Summary
The clinical successes of adaptive transfer of in vitro-expanded antigen-specific CD8+ T cells isolated from patients’ tumors has demonstrated that effector cells of the adaptive immune system can effectively eliminate even large tumor masses. Nevertheless, cancer vaccines that aim to expand such CD8+ T cells in situ have had remarkably little success in spite of numerous attempts. Recent advances in basic immunology have revealed layers of complexity controlling activation and maintenance of adaptive immune responses that are tightly controlled by immunoinhibitory pathways to avoid horror autotoxicus. During tumor progression the activities of negative pathways increase and together with cancer immune evasion tactics presumably prevent induction of an efficacious immune response by cancer vaccines that solely provide more antigen to an already suppressed system. Cancer vaccines may thus need to readjust the imbalance of the cancer patients’ immune system by inhibiting immunoinhibitors; such regimens have shown pre-clinical efficacy and are now entering clinical trials hopefully ending the Kafkaesque futility of cancer vaccines.
Introduction
Vaccines to cancer unlike those to infectious agents face a distinct set of challenges. Vaccines to pathogens in general are given prophylactically to an immunologically naive individual with the goal to induce potent and sustained immune responses that will provide protection against an infection that may occur in the future. For most viruses neutralizing antibodies correlate with protection and they can be induced by attenuated or inactivated virions, virus-like particles, adjuvanted proteins or other types of vaccines.
Vaccines to cancer in contrast are meant as therapeutics for individuals with tumors that are large enough for detection and not curable by other means such as surgery. Cancer vaccines thus unlike preventative vaccines aim to induce immunity in an individual whose immune system has experienced the antigens but is unable to mount immune responses that effectively eliminate the transformed cells. Most tumor-associated antigens (TAA) that could form the basis for therapeutic vaccines are in the cytoplasm or nucleus not readily accessible to antibodies [1]. Cancer vaccines are thus in general designed to elicit T cell responses but for some exceptions where cell surface molecules on tumor cells may serve as specific targets.
Cancer vaccines are theoretically immensely appealing for their potential to replace therapeutics with high toxicity and a relative lack of precision with a highly targeted immune response that very specifically eliminates tumor cells while causing little or no harm to normal cells. Unfortunately, although commonly effective in experimental animals, cancer vaccines have had little success in humans with advanced disease [2]. Notwithstanding, in some types of cancers such as melanoma, adoptive transfer of in vitro expanded CD8+ T cells isolated from the patients’ tumors combined with partial myeloablation can eliminate massive malignancies when transferred back into the patients [3] while in contrast vaccines that aim to induce or expand such CD8+ T cells in vivo are ineffective. Notwithstanding, the success of adoptive T cell transfer clearly shows that the immune system can indeed cause tumor regression and that thus active cancer immunotherapy should be an attainable goal. Lack of success thus far may reflect impairments of the patients’ tumor-specific immune mechanisms that prevent vaccine-induced expansion of high quality tumor antigen-specific CD8+ T cells. Vaccines to cancer thus have to be formulated to not only express TAAs for induction or expansion of a potent T cell response but also to overcome immune dysfunctions and inhibitory pathways evoked during tumor progression.
Status quo
Two vaccines commonly viewed as cancer vaccines have been licensed. Both are prophylactic vaccines against viral proteins, i.e., hepatitis B surface antigen for prevention of hepatocellular carcinoma [4] and virus particles formed by L1, the major capsid protein of human papillomavirus (HPV), to prevent cervical cancer [5]. These two types of vaccines that are highly effective are primarily anti-viral vaccines, which block infections through the induction of neutralizing antibodies. Thus far not a single therapeutic cancer vaccine has been licensed although Provenge, a vaccine based on dendritic cells (DCs) transduced to expressed granulocyte macrophage colont-stimulaying factor (GM-CSF) and prostatic acid phosphatase (PAP) shows a modest survival benefit of ~ 4 months in end-stage prostate cancer patients [6] and is expected to be approved in 2010 for use in the US by the Food and Drug Administration (FDA).
