Abstract
Cytosolic calcium concentration in resting cardiac myocytes locally fluctuates as a result of spontaneous microscopic Ca2+ releases or abruptly rises as a result of an external trigger. These processes, observed as calcium sparks, are fundamental for proper function of cardiac muscle. In this study, we analyze how the characteristics of spontaneous and triggered calcium sparks are related to cardiac ryanodine receptor (RYR) gating. We show that the frequency of spontaneous sparks and the probability distribution of calcium release flux quanta of triggered sparks correspond quantitatively to predictions of an allosteric homotetrameric model of RYR gating. This model includes competitive binding of Ca2+ and Mg2+ ions to the RYR activation sites and allosteric interaction between divalent ion binding and channel opening. It turns out that at rest, RYRs are almost fully occupied by Mg2+. Therefore, spontaneous sparks are most frequently evoked by random openings of the highly populated but rarely opening Mg4RYR and CaMg3RYR forms, whereas triggered sparks are most frequently evoked by random openings of the less populated but much more readily opening Ca2Mg2RYR and Ca3MgRYR forms. In both the spontaneous and the triggered sparks, only a small fraction of RYRs in the calcium release unit manages to open during the spark because of the limited rate of Mg2+ unbinding. This mechanism clarifies the unexpectedly low calcium release flux during elementary release events and unifies the theory of calcium signaling in resting and contracting cardiac myocytes.
INTRODUCTION
In cardiac myocytes, a local increase of the calcium concentration observable as a calcium spark (Cheng et al., 1993) was shown to be an elementary event of calcium release causing cell contraction. It has also been demonstrated that the local calcium release comprises a significant fraction of diastolic calcium leak (Bassani and Bers, 1995) that regulates the diastolic SR calcium content (Lukyanenko et al., 2001) and thus controls the extent of triggered calcium release (Bassani et al., 1995). Under pathological conditions such as heart failure, the increased diastolic calcium spark frequency may decrease SR calcium content (Kubalová et al., 2005; Belevych et al., 2007), thus participating in the reduced contractility of cardiac muscle. Increased diastolic calcium leak in genetic diseases (catecholaminergic polymorphic ventricular tachycardia and arrhythmogenic right ventricular dysplasia) as well as in heart failure and diabetes has been attributed to dysregulation of RYR gating (Durham et al., 2007). RYR dysfunction has also been hypothesized to lead to the generation of calcium waves and arrhythmias (Yano et al., 2006; Paavola et al., 2007). However, the relationship between RYR gating and calcium spark frequency is not clear.
The morphological substrate of calcium sparks is the calcium release unit (CRU), a terminal cistern of the SR. In rat cardiac myocytes, it contains a cluster of ∼182–267 RYR channels on average (Franzini-Armstrong et al., 1999; Soeller et al., 2007). In other mammalian species, it contains substantially less RYRs, ∼130 in mouse (Franzini-Armstrong et al., 1999) and human (Soeller et al., 2007) and ∼90 in dog myocytes (Franzini-Armstrong et al., 1999). The spontaneous calcium sparks are indistinguishable from the triggered sparks on the basis of their amplitude, duration, and size (Cannell et al., 1995). It is generally accepted that spontaneous sparks arise not from spontaneous triggers but from spontaneous openings of RYR channels themselves (Cheng et al., 1993, 1996; Lukyanenko and Györke, 1999). Lukyanenko and Györke (1999) have shown that calcium sparks, identical to those in an intact cell, occur in permeabilized myocytes as well. Moreover, they showed that the frequency of calcium sparks is an increasing function of cytosolic Ca2+ in the diastolic concentration range and that the apparent calcium sensitivity of the CRU activity is similar to that of individual RYRs in the presence of Mg2+ and ATP. These facts strongly indicate that spontaneous calcium sparks are directly related to RYR gating under diastolic conditions. However, there is a lack of quantitative data on the activity of RYRs at low Ca2+ concentration because of their very low open probability. The effort to resolve the mechanism of spark activation directly by their analysis encountered difficulties as well because of the transient and spatially limited nature of sparks and the uncertain relationship between the calcium-dependent fluorescence signal and calcium release flux (for review see Ríos and Brum, 2002; Zahradníková et al., 2007).
It is generally accepted that once a single RYR in the CRU opens, it activates all RYRs of the CRU (Stern, 1992; Zahradníková et al., 2007) because it increases Ca2+ concentration within the dyadic gap to values by an order of magnitude higher than the apparent RYR calcium affinity under physiological conditions (Stern et al., 1999; Fill and Copello, 2002; Valent et al., 2007). Moreover, RYRs may even coordinate their gating via protein–protein interactions (Marx et al., 2001). The amplitude of the calcium release current flowing during a cardiac calcium spark was estimated only roughly, in the range of 1.4–20 pA (for review see Ríos and Brum, 2002). This current range would correspond to 3–40 RYRs simultaneously open during the spark with the experimental estimate of the RYR single-channel calcium current of 0.5 pA under physiological conditions (Mejía-Alvarez et al., 1999; Kettlun et al., 2003). Because the calcium gradient between the SR and the cytosol decreases during the spark (Brochet et al., 2005; Kubalová et al., 2005), RYR single-channel current should decrease with time, increasing this estimate to 5–60 RYRs. Other methods provided the number of simultaneously open RYRs during the spark in the range of 1–20 (Bridge et al., 1999; Lukyanenko et al., 2000; Wang et al., 2004). These estimates might be questioned, as they represent only a small fraction of RYRs in the CRU. However, the alternative proposition that all RYRs in the CRU open in concert, as expected for the CICR mechanism (Fabiato, 1985; Stern, 1992) or for coupled gating (Marx et al., 2001), ends up with a very low single-channel current (<0.1 pA/RYR; for review see Cheng and Lederer, 2008) and is not easily reconciled with the variability of spark amplitudes (Bridge et al., 1999) and with the quantal character of calcium spark release flux at a single release site (Wang et al., 2004).
The regulation of RYR activity by Mg2+ ions (Meissner et al., 1986), which was recently described in bilayer studies (Laver et al., 1997; Copello et al., 2002; Zahradníková et al., 2003), might provide a hint. According to these studies, a substantial fraction of RYRs is occupied by Mg2+ ions at the RYR activation sites and at the independent RYR inhibition sites under diastolic conditions. Therefore, in this study, we address the question of how the interaction of Mg2+ ions with the RYR determines the activity of RYR channels in relation to both the resting and the stimulated calcium sparks. To this end, we used the published experimental data on calcium dependence of spark frequency (Lukyanenko and Györke, 1999), distributions of calcium release flux quanta (Wang et al., 2004), and a model of CRU activity based on recent RYR gating models (Zahradník et al., 2005). We show that these independent experimental data can be explained when Ca2+ binding at the RYR monomer activation site has a positive allosteric effect, whereas Mg2+ binding has a weak negative allosteric effect on RYR transitions to open states. The model of the CRU activity presented here reconciles the low number of RYRs active in a spark with the high number of RYRs in the CRU and the observations on diastolic and systolic calcium signaling in mammalian myocytes.
MATERIALS AND METHODS
The system of equations (see supplemental material) characterizing the Ca2+ and Mg2+ dependence of state probabilities of the RYR gating schemes was solved in Mathematica (version 6.0.1; Wolfram Research) as previously described (Zahradník et al., 2005), thus giving formulae for the steady-state probabilities of RYR occurring in the states of interest. All analyses were performed using the program Origin (version 7.5; OriginLab). Least-squares fitting of Eq. 5 was performed in Origin. Maximum likelihood fitting of Eq. 8 was performed in Mathematica. The errors are given as the standard error of the fit, where applicable.
Derivation of models
RYR gating.
For calculating the open probability of the RYR channel, we used two gating models that were shown to satisfy the calcium dependence of activation of the wild-type cardiac RYR in planar bilayers (Zahradník et al., 2005). The two models of RYR gating, the fully cooperative extended minimal gating (EMG) model (Zahradníková et al., 1999) and the allosteric EMG (aEMG) model (Zahradník et al., 2005), were based on the homotetrameric concept of RYR function. To account explicitly for the effect of Mg2+ on channel activity, the models were extended here to include the competition between magnesium and calcium for the RYR activation sites. Furthermore, we included the steady-state inhibition of the RYR channel by Mg2+ ions as an independent process, as described in Zahradníková et al. (2003). The inhibition site does not distinguish between Ca2+ and Mg2+ ions (Meissner et al., 1986; Laver et al., 1997). However, because [Ca2+] << [Mg2+] during the diastole, we have disregarded Ca2+ binding to this site. Although the models are based on the previously published gating core (Zahradník et al., 2005), we changed the model acronym from EMG to HTG (homotetrameric gating) to underline their homotetrameric concept. The prefix “a” will stay for allosteric.
In the HTG model, the channel opens only when all four divalent ion–binding sites are occupied by calcium (Zahradníková et al., 1999) and, at the same time, the channel is not inhibited by Mg2+ (Zahradníková et al., 2003). The gating scheme of this model was obtained (see supplemental material) by extending the original EMG model for Mg2+ binding, assuming that only one divalent ion can be bound to the activation site of each RYR monomer. Additionally, Mg2+ was assumed to bind to the RYR inhibition site with a Hill slope of 2. Using the same methodology as described previously (Zahradník et al., 2005), the expression for combined Ca2+/Mg2+ dependence of the RYR open probability was derived as
| (1) |
where [Ca] and [Mg] are the free cytosolic concentrations of Ca2+ and Mg2+. The set of equilibrium dissociation constants, Kx, for each channel transition is described in detail in Table I and in the supplemental material. It should be noted that despite the increased complexity of the gating scheme, only one new parameter, KMg, was included in the HTG model.
Table I.
