In the heart, translocation of the S4 voltage-sensing helices of cardiac L-type Ca2+ channels (or 1,4-dihydropyridine receptors [DHPRs]) in response to depolarization of the sarcolemma is the initial event in excitation–contraction (EC) coupling. The movement of the voltage sensors is in turn allosterically coupled to opening of the channel pore. The Ca2+ influx conducted by the L-type channel gates cardiac RYRs (RYR2), thereby eliciting the Ca2+ efflux from the SR that activates the contractile filaments and causes contraction of the myocardium. As in cardiac muscle, EC coupling in skeletal muscle depends on the response of DHPRs to membrane depolarization and on Ca2+ release from the SR via RYRs. The skeletal and cardiac DHPRs have several similarities as well as important differences, which is also the case for the skeletal and cardiac RYRs. Furthermore, unlike cardiac-type EC coupling, which requires the influx of extracellular Ca2+ via the L-type channel, skeletal-type EC coupling does not require such Ca2+ influx. For this reason, it is thought that transmission of the EC coupling signal from the voltage-sensing S4 regions of the skeletal DHPR to the pore of the skeletal RYR (RYR1) depends on conformational coupling between these two multimeric channels (Beam and Horowicz, 2004).
In addition to the orthograde signal (i.e., the EC coupling signal) that is transmitted from the skeletal DHPR to RYR1, a retrograde signal was revealed by the observation that L-type currents of dyspedic (RYR1-null) myotubes were substantially smaller than L-type currents of wild-type myotubes, despite similar membrane expression of the DHPR. Just as orthograde coupling does not depend upon Ca2+ movements through the skeletal DHPR, retrograde, RYR1-dependent enhancement of skeletal L-type current does not depend upon Ca2+ movements via RYR1. Moreover, both orthograde and retrograde coupling depend on the integrity of some of the same structural elements of the DHPR α1S subunit and RYR1. Collectively, these observations suggest that retrograde coupling, like orthograde coupling, is supported by protein–protein contacts linking RYR1 and the DHPR channel complex (Beam and Horowicz, 2004).
Although the functional evidence for conformational coupling described above provides a solid foundation for the notion that protein–protein interactions link the DHPR and RYR1, this idea is most strongly supported by the elegant work of Franzini-Armstrong and colleagues (cf. Takekura et al., 2004). Collectively, these ultrastructural studies revealed that intramembranous particles in the plasma membrane, which appear to represent DHPRs, are arranged into groups of four (“tetrads”) in freeze-fracture replicas of plasma membrane–SR junctions. Moreover, these tetrads are arranged in register with the four subunits of every other RYR1. Subsequent work, showing that the distance between DHPRs within tetrads is decreased by exposure to concentrations of ryanodine sufficient to lock RYR1 in a non-conducting state, almost unequivocally demonstrates that skeletal DHPRs are linked (directly or indirectly) to RYR1s (Paolini et al., 2004). Such links are not thought to exist between cardiac DHPRs and RYR2s because the arrangement of DHPRs into tetrads has not been demonstrated in cardiac muscle.
Over the last 20-odd years, multidisciplinary approaches have generated a wealth of knowledge regarding how skeletal DHPRs and RYRs interact in skeletal muscle. Yet, the basic mechanism of DHPR–RYR1 communication remains elusive. Here, we will assess the current knowledge yielded by these multidisciplinary methods, and we will also discuss the frustrating limitations of these approaches. In addition, we will speculate on what are likely to be important new areas of investigation, including the development of new genetic models and the application of cryo-electron microscopy (EM), x-ray crystallography, and proteomics.
What we do actually know about EC coupling in skeletal muscle?
The skeletal muscle DHPR is a heteromultimeric Ca2+ channel complex consisting of a principle α1S (CaV1.1) subunit and auxiliary β1a, α2δ-1, and γ1 subunits. siRNA knockdown of either α2δ-1 or genetic ablation of γ1 has little effect on EC coupling (Obermair et al., 2008), whereas the absence of either the α1S or the β1a subunit produces an EC coupling–dead phenotype in which mice null for either subunit die perinatally as a consequence of respiratory paralysis. To date, the only junctional protein other than the DHPR α1S and β1a subunits known to be essential for skeletal EC coupling is RYR1. Thus, it seems reasonable that the events that support EC coupling minimally involve intermolecular communication between at least two of these three proteins (Beam and Horowicz, 2004). Still, despite the identification of the key players in skeletal EC coupling, the same mechanistic questions facing investigators 15 years ago persist today: What parts of the DHPR α1S subunit trigger EC coupling? How does the essential DHPR β1a subunit participate in EC coupling? How is the EC coupling signal transmitted from the voltage-sensing regions of the α1S to RYR1?
