Abstract
Off-line analysis and characterization of samples separated by capillary liquid chromatography (LC) has been problematic using conventional approaches to fraction collection. We demonstrate collection of nanoliter fractions by forming plugs of effluent from a 75 μm inner diameter LC column segmented by an immiscible oil such as perfluorodecalin. The plugs are stored in tubing that can then be used to manipulate the samples. Off-line electrospray ionization mass spectrometry (ESI-MS) was used to characterize the samples. ESI-MS was performed by directly pumping the segmented plugs into a nanospray emitter tip. Critical parameters including the choice of oils, ESI voltage, and flow rates that allows successful direct infusion analysis were investigated. Best signals were obtained under conditions in which the oil did not form an electrospray but was siphoned away from the tip. Off-line analysis showed preservation of the chromatogram with no loss of resolution. The method was demonstrated to allow changes in flow rate during the analysis. Specifically, decreases in flow rate were used to allow extended MS analysis time on selected fractions, similar to “peak parking”.
Introduction
Microscale separations methods such as capillary liquid chromatography (LC) and capillary electrophoresis (CE) are well-recognized as powerful methods that can provide numerous advantages including high resolution, high sensitivity, and effective coupling to mass spectrometry (MS).1, 2 A limitation of such methods has been the relative difficulty of collecting fractions for storage and further characterization off-line. This difficulty stems chiefly from the problems of storing and manipulating nanoliter and smaller fractions that would be generated. Conventional methods for fraction collection from a separation method commonly involve transferring samples to wells or vials; however, this approach is limited in practice to fractions no smaller than a few microliters. In this work we demonstrate fraction collection from capillary LC based on flow segmentation i.e., collecting fractions in a tube as plugs or droplets separated by an immiscible oil, followed by off-line electrospray ionization (ESI)-MS of the segmented samples.
Although on-line ESI-MS is generally effective, fraction collection and off-line ESI-MS may be desirable in many situations including when: 1) using off-site mass spectrometers; 2) using multiple mass spectrometers for analysis of a single sample; 3) only a portion of the chromatogram requires MS analysis; and 4) multiplexing slow separations to rapid MS analysis. Off-line analysis is also desirable when certain fractions of a chromatogram require MS analysis time that is longer than the peak width. This latter situation may arise in analysis of complex samples generated from proteomics or metabolomics studies where multiple stages of mass spectrometry (MSn) may be used to gain chemical information on several overlapping or co-eluting compounds. When using on-line analysis, these problems may be avoided by slowing the entire chromatographic separation; however, this unnecessarily increases analysis time and it may dilute compounds. Alternatively, “peak parking” may be used wherein mobile phase flow is stopped or slowed to allow more time to collect mass spectra when compounds of interest elute.3 Peak parking is infrequently used because of the complexity of varying flow rate during chromatographic separation and deleterious effects on the separation.
Off-line analysis provides a convenient approach to avoid these limitations. A commercial system for fraction collection and off-line ESI-MS based on a microfabricated chip has been developed. This system uses fraction collection onto well-plates and requires 1–10 μL fractions for ESI-MS analysis.4
Compartmentalization of effluent into segmented flow has emerged as a novel way to collect fractions from miniaturized separations such as chip electrophoresis5 and capillary LC6. For capillary LC, fractions were collected as segmented flow to facilitate interfacing to CE for 2-dimensional separation. Both of these examples used on-line analysis and did not explore off-line analysis or interface to mass spectrometry.
Performing off-line ESI-MS of fractions required development of a method of interfacing oil-segmented samples to the ionization source. One approach to analyzing segmented flow using ESI-MS has been to use a microfluidic device to extract sample plugs from oil and transfer them to an aqueous stream interfaced with MS.7, 8 This approach has limitations such as unavoidable dilution and spreading of sample zones during extraction and transfer to the ESI emitter tip. As a result, the sensitivity, throughput and quantification possible by this approach are compromised. We have previously reported that sample plugs segmented by air can be directly infused into a metal-coated nano ESI emitter tip to achieve high-throughput, low carry-over between samples, and sensitive ESI-MS analysis.9 Use of air-segmented samples also has limitations however. Segments can merge, allowing mixing of fractions, because of the compressibility of air under pressure required to pump the sample plugs through an ESI emitter. Segments can also merge during storage due to evaporation of the air through Teflon or polydimethylsiloxane containers. In this work, we demonstrate ESI-MS of oil-segmented samples and application to fraction collection from capillary LC with subsequent off-line ESI-MS.
Experimental Section
Chemicals and Reagents
Capillary LC solvents, including acetonitrile, methanol and water were purchased from Burdick & Jackson (Muskegon, MI). FC-72, FC-77, FC-40 and perfluorodecalin were from Sigma-Aldrich. Acetic acid and hydrofluoric acid were purchased from Fisher Scientific (Pittsburgh, PA). Mobile phases were prepared weekly and were filtered with 0.02μm-pore filters (Whatman, Maidstone, England) to remove particulates. Fused silica capillary was from Polymicro Technologies (Phoenix, AZ). Small molecule metabolites samples malate, citrate, phosphoenolpyruvate (PEP) and fructose 1,6-biphosphate (F1,6P), fumarate, succinate and cyclic adenosine monophosphate (cAMP) were from Sigma-Aldrich. Corticotropin releasing factor (CRF) was from Phoenix pharmaceuticals, Inc. (Burlingame, CA).
Sample Preparation
Metabolite sample stock solutions were made in water at 5 mM concentration then stored in −80°C. Samples were then diluted from stock using 80% methanol and 20% water for injection on HILIC column.
Analysis of oil-segmented flows with MS
For initial tests of ESI of oil-segmented flow, segmented samples were made by pumping sample (50 μM cAMP dissolved in 50% acetonitrile 50% ammonium acetate at pH 9.9) and oil into two separate arms of a tee junction with 100 μm i.d. at 500 nL/min using a syringe pump (Fusion 400, Chemyx, Stafford, TX, USA). In this way, ~7 nL sample plugs separated by 7 nL oil plugs were formed and pumped into 150 μm i.d. by 360 μm o.d. high purity perfluoroalkoxy plus (HPFA+) tubing (Upchurch Scientific, Oak Harbor, OR) connected to the third arm of the tee.