Parameters of RYR gating
| Parameter | HTG | aHTG | Comments |
| KCa (µM) | 1.36 | 0.6 | Zahradník et al., 2005 |
| KMg (μM) | 431 ± 12 | 79 ± 8 | Fitted parameter |
| fCa | N/A | 0.046 | Zahradník et al., 2005 |
| fMg | N/A | 1.40 ± 0.05 | Fitted parameter |
| KO00 | N/A | 10,800 | Zahradník et al., 2005 |
| KO40 | 0.00219 | N/A | Zahradník et al., 2005 |
| KOL | 0.5 | 0.5 | Zahradník et al., 2005 |
| KCL | 0.0333 | 0.89 | Zahradník et al., 2005 |
| KCI | 3.0 | 3.0 | Zahradník et al., 2005 |
| KI (μM) | 760 | 760 | Zahradníková et al., 2003 |
| [Mg] (mM) | 1.0 | 1.0 | Lukyanenko and Györke, 1999 |
The corresponding gating schemes are shown in the supplemental material. N/A, not applicable. KCa, calcium dissociation constant of the ion-free closed state C00. KMg, magnesium dissociation constant of the ion-free closed state C00. KO00, dissociation constant of the ion-free open state O00. KO40, dissociation constant of the open state O40. fCa, allosteric factor coupling calcium binding to channel opening. fMg, allosteric factor coupling magnesium binding to channel opening. KOL, dissociation constant of the L-mode open state OL. KCL, dissociation constant of the L-mode closed state CL. KCI, dissociation constant of the inactivated state I. KI, inhibitory constant of Mg2+ ions at the divalent inhibitory site. [Mg], free Mg2+ concentration.
In the allosteric HTG (aHTG) model, which is obtained by extending the original aEMG model (Zahradník et al., 2005) to include Mg2+ binding, the channel can open even without any divalent ion bound to the binding sites if it is not inhibited by Mg2+ at the inhibition site (Zahradníková et al., 2003). Divalent ion binding is coupled to channel opening through allosteric transitions that modulate channel open probability. The gating scheme of this model was also obtained (see supplemental material) by assuming that only one divalent ion can be bound to the activation site of each RYR monomer. Additionally, Mg2+ was assumed to bind to the RYR inhibition site with a Hill slope of 2. The Ca2+/Mg2+ dependence of the RYR open probability is then
| (2) |
The set of equilibrium constants, Kx, and allosteric factors, fx, is described in detail in Table I and in the supplemental material. It should be noted again that the increased complexity of the aHTG gating scheme is described with only two new parameters, KMg and fMg.
Steady-state frequency of sparks.
After activation of calcium release, RYRs become refractory to further activation (Sham et al., 1998; Terentyev et al., 2002; Szentesi et al., 2004; Sobie et al., 2005; and Kubalová, Z., A. Zahradníková, and I. Zahradník. 2001. Biophysical Society 45th Annual Meeting. Abstr. 592). Refractoriness is not fully understood but includes luminal regulation of RYR activity by Ca2+ (Terentyev et al., 2002; Szentesi et al., 2004; Sobie et al., 2005) and activation-dependent inactivation of RYRs (Zahradníková and Zahradník, 1996; Sham et al., 1998; Zahradníková et al., 2007). Therefore, the period between the onsets of two consecutive sparks at a single CRU consists of three components: the duration of release itself (trd), the refractory period (trp), and the idle time (tit). The duration of release (trd) was defined as the mean of the release durations of individual sparks, which have been shown to be approximately equal to the time to the peak of calcium sparks (Smith et al., 1998) or to the full width at half-maximum of calcium spikes (Zahradníková et al., 2007). The refractory period was defined as the mean duration of the refractory periods after individual sparks. The idle time (tit) was defined as the mean duration of the period between recovery from refractoriness and activation of a subsequent spark. During the idle time, the CRU is inactive, neither refractory nor releasing, but ready for activation. Idle time ends by the first RYR opening within the CRU, which demarcates activation of the subsequent spark. According to this mechanism of calcium release activation, each CRU may produce at most a single spark (either spontaneous or triggered) during the period (trd + trp). Activation of additional RYRs in the CRU will be dealt with in the following section, Distribution of active RYRs in sparks, because it does not affect calcium spark frequency.
Taking into consideration that openings of RYR channels are equivalent, the CRU idle time (tit) can be related, in analogy to the closed time of other single-channel ensembles (Kunze et al., 1985), to the mean closed time τC = τO (1/PO − 1) of a single RYR channel and to the number of RYRs in the CRU, nRYR:
| (3) |
where τO is the mean RYR open time that has been estimated under conditions close to physiological by Gaburjakova and Gaburjakova (2006). The probability of RYR opening, PO, can be calculated using Eqs. 1 or 2. The mean number of RYRs per CRU, nRYR, characterizing the size of CRU, has been estimated experimentally (Table II).
Table II.
Parameters of the spark frequency model
| Parameter | HTG | aHTG | Comments |
| τO (ms) | 15 | 15 | Gaburjakova and Gaburjakova, 2006 |
| trd (ms) | 15 | 15 | Brochet et al., 2005; Kubalová et al., 2005; Terentyev et al., 2006; Zahradníková et al., 2007 |
| trp (ms) | 1,300 ± 280 | 713 ± 80 | Fitted parameter |
| nRYR | 182 | 182 | Soeller et al., 2007 |
| νCRU (µm−3) | 1.01 | 1.01 | Soeller et al., 2007 |
τO, mean RYR open time. trd, release duration, approximately equal to the time to peak of calcium spark (Smith et al., 1998). trp, refractory period for calcium release. nRYR, number of RYR channels in the release site. νCRU, the numerical density of calcium release sites.
The frequency of sparks at a single release site is the inverse of the mean period between sparks:
| (4) |
Now, if the number of release sites per unit of volume (numerical density) is νCRU, the frequency of sparks in a volume unit is Φspark:
| (5) |
where PO is expressed using the HTG or aHTG model (Eq. 1 or Eq. 2, respectively). All other parameters are summarized in Tables I and II.
In a typical laser-scanning confocal microscopic experiment, calcium sparks are collected at a random line scanned longitudinally along the myocyte. The volume sampled by the confocal microscope per distance, ℓ = 100 µm of the line scan, Vls, was calculated as
| (6) |
where RY = 0.4 µm and RZ = 0.9 µm (Lukyanenko et al., 1999) is the resolution of the confocal microscope in the y and z directions. This estimate is in approximate accordance with the simulations of the relationship between the spark amplitude and the distance from the focal plane (Smith et al., 1998). The value of Vls = 36 µm3 was used for estimation of the observed spark frequency, FSpark:
| (7) |
Distribution of active RYRs in sparks.
The number of RYRs recruited in a spark by the first (either spontaneous or dihydropyridine receptor [DHPR] triggered) RYR opening is determined by the probability that a single recruited RYR channel will be open during a spark (pO), i.e., the fraction of time a recruited RYR is open during the spark on average, and by the number of RYRs in the CRU (nRYR). The probability, PnO, that the exact number nO of RYR channels out of the total nRYR in the CRU will be open during the spark, i.e., that exactly nO – 1 independent RYR channels will be recruited by the first RYR opening, is given by the binomial distribution
| (8) |
Eq. 8 was used to find the value of pO for a given nRYR in the CRU by fitting the experimental data of Wang et al. (2004) (see Fig. 2).
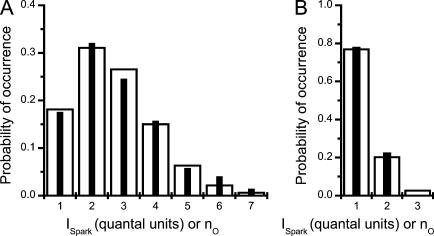
Figure 2.
Approximation of the distribution of calcium release fluxes (ISpark) with the theoretical distribution of the number of open RYRs in sparks. (A) Black bars represent the probability distribution of spark fluxes recorded under control conditions (Wang et al., 2004 and their Fig. 2) expressed in units of the elementary quanta of calcium release flux. White bars represent the probability distribution of the number, nO, of RYRs open during spark, which was calculated using Eq. 8. (B) The same comparison as in A for the sparks measured in the presence of tetracaine (Wang et al., 2004).
Coupling fidelity.
In general, the extent of CRU activation is based on coupling of stochastically operating RYRs to the opening of a channel that increases calcium concentration in their vicinity. That channel can be either a DHPR channel, as in the case of calcium release triggered by excitation, or an RYR channel, as in the case of spontaneous sparks. By definition, coupling fidelity (PCpl) is the probability that a single open channel will activate any channel in the CRU (Zhou et al., 1999), and therefore it is equal to the complement of the probability that no channel will be activated (for an explanation see Bridge et al., 2008).
| (9) |
where the probability, p, is equal to the open probability of RYR channels of the CRU during the coupling process, and the exponent n is equal to the number of RYRs in the CRU that may undergo activation. The probability, p, can be calculated from the known coupling fidelity as
| (10) |
where all symbols have been defined previously.
Eq. 9 allows us to compare the coupling fidelity within the CRU (RRPCpl) with the coupling fidelity between a DHPR opening and activation of the first RYR during an evoked spark (DRPCpl), which has been previously estimated under several conditions (Zhou et al., 1999; Zahradníková et al., 2004; Poláková et al., 2008). Conversely, Eq. 10 allows calculation of the open probability, pO, of the RYRs in the CRU during their activation by DHPR channels from the known DHPR–RYR coupling fidelity, DRPCpl.
Online supplemental material
The supplemental material provides a detailed description of the HTG and aHTG models used for derivation of Eqs. 1 and 2; a description of the calculations of state probabilities of the aHTG model, which was used for calculations depicted in Figs. 3 and 4; and estimation of coupling fidelity between RYRs in the CRU (RRPCpl) based on the procedure used in Poláková et al. (2008). Online supplemental material is available at http://www.jgp.org/cgi/content/full/jgp.200910380/DC1.