In pursuit of answers to these questions, we have almost exhausted traditional experimental approaches such as analysis of chimeric DHPR subunits and chimeric RYRs, application of peptides to isolated RYR1s, and biochemical analysis of binding interactions to investigate communication between the DHPR and RYR1. These approaches have provided invaluable information about DHPR–RYR1 communication, but we must acknowledge the inherent limitations of these methodologies. In particular, functional analysis of chimeric DHPR subunits and chimeric RYRs has proven quite effective in the identification of regions within these proteins that are important for transmission of the EC coupling signal. However, with this approach it is not possible to conclude whether a region of demonstrated importance is an actual site of interaction with other junctional proteins or allosterically affects such interactions. In vitro biochemical methods (including the application of synthetic peptides to RYRs in vesicles or bilayers) can demonstrate direct interactions, but the identified interactions may not have a physiological correlate in vivo.
What regions of the DHPR α1S subunit are important for EC coupling?
Like the α1 subunits of the nine other currently known CaV channels, the DHPR α1S subunit consists of four relatively conserved membrane-bound domains, which are linked by three intracellular loops that, along with the amino and carboxyl termini, are cytoplasmic (Bannister, 2007). The amino terminus is largely dispensable for EC coupling because 37 of its 51 residues can be deleted without much effect. Although not required for EC coupling, the distal carboxyl terminus assists in the junctional targeting of DHPRs. Substantial sequence conservation in the proximal carboxyl terminus with the corresponding regions of other CaV channels has hindered investigation of its role in EC coupling. The α1S I-II loop is essential for EC coupling because it is the site for interaction with the DHPR β1a subunit. The α1S II-IV loop does not appear to play a direct role in EC coupling, but it participates indirectly in the process by influencing DHPR gating.
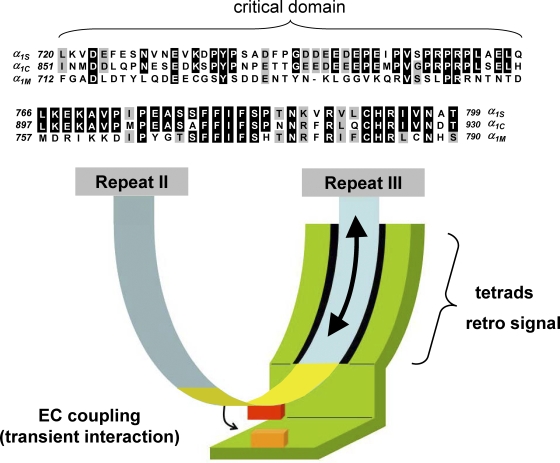
For several years, there has been general agreement among investigators that the II-III loop of the α1S subunit plays a key role in transmitting the EC coupling signal to RYR1 (Bannister, 2007). However, the precise portion of the loop that is involved in this process has been a topic of contention. The experimental strategy of expressing chimeric DHPRs in dysgenic (α1S-null) myotubes has identified a domain (initially described as α1S residues 720–765; Fig. 1, top) in the center of the loop as being essential for skeletal EC coupling (Beam and Horowicz, 2004). In contrast, a synthetic peptide corresponding to an α-helical domain (roughly α1S residues 671–690; the “peptide A” domain) in the amino-terminal portion of the α1S II-III loop activates RYR1 in reconstituted lipid bilayers, which has given rise to the idea that this portion of the α1S II-III loop may interact directly with RYR1. Indeed, a recent study showed binding between peptide A and a fragment of RYR1 in vitro (Cui et al., 2009). However, the physiological implications of this interaction are unclear because several studies have shown that EC coupling can be restored in dysgenic myotubes expressing α1S constructs in which the peptide A domain has been disrupted or even deleted (Ahern et al., 2001a,b; Beam and Horowicz, 2004). Most recently, we have demonstrated that both orthograde and retrograde coupling are supported by four different α1S constructs in which a 56-kD CFP–YFP tandem replaced the peptide A region (Bannister et al., 2009). Thus, it seems unlikely that the peptide A region or immediately adjacent segments of the α1S II-III loop directly participate in protein–protein interactions necessary for bidirectional coupling.