For off-line ESI-MS detection, the HPFA+ tubing containing sample was connected with a Teflon connector to a Pt-coated, fused silica ESI emitter (PicoTip™ EMITTER FS360-50-8, New Objective, Woburn, MA, USA) with 8 μm i.d. at the tip (see Figure 1B). The emitter was mounted into a nanospray ESI source (PV-550, New Objective) interfaced to a linear ion trap (LIT) MS (LTQ, Thermo Fisher Scientific, Waltham, MA). Unless stated otherwise, samples were pumped at 200 nL/min with the emitter tip poised at 1.5 kV. Full scan MS was used in such experiments showing cAMP sample signal at m/z 328. All the other metabolite samples were also detected with negative mode ESI.
Figure 1.
Illustration of scheme for fraction collection from capillary LC and off-line ESI-MS using segmented flow. (A) Segmented flow was generated with a tee junction that connected an oil stream and effluent from capillary LC. (B) Oil-segmented fractions collected could be stored in HPFA+ tubing and then be infused into MS off-line by a syringe pump. (C) Picture of the oil-segmented flow in 150 μm i.d. tubing showing ~400 μm long sample plugs (LC fractions) separated by ~240 μm long oil plugs.
Capillary LC Separations
Fraction collection and off-line ESI MS analysis were performed for two different applications each using a different chromatography mode. The first was separation of polar metabolites by hydrophilic interaction liquid chromatography (HILIC). To prepare capillary HILIC columns, a frit was first made by tapping nonporous silica (Micra Scientific, Inc., Northbrook, IL) into one end of a 15 cm length of 75 μm i.d. fused silica capillary. The particles were briefly heated with a flame to sinter them in place. The capillary was then packed from a slurry of 8 mg Luna NH2 particles (Phenomenex, Torrance, CA) in 4 mL acetone as described elsewhere.10 The ESI emitter tip was pulled from a separate capillary with 10 μm i.d. and 360 μm o.d. using a 2 cycle program (Cycle 1: HEAT 330, FIL void, DELAY 128, PULL void. Cycle 2: HEAT 330, FIL (void), DELAY 128, PULL 125) on Sutter P-2000 pipette puller (Sutter Instruments, Novato, CA). The tip was then etched with 49% hydrofluoric acid for 100 s to create sharp-edged electrospray emitters. Separations were performed using a UPLC pump (NanoAcquity, Waters, Milford, MA). Mobile phase (MP) A was acetonitrile, while MP B was 5 mM Ammonium acetate in water with pH adjusted to 9.9 by NaOH. Separation of metabolites was realized with a linear mobile phase gradient from 30% to 100% MP B over 22 minutes. For on-line detection, the column was interfaced to a triple quadrupole (QQQ) MS (QuattroUltima, Micromass/Waters, Milford, MA) using a Waters Universal NanoFlow Sprayer ESI source. Off-line detection was performed with the LIT.
Malate (m/z = 133), citrate (m/z = 191), PEP (m/z = 167) and F1,6P (m/z = 339), were separated on a 15 cm long HILIC column with 75 μm i.d. at a flow rate 500 nL/min. Full scan MS was utilized on detection of 1 μL injection of 20 μM of these four fully resolved molecules. For MRM detection, another set of metabolites were used, including fumarate (m/z 115), succinate (m/z 117), malate, cAMP and F1,6P, and the sample concentration were lowered to 10 μM due to higher sensitivity with MRM detection compared to full scan analysis. Both the QQQ and LIT MS were operated in negative mode. With QQQ, transitions used for MRM detection of these five metabolites were determined to be: m/z 115→ m/z 71 for fumarate, m/z 117→ m/z 73 for succinate, m/z 133→ m/z 115 for malate, m/z 328→ m/z 134 for cAMP, and m/z 339→ m/z 96 for F1,6P. With LIT MS, daughter ion scans used for MRM of these samples were obtained by setting 5 different scan events to 5 parent ions of different molecules and detecting all daughter ions in a range of 50 to 1000 m/z.
The second application was separation of a tryptic digest of corticotropin-releasing factor (CRF) using reverse phase capillary LC. Instead of using a separate emitter tip, the reverse phase columns were made with integrated emitter tips as described before.11, 12 Columns were then packed with an acetone slurry (10 mg/mL) of 5 μm Atlantis C18 reversed-phase particles (Alltech, Deerfield, IL) at 500 psi to 3 cm length as described elsewhere.13 2 μL of 1 nM of the tryptic CRF samples were injected by WPS-3000TPL autosampler (Dionex, Sunnyvale, CA) in weak mobile phases (2% acetic acid in H2O) to allow the analytes to stack at the head of the column. The capillary LC system utilizes a high pressure (4000 psi) pump (Haskel Inc., Burbank, CA) for sample loading and desalting for 12 min, and a lower pressure (500 psi) micro HPLC pump (MicroPro, Eldex Laboratories, Napa, CA) for gradient separation. MP A was water containing 2% acetic acid, while MP B was methanol with 2% acetic acid. The gradient went from 10% to 90% of MP B for 7 min. Both on-line and off-line detection used the LIT MS, operated in positive mode.
Fraction Collection
For off-line analysis, LC effluent was collected into fractions using the system shown in Figure 1. In this approach, effluent from the column is directed into a tee with an immiscible fluid, typically a perfluorinated oil, flowing through another arm of the tee. As described elsewhere, within a certain flow rate range, alternating and regularly spaced plugs of sample and oil are formed.14–17 Polyether ether ketone (PEEK) tees with 50, 100 and 150 μm i.d. (Valco, Houston, TX) were used for this work. The oil-segmented fractions collected into a 60 cm length of 150 μm i.d. by 360 μm o.d. HPFA+ tubing for storage. A picture of the tubing containing such fractions is shown in Figure 1C.