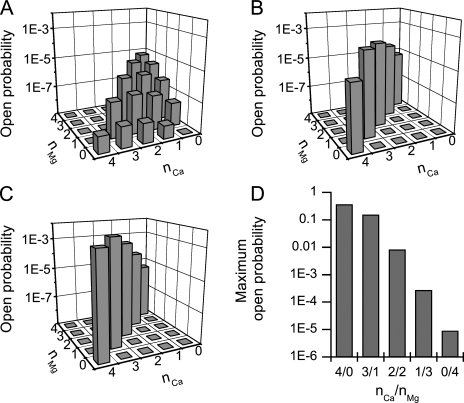
Figure 3.
Contribution of different divalent ion–bound states to RYR open probability. (A) Resting conditions (50 nM Ca2+ and 1 mM Mg2+; Eqs. S18 and S19 of supplemental material). (B) Immediately after opening of the first RYR (100 µM Ca2+ and 1 mM Mg2+) and binding of Ca2+ ions to all free RYR-binding sites (Eqs. S20 and S21 of supplemental material). (C) At the steady state (100 µM Ca2+ and 1 mM Mg2+) after a decrease in the mean number of RYR activation sites occupied by Mg2+ from 3.68 to 3.25 (Eqs. S22 and S23 of supplemental material). (D) The maximum open probabilities of RYRs fully occupied by divalent ions at different Ca2+/Mg2+ ratios (nCa/nMg).
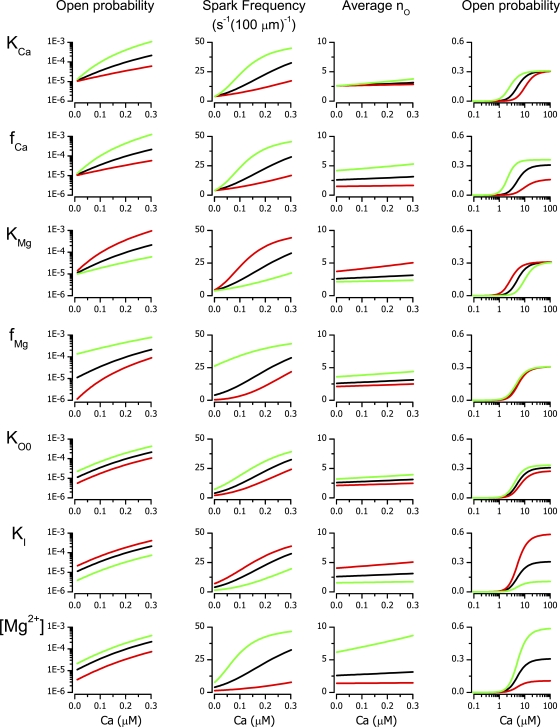
Figure 4.
Simulated calcium dependences of RYR open probability, of calcium spark frequency, and of the mean number of open RYRs. Black lines are the results of simulations with standard parameter values (Tables I and II). The red and green lines correspond to the 200% and 50% change, respectively, of the parameter (indicated on the left) relative to its standard value. The abscissas show the cytosolic calcium concentrations. Ordinates are specified above each column.
RESULTS
In the cell, the relationship between activation of RYRs and the properties of calcium sparks is reflected in the frequency of calcium sparks and in the number of open RYRs that constitute sparks. The conjectures are that the spark frequency in resting myocytes should be quantitatively related to the resting cytosolic calcium concentration, that the number of open RYRs in the spark should be quantitatively related to the calcium concentration in the dyadic gap during the spark, and that both processes can be described by the same calcium dependence of RYR open probability. To test specific hypotheses on calcium-dependent gating of RYR channels within the CRU against experimental data on calcium sparks, we used mathematical relations describing the CRU system, which were derived in the Materials and methods section.
Calcium dependence of spark frequency
The calcium dependence of calcium spark activation was studied in saponin-permeabilized rat myocytes by Lukyanenko and Györke (1999). We used this unique published dataset to test the hypothesis that the spontaneous (or better to say the basal) spark activity at resting free cytosolic calcium concentration corresponds directly to the activity of RYRs. The model of spark frequency (Eq. 5) with either of the two models of RYR gating described in Materials and methods was fitted to the experimental data in the cytosolic Ca2+ range of 0.05–0.25 µM (i.e., under conditions in which no calcium waves were observed). Their comparison is presented in Fig. 1. The parameters of RYR gating models (Table I) were taken as published previously except for KMg and fMg, the new parameters characterizing Mg2+ binding to the RYR activation site, which were optimized by fitting. The parameters characterizing the CRU (Table II) were taken from the literature with the exception of the refractory period, trp, for which quantitative estimates in permeabilized myocytes were not available, and therefore it was optimized as well.
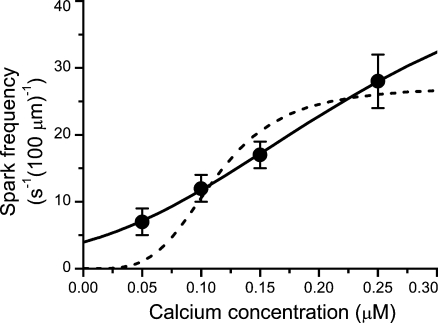
Figure 1.
Approximation of the calcium dependence of spark frequency. Symbols with error bars represent data on permeabilized myocytes from Lukyanenko and Györke (1999). The lines represent the best fits by Eq. 5 with parameters shown in Tables I and II for the aHTG (solid line) and HTG (dashed line) models. Both the experimental and model data were obtained at 1 mM of free Mg2+ in the cytosol. The abscissa shows the cytosolic calcium concentration.
Fitting of the spark frequency model to the experimental data provided a very good result for the aHTG model but a very poor one for the HTG model (Fig. 1). The fit for the HTG model was not improved even when all RYR gating parameters were set free for optimization (unpublished data). Because the HTG model failed to fit the calcium dependence of spark frequency, even though it approximated very well the single wild-type RYR channel gating data (Zahradník et al., 2005), we conclude that the allosteric interaction, the specific feature embedded only in the aHTG model, is essential for relating RYR gating to spark frequency.
There were three parameters in the aHTG and the spark frequency models set free for fitting the data: the microscopic Mg2+ dissociation constant KMg, the allosteric factor fMg, and the refractory period trp. The fitted value of KMg, 79 ± 8 µM, was close to the recently reported value for the apparent KMg of calcium spark inhibition (100 µM; Gusev and Niggli, 2008) and comparable with the value of the apparent KMg obtained from the phenomenological model of Ca–Mg competition (250 ± 150 µM; Zahradníková et al., 2003). The two orders of magnitude difference between KMg and KCa is in accordance with the chemistry of Mg2+ and Ca2+ ions.
The value of the allosteric coupling factor for magnesium, fMg, which is 1.40 ± 0.05, brings about a threefold reduction of the channel open probability between its divalents-free and fully Mg2+-bound activation sites. In contrast, the allosteric factor for calcium, fCa, is much less than one (Zahradník et al., 2005) and provides a 200,000-fold increase of open probability of a fully Ca2+-bound channel over a divalents-free channel. This means that, in the case of Ca2+, there is a strong positive allosteric coupling, whereas in the case of Mg2+, there is a weak negative allosteric coupling between ion binding to the RYR activation sites and opening of the channel.
The value of the refractory period, trp, of 713 ± 80 ms, obtained here by fitting experimental data measured in the presence of EGTA (Lukyanenko and Györke, 1999), is close to the refractory periods observed in the presence of high affinity calcium buffers EGTA (∼1 s [Sham et al., 1998] and 300–400 ms [Terentyev et al., 2002]) or DM-nitrophen (720 ± 32 ms; Szentesi et al., 2004). In the absence of EGTA, the value of trp should be shorter, as high affinity calcium buffers might slow down SR refilling by their slow unbinding of Ca2+ (Sham et al., 1998; and Sham, J.S., L.S. Song, M.D. Stern, E.G. Lakatta, and H. Cheng. 1999. Biophysical Society 43rd Annual Meeting. Abstr. 385). Indeed, the refractory period observed in intact resting myocytes was ∼150 ms (Sobie et al., 2005).
It can be concluded that the observed frequency of spontaneous calcium spark activation is consistent with the theory of RYR gating based on allosteric interaction between binding of divalent ions and channel opening and with the known CRU size, construction, and distribution.
Quantal distribution of calcium release flux
The relation of CRU size to the amplitude of calcium release flux, or in other words, how many RYRs make a spark, is not well known (for reviews see Ríos and Brum, 2002; Cheng and Lederer, 2008). The probability distribution of calcium release fluxes of calcium sparks was studied in intact rat cardiac myocytes by Wang et al. (2004). In these original experiments, calcium sparks were evoked repetitively from a calcium release site under the sarcolemma, and the measured calcium release fluxes were quantified. By comparing the rising phase of the sparks with the kinetics of calcium binding to the indicator fluo-3, Wang et al. (2004) determined spark calcium release fluxes (effectively, the rising rates of the calcium sparks) and revealed their quantal character. They interpreted the quantal character of the calcium release flux as the consequence of dynamic recruitment of small, variable cohorts of RYRs. The number of recruited RYRs was estimated between one and eight (Fig. 2), which is unexpectedly low for a CRU containing up to a few hundred RYRs. We tested the consistency of this interpretation with the aHTG model of RYR gating derived and optimized in this study. To this end, the probability, pO, that a single RYR channel will be open during a spark was estimated and compared with the predictions of the aHTG model. At first, the probability, PnO that the exact number, nO, of independent RYR channels out of the total nRYR in the CRU will open during the spark (Eq. 8) was fitted to the observed distribution of the control dataset of Wang et al. (2004) in the whole range of nO (1 – nRYR) as shown in Fig. 2 A for nO ≤ 8. For a typical CRU containing 182 RYRs (Soeller et al., 2007), the method of maximal likelihood provided a pO of 0.0093, which corresponds to a mean of 2.68 simultaneously open RYRs during a typical spark (i.e., to 1.68 RYRs recruited by one DHPR-triggered RYR opening). The same approach was used to reproduce the distribution of calcium release current amplitudes in the presence of the RYR channel inhibitor tetracaine (Fig. 2 B). The effect of 100 µM tetracaine was fitted with a pO of 0.0014; that is, 15% of control, which corresponded to a mean of 1.27 simultaneously open RYRs (i.e., to 0.27 RYRs recruited by one DHPR-triggered RYR opening).