Figure 1.
Model for the engagement of skeletal muscle EC coupling based on the current literature. (Top) Sequence alignment is shown for rabbit α1S, rabbit α1C, and Musca domestica α1M. The top panel (α1S residues 720–765) represents the critical domain (Beam and Horowicz, 2004). The middle panel (α1S residues 766–799) represents the region of the α1S II-III loop carboxyl-terminal to the critical domain, ending just before repeat III. Sequence is not shown for the relatively divergent amino-terminal portion of the II-III loop (α1S residues 662–719). Residues of α1C or α1M identical to those of α1S are shown boxed in black, and residues conserved with those of α1S are shown boxed in gray. (Bottom) The arc extending from repeat II to repeat III represents the α1S II-III loop; the yellow portion of the loop represents the critical domain (α1S residues 720–765). The green entity represents the junctional interaction partner(s) of the α1S II-III loop. Because the amino-terminal portion of the α1S II-III loop is accessible to large streptavidin probes, it is depicted as being devoid of junctional interaction partners. Because ultrastructural analysis of α1S/α1M chimeras suggests that the carboxyl-terminal portion of the α1S II-III loop supports junctional contacts that are essential for tetrad formation, the carboxyl-terminal portion of the loop is shown as a surface for interaction with other junctional proteins. In addition, the carboxyl-terminal portion of the loop may also serve as a line of communication (large arrow) between repeat III and the critical domain (yellow) in the center of the α1S II-III loop; voltage-induced conformational rearrangements (little arrow) in the critical domain engage SR Ca2+ release via a transient protein–protein interaction between a portion of the critical domain (red box) and another junctional protein (orange box).
In the same study, we used YFP insertions as a means to probe the importance of the carboxyl-terminal region of the α1S II-III loop, which links the critical domain to repeat III. The role of this region of the α1S II-III loop had not been investigated adequately in earlier chimeras because it is highly conserved among α1S, the cardiac α1C isoform, and the Musca domesticus (common housefly) α1M isoform (see Fig. 1, middle). The rationale for this strategy was that YFP insertion would perturb SR Ca2+ release at loci within the α1S II-III loop that are important for EC coupling. In this particular experiment, introduction of a single YFP between residues 785 and 786 in the carboxyl-terminal portion of the loop ablated bidirectional coupling without affecting membrane expression of the channel or significantly distorting the conformation of the critical domain.
The disruption of EC coupling by YFP insertion in the conserved carboxyl-terminal region of the α1S II-III loop suggests that it may be an important site of protein–protein interaction required for signaling. Interestingly, some support for this idea has already been presented in an earlier study by Takekura et al. (2004), in which they identified an α1S/α1M chimera (SkLM) that stands as the one exception to the “tetrad correlate,” whereby all α1S-α1C/α1M chimeric channels require the presence of the α1S critical domain to form tetrads. Specifically, the chimera SkLM (consisting of the entire α1M II-III loop in an otherwise α1S background) forms tetrads, albeit less efficiently than chimeras containing the α1S critical domain, suggesting that additional conserved elements within the II-III loops of α1S and α1M other than the critical domain regions contribute weakly to tetrad formation. Based on the sequence similarity in the carboxyl-terminal regions of α1S and α1M II-III loops (Fig. 1, middle), and the sensitivity of this domain to perturbation by YFP insertion, one might postulate that this region is involved in static interactions with other junctional proteins that are requisite for tetrad formation and consequently support skeletal-type EC coupling. Another possible, but not necessarily exclusive, role for the carboxyl-terminal portion of the α1S II-III loop is that it serves as a conduit for communication between the critical domain and repeat III of the DHPR.
What is the role of the β1a subunit?