Results and Discussion
ESI Conditions for Oil Segmented Flow
Initial studies were directed towards identifying conditions for successful direct infusion ESI-MS of oil-segmented samples. Preliminary studies identified the immiscible fluid used for segmenting samples, electrospray voltage, and infusion flow rate as critical parameters for achieving stable and sensitive direct ESI-MS analysis.
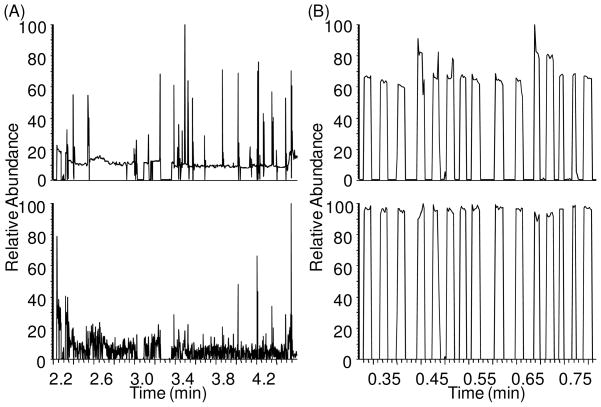
Five different liquids, hexane, FC-72, FC-77, FC-40 and perfluorodecalin (PFD), were evaluated as possible immiscible fluids to segment samples. We observed that hexane, FC-72 and FC-77 all generated a visible electrospray at voltage > −1 kV, which is similar to the lower voltage needed for electrospray of aqueous sample. Attempts to analyze aqueous cAMP samples segmented by these fluids during direct infusion did not yield a series of segments but instead a low and fluctuating ion current as illustrated by the example in Figure 2A. In contrast, FC-40 and PFD did not yield electrospray up to −1.5 kV. Instead, these oils formed droplets at the emitter tip that then migrated along the outside of the tip away from the emitter, presumably due to gravity and interfacial tension effects. With these oils, no signal was observed when the oil plug flowed through the tip (Figure 2B) and only sample signal was detected thus allowing detection of cAMP as a series of discrete current bursts corresponding to the plugs exiting the emitter tip. These results suggest that the electrospray of immiscible segmenting fluid interferes with formation and detection of ions from adjacent aqueous sample plugs. The mechanism for this effect is not clear. The difference in oil performance can be attributed, at least in part, to their viscosity. Higher viscosity fluids are more difficult to electrospray18, 19 and it was the higher viscosity fluids (see Table 1) that could be successfully used in this case.
Figure 2.
(A) TIC (upper) and RIC (lower) of 50 μM cAMP (m/z = 328) sample droplets infused at 200 nL/min with FC-72 as oil phase, showing noisy signal all over the chromatogram and little signal of samples. (B) TIC (upper) and RIC (lower) of the same cAMP sample droplets with PFD as oil phase, showing discrete segmented signals of cAMP sample plugs.
Table 1.
Dynamic viscosities of five tested oils at 300 K and comparison to commonly used ESI solvents water and methanol.
| Hexane | Methanol | FC-72 | Water | FC-77 | FC-40 | PFD | |
|---|---|---|---|---|---|---|---|
| Dynamic viscosity (mPa·s) | 0.3 | 0.56 | 0.64 | 0.89 | 1.3 | 3.5 | 5.1 |
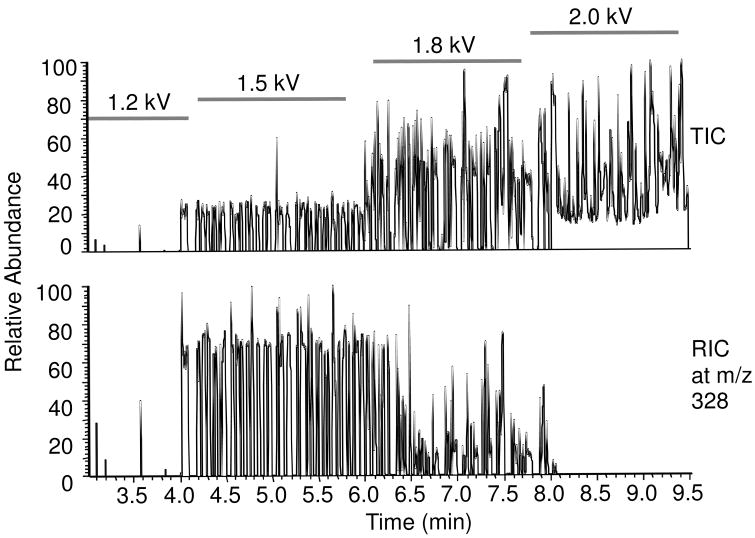
Because PFD did not interfere with spray of sample, further experiments were performed with it as the oil phase. The effect of ESI voltage was tested while infusing a series of aqueous samples of 50 μM cAMP in full scan mode. As illustrated in Figure 3, at voltage less than −1.2 kV no signal for cAMP was observed. At this voltage, neither the aqueous sample nor the oil generated visible electrospray. When the voltage increased to −1.5 kV, signal for the analyte was detected as discrete bursts in the reconstructed ion current (RIC) trace. The total ion current (TIC) revealed a similar pattern showing that no signal was obtained as the oil was pumped through the emitter. In agreement with these observations of the signal, we observed electrospray only for the aqueous plugs in this voltage range. At −1.8 kV, the TIC increased; however, signal for the analyte was reduced in the RIC suggesting that the increase in TIC was due to signal from the oil which begins to electrospray at this voltage. The signal for cAMP also becomes erratic with the onset of oil electrospray. Above −1.8 kV this trend continues and no signal for analyte is detected and the TIC remains noticeably elevated between aqueous plugs. Optimal ESI voltage was thus determined to be around −1.5 kV on our instrument and used for the following experiments. With this ESI voltage and sample flow rate, the signal for oil-segmented samples was not statistically different from samples that were directly infused as a continuous aqueous phase suggesting that the presence of oil segments does not interfere with ESI of the samples.
