Abstract
Background
Cross-sectional studies suggest that prevalence of the metabolic syndrome (MetS) increases from premenopause to postmenopause in women, independent of age. Little is known about why. We hypothesized that the incidence of the MetS increases with progression through menopause and that this increase is explained by the progressive androgenicity of the hormonal milieu.
Methods
This longitudinal, 9-year study of 949 participants in the Study of Women’s Health Across the Nation investigates the natural history of the menopausal transition. Participants of 5 ethnicities at 7 geographic sites were recruited when they were premenopausal or early perimenopausal and were eligible for this study if they (1) reached menopause during the study; (2) had never taken hormone therapy, and (3) did not have diabetes mellitus or the MetS at baseline. The primary outcome was the presence of MetS using National Cholesterol Education Program Adult Treatment Panel III criteria. Secondary outcomes were the components of the MetS.
Results
By the final menstrual period, 13.7% of the women had new-onset MetS. Longitudinal analyses, centered at the final menstrual period, were adjusted for age at menopause, ethnicity, study site, marital status, education, body mass index, smoking, and aging. Odds of developing the MetS per year in perimenopause were 1.45 (95% confidence interval, 1.35-1.56); after menopause, 1.24 (95% confidence interval, 1.18-1.30). These odds were significantly different (P<.001). An increase in bioavailable testosterone or a decrease in sex hormone–binding globulin levels increased the odds.
Conclusions
As testosterone progressively dominates the hormonal milieu during the menopausal transition, the prevalence of MetS increases, independent of aging and other important covariates. This may be a pathway by which cardiovascular disease increases during menopause.
Cardiovascular disease (CVD) is the primary cause of death in women in Western countries. Women tend to develop the disease about 10 years later than men, with a marked increase through the menopausal years.1 Cardiovascular disease is rare among women younger than 45 years, but women older than 55 years are more likely than men to have CVD.2,3 This has led to the hypothesis that changes during the menopausal transition increase the risk of CVD, independent of normal aging.4,5 This hypothesis is supported by studies that show that surgically induced menopause increases the risk of CVD6 and by autopsy studies that show minimal vascular disease before but not after menopause.7
Menopausal status, most commonly defined by changes in menstrual bleeding, is a proxy for changes in sex hormone levels, the most important of which are estradiol and testosterone.8 Sex hormone–binding globulin (SHBG) is a protein that binds testosterone and estradiol and transports them to target organs. Because this binding occurs preferentially to testosterone, SHBG is viewed as an indirect marker of bioavailable androgen.9
A hallmark of the menopausal transition is the dramatic reduction in estradiol levels.8 With this reduction, there is a progressive shift toward androgen dominance in the hormonal milieu.10,11 Although little is known about how this hormonal shift influences CVD risk, available studies suggest a link between androgenicity and CVD risk factors.12-14 These studies are limited, however, because they are small, cross-sectional, and/or primarily conducted in Caucasian samples.
The metabolic syndrome (MetS) is a summary measure of important CVD risk factors that frequently coexist. The syndrome is evident in 20% to 30% of middle-aged women and has been linked to the development of CVD and diabetes.15-17 To our knowledge, there have been no longitudinal studies of the impact of the menopausal transition on the development of the MetS. Menopausal status has been linked to some components of the MetS, but results are inconsistent.18 Most cross-sectional studies show that postmenopausal women are more likely then premenopausal women to have higher triglyceride and lower high-density lipoprotein cholesterol (HDL-C) levels independent of age,18 but some large European studies19,20 show no significant differences in triglyceride or HDL-C levels after adjustment for age. Longitudinal results are also mixed. In 2 small studies in Asia, premenopausal to postmenopausal changes in HDL-C and triglyceride levels were not significant.21,22 In a large longitudinal study of female Japanese survivors of the atomic bomb, menopausal status was associated with an increase in total cholesterol levels, but not in body mass index (BMI) (calculated as weight in kilograms divided by height in meters squared) or in blood pressure.23
There is evidence that specific reproductive hormones are associated with CVD risk factors in premenopausal and postmenopausal Caucasian women.24,25 Increased testosterone and decreased SHBG levels are strongly associated with central adiposity, increased triglycerides, and decreased HDL-C levels in Caucasian women24,25 as well as in an ethnically diverse population.26 Even after age and BMI were adjusted for, low SHBG and high testosterone levels were significantly correlated with the presence of the MetS.27 To our knowledge, there have been no studies of the association between menopause-related changes in sex hormone levels and the MetS.
The purposes of this study were (1) to determine whether the incidence of the MetS increases with progression through menopause, independent of age and other standard CVD risk factors, using a longitudinal design; and (2) to test the hypothesis that testosterone is associated with the development of the MetS and its components.
METHODS
STUDY DESIGN AND PARTICIPANTS
The Study of Women’s Health Across the Nation (SWAN) is a multiethnic, community-based, longitudinal cohort study of the natural history of the menopausal transition in 3302 women enrolled at 7 sites throughout the United States (Boston, Massachusetts; Chicago; Detroit, Michigan; Los Angeles, California; Oakland, California; Newark, New Jersey; and Pittsburgh). The design of the main study has been described elsewhere.28 The cohort at baseline included women of Caucasian, African American, Chinese, Japanese, and Hispanic origins, aged 42 to 52 years, who (1) had an intact uterus and at least 1 ovary, (2) had had at least 1 menstrual period in the preceding 3 months, (3) had used no sex steroid hormone therapy in the preceding 3 months, and (4) were not pregnant.