The question is how this low pO reconciles with the gating of RYRs. Equipped with the allosteric mechanism, a RYR channel can open even without divalent ion binding; that is, only by internal transitions caused by thermal agitation. The aHTG model (Table I) predicts that RYR can open also from Mg2+-bound states with a total PO of 2 × 10−5 (Eq. 2). The contribution of RYRs occupied by different numbers of Mg2+ and Ca2+ ions (i.e., RYR ionic forms CaiMgjRYR with i + j ≤ 4) to the overall steady-state open probability (Fig. 3 A) was expressed as the products of the probability of occurrence and the open probability of these ionic forms (see supplemental material). It was the largest for Mg4RYR (P04 × = 6.2 × 10−6), CaMg3RYR (P13 × = 5.0 × 10−6), and Mg3RYR (P03 × = 2.8 × 10−6) forms.
Immediately after opening of the first RYR, the ionic conditions at the CRU change dramatically. Therefore, steady-state probabilities of channel states cannot be used under these conditions. Calcium reaches saturating concentration (∼100 µM; Valent et al., 2007), and in ∼0.2 ms (Zahradníková et al., 1999) it binds to all free RYR-binding sites (see Fig. 3 B and supplemental material for the contribution of individual states to the open probability). Because the calcium-binding step is much faster than other channel transitions, the increase of open probability caused by calcium binding can be calculated analytically, without having to resort to the differential equations of the model (see supplemental material). The overall open probability resulting from rapid calcium binding is 6 × 10−4, still much less than the estimated pO. The expected level of open probability may be reached only if some Mg2+ ions are liberated from the RYR activation sites because the emerging divalent-free sites can be immediately occupied by Ca2+ and the open probability can be further increased. Even a small fraction of dissociated Mg2+ ions will strongly increase the RYR open probability because PO steeply increases with each Mg2+ replaced by Ca2+ (Fig. 3 D). To maintain consistency with the data of Wang et al. (2004), we expressed the value of pO as a function of the mean fraction of Mg2+ dissociated from an activation site. This non–steady-state calculation could again be performed analytically (see supplemental material) because it calculates the integral of Mg2+ dissociation for the period reflected in the measurements of Wang et al. (2004). To achieve pO = 0.0093, the fraction of RYRs from which one, two, three, or four (n) Mg2+ ions dissociate has to be 0.25n, i.e., 0.25, 0.063, 0.016, and 0.004, respectively (see supplemental material). Therefore, the mean number of Mg2+ ions bound to the RYR would decrease from 3.68 at rest to ∼3.25 during the spark. The contribution of individual ionic forms of RYR to the open probability during the sparks resulting from the aforementioned changes in occupation by Mg2+ is shown in Fig. 3 C.
Another question is how the low value of pO reconciles with the postulated high coupling fidelity between the first RYR opening and the remaining openings (i.e., with the notion that the first RYR opening activates the whole CRU; Stern, 1992; Zahradníková et al., 2007). Using Eq. 9, p = pO = 0.0093 and n = nRYR – 1 = 181, we obtain RRPCpl = 0.82. An alternative estimate of coupling fidelity based on published values of coupling fidelity between DHPR and RYR channels (DRPCpl) and on current amplitudes and open times of these channels (see Eq. S27 of the supplemental material) provided RRPCpl = 0.96, which corresponds to pO = 0.018 (Eq. 10). Although calculation according to Eq. 10 makes no assumptions regarding the distance between the triggering and the triggered RYR, calculation according to Eq. S27 assumes that the triggering and the triggered channels are the nearest neighbors. The values of 0.82 and 0.96 may be viewed as the low and high estimates of the coupling fidelity between RYRs. Both calculations show that the low value of pO during the spark is not at odds with a high RYR–RYR coupling fidelity.
It can be concluded that the observed quantal distribution of spark calcium release flux requires the vast majority of RYR channels to stay closed during the spark. According to the aHTG model with parameters optimized for the description of spontaneous spark frequency, this can be explained by occupation of most RYRs by two or more Mg2+ ions at their activation sites. Calcium release flux during the sparks is generated by the small fraction of RYRs occupied by at most one Mg2+ and three or four Ca2+ ions.
Effects of RYR gating, couplon properties, and cytosolic Mg2+ concentration
Under pathological conditions, the frequency of calcium sparks in the diastole or at rest is increased (Kubalová et al., 2005; Song et al., 2005; Fernández-Velasco et al., 2009), probably as a result of changes in RYR gating (Yano et al., 2006). To elucidate this observation, we have simulated the effects of changes in individual parameters of the model and evaluated their impact on the RYR open probability, on the calcium spark frequency, and on the number of open RYRs in sparks. The parameters of RYR gating estimated previously (Zahradníková et al., 2003; Zahradník et al., 2005) from independent bilayer experiments (Li and Chen, 2001; Zahradníková et al., 2003) or in this work (Table I) were used as standard parameters.
The effect of variation of model parameters is compared in Fig. 4. The analysis suggests that the diastolic/resting open probability can vary by an order of magnitude under the influences that modify parameters affecting RYR gating to half or twice the standard value (Fig. 4, left column). Of note is the effect of Mg2+-related parameters, especially the unexpectedly pronounced sensitivity to fMg that reveals a potential pharmacological target. The extent of simulated Mg2+ concentration changes exceeds normal variability in vivo, except for acute ischemia (Murphy et al., 1989), but should be considered in design and interpretation of experiments in which nonphysiological concentrations of Mg2+ are occasionally used. The frequency of calcium sparks is sensitive to all interventions, especially to changes in allosteric coupling of Mg2+ binding to channel opening and to changes in Mg2+ concentration. There is a close correlation, albeit not linear, between the variation of the open probability and of the frequency of spontaneous sparks. The refractory period does not have a significant impact on the spark frequency at 50 nM cytosolic calcium; however, for cytosolic calcium levels above 100 nM, the prolongation of trp may become a spark frequency limiting factor (unpublished data). In most cases, the higher the cytosolic calcium level is, the more pronounced are the effects of parameter variation, except for the allosteric factor for magnesium, fMg, whose contribution decreases with increasing calcium and even ceases in the systolic Ca2+ concentration range.
The sensitivity of the number of RYRs recruited during the spark to parameter variation is markedly different from that of spark frequency. The value of the mean nO is substantially influenced by fCa, KMg, fMg, KI, and [Mg2+] through their modulation of the distribution of the number of Mg2+ ions bound to the RYRs. The parameter KCa, although affecting the spark frequency, does not have a significant effect on nO. Interestingly, the mean number of open RYRs in a spark is relatively insensitive to the cytosolic Ca level unless Mg2+ concentration is decreased. The refractory period (trp) does not affect nO (unpublished data) because it has no relation to the equilibrium distribution of RYR gating states.
In the high Ca2+ concentration range, at which measurements of RYR activity are typically performed, the effects of parameter variation on PO were accompanied by shifts of the apparent KCa. Again, the role of Mg2+-dependent parameters KMg and KI as well as the effect of cytosolic [Mg2+] were dominant. It is apparent that the effect of parameter variation on PO is substantially different at low and high calcium concentrations. The allosteric factor fMg is the most illustrative in this respect because its variation has no effect in the micromolar calcium concentration range but the strongest effect in the diastolic calcium concentration range. Therefore, conclusions or predictions regarding diastolic open probability and spark frequency cannot be safely made from measurements of RYR activity in the micromolar calcium concentration range.
Variability of the CRU
The variability in the number of DHPR channels per CRU (Inoue and Bridge, 2005) as well as in the quantal substructure of sparks (Wang et al., 2004) indicates that individual CRUs differ in their size. It is clear that the numerical density and the size of CRUs vary within myocytes (Soeller et al., 2007; Hayashi et al., 2009). For many reasons (e.g., as a result of ontogenesis, work load, or diseases), the amount of expressed RYRs, that is, the size of CRU as well as the distribution of RYRs between CRUs, may also vary considerably (Vatner et al., 1994; Dan et al., 2007). Predictably, it should have functional consequences because both νCRU and nRYR affect spark frequency (Eq. 5).
Variation of the total number of RYRs to 200 or 50% of the default value was implemented as variation in the numerical density of CRUs (νCRU), in the size of CRUs (nRYR), or as a proportional variation of both. Simulated effects of changes in the numerical density of CRUs on spark frequency are shown in Fig. 5 A. It should be noted that these changes are accompanied by a change in the mean distance between two CRUs on the same Z line and by increased steepness of the observed cytosolic calcium dependence of spark frequency. The mean distance changed from 0.97 µm (Chen-Izu et al., 2006) to 0.69 and 2.34 µm for increased and decreased CRU density, respectively. Numerically identical results are obtained if, at a constant numerical density of the CRUs, the volume (Vls) sampled by the confocal microscope is varied (Eq. 7).
Figure 5.