Like dysgenic mice, mice lacking the β1a subunit die perinatally, and myotubes harvested from β1-null pups lack voltage-induced Ca2+ release from the SR and have only minimal L-type Ca2+ current (Coronado et al., 2004). The virtually identical phenotypes of β1-null and dysgenic mice were initially explained by the inability of α1S to be trafficked to triad junctions in the absence of β1a (Coronado et al., 2004). However, the presence of DHPRs (although fewer than normal) in freeze-fracture replicas obtained from the muscle of zebrafish β1-null (relaxed) mutants suggests that trafficking α1S subunits to junctions is not the only role of β1a (Schredelseker et al., 2005). In particular, the DHPRs in relaxed muscle are not organized into tetrads, an indication that the links between DHPRs and RYR1 that result in tetrads require the presence of β1a.
Like the other CaVβ isoforms, β1a is a member of the membrane-associated guanylate kinase (MAGUK) family of scaffolding proteins and consists of five distinct domains (D1–D5). The recent crystal structures of the core (i.e., D2–D4 regions) of cardiac β2a and neuronal β3 subunits have provided useful information that can be extrapolated to β1a (cf. Van Petegem et al., 2004). In these structures, the conserved D2 and D4 domains display a fair amount of structural similarity to SH3 domains and guanylate kinase (GK) domains of MAGUK proteins. The structures reveal that the SH3-like domain is almost certainly incapable of interacting with polyproline domains, and the GK-like domain has no kinase function (Van Petegem et al., 2004).
The highly variable amino-terminal D1 and carboxyl-terminal D5 regions may be involved in subtype-specific functions of CaVβ subunits. In the case of β1a, deletion of D1 was found to have little effect on EC coupling (Coronado et al., 2004). However, differences in FRET efficiencies of a CFP–YFP tandem fused to the β1a amino terminus in the presence or absence of RYR1 (i.e., in dyspedic myotubes) seem to indicate that this region lies in close proximity to RYR1 or to structures that are indirectly impacted by the presence of RYR1 (Papadopoulos et al., 2004). Paradoxically, the failure of D5 deletion mutants to support EC coupling in β1-null myotubes has clearly identified the β1a carboxyl terminus as a critical element of β1a function (Coronado et al., 2004), but the FRET efficiency of a CFP–YFP tandem fused to the β1a carboxyl terminus is little affected by the presence of RYR1 (Papadopoulos et al., 2004). Moreover, a biotin acceptor domain tag fused to the carboxyl terminus of β1a is accessible to a large 60-kD streptavidin probe in fixed or nonfixed β1-null myotubes (Lorenzon et al., 2004; Lorenzon and Beam, 2007). In nonfixed cells, EC coupling persists after the binding of streptavidin to the biotin acceptor domain affixed to the β1a carboxyl terminus. These latter observations suggest that the β1a carboxyl terminus probably does not interact directly with RYR1, at least not in the resting state. Even so, purified full-length β1a subunits have been shown to bind a fragment of RYR1 in vitro (Cheng et al., 2005), raising the possibility that a β1a–RYR1 interaction may support tetrad formation or may even possibly be a component of the trigger mechanism for SR Ca2+ release. However, such an interaction between β1a and RYR1 is not sufficient to deliver β1a to triad junctions without α1S expression (Papadopoulos et al., 2004; Leuranguer et al., 2006). Collectively, evidence obtained in live muscle cells indicates that α1S cannot interact with RYR1 in the absence of β1a, and that β1a cannot interact with RYR1 in the absence of α1S. Thus, a high priority goal is to determine how the β1a subunit, and in particular its carboxyl terminus, participates in these reciprocal interactions with α1S and RYR1.
How is the EC coupling signal transmitted from the DHPR to RYR1?