To date, a total of 1644 women in the SWAN cohort have reached natural menopause and were thus eligible for this study. Of these, we excluded 309 (18.8%) because they used hormone therapy before reaching menopause; 312 (19.0%) because they had the MetS at baseline; 29 (1.8%) because they had diabetes mellitus at baseline; 10 (0.6%) because they had missing data on weight at baseline; and 35 (2.1%) because of missing assessments for key variables after the initial examination. Thus, the analytic sample for this report was 949. The only difference between the samples before and after these exclusions was in ethnicity because fewer Japanese and Chinese women were excluded owing to their less frequent use of hormone therapy.
ASSESSMENTS
All participants underwent annual examinations that included interviews, anthropometry, questionnaires, and a blood draw for the assessment of sociodemographic factors, CVD risk factors, and reproductive hormone levels. Owing to budgetary constraints, the blood assays, including those for glucose, HDL-C, and triglyceride levels, were performed every other year only.
Phlebotomy was performed in the morning after an overnight fast. Subjects were scheduled for venipuncture on days 2 to 5 of a spontaneous menstrual cycle within 60 days of the anniversary of the baseline examination date. All assays were performed on an automated analyzer (ACS-180; Bayer Diagnostics Corporation, Tarrytown, New York) using a double-antibody chemiluminescent immunoassay with a solid-phase anti-IgG immunoglobulin conjugated to paramagnetic particles, antiligand antibody, and competitive ligand labeled with dimethylacridinium ester. The estradiol assay modifies the rabbit anti–estradiol-6 ACS-180 immunoassay to increase sensitivity, with a lower limit of detection (LLD) of 1.0 pg/mL (to convert to picomoles per liter, multiply by 3.671). The testosterone assay modifies the rabbit polyclonal antitestosterone ACS-180 immunoassay, with an LLD of 2.19 ng/dL (to convert to nanomoles per liter, multiply by 0.0347). The SHBG assay was developed at the central laboratory at the University of Michigan, Ann Arbor, using rabbit anti-SHBG antibodies, with an LLD of 0.22 μg/mL (to convert to nanomoles per liter, multiply by 8.896). Duplicate estradiol assays were conducted with results reported as the arithmetic mean for each subject, with a coefficient of variation of 3% to 12%. All other assays were single determinations. Bioavailable testosterone level was calculated as T×100/(3.24×SHBG), where T indicates testosterone levels in nanograms per deciliter and SHBG levels are given in micrograms per milliliter.
Standardized protocols were used to measure weight, height, and waist circumference. Height was measured without shoes using a stadiometer. Weight was measured without shoes and with light indoor clothing using scales that were calibrated on a monthly basis to a standard. Waist circumference was measured with the respondent in nonrestrictive undergarments. Blood pressure was measured according to a standardized protocol, with readings taken on the right arm and with the respondent seated with feet flat on the floor for at least 5 minutes before measurement. Respondents had not smoked or consumed any caffeinated beverage within 30 minutes of blood pressure measurement. A standard mercury sphygmomanometer was used to record systolic and diastolic pressures at the first and fifth Korotkoff sounds. Two sequential blood pressure values, with a minimum 2-minute rest between measures, were obtained and averaged.
Total cholesterol and triglyceride levels were analyzed using enzymatic methods (Hitachi 747 analyzer; Boehringer Mannheim Diagnostics, Indianapolis, Indiana) as previously described.29 We isolated HDL-C using heparin–manganese chloride.30
We defined the MetS using the modified National Cholesterol Education Program Adult Treatment Panel III definition with 100 mg/dL as the cut point for glucose level (to convert to millimoles per liter, multiply by 0.0555).1 However, because lower overall adiposity has been associated with an increased risk of type 2 diabetes mellitus, hypertension, and dyslipidemia in Asians,31,32 the waist circumference cut point proposed by the World Health Organization (≥80 cm)33 was used for the Japanese and Chinese participants instead of the cut point used in the National Cholesterol Education Program Adult Treatment Panel III definition (≥88 cm).34
STATISTICAL METHODS
Data were summarized as mean (SD) or number (percentage), as indicated. The women experienced menopause at varying times in the 8 years of follow-up. By aligning each measurement from each woman according to the date of her final menstrual period (FMP), we were able to summarize the accumulated data for a 13-year time scale (from 6 years before to 6 years after the FMP).
The longitudinal data were analyzed as repeated measures, ordered by time relative to the FMP, taking into account the correlation between successive observations within individuals. The BMI values were transformed by natural logarithm before analysis to ensure that the distributions of the residuals (observed values–fitted values) were close to normal. All models were adjusted for aging, age at the FMP, ethnicity, study site, marital status, education, smoking, BMI at baseline, and change in BMI from baseline. To assess the effect of menopause, the first model included 2 terms for aging, one for perimenopause (<1 year after the FMP) and the other for postmenopause (starting 1 year after the FMP). To assess the difference in the β estimates for the 2 aging terms, the adjusted model was rerun with aging and an interaction of menopausal status and aging. In subsequent models, we examined the change in a specific hormone level adjusted for that hormone level at baseline with aging not separated by menopausal status. Baseline hormone measures as well as their changes were standardized by dividing each value by the overall standard deviation to make their influence on the outcomes comparable. We analyzed the MetS using generalized estimating equations. Goodness of fit was assessed by log-likelihood. Changes in components of the MetS, adjusting for baseline values, were analyzed as repeated-measures mixed models with fixed effects only. Allowance for a random intercept did not change results. Goodness of fit was assessed with the Bayes information criterion.35 An autoregressive covariance structure was used in all models to account for the dependence of measures over time.
