment are more likely to self-administer addictive drugs than low responders (LRs), a finding that may result from higher nAChR activity in dopamine neurons of HR rats (Fagen et al. , 2007). Our data show that nicotine activation of nAChRs increases ambulatory activity in an inescapable novel environment only in younger adolescents, an interaction which may play a role in the increased vulnerability of this age group to tobacco addiction.
In contrast to horizontal activity, effects of nicotine on vertical activity are only evident in adults. Since vertical activity is a measure of exploratory behavior, the higher basal activity of younger adolescents may reflect increased novelty seeking (Spear, 2000). Although nicotine also decreased repetitive activity only in adults, basal repetitive activity was lower in adolescents as compared to adults, a finding that reflects dissociation of repetitive and vertical activities. The differences in basal repetitive activity may contribute to the age differences observed in nicotine’s effect, since animals at all three ages show a similar repetitive activity in response to nicotine. The lack of observed sex differences is consistent with a previous finding that sex steroids have little effect on acute nicotine-induced locomotor activity (Kanyt, et al. , 1999), although they do appear to play a role in the increased sensitivity of females to chronic nicotine treatment (Elliott, et al. , 2004, Kanyt, et al., 1999).
The age differences in locomotor responses to nicotine suggest that the underlying neuronal circuitries continue to mature during adolescence. The striatal dopamine system, which is important in motor control and is modulated by nAChRs, is still developing during adolescence (Andersen and Teicher, 2000, Cao et al. , 2007b, Tarazi et al. , 1999). Dopamine neurons within the ventral tegmental area are more excitable in adolescence than in adulthood, and more sensitive to nicotine-induced long-term potentiation (Navidad et al., 2010; Placzek et al., 2009). Furthermore, we have previously shown that the pharmacological properties of the nAChRs that regulate dopamine release from limbic terminals in the ventral striatum change significantly during adolescence (Azam et al., 2007). This finding is consistent with our current observation of differing ambulatory responses to nicotine in early and late adolescence, and with other behavioral studies in both rats and mice that have shown changing effects of nicotine during this developmental period (Adriani et al., 2002; Belluzzi et al., 2004; Kota et al., 2009; Park et al., 2007).
Center time
We have used the center time model, widely used to examine anxiety-like behaviors (Prut and Belzung, 2003), to evaluate the interactions of age and sex on the anxiogenic and/or anxiolytic effects of nicotine. Both baseline anxiety levels and drug effects were age dependent. Unlike locomotor activity, sex differences were observed in both early adolescence and adulthood. The peripubertal period (P38) was again a transition period, with no response to nicotine in either males or females.
Nicotine treatment increased center time in younger adolescents, with more prolonged effects in males than females. These data are consistent with prior findings of anxiolytic effects of nicotine in adolescents of both sexes in a social interaction test (Cheeta et al., 2001). In contrast, using an elevated plus maze paradigm, nicotine was anxiolytic in adolescent males but anxiogenic in females (Elliott et al., 2004). Unlike adolescents, sex differences were observed in baseline center time in adults, with females exhibiting less anxiety than males. Nicotine administration eliminated this sex difference, increasing center time in males, while decreasing it in females. Thus sex differences in nicotine effect on center time in adults may result, at least partially, from these inherent baseline differences. These findings are consistent with previous findings of baseline sex differences in anxiety levels of adult males and females (Ray and Hansen, 2004) , and that nicotine is anxiolytic in adult males (Sienkiewicz-Jarosz et al. , 2000). However, other studies employing different anxiety models have reported that nicotine has anxiogenic effects in both adult males and females (Elliott et al., 2004).
Numerous factors, such as drug dose, animal strain, housing conditions and anxiety test paradigm, may underlie these differences in experimental findings. Given the complexities of anxiety, in which one behavioral model may only test some but not all aspects of anxiety (Schmidt and Muller, 2006), caution is appropriate in interpreting these data. The center time model emphasizes explorative anxiety, which is not always correlated with social anxiety and somatic fear induced anxiety. Future studies will be necessary to examine other aspects of anxiety using different models such as social interaction and the conditioned fear approach.