A cancer vaccine based on an idiotypic antibody mixed with GM-CSF prolongs time to relapse in patients with follicular lymphomas [7] and may be licensed in the near future. Another vaccine using synthetic long peptides of HPV-16 E6 and E7 oncoproteins mixed with incomplete Freund’s adjuvant administrated in women with HPV-16–positive high-grade vulvar intraepithelial neoplasia induced T cell responses in all vaccinated women. Moreover, 79% of the patients showed clinical responses and 5 in 20 patients showed complete regression of their lesions [8]. Despite recent advances, the paucity of potential vaccines is not due to lack of effort but rather reflects a lack of success; the NCI website (http://www.cancer.gov/clinicaltrials) lists a total of 610 completed clinical trials for cancer vaccines and many more are in pre-clinical development.
Cancer and the Immune System
Why has the scientific community failed to generate efficacious cancer vaccines although numerous TAAs that could serve as targets have been identified? One would assume that at the initial stages of tumor development TAAs are largely ignored by the immune system, as they are not presented by mature professional antigen presenting cells (APCs). Once a tumor grows and causes damage to the surrounding tissue or becomes in part necrotic, inflammatory reactions are triggered driving maturation of APCs, which at this stage should present the TAAs and initiate adaptive immune responses that eliminate the tumor. Indeed in healthy individuals T cells are apparently able to eliminate tumor cells early on before diagnosis, as there is ample evidence that some types of cancer, especially those caused by viral oncoproteins, are less common in immunocompetent individuals [9].
The argument could be made that TAAs are as a rule poorly immunogenic. They are commonly differentiation antigens or mutated self-antigens to which the patient’s immune system is tolerant [9]. Others such as those from oncogenic viruses are foreign and should more readily induce potent T cell responses. However many of those are short polypeptides such as the E6 and E7 oncoproteins of HPV that may lack potent epitopes while others such as those from herpes viruses encode proteins that allow them to avoid detection or destruction by the immune system [10]. Tumors actively suppress induction or effector functions of T cells [11]; they secrete inhibitory cytokines such as prostaglandins, TGF-β, IL-10 and vascular endothelial growth factors [12,13], they down-regulate molecules that are part of the antigen presentation pathway such as MHC class I molecules [14] or TAP [15], they up-regulate anti-apoptotic molecules, such as Bcl-X(L), Mcl-1, c-IAP1, XIAP or survivin [16] and molecules that can kill or subvert effector T cells such as FasL [17] or members of the B7 family (PD-L1, B7-H3 or B7-H4, [18]) and expand T cells with immunoinhibitory functions, such as CD4+CD25+FoxP3+ Tregs [19]. In addition TAA-specific CD8+ T cells may lose function over time and differentiate towards exhaustion as a reflection of chronic antigenic exposure similar to T cells found in chronic viral infection [20], although experimental evidences have thus far not demonstrated T cell exhaustion during cancer progression [21].
Animal Models for Pre-Clinical Testing of Cancer Vaccines
Initial pre-clinical testing of cancer vaccines, especially those that aim to induce protective T cell responses is generally conducted in inbred strains of mice, which are genetically well defined and for which reagents for analyses of vaccine immunogenicity are in ample supply. Therapeutic cancer vaccines have thus far been tested mainly in so-called transplantable tumor cell models. Protection achieved in transplantable tumor models most likely paints an unduly optimistic picture as such models fail to take into account that slowly progressing tumors have profound effects on the adaptive immune system.
Advances in molecular biology have allowed for the engineering of transgenic mice, which develop cancers in specific tissues due to overexpression of oncoproteins or deletion of tumor suppressor genes (Mouse Models for Human Cancers Consortium, http://mouse.ncifcrf.gov). Conditional deletions of proteins crucial for cell cycle control allows for a further refinement of such models [22,23]. Models based on cancer-prone mice should allow for a more realistic assessment of the immunogenicity and efficacy of cancer vaccines.
Traditional Cancer Vaccines
A multitude of vaccine prototypes has been tested for induction of cellular immune responses to TAAs [24–27]. These have included peptides, adjuvanted proteins, DNA vaccines and viral replicons, viral or bacterial recombinant vaccines, and antigen- or RNA-pulsed DCs or tumor cells modified to express cytokines, chemokines or other immunomodulatory factors [28]. Vaccines have been combined with immunoactivating agents or with chemotherapy [29] and many of these regimens showed efficacy in animal models but then failed in clinical trials. The justifiable argument has been made that addition of more antigen delivered by a vaccine in context of immunostimulation to a cancer patient who is already bathed in antigen and commonly at the time of immunization has mounted a detectable albeit ineffective TAA-specific T cell response may be imprudent [30].