RESULTS
After exclusions, women in the analytic sample who, by definition, had had an FMP were older than the remainder of the SWAN cohort who were awaiting their FMP. Given their older age, they had a higher Framingham Risk Score, lower HDL-C level, higher glucose level, and higher blood pressure. Comparisons of the 2 samples at baseline revealed that women in the analytic sample had less education and lower testosterone levels, but higher estradiol and SHBG levels.
Table 1 presents the characteristics of the analytic sample at the time of the FMP, separated by a new occurrence of the MetS. In the total sample, the mean age at the FMP was 50.9 years and ranged from 42 to 58 years. The mean BMI was 26.9, indicating that, on average, the women were overweight. At the time of the FMP, 13.7% of the cohort had a new onset of the MetS. Women with the MetS differed from women without the MetS on all variables except ethnicity, study site, smoking, and low-density lipoprotein cholesterol (LDL-C) level. Combining baseline prevalent cases with new-onset cases, 32.7% of the women had the MetS by the time of the FMP.
Table 1. Characteristics of Cohort at FMP, Overall and by MetS at FMP.
| Characteristic | Total Cohort | Cohort Without MetS | Cohort With MetS |
|---|---|---|---|
| All women, No. (%) | 949 (100.0) | 819 (86.3) | 130 (13.7) |
| Ethnicity, No. (%) | |||
| African American | 247 (26.0) | 202 (24.7) | 45 (34.6) |
| Caucasian | 438 (46.2) | 383 (46.8) | 55 (42.3) |
| Chinese | 104 (11.0) | 93 (11.4) | 11 (8.5) |
| Hispanic | 46 (4.9) | 38 (4.6) | 8 (6.2) |
| Japanese | 114 (12.0) | 103 (12.6) | 11 (8.5) |
| Site, No. (%) | |||
| Boston, Massachusetts | 146 (15.4) | 127 (15.5) | 19 (14.6) |
| Chicago, Illinois | 128 (13.5) | 104 (12.7) | 24 (18.5) |
| Los Angeles, California | 179 (18.9) | 163 (19.9) | 16 (12.3) |
| Detroit, Michigan | 132 (13.9) | 110 (13.4) | 22 (16.9) |
| Newark, New Jersey | 70 (7.4) | 61 (7.4) | 9 (6.9) |
| Oakland, California | 165 (17.4) | 149 (18.2) | 16 (12.3) |
| Pittsburgh, Pennsylvania | 129 (13.6) | 105 (12.8) | 24 (18.5) |
| Demographics, No. (%) | |||
| Smokinga | 146 (15.4) | 122 (14.9) | 24 (18.5) |
| Unmarriedb | 301 (31.7) | 255 (31.5) | 46 (35.7) |
| Education level of ≤high schoola,b | 224 (23.6) | 183 (22.4) | 41 (32.0)c |
| CVD risk factors, mean (SD) | |||
| Age, y | 50.9 (2.9) | 50.7 (2.9) | 52.2 (2.3)d |
| Weight, kg | 70.3 (17.5) | 68.0 (15.9) | 82.0 (18.8)d |
| BMI | 26.9 (6.2) | 26.0 (5.7) | 31.5 (6.2)d |
| DBP, mm Hg | 74.0 (9.9) | 73.1 (9.6) | 78.9 (9.6)d |
| Framingham Risk Score | 9.8 (3.5) | 9.4 (3.3) | 11.9 (3.4)d |
| LDL-C level, mg/dL | 120.8 (32.4) | 119.6 (31.5) | 127.7 (36.3)c |
| Physical activity | 7.8 (1.8) | 7.9 (1.8) | 7.5 (1.7)c |
| MetS components, mean (SD) | |||
| Waist, cm | 83.3 (13.4) | 81.2 (12.4) | 94.6 (12.2)d |
| HDL-C level, mg/dL | 64.2 (15.5) | 65.9 (15.4) | 54.0 (12.3)d |
| Glucose level, mg/dL | 89.2 (10.6) | 87.8 (7.4) | 98.5 (20.1)d |
| Triglyceride level, mg/dL | 104.6 (51.1) | 97.3 (41.4) | 149.9 (78.8)d |
| SBP, mm Hg | 116.0 (17.0) | 113.8 (15.7) | 126.7 (18.2)d |
| Hormone levels, median (IQR) | |||
| Testosterone, ng/dL | 38.0 (26.5-51.0) | 37.6 (25.8-50.4) | 42.5 (30.4-53.9)c |
| Bioavailable testosterone, ng/dL | 3.13 (1.78-5.32) | 2.89 (1.58-4.86) | 4.52 (2.48-7.08)d |
| SHBG, μg/mL | 4.88 (3.37-6.80) | 5.06 (3.54-7.21) | 3.83 (2.52-5.46)d |
| Estradiol, pg/mL | 25.5 (14.8-65.0) | 26.6 (14.7-69.5) | 21.8 (14.4-34.8)c |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CVD, cardiovascular disease; DBP, diastolic blood pressure; FMP, final menstrual period; HDL-C, high-density lipoprotein cholesterol; IQR, interquartile range; LDL-C, low-density lipoprotein cholesterol; MetS, metabolic syndrome; SBP, systolic blood pressure; SHBG, sex hormone–binding globulin.