Plasma corticosterone
Significant age and sex differences were seen in nicotine-induced corticosterone release. There was no drug-induced release of corticosterone in either males or females at early or mid-adolescence. However, there was a significant nicotine-induced corticosterone release in adults, with a stronger response in females than males. These data confirm and extend prior findings of lack of nicotine-induced HPA activation in adolescent males aged P27 (Cao et al.,2007a), and of sex differences in drug response in adults (Rhodes et al. , 2004, Rhodes et al., 2001).
The behavioral and endocrine studies were designed to model the early phase of self-administration in which animals are placed in a novel testing environment and monitored for drug responding. Consistent with prior self-administration testing conditions (Belluzzi et al., 2005, Levin et al.,2007, Levin, et al. , 2003, Park et al., 2007), animals were single housed after catheter implantation, a form of social stress that may potentially affect behavioral and hormone responses to nicotine (McCormick and Ibrahim, 2007, McCormick et al. , 2008). Since animals were not habituated to the experimental apparatus, there was an initial stress response as shown by elevated corticosterone levels at 15 minutes after saline injection. Although young animals were exposed to a relatively larger novel environment, as compared to adults, there were no age differences in corticosterone levels in saline-injected controls at any time point. This suggests that there were similar baseline stress responses in adolescent and adult males under our experimental condition. This finding is different from that of earlier studies in which prepubertal males have been shown to demonstrate a significantly prolonged stress response (Goldman et al. , 1973, Romeo et al. , 2006, Vazquez and Akil, 1993). Such previous studies have used stressors more rigorous than novelty, however, which may activate different central neuronal pathways (Herman and Cullinan, 1997).
Since no difference in hormonal response to nicotine was observed following behavioral observations in P28 and P38 animals, we restricted the time-course analysis to P28 and adult. Nicotine had no effect on either adolescent or adult males as compared to controls at 15 minutes after injection. The lack of drug effect is unlikely due to a ceiling effect since stress-induced corticosterone can reach much higher levels in both adults and adolescents (Choi and Kellogg, 1996, Rhodes, et al. , 2001), although the high basal hormonal level may have masked a small drug response. In contrast, nicotine significantly induced corticosterone release in adults but not in adolescents at 30 minutes post-injection, when the effect of the experimental procedure on HPA activation had substantially diminished in both age groups. Such age differences in nicotine effect are independent of basal stress levels and testing environment, since we have previously demonstrated age differences in nicotine-induced corticosterone release and hypothalamic paraventricular nucleus (PVN) c-fos expression in male rats in which basal hormone levels were decreased by daily handling and habituation to the experimental apparatus (Cao et al., 2007a). Our findings are consistent with a recent study that showed lack of PVN activation following a single treatment with nicotine in adolescent males and females under different stressor conditions (McCormick and Ibrahim, 2007). Although previous studies have shown comparable HPA activation by nicotine in adolescent and adult males (Cruz et al., 2008, Matta et al., 1987), differences in experimental parameters such as drug dose and route of drug administration may produce differing results. In the current study, we used a low intravenous nicotine dose that produces blood levels equivalent to that following 3–4 cigarettes (Cao et al., 2007a). To further evaluate these age differences in HPA response, however, studies on the dose effect will be necessary.
Correlations between nicotine end-points
Within-animal correlational analysis of behavioral data and plasma corticosterone levels across ages yielded complex findings. Whereas saline-treated females exhibited no correlations between any of the functional endpoints measured, saline-treated males showed significant positive correlations between initial ambulatory counts and both initial vertical counts and total center time. Nicotine treatment resulted in increased correlations in both sexes, with a significant negative correlation between initial ambulatory counts and corticosterone levels at 30 minutes. In contrast, there were no correlations between anxiety in a novel environment, as indicated by center time, and HPA activation. This lack of correlation has also reported by others for the elevated plus maze model (Marquez et al. , 2006).