Cancer Vaccines that Target Immunoinhibitory Pathways
The outcome of an antigenic stimulus is tightly controlled by positive and negative signals delivered at the molecular and cellular level. Negative and positive signals are supplied by members of the B7 family [31] such as B7.1 (CD80) or B7.2 (CD86), which afford co-stimulation to T cells expressing CD28 while inhibiting those expressing CTLA-4 (CD152). B7-H2 (ICOS-L) imparts both activating and suppressing signals through interactions with ICOS while B7-H1 (PD-L1), B7-DC (PD-L2), B7-H3 (B7RP-2) and B7-H4 (B7x,B7S1) are inhibitory [32–34]. B7 family members are expressed on mature APCs and many can be detected on non-lymphoid cells and within tumors [18]; the latter is assumed to favor tumor progression by reducing efficacious immune responses and potentially expanding inhibitory Tregs. Treatments that combine TAA vaccines with agents that interfere with immunoinhibitory B7 molecules, such as blockers of CTLA-4 or PD-1/PD-L1/L2 interactions have yielded promising pre-clinical results [35,36], and are now undergoing clinical testing [35].
Members of the tumor necrosis factor family, such as the herpesvirus entry mediator (HVEM) modulate immune responses [37]. HVEM triggers activation upon binding to LIGHT or lymphotoxin-α (LTα), while ligation through a distinct HVEM domain to the B and T lymphocyte attenuator (BTLA) or CD160 imposes suppressive signals. HVEM is expressed on APCs and Tregs; interactions between HVEM on APCs and BTLA on T cells dampens the response during antigenic stimulation [38] while binding of BTLA on T effector cells to HVEM on Tregs can cause immunoinhibition at a later stage [39]. CD160 unlike BTLA is not expressed on naïve T cells and presumably affects already activated T cells [40], such as those differentiating towards exhaustion, which is hallmarked by a gradual increase in the expression of immunoinhibitors, such as PD-1, LAG-3, CD160 and others [41]. Interactions between HVEM and BTLA/CD160 can be blocked by the N-terminal domain of the herpes simplex virus (HSV) glycoprotein D (gD, [42,43]). Vaccines that express antigens as fusion proteins with the C terminus of gD induce higher T and B cell responses and may rescue T cells rendered tolerant or ineffective by chronic antigenic exposure unlike those that express solely the antigen [42].
Tumor cells secrete cytokines, such as IL-10 and TGF-β, and chemokines, such as CCL2 or CCL12, which reduce functions of effector T cells and in part augment Treg responses [12,44]. Blockade of such factors results in higher TAA-specific T cell responses and a reduction in Tregs and could be combined with active immunization [45]. Tregs, presumably belonging preferentially to the subset of adaptive Tregs, commonly accumulate within a tumor microenvironment and are associated with poor prognosis. The use of agents that eliminate or inactivate Tregs, such as antibodies to specific surface markers or denileukin diftitox, a fusion protein consisting of IL-2 and diphtheria toxin, have shown pre-clinical and clinical successes and are being combined with active immunotherapy [46].
Cancer Vaccines for the Aged
Another predicament of cancer vaccines needs to be considered. The elderly constitute an increasing portion of the human population and cancer is primarily a disease of the elderly. Aging is accompanied by immunosenescence, a general impairment of most aspects of innate and adaptive immunity, resulting in an increased susceptibility to infectious agents and presumably cancer and an inability to mount protective immune responses to vaccines [47].
Primary B cell responses in the elderly are commonly low and short-lived [48], germinal center formation is decreased, antigen transport is impaired and the follicular dendritic cells’ capacity to form antigen depots is reduced [49,50]. Auto-antibodies are more common and the B cell repertoire is less diverse. T cells show clonal senescence, their potential for expansion and their ability to produce certain cytokines or to respond to cytokines decreases. The proportion of T cells with a memory cell phenotype increases while numbers of naïve T cells decrease. CD4+ T cells, show reduced expression of essential co-stimulatory receptors [51–53]. Stimulation with new antigens appears to result in shortened immunological memory [54]. The T cell repertoire looses diversity [55,56]. Effects of aging on inhibitory pathways of the immune system remain understudied.