SI conversion factors: To convert estradiol to picomoles per liter, multiply by 3.671; glucose to millimoles per liter, by 0.0555; testosterone to nanomoles per liter, by 0.0347; SHBG to nanomoles per liter, by 8.896; and triglyceride to millimoles per liter, by 0.0113.
Assessed at baseline.
Percentages are based on different totals because of missing values.
P≤.05.
P≤.001.
Figure 1 shows the odds of developing the MetS during the course of the menopausal transition, after adjustment for ethnicity, site, baseline BMI, change in BMI, education, marital status, smoking, age at FMP, and aging.
Figure 1.
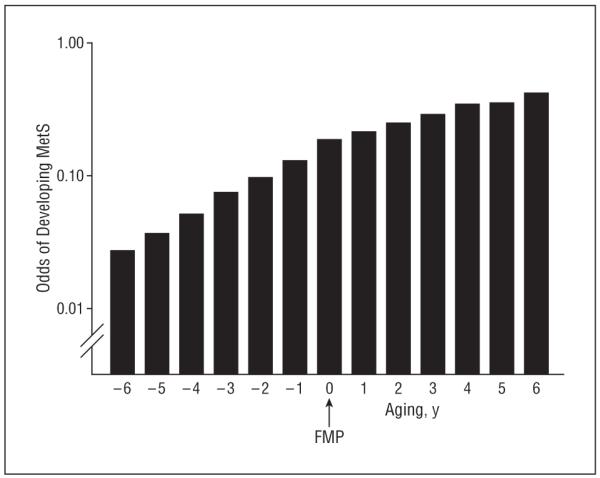
Odds of developing the metabolic syndrome (MetS) by aging after adjustment for standard risk factors, including age at final menstrual period (FMP), ethnicity, study site, education, marital status, smoking, baseline body mass index (BMI), and change in BMI from baseline.
Table 2 presents multivariate predictors of the development of the MetS. Odds of developing the MetS were 1.45 (95% confidence interval, 1.35-1.56) per year during the perimenopausal period and 1.24 (95% confidence interval, 1.18-1.30) per year in the postmenopausal years. The difference between these odds ratios was highly significant (P<.001).
Table 2. Multivariate Predictors of the Development of the MetSa.
| Variable | OR (95% CI) | P Value |
|---|---|---|
| Ethnicity | ||
| African American | 0.74 (0.45-1.22) | .24 |
| Chinese | 2.56 (0.90-7.27) | .08 |
| Hispanic | 2.64 (0.52-13.47) | .24 |
| Japanese | 2.57 (0.88-7.47) | .08 |
| Caucasian | 1 [Reference] | |
| Site | ||
| Boston, Massachusetts | 0.68 (0.34-1.36) | .28 |
| Los Angeles, California | 0.67 (0.23-1.96) | .47 |
| Detroit, Michigan | 0.60 (0.31-1.19) | .14 |
| Newark, New Jersey | 0.38 (0.08-1.83) | .23 |
| Oakland, California | 0.59 (0.22-1.57) | .29 |
| Pittsburgh, Pennsylvania | 1.00 (0.51-1.97) | >.99 |
| Chicago, Illinois | 1 [Reference] | |
| Baseline BMIb | 3.34 (2.72-4.10) | <.001 |
| Δ BMIb | 1.49 (1.32-1.67) | <.001 |
| Smoking | 2.44 (1.52-3.89) | <.001 |
| Education of ≥high school | 1.70 (1.10-2.64) | .02 |
| Unmarried | 1.46 (0.95-2.24) | .08 |
| Age at final menstrual period | 1.17 (1.09-1.25) | <.001 |
| Perimenopausal aging | 1.45 (1.35-1.56) | <.001 |
| Postmenopausal aging | 1.24 (1.18-1.30) | <.001 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CI, confidence interval; Δ, change; MetS, metabolic syndrome; OR, odds ratio.
Excludes women with the MetS at baseline.
Indicates log-transformation used to ensure normality of residuals.
Table 3 compares the results of 3 multivariate models, each focusing on a different hormone. Changes in total estradiol and total testosterone levels were unrelated to the development of the MetS. The change in bioavailable testosterone level was significantly related to the change in the MetS and was essentially interchangeable with the results for change in SHBG levels. For every 1-SD increase in bioavailable testosterone levels, the odds of developing the MetS increased by 10%. For every 1-SD decrease in SHBG level, the odds of developing the MetS increased by 13%.
Table 3. Comparison of 4 Hormone Measures as Predictors of the Development of the MetSa After Adjustment for Standard Risk Factors.
| Hormone Measure | OR (95% CI) | P Value | Log-Likelihood |
|---|---|---|---|
| Bioavailable | |||
| testosterone level | |||
| Δ | 1.10 (1.01-1.20) | .02 | −1430.6 |
| Baseline | 1.34 (1.11-1.62) | .002 | |
| SHBG level | |||
| Δ | 0.87 (0.81-0.93) | <.001 | −1427.5 |
| Baseline | 0.82 (0.69-0.98) | .03 | |
| Estradiol level | |||
| Δ | 0.97 (0.88-1.06) | .49 | −1437.2 |
| Baseline | 0.92 (0.75-1.12) | .40 | |
| Testosterone level | |||
| Δ | 0.96 (0.86-1.06) | .40 | −1442.5 |
| Baseline | 1.16 (0.94-1.42) | .16 |
Abbreviations: Δ, change; MetS, metabolic syndrome; SHBG, sex hormone–binding globulin.