A model illustrating the essential roles of the critical domain and carboxyl-terminal region of the α1S II-III loop in communication with RYR1 is presented in Fig. 1. In this model, the α1S II-III loop (represented by the blue/yellow arc) is juxtaposed with one or more junctional interaction partners (represented collectively by the green moiety). Because the amino-terminal portion of the α1S II-III loop is accessible to large streptavidin probes (Lorenzon et al., 2004; Lorenzon and Beam, 2007), it is depicted as being devoid of junctional interaction partners. This idea is further supported by the observation that the amino-terminal portion of the loop can also accommodate the introduction of CFP–YFP tandem and remain fully functional (Papadopoulos et al., 2004; Bannister et al., 2009). On the other hand, ultrastructural analysis of the SkLM chimera suggests that the carboxyl-terminal portion of the α1S II-III loop supports resting junctional interactions that are important for tetrad formation (Takekura et al., 2004; see above). As portrayed by the rightward-directed arrow in Fig. 1, the EC coupling signal produced by depolarization is propagated from the voltage-sensing regions of DHPR repeat III to the critical domain (yellow portion of α1S II-III loop). The resultant conformational rearrangements in the critical domain (small arrow) facilitate a transient, localized protein–protein interaction of some portion of the critical domain (red box) with another junctional protein (orange box) that engages SR Ca2+ release from RYR1. The conformational rearrangements of the critical domain that elicit EC coupling do not appear to be required for the retrograde enhancement of L-type current because streptavidin binding near the critical domain ablates the former with little effect on the latter (Lorenzon and Beam, 2007). Thus, the model indicates that the conserved, carboxyl-terminal portion of the loop may be important not only for tetrad formation, but also for retrograde signaling (rightward-directed arrow).
Even after years of experimentation, we cannot state that EC coupling is mediated by a direct interaction between the critical domain and RYR1 because the only evidence for such an interaction exists in the form of weak binding between the critical domain and an RYR1 fragment in a yeast two-hybrid assay (Proenza et al., 2002). Moreover, a small component of EC coupling in dysgenic myotubes was restored by a modified α1S subunit lacking both the peptide A domain and the critical domain (Ahern et al., 2001b), and tetrads are not formed in relaxed zebrafish junctions despite the presence of the intact α1S II-III loop (Schredelseker et al., 2005).
There are portions of the model that are intentionally vague because of the substantial gaps in the current knowledge of the basic mechanism of EC coupling. First, the identities of the junctional proteins that are postulated to be directly contacted by the α1S II-III loop are not known and are indicated by the unlabeled green moiety in Fig. 1. Relatively large segments of RYR1 have been identified as being important for bidirectional signaling in studies that have examined the functional properties of chimeric RYRs (Beam and Horowicz, 2004). However, given the poorly understood folding of the myoplasmic foot region of RYR1, these domains may only support conformational coupling allosterically. Thus, the green moiety could include components of RYR1, β1a, or other yet-to-be identified proteins.
How do we get to the answers?
Although the model shown in Fig. 1 conveys the impression that we are starting to understand the basic mechanics of communication between the voltage sensor and the critical domain, the nature of communication between the DHPR and RYR1 remains enigmatic. In light of their limitations, the traditional techniques will have to be used in conjunction with more inventive strategies.
The generation of engineered mice will expand the use of freeze-fracture and traditional thin-section EM in that these preparations may now be explored with antibodies or other probes. Visualization of the location of engineered tags with secondary conjugates will enable the spatial mapping of DHPR structures and also the provide information regarding the orientation of DHPRs within tetrads. Likewise, the introduction of exogenous sequence into either RYR1 or DHPR subunits in engineered mice will provide the material necessary for proteomic studies designed to reveal potential intermolecular interactions that link the DHPR and RYR1, and also in the identification of other junctional proteins.
Single-particle 3-D reconstructions of cryo-EM images of RYR1 and the structurally similar RYR2 have been already quite useful in piecing together a more detailed picture of the triad junction. The most current reconstructions of RYR1 have been refined to ∼10-Å resolution, and both open and closed states of the SR channel have been resolved through pharmacological manipulation (Samsó et al., 2009). Although crystal structures for fragments of either RYR isoform have been generated, such high resolution structure for complete RYRs will be extremely difficult to achieve considering the immensity of the proteins and their dual membrane-bound and cytoplasmic nature. On the other hand, a crystal structure of a CaV family α1 subunit may be a more reasonable objective considering that atomic structures have been presented for other eukaryotic voltage-gated channels (e.g., Shaker KV channels). As noted above, the structures of CaV β subunits have revealed the nature of interaction between the I-II loop of α1 subunits and the GK domain of β subunits. One may imagine that once the crystal structure for a CaV α1 subunit is produced, docking of the β to the α1 will at the very least enable investigators to establish the correct orientation of DHPRs relative to the RYR1 tetramer within reconstructed tetrads. In the meantime, cryo-EM structures of modified DHPRs purified from knock-in or transgenic mice hold great potential to provide information about DHPR structure. For instance, the location of modified structural elements of α1S or β1a (e.g., toxin binding sites, biotin acceptor domains) in the cryostructure can be pinpointed by the presence of cognate probes. In addition, side-by-side comparison of the skeletal DHPR cryostructure with cryostructures of other CaV channels (e.g., CaV3.1; Walsh et al., 2009) may prove useful in identifying elements that are unique to the DHPR and therefore may be involved in its unique ability to interact functionally with RYR1. When used in combination with freeze-fracture and thin-section microscopy, the information provided by 3-D cryo-EM reconstructions may make it possible to infer sites of direct contact between RYR1 and the DHPR channel complex.