Mouse studies have shown that expression levels of co-inhibitory molecules, such as PD-1, ICOS, CTLA-4, Tim-3, LAG-3 and KLRG-1, increase on CD4+ T cells with aging [57,58]. Our studies did not reveal significant differences in imunoinhibitory markers on antigen-specific CD8+ T cells [59] and vaccines that expressed gD together with the antigen enhanced CD8+ T cell responses in young as well as in aged mice. Although numbers of regulatory T cells appear to increase with age [60], auto-immunity is more common suggesting that inhibitory pathways may become impaired [61]. The causes underlying immunosenescence remain understudied and a better understanding of the effects of aging on inhibitory pathways may affect the efficacy of vaccines in their most common target population. It should also be pointed out that the elderly are a very heterogeneous population, ranging from those that are healthy and functionally independent to those that are frail and functionally impaired. Accordingly, immune responses in the aged are by far more variable than those in young individuals, which add an additional layer of complexity to the interpretation of vaccine trials in such cohorts.
Conclusion
Considering the profound effects of progressing tumors on the adaptive immune responses, which in many aspects resembles that of chronic infection to which therapeutic vaccines are direly needed but thus far elusive as well, cancer vaccines need to be formulated to not only provide TAAs but also agents that readjust a system whose equilibrium is tilted strongly towards suppression of the responses the vaccines seek to expand. Genetically engineered mice that show a high incidence of predictable cancers may aid the intelligent design of such vaccine.
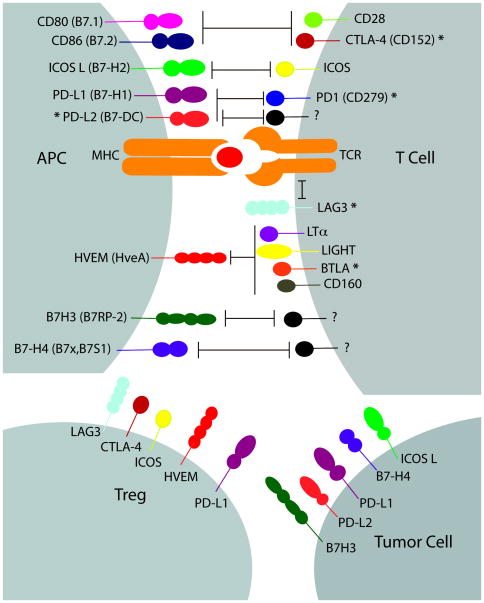
Figure 1.
T cell activation requires two signals; signal one is delivered through the T-cell receptor (TCR) by its engagement with a peptide–major histocompatibility complex (MHC), and signal two is delivered by engagement of either B7 family members or other co-stimulatory/co-inhibitory molecules, which are involved in fine-tuning T cell responses [31]. The binding of CD80 (B7.1) and CD86 (B7.2) to CD28 or CTLA-4 can lead to co-stimulation or co-inhibition signaling, respectively. Engagement of inducible costimulatory (ICOS) molecule and its ligand ICOSL can provide both negative and positive signals. The PD-1:PD-L1/PD-L2 pathway has inhibition effects on T cells. PD-L2 has also been shown to mediate co-stimulatory signals; however, its receptor has not been identified [31]. Lymphocyte-activation gene 3 (LAG-3) has been associated with inhibitory signaling through interactions with the TCR–CD3 complex [62]. HVEM can send positive or negative signals upon interaction with LIGHT and LTα or BTLA and CD160, respectively [63]. Recent studies have shown B7-H3 as a co-stimulator and co-inhibitor of T cell responses, while B7-H4 is a negative regulator of T cell responses. The receptors for B7-H3 and H7-H4 have not yet been identified [31]. Secondary signaling provided by B7 family members are also involved in the network of interactions between the tumor–stroma tissue and T cells. Human tumor cells have been reported to express co-inhibitory molecules, such as ICOSL, B7-H3, B7-H4, PD-L1 and PD-L2 [18,64,65]. Tregs also express co-inhibitory molecules, such as LAG-3, CTLA-4, ICOS, HVEM and PD-L1 [18]. *Blockage of these molecules has been shown to enhance in vivo anti-tumor immunity [36,42,62,64,65].