Excludes women with the MetS at baseline. Covariates include age (P≤.001), age at final menstrual period (P≤.001), ethnicity, study site, baseline body mass index (BMI) (P≤.001), change in BMI from baseline (P≤.001), baseline education level (P≤.05), marital status, and smoking (P≤.001). Significance levels of these covariates do not vary across models. Baseline values of the respective hormone were included.
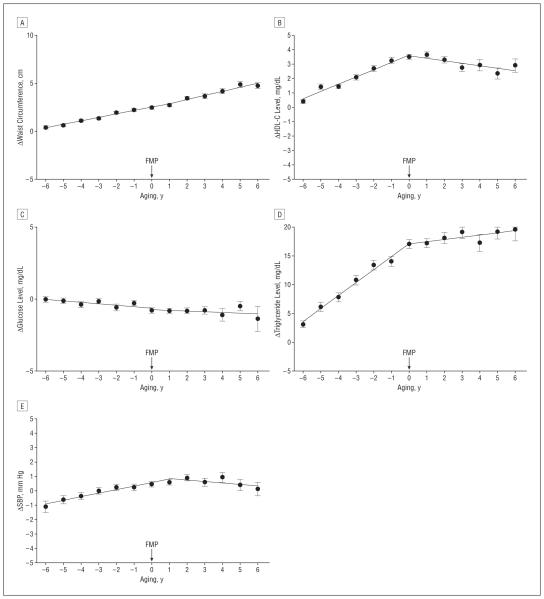
Figure 2 shows the change in the MetS components with the transition from perimenopause to postmenopause, after adjusting for covariates. Waist circumference increased with aging even after controlling for BMI (P<.001) and accelerated after menopause (P=.04). Levels of HDL-C and triglycerides show a similar pattern over time; both increased significantly during the perimenopausal period but stabilized afterward. Glucose level decreased slightly but significantly with aging during the entire study period (P<.001); systolic blood pressure increased but not significantly (P=.07) with aging; and there was no effect of menopause on glucose level or blood pressure.
Figure 2.
Changes in components of the metabolic syndrome by aging after adjustment for standard risk factors, including baseline outcome, age at final menstrual period (FMP), ethnicity, study site, education, marital status, smoking, baseline body mass index (BMI), and change (Δ) in BMI from baseline. Components include waist circumference (A), high-density lipoprotein cholesterol (HDL-C) levels (B), glucose levels (C), triglyceride levels (D), and systolic blood pressure (E). To convert glucose to millimoles per liter, multiply by 0.0555; HDL-C to millimoles per liter, multiply by 0.0259; and triglyceride to millimoles per liter, multiply by 0.0113.
Table 4 presents a comparison of 4 hormone measures as predictors of change in components of the MetS. Changes in bioavailable testosterone and SHBG levels were associated with changes in waist circumference and in HDL-C and glucose levels.
Table 4. Comparison of 4 Hormone Measures as Predictors of Change in Components of the MetS, After Adjustment for Standard Risk Factorsa.
| Outcome, Standardized β Coefficient |
|||||
|---|---|---|---|---|---|
| Measure | Δ Waist Circumference |
Δ HDL-C Level |
Δ Glucose Level |
Δ Triglyceride Level |
Δ SBP |
| Estradiol level | |||||
| Δ | −0.04 | 0.69b | −0.30 | 0.17 | −0.41c |
| Baseline | 0.10 | 0.14 | −0.25 | −2.40c | −0.15 |
| Testosterone level | |||||
| Δ | −0.03 | 0.20 | 0.75b | 1.92d | −0.04 |
| Baseline | −0.01 | −0.03 | −0.04 | 0.94 | 0.49d |
| SHBG level | |||||
| Δ | −0.21b | 0.81b | −0.40d | 0.09 | −0.23 |
| Baseline | −0.10 | 0.37 | −0.07 | −0.54 | 0.13 |
| Bioavailable testosterone level | |||||
| Δ | 0.14c | −0.48c | 0.81b | 1.14 | 0.12 |
| Baseline | 0.08 | −0.35 | 0.01 | 0.89 | 0.23 |
Abbreviations: Δ, change; HDL-C, high-density lipoprotein cholesterol; MetS, metabolic syndrome; SBP, systolic blood pressure; SHBG, sex hormone–binding globulin.
Covariates are the baseline outcome (P≤.001), age at final menstrual period (P≤.001), aging (P≤.001), ethnicity, study site, education, marital status, smoking, baseline body mass index (BMI) (P≤.001), and change in BMI from baseline (P≤.001). Significance levels of these covariates do not vary across models.
P≤.001.
P≤.01.
P≤.05.
COMMENT
To our knowledge, this is the first study to show that the incidence of the MetS increased progressively from 6 years before to 6 years after the FMP, independent of aging and known CVD risk factors. Previous epidemiologic studies have suggested that an association exists between menopause and the MetS, but they were limited by their small size, their cross-sectional design,27 and/or their ethnically homogeneous samples.15,36 The use of the SWAN cohort to examine this link provided the opportunity to examine this question using a rigorous, longitudinal design and a large, multiethnic cohort.