Simulated calcium dependence of calcium spark frequency (FSpark) and its variation with the size and distribution of CRUs. (A) The effect of varying the numerical density of CRUs (νCRU) at a constant CRU size (nRYR). (B) The effect of varying the CRU size (nRYR) at a constant numerical density of CRUs (νCRU). (C) The effect of varying the overall number of RYRs by changing νCRU and nRYR by the same extent. Parameters corresponding to the overall number of RYRs equal to 100%, 200%, and 50% of control are shown as black, red, and green lines, respectively. (D) The effect of varying the numerical density of CRUs (νCRU) at a constant overall number of RYRs (i.e., by changing the size of the CRUs [nRYR] in the direction opposite to that in C). The red line shows the case when νCRU was increased to 200% and, at the same time, the CRU size (nRYR) was decreased to 50% of the default value. The green line shows the case when νCRU was decreased to 50% and the CRU size (nRYR) was increased to 200% of the default value. Abscissas show the cytosolic calcium concentrations.
The effect of varying the mean number of RYRs per CRU, i.e., the size of CRU, is shown in Fig. 5 B. In this case, neither the distance between CRUs nor the steepness of the cytosolic calcium dependence of spark frequency is changed. Changes in the size of CRUs lead to a much less prominent alteration of spark frequency than changes in the numerical density of CRUs.
A proportional change in both νCRU and nRYR to √2 and √(1/2) of their original values, respectively, also results in a change of the mean distance between two CRUs on the same Z line, in this case to 0.82 µm and 1.15 µm, respectively. The effect on spark frequency is intermediate (Fig. 5 C).
The effect of the clustering of RYRs into CRUs of various sizes is shown in Fig. 5 D. In this case, the total number of RYRs was kept constant. The distance between CRUs is changed from 0.97 µm to 0.69 and 2.34 µm. It can be seen that redistribution of RYRs has very little effect at low cytosolic Ca2+ values, but the effect becomes prominent at elevated Ca2+.
It transpires that increasing the size of CRUs represents a safer strategy for regulation of SR calcium content than increasing the number of CRUs because the latter also may cause an increased danger of wave propagation as a result of the reduced distances among neighboring CRUs.
DISCUSSION
This study scrutinizes the relation between calcium sparks and RYR gating in cardiac myocytes. The results revealed that the allosteric principle of RYR gating is at the heart of the calcium signaling system generating local calcium release that is known to regulate both the SR calcium content at rest and the rate of calcium release upon stimulation. Introduction of this principle enabled theoretical description of the calcium dependence of spontaneous calcium spark frequency and of the quantal distribution of calcium spark release fluxes based on gating of independent RYRs in the CRU.
Spark frequency
To study the relation of RYR activity to spark frequency, we used a theoretical approach based on experimental indications (Cheng et al., 1993; Lukyanenko and Györke, 1999) that the basal frequency of calcium sparks is directly related to RYR gating. With the assumption that calcium sparks arise upon the first opening of any RYR in the CRU, we have derived an equation for spark frequency (Eq. 5) that accounts for the open probability of RYRs and for the density and size of the CRUs. In addition to RYR open probability, the equation for the spark frequency uses five parameters, four of which were taken or calculated from previously published experimental data (Table II). With this equation, the task was to solve the problem of RYR activity under resting cytoplasmic conditions. Previously (Zahradník et al., 2005), we have solved RYR models for the calcium dependence of RYR gating for diverse sets of experimental data in the high calcium concentration range in the absence of magnesium. In the present study, we extended those RYR models for Mg2+ binding to account for the resting cytosolic conditions at which the calcium spark recordings take place. In the resulting HTG and aHTG models, six out of seven and seven out of nine RYR gating parameters, respectively, were taken as estimated in previous studies (Table I).
Approximation of the experimental calcium dependence of spark frequency with the theoretical equation (Fig. 1) was acceptable only if RYR gating was described by the aHTG model (Eq. 2); that is, only if Mg2+ and Ca2+ ions compete for the RYR activation site at each RYR monomer and only if ion binding is allosterically coupled to the channel opening. If the RYR states corresponding to the allosteric gating transitions were not considered, i.e., such as in the HTG model, unacceptable deviation from the experimental data was obtained (Fig. 1). Additionally, the calcium dependence of the HTG open probability in the presence of Mg2+ (unpublished data) deviated strongly from the experimentally observed values (Laver et al., 1997; Györke and Györke, 1998; Zahradníková et al., 2003). These results underline the central role of the allosteric principle in the regulation of RYR open probability at resting/diastolic calcium concentrations and provide grounds for understanding the failing RYR function in diseased myocardium.
Parameter analysis of the aHTG model revealed (Fig. 4) that under resting cytosolic conditions, the changes in parameter values and in cytosolic Mg2+ concentration may alter the frequency of sparks substantially, especially at increased diastolic calcium levels. The basal spark frequency does not reach zero even in the complete absence of calcium in the cytosol, but it is greatly reduced when fMg and [Mg2+] are increased or when KI is decreased. The effect of cytosolic Mg2+ on the resting calcium spark frequency, which is consistent with our simulations, has been experimentally documented (Lukyanenko et al., 2001; Gusev and Niggli, 2008).
RYR gating models that do not include Mg2+ binding to the RYR activation site (e.g., the aEMG model; Zahradník et al., 2005) may also fit the experimental data on calcium dependence of spark frequency if KCa and either KO00 or nRYR are left free for optimization and if Ca2+ binding is kept allosterically coupled to channel opening (unpublished data) because the allosteric coupling of Mg2+ binding to channel opening is very low. However, such models would not only miss the experimentally established effects of Mg2+ on RYR activity but, and even more importantly, models lacking Mg2+ binding would fail to explain the quantal distribution of the spark release flux (see section The number of RYRs open during the spark).
Incorporation of Mg2+ inhibition into the model (Laver et al., 1997; Zahradníková et al., 2003) provided an inhibitory mechanism that has a striking effect on the maximum RYR open probability at calcium levels corresponding to an activated CRU. The Mg2+ inhibition site on the RYR also has a suppressing effect on spark frequency (see the effect of KI variation in Fig. 4) and therefore represents an important stabilizing factor.
The size of the CRU
Recently, it has been reported that in the mouse ventricle, there is a high variability in the size of the CRUs, and the occurrence of a large number of closely spaced small CRUs has been described using electron tomography (Hayashi et al., 2009). New data from Baddeley et al. (2009) also suggest that at least some CRUs may be substantially smaller than previously assumed. To assess the effect of CRU size, we have analyzed the dependence of the fitted model parameters KMg, fMg, and trp on CRU size for two estimates of the total number of RYRs per cell, which were previously considered as the upper and lower estimates (Soeller et al., 2007; i.e., 182 and 78 RYRs per CRU, corresponding to 5.5 × 106 and 2.4 × 106 RYRs per 30-pl cell). The analysis of simulations revealed that for a given total number of RYRs, the estimates of RYR gating parameters are independent of CRU size, whereas the refractory period, trp, is inversely proportional to the size of CRU (i.e., directly proportional to the numerical density of CRU; unpublished data). These relations follow from Eq. 5, taking into consideration that trp >> trd. Therefore, an increase of the number of CRUs at the expense of their size would result in a proportional increase of trp for the observed calcium dependence of spark frequency. Reducing the size of the CRUs to less than ∼30–50 RYRs would result in very large refractory periods that are not realistic. Thus, we propose that the groups of closely spaced small RYR clusters separated by ∼20-nm gaps (comparable with the size of an RYR) that were observed in mice (Hayashi et al., 2009) might behave as functionally coupled unless the spark frequency in mice cardiac myocytes is not substantially higher than in rats.
The number of RYRs open during a spark
The agreement between the data of Wang et al. (2004) and the aHTG model prediction regarding the RYR open probability during a spark (Fig. 2) is acceptable when partial Mg2+ unbinding is allowed. Although the rate of Mg2+ unbinding is not known, there are indications that it should be in the millisecond range (Zahradníková et al., 2003); that is, comparable with the time scale of spark duration. Acceptance of the aHTG model bears the conclusion that during the spark, a very substantial fraction of RYRs remains shielded with Mg2+ ions and has a very low open probability. Consequently, a typical spark should be produced by a small randomly activated cohort of RYRs. This subgroup need not be clustered together because the calcium level that increased as a result of the first RYR channel activation is well above the calcium dissociation constant at any RYR of the typical CRU (Valent et al., 2007).
Calcium current flowing through a single RYR channel as well as the calcium release flux through a single CRU might not be constant (Song et al., 1998; Stern et al., 1999; Brochet et al., 2005; Kubalová et al., 2005; Zahradníková et al., 2007), and therefore the assumption of Wang et al. (2004) about the linear relationship between the extrapolated amplitude of the fluorescent signal and the calcium release flux might not hold for the whole duration of the spark. If we assume that the measurements of Wang et al. (2004) reflect the time when the peak of release flux is maximal (6.67 ms as estimated in Zahradníková et al., 2007), the rate constant of Mg2+ unbinding required for the observed 25% dissociation of Mg2+ from the activation sites would be 43 s−1. This value is three times less than that for Mg2+ unbinding from ATP, a compound with affinity to Mg2+ similar to that of the RYR channel (kON = 1.5 × 106 M−1 s−1, kOFF = 150 s−1, and KMg = 100 µM; Baylor and Hollingworth, 1998). This slow rate of dissociation results in recruitment of the mean number of 1.68 RYRs by the first RYR opening (one to seven additional RYRs in individual instances; Fig. 2).
Simulations using the aHTG model have revealed that the RYR gating parameters and the cytosolic Mg2+ affect the number of open RYRs during a spark independently of their effect on the spark frequency. This could not be resolved experimentally because the change in spark frequency induces a secondary change of calcium load of the SR (Lukyanenko et al., 2001) that feeds back on both the RYR open probability and on the single-channel release flux. However, it has been shown that the spark amplitude increases upon removal of Mg2+ (Gusev and Niggli, 2008) and decreases upon the increase of Mg2+ (Lukyanenko et al., 2001), which is in agreement with the simulations in which changes in cytosolic Mg2+ concentration had a prominent effect on nO in the observed directions (Fig. 4). Therefore, it is very likely that the number of open RYRs participates substantially in the variation of the spark amplitude with cytosolic Mg2+ concentration.