Acknowledgments
The authors would like to thank Christina Cole for help in preparation of the manuscript. The authors have filed a patent for the use of HSV gD as a vaccine adjuvant. Otherwise the authors have no financial interests associated with the work presented.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Parmiani G, De Filippo A, Novellino L, Castelli C. Unique human tumor antigens: immunobiology and use in clinical trials. J Immunol. 2007;178:1975–1979. doi: 10.4049/jimmunol.178.4.1975. [DOI] [PubMed] [Google Scholar]
- 2.Lizee G, Cantu MA, Hwu P. Less yin, more yang: confronting the barriers to cancer immunotherapy. Clin Cancer Res. 2007;13:5250–5255. doi: 10.1158/1078-0432.CCR-07-1722. [DOI] [PubMed] [Google Scholar]
- 3.Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, Royal RE, Kammula U, White DE, Mavroukakis SA, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang MH, You SL, Chen CJ, Liu CJ, Lee CM, Lin SM, Chu HC, Wu TC, Yang SS, Kuo HS, et al. Decreased incidence of hepatocellular carcinoma in hepatitis B vaccinees: a 20-year follow-up study. J Natl Cancer Inst. 2009;101:1348–1355. doi: 10.1093/jnci/djp288. [DOI] [PubMed] [Google Scholar]
- 5.Ault KA. Effect of prophylactic human papillomavirus L1 virus-like-particle vaccine on risk of cervical intraepithelial neoplasia grade 2, grade 3, and adenocarcinoma in situ: a combined analysis of four randomised clinical trials. Lancet. 2007;369:1861–1868. doi: 10.1016/S0140-6736(07)60852-6. [DOI] [PubMed] [Google Scholar]
- 6.Drake CG. Immunotherapy for prostate cancer: walk, don’t run. J Clin Oncol. 2009;27:4035–4037. doi: 10.1200/JCO.2009.22.2299. [DOI] [PubMed] [Google Scholar]
- 7.Schuster SJ, Neelapu SS, Gause BL, Muggia FM, Gockerman JP, Sotomayor EM, Winter JN, Flowers CR, Stergiou AM, Kwak LW, et al. Idiotype vaccine therapy (BiovaxID) in follicular lymphoma in first complete remission: Phase III clinical trial results. J Clin Oncol (Meeting Abstracts) 2009;27:2. [Google Scholar]
- 8.Kenter GG, Welters MJ, Valentijn AR, Lowik MJ, Berends-van der Meer DM, Vloon AP, Essahsah F, Fathers LM, Offringa R, Drijfhout JW, et al. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. N Engl J Med. 2009;361:1838–1847. doi: 10.1056/NEJMoa0810097. [DOI] [PubMed] [Google Scholar]
- 9.McMahan RH, Slansky JE. Mobilizing the low-avidity T cell repertoire to kill tumors. Semin Cancer Biol. 2007;17:317–329. doi: 10.1016/j.semcancer.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coscoy L. Immune evasion by Kaposi’s sarcoma-associated herpesvirus. Nat Rev Immunol. 2007;7:391–401. doi: 10.1038/nri2076. [DOI] [PubMed] [Google Scholar]
- 11.Peggs KS, Quezada SA, Allison JP. Cell intrinsic mechanisms of T-cell inhibition and application to cancer therapy. Immunol Rev. 2008;224:141–165. doi: 10.1111/j.1600-065X.2008.00649.x. [DOI] [PubMed] [Google Scholar]
- 12.Lazar-Molnar E, Hegyesi H, Toth S, Falus A. Autocrine and paracrine regulation by cytokines and growth factors in melanoma. Cytokine. 2000;12:547–554. doi: 10.1006/cyto.1999.0614. [DOI] [PubMed] [Google Scholar]
- 13.Ohm JE, Carbone DP. VEGF as a mediator of tumor-associated immunodeficiency. Immunol Res. 2001;23:263–272. doi: 10.1385/IR:23:2-3:263. [DOI] [PubMed] [Google Scholar]
- 14.Ruiz-Cabello F, Nevot MA, Garrido F. MHC class I and II gene expression on human tumors. Adv Exp Med Biol. 1988;233:119–128. doi: 10.1007/978-1-4899-5037-6_14. [DOI] [PubMed] [Google Scholar]
- 15.Seliger B, Maeurer MJ, Ferrone S. TAP off--tumors on. Immunol Today. 1997;18:292–299. doi: 10.1016/s0167-5699(97)01052-9. [DOI] [PubMed] [Google Scholar]