Menopause-related testosterone predominance appears to be implicated as a key hormonal change that is associated with the incidence of the MetS, independent of aging and other standard CVD risk factors. Moreover, testosterone predominance appears to be associated with change in 3 of the 5 components of the MetS. To the best of our knowledge, this is the first longitudinal study to identify hormonal changes associated with the development of the MetS in women.
It was previously thought that estrogen exerted a direct positive effect on CVD risk in women, a benefit that was lost as women transitioned from a premenopausal to a postmenopausal state and experienced a loss of estrogen.8,37 However, these data show that the change in estrogen level is, at best, a weak and nonsignificant predictor of MetS risk. A more likely story is that the progressive androgenicity of the hormonal milieu exerts a direct negative effect on CVD risk. This story is consistent with data from clinical trials showing that the replacement of estrogen does not protect against CVD.38 It is also biologically plausible, based on mounting evidence from cross-sectional clinical studies showing that testosterone is associated with insulin resistance, hyperinsulinemia, low HDL-C levels, high blood levels of glucose and triglycerides, and diabetes mellitus24,25,39 and from epidemiologic data showing that androgens are associated with hemostatic and inflammatory markers.14
The finding reported herein that SHBG is interchangeable with bioavailable testosterone as a predictor of the MetS is consistent with the view that SHBG is a marker of the balance of testosterone relative to estrogen levels. Indeed, in our data, we found a strong negative correlation between SHBG and bioavailable testosterone levels and a strong positive correlation between SHBG and estradiol levels. It is possible, however, that SHBG also has independent functions in the cell that go beyond its role as a marker of bioavailable testosterone. This is an important area of study that is currently under way.11
Strengths of this study are the longitudinal design, the large multiethnic cohort, and the long follow-up through menopause. By anchoring the data at the FMP, we were able to use a more precise definition of time in the menopausal transition. A limitation of the study may be its rep-resentativeness because all of the women lived in major metropolitan areas. Another limitation is that the frequency of glucose, HDL-C, and triglyceride level measurements was every other year, rather than the annual assessments available for all of the other factors. However, our method of analysis is robust in handling this problem. Most of our women were overweight, raising the question of whether results would be different in women of normal weight. Our findings did not change when only women with a BMI of less than 25 at baseline underwent analysis.
This longitudinal study of the largest cohort of middle-aged women undergoing the menopausal transition to date shows that the prevalence of the MetS increases significantly during the perimenopausal and early postmenopausal years, independent of aging and other known CVD risk factors such as weight gain and smoking. Levels of bioavailable testosterone and, alternatively, SHBG, emerged as independent predictors, after controlling for aging and CVD risk factors. The temporality of the association between menopause and CVD risk has been questioned.40 However, these data suggest the hypothesis that progression through menopause, with the associated decrease in estrogen level, results in a progressively androgen-dominated hormonal milieu, which then increases the MetS risk. Further investigation of the role of androgens as CVD risk factors or a CVD consequence is needed.
Acknowledgments
Funding/Support: This study was supported by grants NR004061, AG012505, AG012535, AG012531, AG012539, AG012546, AG012553, AG012554, and AG012495 to SWAN from the NIH, Department of Health and Human Services, through the National Institute on Aging, the National Institute of Nursing Research, and the NIH Office of Research on Women’s Health.
Footnotes
Author Contributions: Dr Janssen had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Janssen, Powell, Lasley, and Sutton-Tyrrell. Acquisition of data: Powell. Analysis and interpretation of data: Janssen, Powell, Crawford, and Lasley. Drafting of the manuscript: Janssen, Powell, and Lasley. Critical revision of the manuscript for important intellectual content: Powell, Crawford, Lasley, and Sutton-Tyrrell. Statistical analysis: Janssen and Crawford. Obtained funding: Powell and Sutton-Tyrrell. Administrative, technical, and material support: Powell. Study supervision: Powell, Lasley, and Sutton-Tyrrell.
Financial Disclosure: None reported.
Additional Contributions: We thank the study staff at each site and all the women who participated in the SWAN.
Participating Centers: Clinical centers (principal investigators) in the SWAN include the University of Michigan, Ann Arbor (MaryFran Sowers, PhD); Massachusetts General Hospital, Boston (Robert Neer, MD, 1994-1999; Joel Finkelstein, MD, 1999-present); Rush University, Rush University Medical Center, Chicago (Lynda Powell, PhD); University of California, Davis/Kaiser (Ellen Gold, PhD); University of California, Los Angeles (Gail Greendale, MD); University of Medicine and Dentistry, New Jersey Medical School, Newark (Gerson Weiss, MD, 1994-2004; Nanette Santoro, MD, 2004-present); and the University of Pittsburgh (Karen Matthews, PhD). The National Institutes of Health (NIH) program offices included the National Institute on Aging, Bethesda, Maryland (program officers, Marcia Ory, PhD, 1994-2001; Sherry Sherman, PhD, 1994-present), and the National Institute of Nursing Research, Bethesda. The central laboratory was at the University of Michigan (Daniel McConnell, PhD, Central Ligand Assay Satellite Services). The coordinating centers (principal investigators) were the New England Research Institutes, Watertown, Massachusetts (Sonja McKinlay, PhD, 1995-2001), and the University of Pittsburgh (Kim Sutton-Tyrrell, DrPH, 2001-present). Chris Gallagher, PhD, and Susan Johnson, MD, were Steering Committee chairs.