Comparison of spontaneous and triggered calcium sparks
Our results reveal that spontaneous and triggered calcium sparks do not emerge from the same RYR states. Spontaneous sparks originate in 75% from a random opening of an RYR occupied by three to four Mg2+ ions at the activation site. The very low maximum open probability of these channel forms (9 × 10−6 for Mg4RYR and 2.6 × 10−4 for CaMg3RYR; Fig. 3 D) determines the very low RYR open probability (2 × 10−5) and the very low probability of a single CRU activation (∼0.003 for a CRU containing 182 RYRs; calculated as trd/fSpark) under diastolic conditions. In contrast, sparks triggered by increased calcium in the dyadic gap occur with relatively high probability. The DHPR–RYR coupling fidelity is in the range of 0.05–0.7 (Zhou et al., 1999; Wang et al., 2004; Poláková et al., 2008), which corresponds to an RYR open probability of 0.0005–0.007 for a CRU containing 182 RYRs (Eq. 10). Eq. 10 also allows examination of the role of individual RYR ionic forms during activation of the calcium spark by a DHPR opening. Comparison of the contributions of individual ionic forms of RYR to the overall open probability at pO = 0.0006 and 0.0093, the pO values similar to those of triggered RYR openings at low and high coupling fidelity (Fig. 3, A and B, respectively), allowing approximate estimation of the ionic composition of RYRs that open in response to the DHPR trigger. At the lowest DHPR–RYR coupling fidelities (p = 0.0006; Fig. 3 A), the first triggered RYR opening is most often from an RYR occupied by one or two Mg2+ ions and two or three Ca2+ ions, whereas at the highest DHPR–RYR coupling fidelities (p = 0.0093; Fig. 3 B), it is most often from an RYR occupied by one Mg2+ and three Ca2+ ions. These numbers are only approximate because they do not take into account the repeated openings of multiple DHPRs and the resulting reequilibration of RYR states after subthreshold calcium increase in the dyadic gap that occur at low coupling fidelity (Zahradníková et al., 2003, 2004; Poláková et al., 2008) and that participate in the effect of the recent history of DHPR openings on coupling fidelity (Zahradníková et al., 2004).
After opening of the first RYR in a CRU, the difference between spontaneous and triggered CRU activation ceases. This is caused by the high single RYR channel calcium flux that provides saturating Ca2+ concentration for RYR activation in the gap of the dyadic junction. The increased junctional calcium level strongly increases the probability of RYR openings that manifests as high RYR–RYR coupling fidelity within a CRU (0.92–0.96; see Quantal distribution of calcium release flux in Results). Although much higher than the fidelity of DHPR–RYR coupling, for kinetic reasons it will result in a limited and variable number of open RYRs during the spark.
General considerations
The general validity of this model of CRU activation can be tested by comparing the relative amounts of calcium provided with the cytoplasm by influx via DHPR channels and by calcium release via RYR channel. Assuming 2.68 open RYRs per spark with a calcium flux amplitude of 0.5 pA per channel (Kettlun et al., 2003) and with an open time of 15 ms (Gaburjakova and Gaburjakova, 2006), the calcium charge of an average spark is QRYR = 20.1 fC. Assuming seven RYR channels per DHPR channel (Bers and Stiffel, 1993), a DHPR open probability of 0.1 (Rose et al., 1992), iDHPR = 0.084 pA at 0 mV (calculated using Eq. S25 in supplemental material), and assuming that during stimulation each CRU generates a single spark that will inactivate its adjoining DHPR channels (Zahradníková et al., 2004) within the duration of the spark, the integral of calcium current through DHPRs of one couplon is QDHPR = 3.3 fC. At a CRU density of 1 µm−3 (Soeller et al., 2007), the amount of calcium entering the cytoplasm upon activation of all CRUs is thus 120 µmol/liter, in accordance with the experimental observations (Bers, 2001). Thus, our model predicts that the contribution of calcium influx and calcium release to the systolic calcium elevation is 14% and 86%, respectively, which is quite consistent with the experimental estimates (Bassani et al., 1994).
In-depth analysis of calcium sparks, allowed by the presented theory, provides common ground for interpretation of experimental observations as different as spontaneous calcium sparks and triggered calcium release in cardiac myocytes. To summarize, the major findings of this work give independent evidence that the elementary quantum of calcium release flux observed by Wang et al. (2004) corresponds to the opening of a single RYR channel; they resolve the discrepancy between the large number of RYRs in the CRU (Franzini-Armstrong et al., 1999; Soeller et al., 2007), the high maximum open probability of RYRs (Li and Chen, 2001; Gaburjakova and Gaburjakova, 2006; Qin et al., 2008), the high calcium concentration in the dyadic space when an RYR channel opens (Valent et al., 2007), and the modest number of RYRs simultaneously open during calcium sparks (Bridge et al., 1999; Lukyanenko et al., 2000; Wang et al., 2004) that has not been predicted by the CICR mechanism. These findings also substantiate the observed occurrence of calcium sparks in resting myocytes (Lukyanenko et al., 2000). All of these observations are based on the allosteric coupling of binding of divalent ions to RYR opening and on Ca2+/Mg2+ competition.
Limitations
Most parameters of the models (Zahradník et al., 2005) used in this study were originally obtained by fitting experimental data from expressed RYR channels in planar bilayers in the absence of calsequestrin, triadin, and junctin (Li and Chen, 2001). However, the calcium sensitivity and maximum open probability of expressed RYR channels (Li and Chen, 2001) were similar to those observed after maximal activation by luminal Ca2+ in the presence of all auxiliary proteins (Qin et al., 2008). At the same time, the calcium sparks analyzed in this work were recorded in permeabilized myocytes, which showed calcium waves in the absence of added EGTA, and the properties of which resembled intact myocytes exposed to 5 mM external Ca2+ (Lukyanenko and Györke, 1999). Therefore, the fitted parameters of the presented model of spark frequency should be considered to represent the conditions of a very high SR calcium load.
The calculation of the volume sampled by the confocal line scan is quite simplified because we assumed that sparks are fully detectable when they are positioned below half-magnitude of the point spread function and undetectable otherwise. The fraction of detected sparks, even if they are in the focal plane, is not known with certainty because it has been usually assumed that all sparks have equal amplitude (Smith et al., 1998). The observed sevenfold variation in the calcium release flux of sparks (Wang et al., 2004) must result in unequal amplitude of sparks as well, even if the relationship between the release flux and the amplitude of sparks is not straightforward (for review see Ríos and Brum, 2002; Wang et al., 2004). It is obvious that for the same calcium dependence of spark frequency, a change in the sampled volume (i.e., in the number of CRUs included in the calculation) requires either a change in the number of RYRs per CRU or in the refractory period (see The size of the CRU). On the other hand, it should be pointed out that the value of the sampled volume used in the calculations has no significant effect on the estimated RYR gating parameters.
The CRU system was modeled upon several suppositions that may have impact on quantitative but not on qualitative model predictions. For comparison, if the size of CRU was 267, as estimated by Franzini-Armstrong et al. (1999), the parameters of the aHTG and spark frequency models would not change significantly, but the estimated pO would be 0.009 and 0.0019, respectively, for the control and tetracaine experiments of Wang et al. (2004). Furthermore, the aHTG model was developed upon data on RYRs in vitro (Li and Chen, 2001; Zahradník et al., 2005) or in permeabilized cells (Lukyanenko and Györke, 1999), whereas data on spark calcium fluxes were obtained in intact cells (Wang et al., 2004). Therefore, the extent of Mg2+ unbinding during the spark, which was determined in this study, has to be considered as only approximate.
It has been shown that luminal calcium affects the maximum open probability of RYR channels as well as their apparent calcium sensitivity (Györke and Györke, 1998; Györke and Terentyev, 2008; Qin et al., 2008). At the same time, luminal Ca2+ has been shown to be a critical regulator of diastolic calcium spark frequency (Lukyanenko et al., 2001; Terentyev et al., 2002). In the aHTG model, control of RYR activity by the steady-state or diastolic luminal calcium may be achieved by changes of either of the allosteric factors fCa or fMg or by changes of the dissociation constant of the calcium-free open state, KO00, but not by changes in KI or KCa. To distinguish between the possible mechanisms, single-channel data at very low open probabilities (i.e., in the cytosolic Ca2+ range below 0.2 µM) should be acquired, which is not an easily feasible goal (Fig. 4, left column). Additionally, the prespark level of luminal calcium and the kinetics of its decline during the spark have been shown to affect the refractoriness of the CRU (Terentyev et al., 2002, Szentesi et al., 2004) as well as spark duration (Terentyev et al., 2002). Because changes in RYR gating affect termination and refractoriness of the CRU (Domeier et al., 2009), the gating parameters of the RYR and the parameters trp and trd may not be fully independent. However, the relationships between RYR gating and termination properties of the CRU are not at present sufficiently understood to enable quantification.
Physiological significance
This study provides a platform for interpreting the effect of physiological and pathophysiological modulations affecting RYR channels on the activation of spontaneous calcium sparks. From this viewpoint, the spontaneous activation of a single calcium spark reflects the spontaneous opening of a single RYR in the CRU at resting/diastolic cytosolic calcium concentration. The relatively high frequency of calcium sparks with respect to the opening frequency of a single RYR is caused by numerous RYRs in the release unit. The number of RYRs that activate during a calcium spark is a fairly small fraction of all RYRs present in the CRU because of the prevalence of multiple Mg2+-bound RYRs and because of the slow Mg2+ unbinding kinetics. This has important ramifications for the process of calcium release termination: the low number of RYRs activated during the spark and the shielding effect of cytosolic magnesium dampen the positive feedback inherent in the calcium-induced calcium release mechanism and thus increase the room for stochastic attrition of RYR activity.