Figure 3.
(A) TIC (upper panel) and RIC (lower panel) of oil segmented droplets of 50 μM cAMP sample infused at 200 nL/min, with different spray voltage from 1.2 to 2.0 kV. (B) Oil coming at the tip at 1.5 kV just dripped off the tip. (C) Oil underwent ESI at 2.0 kV. When the oil sprayed, the TIC signals were higher due to more signal of oil, but the RIC for aqueous samples were lower, which means the spray of oil interfered with the sample ions.
These results further support the conclusion that detection of samples in the aqueous fractions is best if oil does not generate electrospray. For a given oil, the results will be obtained in the range that the aqueous sample generates electrospray but the oil does not. For low viscosity oils such as FC-72 and FC-77, there are no voltages that generate only aqueous spray so these oils did not yield good results under any conditions.
The nano-ESI-MS signal of such sample plugs perfused at 200 nL/min had a RSD for sample plug widths of 38% (n = 30). This variability is not due to variation in plug widths because the RSD of plug lengths generated in the tee junction was 3% as measured by visual observation under a microscope. The variability also is not due to complete coalescence of plugs within the ESI tip because the number of plugs generated always equaled the number detected by MS. Thus, it appears that this variation is cause by flow through the emitter tip. Possible causes include: 1) partial coalescence of plugs; 2) fluctuations in flow rate associated with segmented flow through the emitter. Data obtained during fraction collection by LC argue against the former case as discussed below. The potential effects of this plug width variation on quantative LC-MS have yet to be determined; however, we observe that there was little effect on peak heights.
Effect of Flow Rate
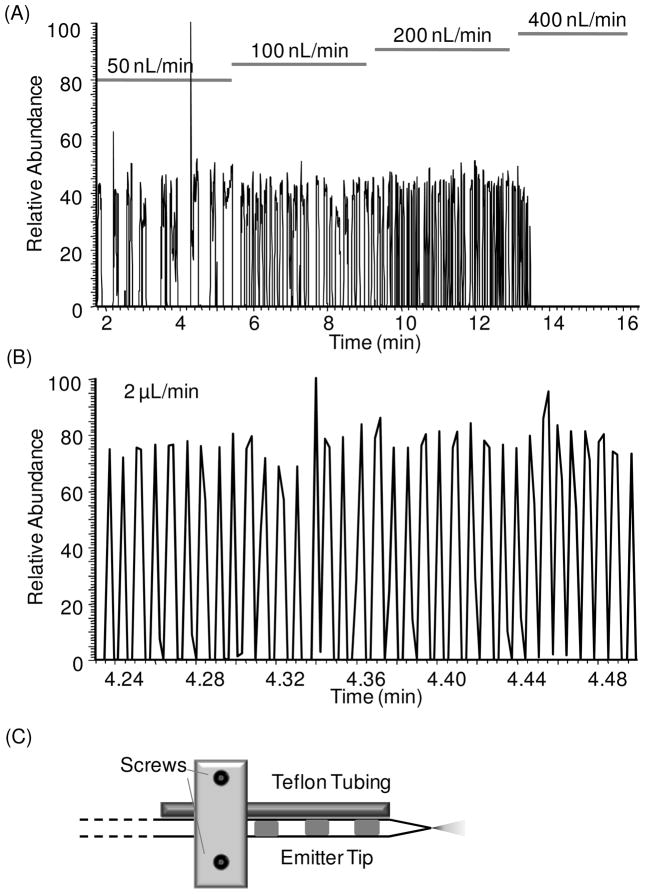
To explore the influence of infusion flow rate, we monitored ESI signal for cAMP from a series of plugs while varying the infusion flow rate. As shown in Figure 4A, increasing flow rate from 50 nL/min to 200 nL/min, had little effect on the signal magnitude, except samples were introduced more rapidly allowing higher throughput. At flow rate lower than 400 nL/min, the traces are stable with occasional spikes which had inconsequential influence on average peak heights. (Occasional dips in signal may be due to flow instability with this type of experiment. At 50 nL/min some instability may be associated with the emitter tip as this is the lower limit recommended for the tips used. All signals shown are raw signals without filtering.) As the flow rate was increased to 400 nL/min however, signal was eliminated. Observation of the emitter tip revealed that this loss of signal coincided with accumulation of oil on the tip. Thus, at the higher flow rates oil phase exiting the tip was not removed fast enough and blocked the emitter tip.
Figure 4.
(A) RIC of oil segmented droplets of 50 μM cAMP sample infused at different flow rate from 50 to 400 nL/min. At 400 nL/min, no signal was seen because oil accumulated at the emitter tip too fast to be removed so it blocked the voltage causing no signal. (B) RIC of oil segmented droplets infused at 2 μL/min. In this case, with a side Teflon tubing to extract oil out (shown in C), such high flow rate could be used and fast detection of droplet signal was achieved. This chromatogram showed detection of 35 droplets in 0.26 min, which is a frequency at about 2.2 Hz.
To prevent oil accumulation on the emitter tip, we attempted to siphon the oil away from the tip by placing a 20 cm length of 50 μm i.d. Teflon tubing next to the emitter about 1 mm from the tip as shown in Figure 4C. As oil droplets emerged from the tip, they migrated away from the orifice as described above, and were then siphoned into the Teflon tubing. In this way, oil did not accumulate on the tip. In this case, alternating 10 nL aqueous and oil plugs could be infused at a flow rates up to 2 μL/min without loss of signal (Figure 4B). With the Teflon siphon tubing, the stability of spray of oil-segmented flow could be maintained from 20 to 2000 nL/min.