Conclusions
In the present study, we have provided evidence that early adolescence is a period of distinct behavioral and endocrine responses to nicotine as compared to adulthood. For some functional endpoints, such as horizontal activity and center time, there were unique effects of nicotine during this period. For others, such as vertical and repetitive activities and corticosterone release, it was a period of blunted response in comparison to adulthood. Mid-adolescence is also a unique period in which no responses to nicotine were seen in any of the paradigms examined. These differing drug responses of early and mid-adolescence likely reflect maturation of nAChRs and their underlying neuronal circuitry, which differ depending on the functional endpoint examined. These differences in pharmacological response may also relate to the clinical observation that younger adolescents are more vulnerable to the initiation of smoking as compared to older teens (Chen and Millar, 1998). Observed sex differences in response to nicotine were dependent on the functional endpoint examined and age, with most differences appearing after the reproductive system matures. Together the present studies demonstrate that adolescents respond to nicotine quite differently from adults. It is therefore important to study the unique mechanisms underlying initiation of smoking during adolescence in order to develop more effective prevention strategies and cessation therapies for smoking.
ACKNOWLEDGEMENTS
This study is supported by PHS grant DA 19138.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adriani W, Macrì S, Pacifici R, Laviola G. Peculiar vulnerability to nicotine oral self-administration in mice during early adolescence. Neuropsychopharmacology. 2002;27:212–224. doi: 10.1016/S0893-133X(02)00295-6. [DOI] [PubMed] [Google Scholar]
- al'Absi M. Hypothalamic-pituitary-adrenocortical responses to psychological stress and risk for smoking relapse. Int J Psychophysiol. 2006;59:218–227. doi: 10.1016/j.ijpsycho.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Teicher MH. Sex differences in dopamine receptors and their relevance to ADHD. Neurosci Biobehav Rev. 2000;24:137–141. doi: 10.1016/s0149-7634(99)00044-5. [DOI] [PubMed] [Google Scholar]
- Balfour DJ, Khullar AK, Longden A. Effects of nicotine on plasma corticosterone and brain amines in stressed and unstressed rats. Pharmacol Biochem Behav. 1975;3:179–184. doi: 10.1016/0091-3057(75)90145-8. [DOI] [PubMed] [Google Scholar]
- Belluzzi JD, Lee AG, Oliff HS, Leslie FM. Age-dependent effects of nicotine on locomotor activity and conditioned place preference in rats. Psychopharmacology (Berl) 2004;174:389–395. doi: 10.1007/s00213-003-1758-6. [DOI] [PubMed] [Google Scholar]
- Belluzzi JD, Wang R, Leslie FM. Acetaldehyde enhances acquisition of nicotine self-administration in adolescent rats. Neuropsychopharmacology. 2005;30:705–712. doi: 10.1038/sj.npp.1300586. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Porchet H, Jacob IP. Pharmacokinetics, metabolism, and pharmacodynamics of nicotine. In: Wonnacott S RM, Stolerman IP, editors. Nicotine Pharmacology: Molecular, Celllular, and Behavioral Aspects. Oxford: Oxford University Press; 1990. pp. 112–151. [Google Scholar]
- Caggiula AR, Donny EC, Epstein LH, Sved AF, Knopf S, Rose C, et al. The role of corticosteroids in nicotine's physiological and behavioral effects. Psychoneuroendocrinology. 1998;23:143–159. doi: 10.1016/s0306-4530(97)00078-4. [DOI] [PubMed] [Google Scholar]