- 16.Fabregat I. Dysregulation of apoptosis in hepatocellular carcinoma cells. World J Gastroenterol. 2009;15:513–520. doi: 10.3748/wjg.15.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Whiteside TL. Tumor-induced death of immune cells: its mechanisms and consequences. Semin Cancer Biol. 2002;12:43–50. doi: 10.1006/scbi.2001.0402. [DOI] [PubMed] [Google Scholar]
- 18.Seliger B, Marincola FM, Ferrone S, Abken H. The complex role of B7 molecules in tumor immunology. Trends Mol Med. 2008;14:550–559. doi: 10.1016/j.molmed.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piersma SJ, Welters MJ, van der Burg SH. Tumor-specific regulatory T cells in cancer patients. Hum Immunol. 2008;69:241–249. doi: 10.1016/j.humimm.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 20.Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol. 2003;77:4911–4927. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wick M, Dubey P, Koeppen H, Siegel CT, Fields PE, Chen L, Bluestone JA, Schreiber H. Antigenic cancer cells grow progressively in immune hosts without evidence for T cell exhaustion or systemic anergy. J Exp Med. 1997;186:229–238. doi: 10.1084/jem.186.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kathryn M, Alan RC. New approaches for modelling cancer mechanisms in the mouse. The Journal of Pathology. 2005;205:181–193. doi: 10.1002/path.1698. [DOI] [PubMed] [Google Scholar]
- 23.Ostrand-Rosenberg S. Animal models of tumor immunity, immunotherapy and cancer vaccines. Curr Opin Immunol. 2004;16:143–150. doi: 10.1016/j.coi.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 24.Draper SJ, Heeney JL. Viruses as vaccine vectors for infectious diseases and cancer. Nat Rev Microbiol. 8:62–73. doi: 10.1038/nrmicro2240. [DOI] [PubMed] [Google Scholar]
- 25.Itoh K, Yamada A, Mine T, Noguchi M. Recent advances in cancer vaccines: an overview. Jpn J Clin Oncol. 2009;39:73–80. doi: 10.1093/jjco/hyn132. [DOI] [PubMed] [Google Scholar]
- 26.Mohebtash M, Gulley JL, Madan RA, Ferrara T, Arlen PM. Cancer vaccines: current directions and perspectives in prostate cancer. Curr Opin Mol Ther. 2009;11:31–36. [PMC free article] [PubMed] [Google Scholar]
- 27.Murthy V, Moiyadi A, Sawant R, Sarin R. Clinical considerations in developing dendritic cell vaccine based immunotherapy protocols in cancer. Curr Mol Med. 2009;9:725–731. doi: 10.2174/156652409788970689. [DOI] [PubMed] [Google Scholar]
- 28.Jinushi M, Tahara H. Cytokine gene-mediated immunotherapy: current status and future perspectives. Cancer Sci. 2009;100:1389–1396. doi: 10.1111/j.1349-7006.2009.01202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baxevanis CN, Perez SA, Papamichail M. Combinatorial treatments including vaccines, chemotherapy and monoclonal antibodies for cancer therapy. Cancer Immunol Immunother. 2009;58:317–324. doi: 10.1007/s00262-008-0576-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Curiel TJ. Tregs and rethinking cancer immunotherapy. J Clin Invest. 2007;117:1167–1174. doi: 10.1172/JCI31202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 32.Keir ME, Francisco LM, Sharpe AH. PD-1 and its ligands in T-cell immunity. Curr Opin Immunol. 2007;19:309–314. doi: 10.1016/j.coi.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 33.van Berkel ME, Oosterwegel MA. CD28 and ICOS: similar or separate costimulators of T cells? Immunol Lett. 2006;105:115–122. doi: 10.1016/j.imlet.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 34.Yi KH, Chen L. Fine tuning the immune response through B7-H3 and B7-H4. Immunol Rev. 2009;229:145–151. doi: 10.1111/j.1600-065X.2009.00768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sarnaik AA, Weber JS. Recent advances using anti-CTLA-4 for the treatment of melanoma. Cancer J. 2009;15:169–173. doi: 10.1097/PPO.0b013e3181a7450f. [DOI] [PubMed] [Google Scholar]