REFERENCES
- 1.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285(19):2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 2.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the Third National Health and Nutrition Examination Survey. JAMA. 2002;287(3):356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 3.Rosamond W, Flegal K, Friday G, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115(5):e69–e171. doi: 10.1161/CIRCULATIONAHA.106.179918. published correction appears in Circulation. 2007;115(5):e172. doi:10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 4.Kannel WB. Metabolic risk factors for coronary heart disease in women: perspective from the Framingham Study. Am Heart J. 1987;114(2):413–419. doi: 10.1016/0002-8703(87)90511-4. [DOI] [PubMed] [Google Scholar]
- 5.Kannel WB, Hjortland MC, McNamara PM, Gordon T. Menopause and risk of cardiovascular disease. Ann Intern Med. 1976;85(4):447–452. doi: 10.7326/0003-4819-85-4-447. [DOI] [PubMed] [Google Scholar]
- 6.Gohlke-Bärwolf C. Coronary artery disease: is menopause a risk factor? Basic Res Cardiol. 2000;95(suppl 1):I77–I83. doi: 10.1007/s003950070014. [DOI] [PubMed] [Google Scholar]
- 7.Tejada C, Strong JP, Montenegro MR, Restrepo C, Solberg LA. Distribution of coronary and aortic atherosclerosis by geographic location, race, and sex. Lab Invest. 1968;18(5):509–526. [PubMed] [Google Scholar]
- 8.Yen S, Jaffe R, Barbieri R. Reproductive Endocrinology: Physiology, Pathophysiology, and Clinical Management. 5th ed. WB Saunders Co; Philadelphia, PA: 1999. [Google Scholar]
- 9.Anderson DC. Sex-hormone binding globulin. Clin Endocrinol (Oxf) 1974;3(1):69–96. doi: 10.1111/j.1365-2265.1974.tb03298.x. [DOI] [PubMed] [Google Scholar]
- 10.Burger HG, Dudley EC, Cui J, Dennerstein L, Hopper JL. A prospective longitudinal udy of serum testosterone, dehydroepiandrosterone sulfate, and sex hormone–binding globulin levels through the menopause transition. J Clin Endocrinol Metab. 2000;85(8):2832–2838. doi: 10.1210/jcem.85.8.6740. [DOI] [PubMed] [Google Scholar]
- 11.Lasley BL, Santoro N, Randolf JF, et al. The relationship of circulating dehydroepiandrosterone, testosterone, and estradiol to stages of the menopausal transition and ethnicity. J Clin Endocrinol Metab. 2002;87(8):3760–3767. doi: 10.1210/jcem.87.8.8741. [DOI] [PubMed] [Google Scholar]
- 12.Do KA, Green A, Guthrie JR, Dudley EC, Burger HG, Dennerstein L. Longitudinal study of risk factors for coronary heart disease across the menopausal transition. Am J Epidemiol. 2000;151(6):584–593. doi: 10.1093/oxfordjournals.aje.a010246. [DOI] [PubMed] [Google Scholar]
- 13.Guthrie JR, Taffe JR, Lehert P, Burger HG, Dennerstein L. Association between hormonal changes at menopause and the risk of a coronary event: a longitudinal study. Menopause. 2004;11(3):315–322. doi: 10.1097/01.gme.0000094208.15096.62. [DOI] [PubMed] [Google Scholar]
- 14.Sowers MR, Jannausch M, Randolph JF, et al. Androgens are associated with hemostatic and inflammatory factors among women at the mid-life. J Clin Endocrinol Metab. 2005;90(11):6064–6071. doi: 10.1210/jc.2005-0765. [DOI] [PubMed] [Google Scholar]
- 15.Bonora E, Kiechl S, Willeit J, et al. Bruneck Study. Carotid atherosclerosis and coronary heart disease in the metabolic syndrome: prospective data from the Bruneck Study. Diabetes Care. 2003;26(4):1251–1257. doi: 10.2337/diacare.26.4.1251. [DOI] [PubMed] [Google Scholar]
- 16.Isomaa B, Almgren P, Tuomi T, et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001;24(4):683–689. doi: 10.2337/diacare.24.4.683. [DOI] [PubMed] [Google Scholar]
- 17.Malik S, Wong ND, Franklin SS, et al. Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation. 2004;110(10):1245–1250. doi: 10.1161/01.CIR.0000140677.20606.0E. [DOI] [PubMed] [Google Scholar]
- 18.Carr MC. The emergence of the metabolic syndrome with menopause. J Clin Endocrinol Metab. 2003;88(6):2404–2411. doi: 10.1210/jc.2003-030242. [DOI] [PubMed] [Google Scholar]
- 19.de Aloysio D, Gambacciani M, Meschia M, et al. The effect of menopause on blood lipid and lipoprotein levels. Atherosclerosis. 1999;147(1):147–153. doi: 10.1016/s0021-9150(99)00315-9. [DOI] [PubMed] [Google Scholar]
- 20.Peters HW, Westendorp ICD, Hak AE, et al. Menopausal status and risk factors for cardiovascular disease. J Intern Med. 1999;246(6):521–528. doi: 10.1046/j.1365-2796.1999.00547.x. [DOI] [PubMed] [Google Scholar]
- 21.Fukami K, Koike K, Hirota K, Yoshikawa H, Miyake A. Perimenopausal changes in serum lipids and lipoproteins: a 7-year longitudinal study. Maturitas. 1995;22(3):193–197. doi: 10.1016/0378-5122(95)00927-d. [DOI] [PubMed] [Google Scholar]