- Cam GR, Bassett JR. The effect of acute nicotine administration on plasma levels of the thyroid hormones and corticosterone in the rat. Pharmacol Biochem Behav. 1983;19:559–561. doi: 10.1016/0091-3057(83)90135-1. [DOI] [PubMed] [Google Scholar]
- Cao J, Belluzzi JD, Loughlin SE, Keyler DE, Pentel PR, Leslie FM. Acetaldehyde, a major constituent of tobacco smoke, enhances behavioral, endocrine, and neuronal responses to nicotine in adolescent and adult rats. Neuropsychopharmacology. 2007a;32:2025–2035. doi: 10.1038/sj.npp.1301327. [DOI] [PubMed] [Google Scholar]
- Cao J, Lotfipour S, Loughlin SE, Leslie FM. Adolescent maturation of cocaine-sensitive neural mechanisms. Neuropsychopharmacology. 2007b;32:2279–2289. doi: 10.1038/sj.npp.1301349. [DOI] [PubMed] [Google Scholar]
- Cheeta S, Irvine EE, Tucci S, Sandhu J, File SE. In adolescence, female rats are more sensitive to the anxiolytic effect of nicotine than are male rats. Neuropsychopharmacology. 2001;25:601–607. doi: 10.1016/S0893-133X(01)00258-5. [DOI] [PubMed] [Google Scholar]
- Chen H, Matta SG, Sharp BM. Acquisition of Nicotine Self-Administration in Adolescent Rats Given Prolonged Access to the Drug. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301135. [DOI] [PubMed] [Google Scholar]
- Chen J, Millar WJ. Age of smoking initiation: implications for quitting. Health reports / Statistics Canada, Canadian Centre for Health Information = Rapports sur la sante / Statistique Canada, Centre canadien d'information sur la sante. 1998;9:39–46. (Eng); 39–48(Fre) [PubMed] [Google Scholar]
- Choi S, Kellogg CK. Adolescent development influences functional responsiveness of noradrenergic projections to the hypothalamus in male rats. Brain Res Dev Brain Res. 1996;94:144–151. doi: 10.1016/s0165-3806(96)80005-8. [DOI] [PubMed] [Google Scholar]
- Critchlow V, Liebelt RA, Bar-Sela M, Mountcastle W, Lipscomb HS. Sex difference in resting pituitary-adrenal function in the rat. The American journal of physiology. 1963;205:807–815. doi: 10.1152/ajplegacy.1963.205.5.807. [DOI] [PubMed] [Google Scholar]
- Cruz FC, DeLucia R, Planeta CS. Effects of chronic stress on nicotine-induced locomotor activity and corticosterone release in adult and adolescent rats. Addict Biol. 2008;13:63–69. doi: 10.1111/j.1369-1600.2007.00080.x. [DOI] [PubMed] [Google Scholar]
- DiFranza JR. Hooked from the first cigarette. Sci Am. 2008;298:82–87. doi: 10.1038/scientificamerican0508-82. [DOI] [PubMed] [Google Scholar]
- DiFranza JR, Wellman RJ, Sargent JD, Weitzman M, Hipple BJ, Winickoff JP. Tobacco promotion and the initiation of tobacco use: assessing the evidence for causality. Pediatrics. 2006;117:e1237–e1248. doi: 10.1542/peds.2005-1817. [DOI] [PubMed] [Google Scholar]
- Elliott BM, Faraday MM, Phillips JM, Grunberg NE. Effects of nicotine on elevated plus maze and locomotor activity in male and female adolescent and adult rats. Pharmacol Biochem Behav. 2004;77:21–28. doi: 10.1016/j.pbb.2003.09.016. [DOI] [PubMed] [Google Scholar]
- Fagen ZM, Mitchum R, Vezina P, McGehee DS. Enhanced nicotinic receptor function and drug abuse vulnerability. J Neurosci. 2007;27:8771–8778. doi: 10.1523/JNEUROSCI.2017-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman L, Winget C, Hollingshead GW, Levine S. Postweaning development of negative feedback in the pituitary-adrenal system of the rat. Neuroendocrinology. 1973;12:199–211. doi: 10.1159/000122169. [DOI] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- Hooks MS, Jones GH, Smith AD, Neill DB, Justice JB., Jr Response to novelty predicts the locomotor and nucleus accumbens dopamine response to cocaine. Synapse. 1991;9:121–128. doi: 10.1002/syn.890090206. [DOI] [PubMed] [Google Scholar]