This study shows that the flat calcium dependence of calcium spark frequency is caused by the very low but significant occurrence of Mg2+-bound RYR open states, the existence of which is a direct consequence of the allosteric mechanism included in the aHTG model. This allosteric principle enables a very smooth control of SR calcium load by regulating the frequency of spontaneous sparks at the low diastolic levels of 50–100 nM Ca2+.
Cellular responses of cardiac myocytes at the level of local calcium release that may result from physiological or pathological stimuli (workload, development, hypoxia, energetic starvation, and morphological remodeling) or from mutations in the RYR protein itself (such as in catecholaminergic polymorphic ventricular tachycardia and arrhythmogenic right ventricular dysplasia diseases) share many common features. These may include changes in the characteristics of RYR channels (divalent ion sensitivity, strength of allosteric coupling, and intrinsic opening tendency), in the intracellular environment (cytosolic concentration of free Ca2+ and Mg2+), or in cellular morphology (density and size of CRUs) treated in this study. In healthy cells, the convergence of cellular responses may contribute to the robustness of physiological behavior as numerous influences that make up the changes in contractility come to the local calcium release by seemingly unspecific pathways. In pathologies, the convergence of cellular responses implies that defects of different origin having the same consequences may have the same treatment, i.e., an intervention that compensates for the defective molecular mechanism indirectly. In other words, the RYR may represent a potential pharmacological target for drugs that may potently intervene with the diastolic open probability of RYRs and thus control the frequency of spontaneous calcium sparks, in effect optimizing the calcium load of the SR and thus improving the priming of myocytes for contraction.
Conclusions
In brief, this study shows that a spontaneous calcium release event (calcium spark) in resting cardiac myocytes results from the spontaneous opening of a single RYR channel, which activates the fraction of RYRs in the CRU that are kinetically available to respond to the local calcium increase. That this is a small fraction of the channels in the couplon explains the low mean number of channels involved in a spark. For the same reason, individual stimulated sparks differ widely in their release flux amplitude as a result of the limited number of RYR channels capable of rapid opening and their ensuing random variation. The frequency of spontaneous calcium sparks and the coupling fidelity of stimulated calcium release are shaped by allosteric coupling of divalent ion binding to RYR channel opening. This mechanism may help to explain the physiological regulation or pathological malfunction of calcium cycling during the diastole and is compatible with the molecular mechanism of the stimulated calcium release during the systole.
Acknowledgments
This work was supported by European Union contracts LSHM-CT-2005–018802/CONTICA and LSHM-CT-2005-018833/EUGeneHeart; by the grants APVT-51-31104, APVV-0139-06/SaFRYR, VEGA 2/0102/08, and VEGA 2/0190/10; and by the European Science Foundation program FuncDyn.
Edward N. Pugh Jr. served as editor.
Footnotes
Abbreviations used in this paper:
- aEMG
- allosteric EMG
- aHTG
- allosteric HTG
- CRU
- calcium release unit
- DHPR
- dihydropyridine receptor
- EMG
- extended minimal gating
- HTG
- homotetrameric gating
References
- Baddeley D., Jayasinghe I.D., Cremer C., Cannell M.B., Soeller C. 2009. Light-induced dark states of organic fluochromes enable 30 nm resolution imaging in standard media. Biophys. J. 96:L22–L24 10.1016/j.bpj.2008.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassani R.A., Bers D.M. 1995. Rate of diastolic Ca release from the sarcoplasmic reticulum of intact rabbit and rat ventricular myocytes. Biophys. J. 68:2015–2022 10.1016/S0006-3495(95)80378-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassani J.W.M., Bassani R.A., Bers D.M. 1994. Relaxation in rabbit and rat cardiac cells: species-dependent differences in cellular mechanisms. J. Physiol. 476:279–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassani J.W.M., Yuan W.L., Bers D.M. 1995. Fractional SR Ca release is regulated by trigger Ca and SR Ca content in cardiac myocytes. Am. J. Physiol. 268:C1313–C1319 [DOI] [PubMed] [Google Scholar]
- Baylor S.M., Hollingworth S. 1998. Model of sarcomeric Ca2+ movements, including ATP Ca2+ binding and diffusion, during activation of frog skeletal muscle. J. Gen. Physiol. 112:297–316 10.1085/jgp.112.3.297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belevych A., Kubalová Z., Terentyev D., Hamlin R.L., Carnes C.A., Györke S. 2007. Enhanced ryanodine receptor-mediated calcium leak determines reduced sarcoplasmic reticulum calcium content in chronic canine heart failure. Biophys. J. 93:4083–4092 10.1529/biophysj.107.114546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bers D.M. 2001. Excitation-Contraction Coupling and Cardiac Contractile Force. Second edition Kluwer Academic Publishers, Boston: 427 pp [Google Scholar]
- Bers D.M., Stiffel V.M. 1993. Ratio of ryanodine to dihydropyridine receptors in cardiac and skeletal muscle and implications for E-C coupling. Am. J. Physiol. 264:C1587–C1593 [DOI] [PubMed] [Google Scholar]
- Bridge J.H., Ershler P.R., Cannell M.B. 1999. Properties of Ca2+ sparks evoked by action potentials in mouse ventricular myocytes. J. Physiol. 518:469–478 10.1111/j.1469-7793.1999.0469p.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridge J.H., Torres N.S., Sobie E.A. 2008. New insights into the structure and function of couplons. J. Physiol. 586:3735 10.1113/jphysiol.2008.159509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brochet D.X., Yang D., Di Maio A., Lederer W.J., Franzini-Armstrong C., Cheng H. 2005. Ca2+ blinks: rapid nanoscopic store calcium signaling. Proc. Natl. Acad. Sci. USA. 102:3099–3104 10.1073/pnas.0500059102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannell M.B., Cheng H., Lederer W.J. 1995. The control of calcium release in heart muscle. Science. 268:1045–1049 10.1126/science.7754384 [DOI] [PubMed] [Google Scholar]
- Cheng H., Lederer W.J. 2008. Calcium sparks. Physiol. Rev. 88:1491–1545 10.1152/physrev.00030.2007 [DOI] [PubMed] [Google Scholar]
- Cheng H., Lederer W.J., Cannell M.B. 1993. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 262:740–744 10.1126/science.8235594 [DOI] [PubMed] [Google Scholar]
- Cheng H., Lederer M.R., Lederer W.J., Cannell M.B. 1996. Calcium sparks and [Ca2+]i waves in cardiac myocytes. Am. J. Physiol. 270:C148–C159 [DOI] [PubMed] [Google Scholar]
- Chen-Izu Y., McCulle S.L., Ward C.W., Soeller C., Allen B.M., Rabang C., Cannell M.B., Balke C.W., Izu L.T. 2006. Three-dimensional distribution of ryanodine receptor clusters in cardiac myocytes. Biophys. J. 91:1–13 10.1529/biophysj.105.077180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copello J.A., Barg S., Sonnleitner A., Porta M., Diaz-Sylvester P., Fill M., Schindler H., Fleischer S. 2002. Differential activation by Ca2+, ATP and caffeine of cardiac and skeletal muscle ryanodine receptors after block by Mg2+. J. Membr. Biol. 187:51–64 10.1007/s00232-001-0150-x [DOI] [PubMed] [Google Scholar]
- Dan P., Lin E., Huang J., Biln P., Tibbits G.F. 2007. Three-dimensional distribution of cardiac Na+-Ca2+ exchanger and ryanodine receptor during development. Biophys. J. 93:2504–2518 10.1529/biophysj.107.104943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domeier T.L., Blatter L.A., Zima A.V. 2009. Alteration of sarcoplasmic reticulum Ca2+ release termination by ryanodine receptor sensitization and in heart failure. J. Physiol. 587:5197–5209 10.1113/jphysiol.2009.177576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham W.J., Wehrens X.H., Sood S., Hamilton S.L. 2007. Diseases associated with altered ryanodine receptor activity. Subcell. Biochem. 45:273–321 10.1007/978-1-4020-6191-2_10 [DOI] [PubMed] [Google Scholar]
- Fabiato A. 1985. Time and calcium dependence of activation and inactivation of calcium-induced release of calcium from the sarcoplasmic reticulum of a skinned canine cardiac Purkinje cell. J. Gen. Physiol. 85:247–289 10.1085/jgp.85.2.247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Velasco M., Rueda A., Rizzi N., Benitah J.P., Colombi B., Napolitano C., Priori S.G., Richard S., Gómez A.M. 2009. Increased Ca2+ sensitivity of the ryanodine receptor mutant RyR2R4496C underlies catecholaminergic polymorphic ventricular tachycardia. Circ. Res. 104:201–209: 12p: 209 10.1161/CIRCRESAHA.108.177493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fill M., Copello J.A. 2002. Ryanodine receptor calcium release channels. Physiol. Rev. 82:893–922 [DOI] [PubMed] [Google Scholar]
- Franzini-Armstrong C., Protasi F., Ramesh V. 1999. Shape, size, and distribution of Ca(2+) release units and couplons in skeletal and cardiac muscles. Biophys. J. 77:1528–1539 10.1016/S0006-3495(99)77000-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaburjakova J., Gaburjakova M. 2006. Comparison of the effects exerted by luminal Ca2+ on the sensitivity of the cardiac ryanodine receptor to caffeine and cytosolic Ca2+. J. Membr. Biol. 212:17–28 10.1007/s00232-006-7018-z [DOI] [PubMed] [Google Scholar]