Conclusion
The application of novel genetic systems and proteomic strategies will contribute useful information to our knowledge of the basic mechanism of EC coupling incrementally, but the seismic changes in our perspective will come in the wake of high resolution structures of the DHPR and RYR1. As our knowledge of the structure of each channel complex grows, the interface between DHPR and RYR1 will begin to reveal itself. This structural information, combined with rigorous multidisciplinary validation of sites of interaction between the DHPR and RYR1, will complete the circle in which functional and structural approaches merge in the quest to answer EC coupling’s most persistent questions.
Acknowledgments
We thank Dr. D.C. Sheridan for helpful discussion regarding the β1a subunit. We apologize to those colleagues whose works were not cited directly in adherence to the limited number of references.
This work was supported in part by grants from the National Institutes of Health (AR055104 and AR44750 to K.G. Beam) and the Muscular Dystrophy Association (MDA4319 to K.G. Beam and MDA4155 to R.A. Bannister).
Footnotes
Abbreviations used in this paper:
- DHPR
- 1,4-dihydropyridine receptor
- EC
- excitation–contraction
- EM
- electron microscopy
- GK
- guanylate kinase
References
- Ahern C.A., Arikkath J., Vallejo P., Gurnett C.A., Powers P.A., Campbell K.P., Coronado R. 2001a. Intramembrane charge movements and excitation-contraction coupling expressed by two-domain fragments of the Ca2+ channel. Proc. Natl. Acad. Sci. USA. 98:6935–6940 10.1073/pnas.111001898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahern C.A., Bhattacharya D., Mortenson L., Coronado R. 2001b. A component of excitation-contraction coupling triggered in the absence of the T671-L690 and L720-Q765 regions of the II-III loop of the dihydropyridine receptor α1s pore subunit. Biophys. J. 81:3294–3307 10.1016/S0006-3495(01)75963-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannister R.A. 2007. Bridging the myoplasmic gap: recent developments in skeletal muscle excitation-contraction coupling. J. Muscle Res. Cell Motil. 28:275–283 10.1007/s10974-007-9118-5 [DOI] [PubMed] [Google Scholar]
- Bannister R.A., Papadopoulos S., Haarmann C.S., Beam K.G. 2009. Effects of inserting fluorescent proteins into the α1S II-III loop: insights into excitation–contraction coupling. J. Gen. Physiol. 134:35–51 10.1085/jgp.200910241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beam K.G., Horowicz P. 2004. Excitation-contraction coupling in skeletal muscle. Myology. Engel A.G., Franzini-Armstrong C., McGraw Hill, New York: 257–280 [Google Scholar]
- Cheng W., Altafaj X., Ronjat M., Coronado R. 2005. Interaction between the dihydropyridine receptor Ca2+ channel β-subunit and ryanodine receptor type 1 strengthens excitation-contraction coupling. Proc. Natl. Acad. Sci. USA. 102:19225–19230 10.1073/pnas.0504334102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coronado R., Ahern C.A., Sheridan D.C., Cheng W., Carbonneau L., Bhattacharya D. 2004. Functional equivalence of dihydropyridine receptor α1S and β1a subunits in triggering excitation-contraction coupling in skeletal muscle. Biol. Res. 37:565–575 10.4067/S0716-97602004000400010 [DOI] [PubMed] [Google Scholar]