- Isiegas C, Mague SD, Blendy JA. Sex differences in response to nicotine in C57Bl/6:129SvEv mice. Nicotine Tob Res. 2009;11:851–858. doi: 10.1093/ntr/ntp076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel DB, Logan JA. Patterns of drug use from adolescence to young adulthood: I. Periods of risk for initiation, continued use, and discontinuation. Am J Public Health. 1984;74:660–666. doi: 10.2105/ajph.74.7.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanyt L, Stolerman IP, Chandler CJ, Saigusa T, Pogun S. Influence of sex and female hormones on nicotine-induced changes in locomotor activity in rats. Pharmacol Biochem Behav. 1999;62:179–187. doi: 10.1016/s0091-3057(98)00140-3. [DOI] [PubMed] [Google Scholar]
- Klebaur JE, Bevins RA, Segar TM, Bardo MT. Individual differences in behavioral responses to novelty and amphetamine self-administration in male and female rats. Behav Pharmacol. 2001;12:267–275. doi: 10.1097/00008877-200107000-00005. [DOI] [PubMed] [Google Scholar]
- Kota D, Martin BR, Damaj MI. Age-dependent differences in nicotine reward and withdrawal in female mice. Psychopharmacology (Berl) 2008;198:201–210. doi: 10.1007/s00213-008-1117-8. [DOI] [PubMed] [Google Scholar]
- Kota D, Martin BR, Robinson SE, Damaj MI. Nicotine dependence and reward differ between adolescent and adult male mice. J Pharmacol Exp Ther. 2007;322:399–407. doi: 10.1124/jpet.107.121616. [DOI] [PubMed] [Google Scholar]
- Leslie FM, Loughlin SE, Wang R, Perez L, Lotfipour S, Belluzzia JD. Adolescent development of forebrain stimulant responsiveness: insights from animal studies. Annals of the New York Academy of Sciences. 2004;1021:148–159. doi: 10.1196/annals.1308.018. [DOI] [PubMed] [Google Scholar]
- Levin ED, Lawrence SS, Petro A, Horton K, Rezvani AH, Seidler FJ, Adolescent vs, et al. adult-onset nicotine self-administration in male rats: duration of effect and differential nicotinic receptor correlates. Neurotoxicol Teratol. 2007;29:458–465. doi: 10.1016/j.ntt.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Rezvani AH, Montoya D, Rose JE, Swartzwelder HS. Adolescent-onset nicotine self-administration modeled in female rats. Psychopharmacology (Berl) 2003;169:141–149. doi: 10.1007/s00213-003-1486-y. [DOI] [PubMed] [Google Scholar]
- Lynch WJ. Sex and ovarian hormones influence vulnerability and motivation for nicotine during adolescence in rats. Pharmacol Biochem Behav. 2009;94:43–50. doi: 10.1016/j.pbb.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez C, Nadal R, Armario A. Influence of reactivity to novelty and anxiety on hypothalamic-pituitary-adrenal and prolactin responses to two different novel environments in adult male rats. Behav Brain Res. 2006;168:13–22. doi: 10.1016/j.bbr.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Marshall L, Schooley M, Ryan H, Cox P, Easton A, Healton C, et al. Youth tobacco surveillance--United States, 2001–2002. MMWR Surveill Summ. 2006;55:1–56. [PubMed] [Google Scholar]
- Matta SG, Beyer HS, McAllen KM, Sharp BM. Nicotine elevates rat plasma ACTH by a central mechanism. J Pharmacol Exp Ther. 1987;243:217–226. [PubMed] [Google Scholar]