At the highest flow rate used, the droplets were analyzed at a rate of 2.2 Hz. While high-throughput sample analysis was not the focus of this work, these results do suggest that ESI-MS of segmented flow may be a useful route to high-throughput analysis. Higher flow rates were not attempted because the throughput became limited by the MS scan rate, which was 0.13 s per scan for this experiment. To reach higher throughput, a faster detector, such as a time-of-flight MS, would be needed.
Fraction collection from capillary LC by oil-segmented flow
Fractions from a capillary LC column were formed by pumping column effluent into a tee with oil flowing perpendicular to the mobile phase as illustrated in Figure 1(A). It is possible vary the fraction size by varying the relative flow rates and tee dimensions. Using a 100 μm i.d. tee, 500 nL/min mobile phase flow, and 300 nL/min oil flow generated ~7 nL LC fraction plugs segmented by ~5 nL oil plugs (Figure 1C). When using tees with 50 and 150 μm i.d., the sample droplet sizes were 2 nL and 35 nL respectively. For this work, we used 7 nL droplets which generated 5 to 18 fractions per chromatographic peak depending on the separation. Consistent sample plug sizes (RSD of 4% for 30 plugs visually observed) were obtained for all fractions collected under our LC separation conditions. No obvious difference was observed for sample plugs generated at the beginning of gradient with 70% acetonitrile and at the end of gradient with 0% acetonitrile.
Detection of LC Separated Components Offline
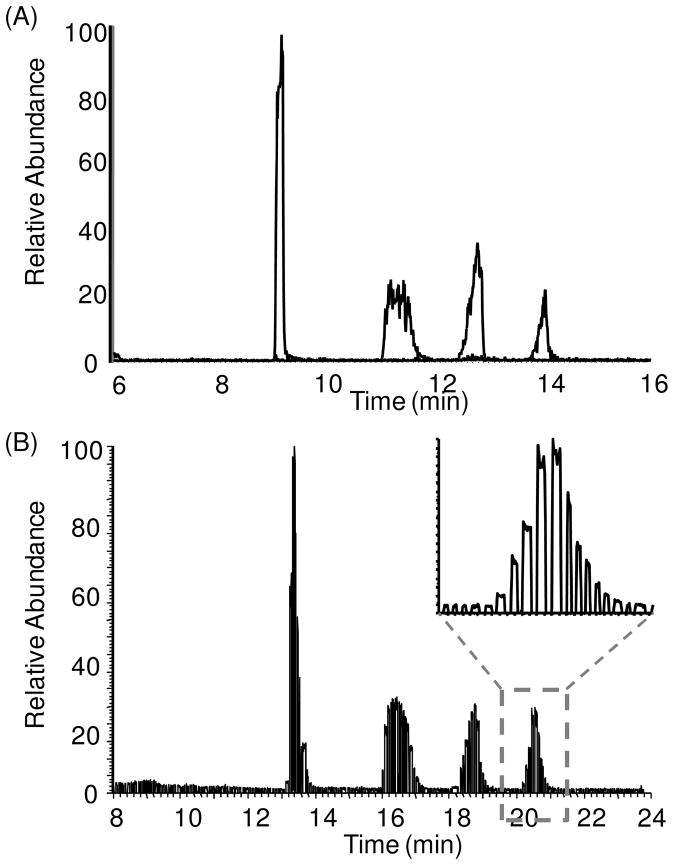
To compare off-line detection of fractions with on-line LC-MS detection, we analyzed a 20 μM mixture of 4 small molecule metabolites (malate, citrate, PEP and F1,6P) using HILIC interfaced to MS both on-line and off-line. For on-line analysis, the components were detected by full scan with a QQQ MS (Figure 5A). For off-line analysis, the fractions were collected as segmented plugs and 1 hour later infused through a nanoESI emitter tip to a LIT MS operated in full scan mode. In the off-line trace (Figure 5B), the individual LC peaks were cleaved into 10–18 fractions. This number of fractions is sufficient to prevent loss of resolution.20 As discussed above, it is possible to adjust conditions to yield different fraction volumes depending upon the experiment.
Figure 5.
Overlap of RICs for 4 metabolite components. (A) On-line detection of 4 sample on micromass QQQ MS, showing peaks of malate, citrate, PEP and F1,6P in a row. (B) Raw RICs of the 4 sample in droplet format obtained using the LIT MS. Using the same flow rate at 500 nL/min, it took 16 min to analyze 10 min of LC effluent because the oil in the final segmented flow accounts for 3/8 of total volume. A zoomed look of the detection of fractions over the F1,6P peak was shown.
In comparing on-line and off-line analysis, the peak shapes and relative sizes are the same indicating no extra-column band broadening occurred during fraction storage and analysis. The results support the conclusion that cross-contamination between plugs is low enough to be inconsequential, at least for these examples. Carry-over between plugs would have resulted in peak tailing in the off-line mass chromatograms as the lower concentration plugs and the trailing edge of the peak would be contaminated by the higher concentrations preceding it; however, not extra tailing is observed in the peaks. This observation is in agreement with our previous report showing low carry-over between peptide samples.9 Further study with different samples and LC methods is required to determine the generality of this conclusion. These results also support the idea that the fractions collected were small enough, and created with sufficiently low mixing during formation, as to prevent extra-column band broadening. (If necessary, smaller plugs could be generated to avoid such effects if they occur.) Resolution is also unaffected, e.g. resolution (Rs) for citrate and PEP was calculated to be 2.0 for both on-line and off-line detection. The most obvious difference in the traces is that the overall times for all four sample peaks are longer in the segmented flow sample (5 min for off-line compared to 8 min for on-line). This difference occurs because the flow rates were kept the same in both methods at 500 nL/min; but, the ratio of oil to sample volume is 3:5, so that infusion of the oil added 3/5 analysis time compared to sample analysis time in the off-line detection. These results illustrate that detection of the chromatogram was unaffected by the storage of those samples in oil-segmented flow and that capillary LC separated components can be preserved for additional analysis off-line. In these experiments, we stored samples for 1–2 h before MS analysis. On-going work in our laboratory is evaluating the potential for longer term storage of collected fractions.