- 36.Wong RM, Scotland RR, Lau RL, Wang C, Korman AJ, Kast WM, Weber JS. Programmed death-1 blockade enhances expansion and functional capacity of human melanoma antigen-specific CTLs. Int Immunol. 2007;19:1223–1234. doi: 10.1093/intimm/dxm091. [DOI] [PubMed] [Google Scholar]
- 37.del Rio ML, Lucas CL, Buhler L, Rayat G, Rodriguez-Barbosa JI. HVEM/LIGHT/BTLA/CD160 cosignaling pathways as targets for immune regulation. J Leukoc Biol. 2009:jlb.0809590. doi: 10.1189/jlb.0809590. [DOI] [PubMed] [Google Scholar]
- 38.Sedy JR, Gavrieli M, Potter KG, Hurchla MA, Lindsley RC, Hildner K, Scheu S, Pfeffer K, Ware CF, Murphy TL, et al. B and T lymphocyte attenuator regulates T cell activation through interaction with herpesvirus entry mediator. Nat Immunol. 2005;6:90–98. doi: 10.1038/ni1144. [DOI] [PubMed] [Google Scholar]
- 39.Tao R, Wang L, Murphy KM, Fraser CC, Hancock WW. Regulatory T cell expression of herpesvirus entry mediator suppresses the function of B and T lymphocyte attenuator-positive effector T cells. J Immunol. 2008;180:6649–6655. doi: 10.4049/jimmunol.180.10.6649. [DOI] [PubMed] [Google Scholar]
- 40.Cai G, Anumanthan A, Brown JA, Greenfield EA, Zhu B, Freeman GJ. CD160 inhibits activation of human CD4+ T cells through interaction with herpesvirus entry mediator. Nat Immunol. 2008;9:176–185. doi: 10.1038/ni1554. [DOI] [PubMed] [Google Scholar]
- 41.Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DA, Wherry EJ. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lasaro MO, Tatsis N, Hensley SE, Whitbeck JC, Lin SW, Rux JJ, Wherry EJ, Cohen GH, Eisenberg RJ, Ertl HC. Targeting of antigen to the herpesvirus entry mediator augments primary adaptive immune responses. Nat Med. 2008;14:205–212. doi: 10.1038/nm1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Montgomery RI, Warner MS, Lum BJ, Spear PG. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell. 1996;87:427–436. doi: 10.1016/s0092-8674(00)81363-x. [DOI] [PubMed] [Google Scholar]
- 44.Fridlender ZG, Buchlis G, Kapoor V, Cheng G, Sun J, Singhal S, Crisanti C, Wang LC, Heitjan D, Snyder LA, et al. CCL2 blockade augments cancer immunotherapy. Cancer Res. 70:109–118. doi: 10.1158/0008-5472.CAN-09-2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rech AJ, Vonderheide RH. Clinical use of anti-CD25 antibody daclizumab to enhance immune responses to tumor antigen vaccination by targeting regulatory T cells. Ann N Y Acad Sci. 2009;1174:99–106. doi: 10.1111/j.1749-6632.2009.04939.x. [DOI] [PubMed] [Google Scholar]
- 46.Ruter J, Barnett BG, Kryczek I, Brumlik MJ, Daniel BJ, Coukos G, Zou W, Curiel TJ. Altering regulatory T cell function in cancer immunotherapy: a novel means to boost the efficacy of cancer vaccines. Front Biosci. 2009;14:1761–1770. doi: 10.2741/3338. [DOI] [PubMed] [Google Scholar]
- 47.McElhaney JE, Effros RB. Immunosenescence: what does it mean to health outcomes in older adults? Curr Opin Immunol. 2009;21:418–424. doi: 10.1016/j.coi.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ginaldi L, De Martinis M, D’Ostilio A, Marini L, Loreto MF, Corsi MP, Quaglino D. The immune system in the elderly: I. Specific humoral immunity. Immunol Res. 1999;20:101–108. doi: 10.1007/BF02786466. [DOI] [PubMed] [Google Scholar]
- 49.Aydar Y, Balogh P, Tew JG, Szakal AK. Follicular dendritic cells in aging, a “bottle-neck” in the humoral immune response. Ageing Res Rev. 2004;3:15–29. doi: 10.1016/j.arr.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 50.Zheng B, Han S, Takahashi Y, Kelsoe G. Immunosenescence and germinal center reaction. Immunol Rev. 1997;160:63–77. doi: 10.1111/j.1600-065x.1997.tb01028.x. [DOI] [PubMed] [Google Scholar]