- Matta SG, Fu Y, Valentine JD, Sharp BM. Response of the hypothalamo-pituitary-adrenal axis to nicotine. Psychoneuroendocrinology. 1998;23:103–113. doi: 10.1016/s0306-4530(97)00079-6. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Ibrahim FN. Locomotor activity to nicotine and Fos immunoreactivity in the paraventricular nucleus of the hypothalamus in adolescent socially-stressed rats. Pharmacol Biochem Behav. 2007;86:92–102. doi: 10.1016/j.pbb.2006.12.012. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Mathews IZ. HPA function in adolescence: role of sex hormones in its regulation and the enduring consequences of exposure to stressors. Pharmacol Biochem Behav. 2007;86:220–233. doi: 10.1016/j.pbb.2006.07.012. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Smith C, Mathews IZ. Effects of chronic social stress in adolescence on anxiety and neuroendocrine response to mild stress in male and female rats. Behav Brain Res. 2008;187:228–238. doi: 10.1016/j.bbr.2007.09.005. [DOI] [PubMed] [Google Scholar]
- McQuown SC, Belluzzi JD, Leslie FM. Low dose nicotine treatment during early adolescence increases subsequent cocaine reward. Neurotoxicol Teratol. 2007;29:66–73. doi: 10.1016/j.ntt.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill MF, Dourish CT, Iversen SD. Evidence for an involvement of D1 and D2 dopamine receptors in mediating nicotine-induced hyperactivity in rats. Psychopharmacology (Berl) 1991;104:343–350. doi: 10.1007/BF02246034. [DOI] [PubMed] [Google Scholar]
- Park MK, Belluzzi JD, Han SH, Cao J, Leslie FM. Age, sex and early environment contribute to individual differences in nicotine/acetaldehyde-induced behavioral and endocrine responses in rats. Pharmacol Biochem Behav. 2007;86:297–305. doi: 10.1016/j.pbb.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Park MK, Loughlin SE, Leslie FM. Gestational nicotine-induced changes in adolescent neuronal activity. Brain research. 2006;1094:119–126. doi: 10.1016/j.brainres.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Pauly JR, Ullman EA, Collins AC. Adrenocortical hormone regulation of nicotine sensitivity in mice. Physiol Behav. 1988;44:109–116. doi: 10.1016/0031-9384(88)90353-8. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Donny E, Caggiula AR. Sex differences in nicotine effects and self-administration: review of human and animal evidence. Nicotine Tob Res. 1999;1:301–315. doi: 10.1080/14622299050011431. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminiere JM, Le Moal M, Simon H. Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989;245:1511–1513. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deroche V, Rouge-Pont F, Le Moal M. Behavioral and biological factors associated with individual vulnerability to psychostimulant abuse. NIDA Res Monogr. 1998;169:105–133. [PubMed] [Google Scholar]
- Picciotto MR, Brunzell DH, Caldarone BJ. Effect of nicotine and nicotinic receptors on anxiety and depression. Neuroreport. 2002;13:1097–1106. doi: 10.1097/00001756-200207020-00006. [DOI] [PubMed] [Google Scholar]
- Placzek AN, Zhang TA, Dani JA. Age dependent nicotinic influences over dopamine neuron synaptic plasticity. Biochem Pharmacol. 2009;78:686–692. doi: 10.1016/j.bcp.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. European journal of pharmacology. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- Ray J, Hansen S. Temperament in the rat: sex differences and hormonal influences on harm avoidance and novelty seeking. Behav Neurosci. 2004;118:488–497. doi: 10.1037/0735-7044.118.3.488. [DOI] [PubMed] [Google Scholar]