- Cui Y., Tae H.S., Norris N.C., Karunasekara Y., Pouliquin P., Board P.G., Dulhunty A.F., Casarotto M.G. 2009. A dihydropyridine receptor α1s loop region critical for skeletal muscle contraction is intrinsically unstructured and binds to a SPRY domain of the type 1 ryanodine receptor. Int. J. Biochem. Cell Biol. 41:677–686 10.1016/j.biocel.2008.08.004 [DOI] [PubMed] [Google Scholar]
- Leuranguer V., Papadopoulos S., Beam K.G. 2006. Organization of calcium channel β1a subunits in triad junctions in skeletal muscle. J. Biol. Chem. 281:3521–3527 10.1074/jbc.M509566200 [DOI] [PubMed] [Google Scholar]
- Lorenzon N.M., Beam K.G. 2007. Accessibility of targeted DHPR sites to streptavidin and functional effects of binding on EC coupling. J. Gen. Physiol. 130:379–388 10.1085/jgp.200609730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzon N.M., Haarmann C.S., Norris E.E., Papadopoulos S., Beam K.G. 2004. Metabolic biotinylation as a probe of supramolecular structure of the triad junction in skeletal muscle. J. Biol. Chem. 279:44057–44064 10.1074/jbc.M405318200 [DOI] [PubMed] [Google Scholar]
- Obermair G.J., Tuluc P., Flucher B.E. 2008. Auxiliary Ca2+ channel subunits: lessons learned from muscle. Curr. Opin. Pharmacol. 8:311–318 10.1016/j.coph.2008.01.008 [DOI] [PubMed] [Google Scholar]
- Paolini C., Fessenden J.D., Pessah I.N., Franzini-Armstrong C. 2004. Evidence for conformational coupling between two calcium channels. Proc. Natl. Acad. Sci. USA. 101:12748–12752 10.1073/pnas.0404836101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos S., Leuranguer V., Bannister R.A., Beam K.G. 2004. Mapping sites of potential proximity between the dihydropyridine receptor and RyR1 in muscle using a cyan fluorescent protein-yellow fluorescent protein tandem as a fluorescence resonance energy transfer probe. J. Biol. Chem. 279:44046–44056 10.1074/jbc.M405317200 [DOI] [PubMed] [Google Scholar]
- Proenza C., O’Brien J.J., Nakai J., Mukherjee S., Allen P.D., Beam K.G. 2002. Identification of a region of RyR1 that participates in allosteric coupling with the α1S (CaV1.1) II-III loop. J. Biol. Chem. 277:6530–6535 10.1074/jbc.M106471200 [DOI] [PubMed] [Google Scholar]
- Samsó M., Feng W., Pessah I.N., Allen P.D. 2009. Coordinated movement of cytoplasmic and transmembrane domains of RyR1 upon gating. PLoS Biol. 7:e85 10.1371/journal.pbio.1000085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schredelseker J., Di Biase V., Obermair G.J., Felder E.T., Flucher B.E., Franzini-Armstrong C., Grabner M. 2005. The β1a subunit is essential for the assembly of dihydropyridine-receptor arrays in skeletal muscle. Proc. Natl. Acad. Sci. USA. 102:17219–17224 10.1073/pnas.0508710102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takekura H., Paolini C., Franzini-Armstrong C., Kugler G., Grabner M., Flucher B.E. 2004. Differential contribution of skeletal and cardiac II-III loop sequences to the assembly of dihydropyridine-receptor arrays in skeletal muscle. Mol. Biol. Cell. 15:5408–5419 10.1091/mbc.E04-05-0414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Petegem F., Clark K.A., Chatelain F.C., Minor D.L., Jr 2004. Structure of a complex between a voltage-gated calcium channel β-subunit and an α-subunit domain. Nature. 429:671–675 10.1038/nature02588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh C.P., Davies A., Butcher A.J., Dolphin A.C., Kitmitto A. 2009. Three-dimensional structure of CaV3.1: comparison with the cardiac L-type voltage-gated calcium channel monomer architecture. J. Biol. Chem. 284:22310–22321 10.1074/jbc.M109.017152 [DOI] [PMC free article] [PubMed] [Google Scholar]