- 51.Eaton SM, Burns EM, Kusser K, Randall TD, Haynes L. Age-related defects in CD4 T cell cognate helper function lead to reductions in humoral responses. J Exp Med. 2004;200:1613–1622. doi: 10.1084/jem.20041395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lazuardi L, Jenewein B, Wolf AM, Pfister G, Tzankov A, Grubeck-Loebenstein B. Age-related loss of naive T cells and dysregulation of T-cell/B-cell interactions in human lymph nodes. Immunology. 2005;114:37–43. doi: 10.1111/j.1365-2567.2004.02006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang X, Stedra J, Cerny J. Relative contribution of T and B cells to hypermutation and selection of the antibody repertoire in germinal centers of aged mice. J Exp Med. 1996;183:959–970. doi: 10.1084/jem.183.3.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Effros RB, Walford RL. The immune response of aged mice to influenza: diminished T-cell proliferation, interleukin 2 production and cytotoxicity. Cell Immunol. 1983;81:298–305. doi: 10.1016/0008-8749(83)90237-x. [DOI] [PubMed] [Google Scholar]
- 55.Franceschi C, Bonafe M, Valensin S. Human immunosenescence: the prevailing of innate immunity, the failing of clonotypic immunity, and the filling of immunological space. Vaccine. 2000;18:1717–1720. doi: 10.1016/s0264-410x(99)00513-7. [DOI] [PubMed] [Google Scholar]
- 56.Yager EJ, Ahmed M, Lanzer K, Randall TD, Woodland DL, Blackman MA. Age-associated decline in T cell repertoire diversity leads to holes in the repertoire and impaired immunity to influenza virus. J Exp Med. 2008;205:711–723. doi: 10.1084/jem.20071140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shimada Y, Hayashi M, Nagasaka Y, Ohno-Iwashita Y, Inomata M. Age-associated up-regulation of a negative co-stimulatory receptor PD-1 in mouse CD4+ T cells. Exp Gerontol. 2009;44:517–522. doi: 10.1016/j.exger.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 58.Channappanavar R, Twardy BS, Krishna P, Suvas S. Advancing age leads to predominance of inhibitory receptor expressing CD4 T cells. Mech Ageing Dev. 2009;130:709–712. doi: 10.1016/j.mad.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 59.DiMenna L, Latimer B, Parzych E, Haut L, Töpfer K, Abdulla S, Yu H, Manson B, Giles-Davis W, Zhou D, et al. Augmentation of primary influenza A virus specific CD8+ T cell responses in aged mice through blockade of an immunoinhibitory pathway. J Immunol. doi: 10.4049/jimmunol.0903808. (in press) [DOI] [PubMed] [Google Scholar]
- 60.Gregg R, Smith CM, Clark FJ, Dunnion D, Khan N, Chakraverty R, Nayak L, Moss PA. The number of human peripheral blood CD4+ CD25high regulatory T cells increases with age. Clin Exp Immunol. 2005;140:540–546. doi: 10.1111/j.1365-2249.2005.02798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hwang KA, Kim HR, Kang I. Aging and human CD4(+) regulatory T cells. Mech Ageing Dev. 2009;130:509–517. doi: 10.1016/j.mad.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grosso JF, Kelleher CC, Harris TJ, Maris CH, Hipkiss EL, De Marzo A, Anders R, Netto G, Getnet D, Bruno TC, et al. LAG-3 regulates CD8+ T cell accumulation and effector function in murine self- and tumor-tolerance systems. J Clin Invest. 2007;117:3383–3392. doi: 10.1172/JCI31184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lasaro MO, Ertl HC. Potentiating vaccine immunogenicity by manipulating the HVEM/BTLA pathway and other co-stimulatory and co-inhibitory signals of the immune system. Hum Vaccin. 2009;5:6–14. doi: 10.4161/hv.5.1.6399. [DOI] [PubMed] [Google Scholar]
- 64.Korman AJ, Peggs KS, Allison JP. Checkpoint blockade in cancer immunotherapy. Adv Immunol. 2006;90:297–339. doi: 10.1016/S0065-2776(06)90008-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8:467–477. doi: 10.1038/nri2326. [DOI] [PubMed] [Google Scholar]