By measuring the peak widths of the ion current signal of off-line detection of the fractions, it also showed no difference for sample plugs at high or low organic concentrations, with average peak widths at 0.036 min (n = 26) and 0.035 min (n = 26), respectively. But the RSDs of peak widths for different sample plugs were higher to 33% (n = 26) for plugs in high organic solution or 37% (n = 26) for ones in low organic solution. This RSD was similar to the RSD when detecting standard sample plugs, meaning the additional variability is not due to the separation and the fraction collection procedure, but is still contributed to the process of nano-ESI on oil segmented flow as described before.
Peak parking
We next tested the off-line system for extending the MS analysis time of selected components, analogous to peak-parking, for two examples. The first was to obtain multiple MS2 spectra (i.e., multiple reaction monitoring) for co-eluting peaks using a relatively slow mass spectrometer. For complex samples, MRM is a common method for simultaneous detection and quantification of targeted components.22–24 Triple quadrupole MS is generally used for MRM detection because of its ability to rapidly switch between different MS-MS transitions; however, quadrupole ion traps can be advantageous for MRM because they usually have better full scan sensitivity in MS2, and can be used for MSn analysis, which cannot be done by QQQ. A limitation of this approach is that MRM on an ion trap is relatively slow due to longer scan time. For demonstration of off-line ESI-MS with MRM, we analyzed a test mixture of five metabolites, fumarate, succinate, malate, cAMP and F1,6P at 10 μM each. Fumarate, succinate and malate were allowed to co-elute to illustrate the challenge of MRM for co-eluting compounds. In the experiment, fractions were collected at 0.84 s intervals corresponding to 7 nL samples (flow rate was 500 nL/min).
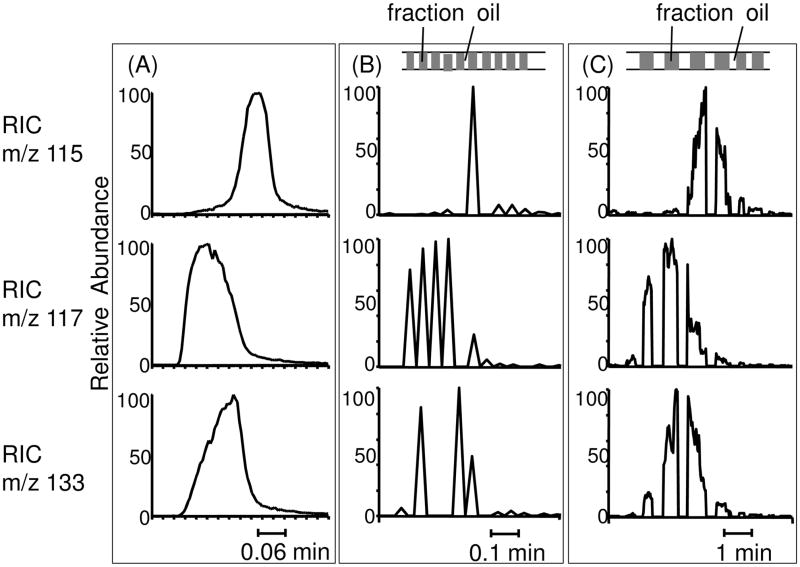
On-line detection of the three co-eluting compounds gave RICs as shown in Figure 6A. In the first case of off-line detection, the sample was analyzed by pumping the fractions at 500 nL/min while monitoring MS-MS transitions on a linear ion trap for all 5 analytes yielding the RICs shown in Figure 6B. Under this condition, the total time for the 3 co-eluting analytes was about 30 s but the MRM scan time was 1.8 s for each point of one analyte; therefore, it was possible to only obtain 1 scan for each MS-MS transition over a sample plug as illustrated in Figure 6B. Furthermore, not all compounds could be detected in each plug so for some sample plugs, no signal of a particular compound was detected. For example, the middle RIC in Figure 6B showed a total of 6 spikes, which were 6 points detected for succinate (m/z 117) peak. However, no signal was detected between the fourth and fifth spike, while a sample plug was seen at the same time, indicating a missing signal for that plug.
Figure 6.
Comparison of RICs of 3 co-eluting components fumarate (m/z 115), succinate (m/z 117) and malate (m/z 133) without and with peak parking. Different time scales for three groups of chromatograms were marked at the bottom of each figure. (A) On-line detection of the 3 compounds with QQQ-MS. (B) Off-line detection of the 3 compounds in segmented flow at 500 nL/min, the same flow rate as the original on-line detection. These peaks were narrow, resulting in only 1–5 scans covering each sample peak. Top figure showed rough sample droplets distribution. (C) Off-line detection of the 3 compounds in segmented flow by reducing flow rate to 50 nL/min right before the three peaks, resulting in more scan numbers over each sample peak.
The off-line experiment was then repeated but the flow rate was reduced from 500 nL/min to 50 nL/min during the detection of the co-eluting peak (Figure 6C). Under this condition, the peak width, and detection time, is increased by a factor of 10. This allows many more scans to be acquired per sample plug and per chromatographic band. For succinate, only 6 scans with S/N > 3 were obtained at 500 nL/min as shown in Figure 6B, while over 80 scans were obtained with the reduced flow rate as shown in Figure 6C. With the greater scan number, it was also possible to detect the analyte in all the plugs. Meanwhile, the advantages of capillary LC are preserved such as high resolution, improved sample concentration and increased ionization efficiency.