- 22.Torng PL, Su TC, Sung FC, et al. Effects of menopause on intraindividual changes in serum lipids, blood pressure, and body weight: the Chin-Shan Community Cardiovascular Cohort Study. Atherosclerosis. 2002;161(2):409–415. doi: 10.1016/s0021-9150(01)00644-x. [DOI] [PubMed] [Google Scholar]
- 23.Akahoshi M, Soda M, Nakashima E, Shimaoka K, Seto S, Yano K. Effects of menopause on trends of serum cholesterol, blood pressure, and body mass index. Circulation. 1996;94(1):61–66. doi: 10.1161/01.cir.94.1.61. [DOI] [PubMed] [Google Scholar]
- 24.Haffner SM, Valdez RA. Endogenous sex hormones: impact on lipids, lipoproteins, and insulin. Am J Med. 1995;98(1A):40S–47S. doi: 10.1016/s0002-9343(99)80058-8. [DOI] [PubMed] [Google Scholar]
- 25.Pugeat M, Moulin P, Cousin P, et al. Interrelations between sex hormone–binding globulin (SHBG), plasma lipoproteins and cardiovascular risk. J Steroid Biochem Mol Biol. 1995;53(1-6):567–572. doi: 10.1016/0960-0760(95)00102-6. [DOI] [PubMed] [Google Scholar]
- 26.Sutton-Tyrrell K, Wildman RP, Matthews KA, et al. SWAN Investigators Sex hormone–binding globulin and the free androgen index are related to cardiovascular risk factors in multiethnic premenopausal and perimenopausal women enrolled in the Study of Women Across the Nation (SWAN) Circulation. 2005;111(10):1242–1249. doi: 10.1161/01.CIR.0000157697.54255.CE. [DOI] [PubMed] [Google Scholar]
- 27.Santoro N, Torrens J, Crawford S, et al. Correlates of circulating androgens in mid-life women: the Study of Women’s Health Across the Nation. J Clin Endocrinol Metab. 2005;90(8):4836–4845. doi: 10.1210/jc.2004-2063. [DOI] [PubMed] [Google Scholar]
- 28.Sowers MC, Sternfeld B, Morganstein D, et al. SWAN: a multicenter, multiethnic, community-based cohort study of women and the menopausal transition. In: Lobo RMR, Kelsey J, editors. Menopause: Biology and Pathobiology. Academic Press; San Diego, CA: 2000. pp. 175–188. [Google Scholar]
- 29.Steiner P, Freidel J, Bremner W, Stein E. Standardization of micromethods for plasma cholesterol, triglyceride and HDL-cholesterol with the lipid clinics’ methodology [abstract] J Clin Chem Clin Biochem. 1981;19:850. [Google Scholar]
- 30.Warnick GR, Albers JJ. A comprehensive evaluation of the heparin-manganese precipitation procedure for estimating high density lipoprotein cholesterol. J Lipid Res. 1978;19(1):65–76. [PubMed] [Google Scholar]
- 31.Deurenberg-Yap M, Yian T, Kai C, Deurenberg P, van Staveren WA. Manifestation of cardiovascular risk factors at low levels of body mass index and waist-to-hip ratio in Singaporean Chinese. Asia Pac J Clin Nutr. 1999;8(3):177–183. doi: 10.1046/j.1440-6047.1999.00091.x. [DOI] [PubMed] [Google Scholar]
- 32.Ko GT, Chan J, Cockram C, Woo J. Prediction of hypertension, diabetes, dyslipidaemia or albuminuria using simple anthropometric indexes in Hong Kong Chinese. Int J Obes Relat Metab Disord. 1999;23(11):1136–1142. doi: 10.1038/sj.ijo.0801043. [DOI] [PubMed] [Google Scholar]
- 33.World Health Organization. International Association for the Study of Obesity. International Obesity Taskforce [Accessed December 21, 2006];The Asia-Pacific perspective: redefining obesity and its treatment. http://www.diabetes.com.au/pdf/obesity_report.pdf.
- 34.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C, American Heart Association. National Heart, Lung, and Blood Institute Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109(3):433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 35.Gurka MJ. Selecting the best linear mixed model under REML. Am Stat. 2006;60(1):19–26. [Google Scholar]
- 36.Ainy E, Mirmiran P, Zahedi Asl S, Azizi F. Prevalence of metabolic syndrome during menopausal transition Tehranian women: Tehran Lipid and Glucose Study (TLGS) Maturitas. 2007;58(2):150–155. doi: 10.1016/j.maturitas.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 37.Burger HG. The endocrinology of the menopause. Maturitas. 1996;23(2):129–136. doi: 10.1016/0378-5122(95)00969-8. [DOI] [PubMed] [Google Scholar]
- 38.Rozenbaum H. Critique of the evidence from large trials of hormone replacement therapy. Int Congr Ser. 2004;1266:139–150. [Google Scholar]
- 39.Ding EL, Song Y, Malik VS, Liu S. Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2006;295(11):1288–1299. doi: 10.1001/jama.295.11.1288. [DOI] [PubMed] [Google Scholar]
- 40.Kok HS, van Asselt KM, van der Schouw YT, et al. Heart disease risk determines menopausal age rather than the reverse. J Am Coll Cardiol. 2006;47(10):1976–1983. doi: 10.1016/j.jacc.2005.12.066. [DOI] [PubMed] [Google Scholar]