- Rhodes ME, Kennell JS, Belz EE, Czambel RK, Rubin RT. Rat estrous cycle influences the sexual diergism of HPA axis stimulation by nicotine. Brain Res Bull. 2004;64:205–213. doi: 10.1016/j.brainresbull.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Rhodes ME, O'Toole SM, Czambel RK, Rubin RT. Male-female differences in rat hypothalamic-pituitary-adrenal axis responses to nicotine stimulation. Brain Res Bull. 2001;54:681–688. doi: 10.1016/s0361-9230(01)00488-9. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Bellani R, Karatsoreos IN, Chhua N, Vernov M, Conrad CD, et al. Stress History and Pubertal Development Interact to Shape Hypothalamic-Pituitary-Adrenal Axis Plasticity 10.1210/en.2005-1432. Endocrinology. 2006;147:1664–1674. doi: 10.1210/en.2005-1432. [DOI] [PubMed] [Google Scholar]
- Schmidt MV, Muller MB. Animal models of anxiety. Nervous system disorders. 2006;3:369–374. [Google Scholar]
- Schochet TL, Kelley AE, Landry CF. Differential behavioral effects of nicotine exposure in adolescent and adult rats. Psychopharmacology (Berl) 2004;175:265–273. doi: 10.1007/s00213-004-1831-9. [DOI] [PubMed] [Google Scholar]
- Sienkiewicz-Jarosz H, Czlonkowska AI, Siemiatkowski M, Maciejak P, Szyndler J, Plaznik A. The effects of physostigmine and cholinergic receptor ligands on novelty-induced neophobia. J Neural Transm. 2000;107:1403–1412. doi: 10.1007/s007020070004. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Stanton WR, McClelland M, Elwood C, Ferry D, Silva PA. Prevalence, reliability and bias of adolescents' reports of smoking and quitting. Addiction (Abingdon, England) 1996;91:1705–1714. [PubMed] [Google Scholar]
- Suto N, Austin JD, Vezina P. Locomotor response to novelty predicts a rat's propensity to self-administer nicotine. Psychopharmacology (Berl) 2001;158:175–180. doi: 10.1007/s002130100867. [DOI] [PubMed] [Google Scholar]
- Tarazi FI, Tomasini EC, Baldessarini RJ. Postnatal development of dopamine D1-like receptors in rat cortical and striatolimbic brain regions: An autoradiographic study. Dev Neurosci. 1999;21:43–49. doi: 10.1159/000017365. [DOI] [PubMed] [Google Scholar]
- Torres OV, Natividad LA, Tejeda HA, Van Weelden SA, O'Dell LE. Female rats display dose-dependent differences to the rewarding and aversive effects of nicotine in an age-, hormone-, and sex-dependent manner. Psychopharmacology (Berl) 2009;206:303–312. doi: 10.1007/s00213-009-1607-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres OV, Tejeda HA, Natividad LA, O'Dell LE. Enhanced vulnerability to the rewarding effects of nicotine during the adolescent period of development. Pharmacol Biochem Behav. 2008;90:658–663. doi: 10.1016/j.pbb.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vastola BJ, Douglas LA, Varlinskaya EI, Spear LP. Nicotine-induced conditioned place preference in adolescent and adult rats. Physiol Behav. 2002;77:107–114. doi: 10.1016/s0031-9384(02)00818-1. [DOI] [PubMed] [Google Scholar]
- Vazquez DM, Akil H. Pituitary-adrenal response to ether vapor in the weanling animal: characterization of the inhibitory effect of glucocorticoids on adrenocorticotropin secretion. Pediatr Res. 1993;34:646–653. doi: 10.1203/00006450-199311000-00017. [DOI] [PubMed] [Google Scholar]
- Wilmouth CE, Spear LP. Adolescent and adult rats' aversion to flavors previously paired with nicotine. Annals of the New York Academy of Sciences. 2004;1021:462–464. doi: 10.1196/annals.1308.065. [DOI] [PubMed] [Google Scholar]
- Zheng XG, Tan BP, Luo XJ, Xu W, Yang XY, Sui N. Novelty-seeking behavior and stress-induced locomotion in rats of juvenile period differentially related to morphine place conditioning in their adulthood. Behav Processes. 2004;65:15–23. doi: 10.1016/s0376-6357(03)00151-7. [DOI] [PubMed] [Google Scholar]