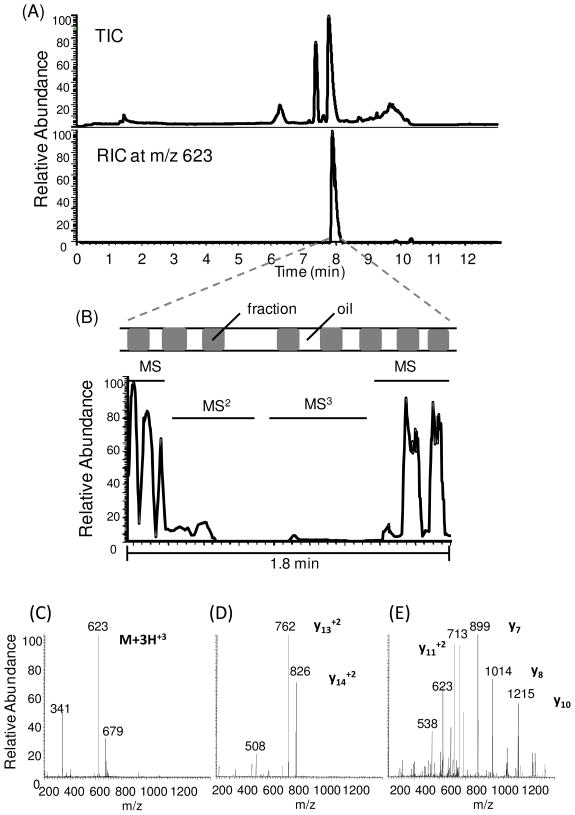
As a second demonstration of the utility of off-line analysis for peak parking, we examined acquiring multiple spectra for compound identification using analysis of a tryptic digest of the peptide CRF as an example. In the separation of CRF tryptic peptides, the flow rate of LC separation was reduced to 100 nL/min to reach better nano-ESI sensitivity. So the oil flow rate was lowered to 60 nL/min to maintain a fixed ratio at 5:3 as well. Compared to the experiment above, despite of different flow rates, droplet sizes were the same at 7 nL. With on-line separation at 100 nL/min and full scan MS, the most dominant peak in the chromatogram corresponds to the fragment with m/z 623 (Figure 7A), but the peak was only about 0.3 min wide which was insufficient to acquire multiple stages of MS with optimized CID manually. To confirm the sequence of this fragment peptide, fractions were collected and off-line ESI analysis performed at 100 nL/min. During elution of the peak of interest, the flow rate was reduced to 25 nL/min. In this way, a 0.3 min wide peak was extended to about 1.8 min width which allowed manual selection of parent ions for MS2 and MS3 analysis. During this time, a series of 8 fractions (i.e., sample plugs) were pumped through the emitter. The parking event was terminated after the MS3 analysis was accomplished. With the spectra, we found the most abundant tryptic fragment of CRF is the peptide CRF1–16 with sequence SEEPPISLDLTFHLLR by comparison with Protein Prospector MS-product database. (This software is freely available on the web at http://prospector2.ucsf.edu/.)
Figure 7.
(A) TIC and RIC of trypsin digested CRF. RIC showed the peak of the most abundant fragment peptide at m/z 623. (B) The expanded region of the TIC corresponding to the peak parking event initiated when first peak at m/z 623 was seen for MS detection of segmented flow of the separation. MS2 and MS3 analyses were performed manually by selecting the most abundant parent ion. Sample droplet distribution was indicated, which was uneven due to unstable perfusion flow rate at 25 nL/min generating by the syringe pump. TIC for MS2 and MS3 were lower compared to MS signal. (C),(D), and (E) show mass spectra corresponding to the MS, MS2 and MS3 event respectively in the peak parking region.
Our system offers a simple alternative to on-line peak parking. To achieve peak parking with on-line capillary LC-MS, specially designed LC-MS systems were needed to be used that allow the flow rate to be reduced during separation.3, 25, 26 Thus, when a peak of interest elutes into the MS, the LC flow rate is switched from normal to reduced flow for the extension of analysis time for selected peaks. While this approach is feasible, it has several difficulties. Successful flow rate switching for gradients at low flow rates requires considerable engineering of the flow system. Also, because larger emitter tips yield unstable sprays under these conditions, the best results have typically been obtained from small emitter tips (1–2 μm), which are unfortunately the easiest to be clogged.26 With the off-line approach however, it was easy to change the flow rate for peak parking by only changing the flow rate of the syringe pump for infusion of the segmented flow into MS. These flow rate changes had little effect on signal intensity over a range of 20 nL/min to 2 μL/min. By decoupling the separation and MS detection, it is possible to maintain the optimal flow rate for separation and MS analysis.
The system described here is also a useful alternative to collecting fractions in a multi-well plate. A primary advantage for this approach is the ease of collecting, manipulating, and analyzing nanoliter volume fractions which is extremely difficult when using multi-well plates.27
Other applications of the fraction collection and off-line analysis can be envisioned. By splitting plugs, using established methods,21, 28 it would be possible to analyze plugs by different mass spectrometers, NMR29, a second dimension of separation6, or other methods. Furthermore, plugs could be stored as long as they are stable for later analysis or re-analysis. The system may also be useful for multiplexing a MS. If the chromatographic separation is relatively slow, it may be possible to perform several separations in parallel and then rapidly infuse them into a fast scanning MS, e.g. TOF-MS, for improved throughput.
Conclusions
In this study, we have established a method for direct ESI-MS analysis of oil-segmented flow. When coupled with fraction collection from capillary LC, the method allows off-line ESI MS analysis with no extra column band broadening and no mixing of fractions collected. The system was shown to yield mass chromatograms that are equivalent to on-line analysis. With off-line analysis however, it is possible to better match the MS analysis time to the chromatographic peak widths. In this case, we demonstrated the equivalent of peak parking wherein flow rate is slowed for longer MS analysis of selected fractions. The system was demonstrated to be suitable for both reverse phase and HILIC separations. The method illustrates a general approach for preserving low volume components from microscale separation for further manipulation and study. Other applications are possible such as performing multiple assays on collected fractions. The capability of segmented flow ESI-MS for analysis rates over 2 Hz was also demonstrated. This suggests the potential for using ESI-MS for high-throughput screening in drug discovery and other applications.
Acknowledgments
This work was supported by NIH grant R37 EB003320 (R.T.K.). New Objective kindly donated the electrospray source and emitter tips used for many of the experiments.
References
- 1.Meiring HD, van der Heeft E, ten Hove GJ, de Jong A. J Sep Sci. 2002;25:557–568. [Google Scholar]
- 2.Simpson DC, Smith RD. Electrophoresis. 2005;26:1291–1305. doi: 10.1002/elps.200410132. [DOI] [PubMed] [Google Scholar]
- 3.Davis MT, Stahl DC, Hefta SA, Lee TD. Anal Chem. 1995;67:4549–4556. doi: 10.1021/ac00120a019. [DOI] [PubMed] [Google Scholar]
- 4.Van Pelt CK, Zhang S, Fung E, Chu IH, Liu TT, Li C, Korfmacher WA, Henion J. Rapid Commun Mass Spectrom. 2003;17:1573–1578. doi: 10.1002/rcm.1087. [DOI] [PubMed] [Google Scholar]
- 5.Edgar JS, Milne G, Zhao YQ, Pabbati CP, Lim DSW, Chiu DT. Angew Chem, Int Ed. 2009;48:2719–2722. [Google Scholar]
- 6.Niu XZ, Zhang B, Marszalek RT, Ces O, Edel JB, Klug DR, Demello AJ. Chem Commun. 2009:6159–6161. doi: 10.1039/b918100h. [DOI] [PubMed] [Google Scholar]
- 7.Fidalgo LM, Whyte G, Ruotolo BT, Benesch JLP, Stengel F, Abell C, Robinson CV, Huck WTS. Angew Chem, Int Ed. 2009;48:3665–3668. doi: 10.1002/anie.200806103. [DOI] [PubMed] [Google Scholar]
- 8.Kelly RT, Page JS, Marginean I, Tang K, Smith RD. Angew Chem, Int Ed. 2009;48:6832–6835. doi: 10.1002/anie.200902501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pei J, Li Q, Lee MS, Valaskovic GA, Kennedy RT. Anal Chem. 2009;81:6558–6561. doi: 10.1021/ac901172a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kennedy RT, Jorgenson JW. Anal Chem. 1989;61:1128–1135. doi: 10.1021/ac00180a012. [DOI] [PubMed] [Google Scholar]
- 11.Haskins WE, Wang Z, Watson CJ, Rostand RR, Witowski SR, Powell DH, Kennedy RT. Anal Chem. 2001;73:5005–5014. doi: 10.1021/ac010774d. [DOI] [PubMed] [Google Scholar]
- 12.Li Q, Zubieta JK, Kennedy RT. Anal Chem. 2009;81:2242–2250. doi: 10.1021/ac802391b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valaskovic GA, Kelleher NL, Little DP, Aaserud DJ, McLafferty FW. Anal Chem. 1995;67:3802–3805. doi: 10.1021/ac00116a030. [DOI] [PubMed] [Google Scholar]
- 14.Thorsen T, Roberts RW, Arnold FH, Quake SR. Phys Rev Lett. 2001;86:4163–4166. doi: 10.1103/PhysRevLett.86.4163. [DOI] [PubMed] [Google Scholar]
- 15.Tice JD, Song H, Lyon AD, Ismagilov RF. Langmuir. 2003;19:9127–9133. [Google Scholar]
- 16.Okushima S, Nisisako T, Torii T, Higuchi T. Langmuir. 2004;20:9905–9908. doi: 10.1021/la0480336. [DOI] [PubMed] [Google Scholar]
- 17.Garstecki P, Fuerstman MJ, Stone HA, Whitesides GM. Lab Chip. 2006;6:437–446. doi: 10.1039/b510841a. [DOI] [PubMed] [Google Scholar]
- 18.Kostiainen R, Bruins AP. Rapid Commun Mass Spectrom. 1996;10:1393–1399. doi: 10.1002/rcm.1408. [DOI] [PubMed] [Google Scholar]
- 19.Kostiainen R, Kauppila TJ. J Chromatogr A. 2009;1216:685–699. doi: 10.1016/j.chroma.2008.08.095. [DOI] [PubMed] [Google Scholar]
- 20.Murphy RE, Schure MR, Foley JP. Anal Chem. 1998;70:1585–1594. [Google Scholar]
- 21.Link DR, Anna SL, Weitz DA, Stone HA. Phys Rev Lett. 2004;92:054503. doi: 10.1103/PhysRevLett.92.054503. [DOI] [PubMed] [Google Scholar]
- 22.Leitner A, Zollner P, Lindner W. J Chromatogr A. 2001;939:49–58. doi: 10.1016/s0021-9673(01)01331-0. [DOI] [PubMed] [Google Scholar]
- 23.Berthiller F, Schuhmacher R, Buttinger G, Krska R. J Chromatogr A. 2005;1062:209–216. doi: 10.1016/j.chroma.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 24.Kirchherr H, Kuhn-Velten WN. J Chromatogr B. 2006;843:100–113. doi: 10.1016/j.jchromb.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 25.Davis MT, Lee TD. J Am Soc Mass Spectrom. 1998;9:194–201. doi: 10.1016/S1044-0305(97)00282-1. [DOI] [PubMed] [Google Scholar]
- 26.Davis MT, Lee TD. J Am Soc Mass Spectrom. 1997;8:1059–1069. [Google Scholar]
- 27.Doneanu CE, Griffin DA, Barofsky EL, Barofsky DF. J Am Soc Mass Spectrom. 2001;12:1205–1213. doi: 10.1016/S1044-0305(01)00307-5. [DOI] [PubMed] [Google Scholar]
- 28.Menetrier-Deremble L, Tabeling P. Physical Review E. 2006;74:035303. doi: 10.1103/PhysRevE.74.035303. [DOI] [PubMed] [Google Scholar]
- 29.Kautz RA, Goetzinger WK, Karger BL. J Com Chem. 2005;7:14–20. doi: 10.1021/cc0498940. [DOI] [PubMed] [Google Scholar]