- Gusev K., Niggli E. 2008. Modulation of the local SR Ca2+ release by intracellular Mg2+ in cardiac myocytes. J. Gen. Physiol. 132:721–730 10.1085/jgp.200810119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Györke I., Györke S. 1998. Regulation of the cardiac ryanodine receptor channel by luminal Ca2+ involves luminal Ca2+ sensing sites. Biophys. J. 75:2801–2810 10.1016/S0006-3495(98)77723-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Györke S., Terentyev D. 2008. Modulation of ryanodine receptor by luminal calcium and accessory proteins in health and cardiac disease. Cardiovasc. Res. 77:245–255 10.1093/cvr/cvm038 [DOI] [PubMed] [Google Scholar]
- Hayashi T., Martone M.E., Yu Z., Thor A., Doi M., Holst M.J., Ellisman M.H., Hoshijima M. 2009. Three-dimensional electron microscopy reveals new details of membrane systems for Ca2+ signaling in the heart. J. Cell Sci. 122:1005–1013 10.1242/jcs.028175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M., Bridge J.H. 2005. Variability in couplon size in rabbit ventricular myocytes. Biophys. J. 89:3102–3110 10.1529/biophysj.105.065862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettlun C., González A., Ríos E., Fill M. 2003. Unitary Ca2+ current through mammalian cardiac and amphibian skeletal muscle ryanodine receptor channels under near-physiological ionic conditions. J. Gen. Physiol. 122:407–417 10.1085/jgp.200308843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubalová Z., Terentyev D., Viatchenko-Karpinski S., Nishijima Y., Györke I., Terentyeva R., da Cuñha D.N., Sridhar A., Feldman D.S., Hamlin R.L., et al. 2005. Abnormal intrastore calcium signaling in chronic heart failure. Proc. Natl. Acad. Sci. USA. 102:14104–14109 10.1073/pnas.0504298102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunze D.L., Lacerda A.E., Wilson D.L., Brown A.M. 1985. Cardiac Na currents and the inactivating, reopening, and waiting properties of single cardiac Na channels. J. Gen. Physiol. 86:691–719 10.1085/jgp.86.5.691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laver D.R., Baynes T.M., Dulhunty A.F. 1997. Magnesium inhibition of ryanodine-receptor calcium channels: evidence for two independent mechanisms. J. Membr. Biol. 156:213–229 10.1007/s002329900202 [DOI] [PubMed] [Google Scholar]
- Li P., Chen S.R. 2001. Molecular basis of Ca2+ activation of the mouse cardiac Ca2+ release channel (ryanodine receptor). J. Gen. Physiol. 118:33–44 10.1085/jgp.118.1.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukyanenko V., Györke S. 1999. Ca2+ sparks and Ca2+ waves in saponin-permeabilized rat ventricular myocytes. J. Physiol. 521:575–585 10.1111/j.1469-7793.1999.00575.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukyanenko V., Subramanian S., Györke I., Wiesner T.F., Györke S. 1999. The role of luminal Ca2+ in the generation of Ca2+ waves in rat ventricular myocytes. J. Physiol. 518:173–186 10.1111/j.1469-7793.1999.0173r.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukyanenko V., Györke I., Subramanian S., Smirnov A., Wiesner T.F., Györke S. 2000. Inhibition of Ca(2+) sparks by ruthenium red in permeabilized rat ventricular myocytes. Biophys. J. 79:1273–1284 10.1016/S0006-3495(00)76381-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukyanenko V., Viatchenko-Karpinski S., Smirnov A., Wiesner T.F., Györke S. 2001. Dynamic regulation of sarcoplasmic reticulum Ca(2+) content and release by luminal Ca(2+)-sensitive leak in rat ventricular myocytes. Biophys. J. 81:785–798 10.1016/S0006-3495(01)75741-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx S.O., Gaburjakova J., Gaburjakova M., Henrikson C., Ondrias K., Marks A.R. 2001. Coupled gating between cardiac calcium release channels (ryanodine receptors). Circ. Res. 88:1151–1158 10.1161/hh1101.091268 [DOI] [PubMed] [Google Scholar]
- Meissner G., Darling E., Eveleth J. 1986. Kinetics of rapid Ca2+ release by sarcoplasmic reticulum. Effects of Ca2+, Mg2+, and adenine nucleotides. Biochemistry. 25:236–244 10.1021/bi00349a033 [DOI] [PubMed] [Google Scholar]
- Mejía-Alvarez R., Kettlun C., Ríos E., Stern M., Fill M. 1999. Unitary Ca2+ current through cardiac ryanodine receptor channels under quasi-physiological ionic conditions. J. Gen. Physiol. 113:177–186 10.1085/jgp.113.2.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy E., Steenbergen C., Levy L.A., Raju B., London R.E. 1989. Cytosolic free magnesium levels in ischemic rat heart. J. Biol. Chem. 264:5622–5627 [PubMed] [Google Scholar]
- Paavola J., Viitasalo M., Laitinen-Forsblom P.J., Pasternack M., Swan H., Tikkanen I., Toivonen L., Kontula K., Laine M. 2007. Mutant ryanodine receptors in catecholaminergic polymorphic ventricular tachycardia generate delayed after depolarizations due to increased propensity to Ca2+ waves. Eur. Heart J. 28:1135–1142 10.1093/eurheartj/ehl543 [DOI] [PubMed] [Google Scholar]
- Poláková E., Zahradníková A., Jr., Pavelková J., Zahradník I., Zahradníková A. 2008. Local calcium release activation by DHPR calcium channel openings in rat cardiac myocytes. J. Physiol. 586:3839–3854 10.1113/jphysiol.2007.149989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J., Valle G., Nani A., Nori A., Rizzi N., Priori S.G., Volpe P., Fill M. 2008. Luminal Ca2+ regulation of single cardiac ryanodine receptors: insights provided by calsequestrin and its mutants. J. Gen. Physiol. 131:325–334 10.1085/jgp.200709907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ríos E., Brum G. 2002. Ca2+ release flux underlying Ca2+ transients and Ca2+ sparks in skeletal muscle. Front. Biosci. 7:d1195–d1211 [DOI] [PubMed] [Google Scholar]
- Rose W.C., Balke C.W., Wier W.G., Marban E. 1992. Macroscopic and unitary properties of physiological ion flux through L-type Ca2+ channels in guinea-pig heart cells. J. Physiol. 456:267–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sham J.S., Song L.S., Chen Y., Deng L.H., Stern M.D., Lakatta E.G., Cheng H. 1998. Termination of Ca2+ release by a local inactivation of ryanodine receptors in cardiac myocytes. Proc. Natl. Acad. Sci. USA. 95:15096–15101 10.1073/pnas.95.25.15096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G.D., Keizer J.E., Stern M.D., Lederer W.J., Cheng H. 1998. A simple numerical model of calcium spark formation and detection in cardiac myocytes. Biophys. J. 75:15–32 10.1016/S0006-3495(98)77491-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobie E.A., Song L.S., Lederer W.J. 2005. Local recovery of Ca2+ release in rat ventricular myocytes. J. Physiol. 565:441–447 10.1113/jphysiol.2005.086496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeller C., Crossman D., Gilbert R., Cannell M.B. 2007. Analysis of ryanodine receptor clusters in rat and human cardiac myocytes. Proc. Natl. Acad. Sci. USA. 104:14958–14963 10.1073/pnas.0703016104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L.S., Sham J.S., Stern M.D., Lakatta E.G., Cheng H. 1998. Direct measurement of SR release flux by tracking ‘Ca2+ spikes’ in rat cardiac myocytes. J. Physiol. 512:677–691 10.1111/j.1469-7793.1998.677bd.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L.S., Pi Y., Kim S.J., Yatani A., Guatimosim S., Kudej R.K., Zhang Q., Cheng H., Hittinger L., Ghaleh B., et al. 2005. Paradoxical cellular Ca2+ signaling in severe but compensated canine left ventricular hypertrophy. Circ. Res. 97:457–464 10.1161/01.RES.0000179722.79295.d4 [DOI] [PubMed] [Google Scholar]
- Stern M.D. 1992. Theory of excitation-contraction coupling in cardiac muscle. Biophys. J. 63:497–517 10.1016/S0006-3495(92)81615-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern M.D., Song L.S., Cheng H., Sham J.S., Yang H.T., Boheler K.R., Ríos E. 1999. Local control models of cardiac excitation-contraction coupling. A possible role for allosteric interactions between ryanodine receptors. J. Gen. Physiol. 113:469–489 10.1085/jgp.113.3.469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szentesi P., Pignier C., Egger M., Kranias E.G., Niggli E. 2004. Sarcoplasmic reticulum Ca2+ refilling controls recovery from Ca2+-induced Ca2+ release refractoriness in heart muscle. Circ. Res. 95:807–813 10.1161/01.RES.0000146029.80463.7d [DOI] [PubMed] [Google Scholar]
- Terentyev D., Viatchenko-Karpinski S., Valdivia H.H., Escobar A.L., Györke S. 2002. Luminal Ca2+ controls termination and refractory behavior of Ca2+-induced Ca2+ release in cardiac myocytes. Circ. Res. 91:414–420 10.1161/01.RES.0000032490.04207.BD [DOI] [PubMed] [Google Scholar]
- Terentyev D., Nori A., Santoro M., Viatchenko-Karpinski S., Kubalová Z., Györke I., Terentyeva R., Vedamoorthyrao S., Blom N.A., Valle G., et al. 2006. Abnormal interactions of calsequestrin with the ryanodine receptor calcium release channel complex linked to exercise-induced sudden cardiac death. Circ. Res. 98:1151–1158 10.1161/01.RES.0000220647.93982.08 [DOI] [PubMed] [Google Scholar]
- Valent I., Zahradníková A., Pavelková J., Zahradník I. 2007. Spatial and temporal Ca2+, Mg2+, and ATP2- dynamics in cardiac dyads during calcium release. Biochim. Biophys. Acta. 1768:155–166 10.1016/j.bbamem.2006.08.020 [DOI] [PubMed] [Google Scholar]
- Vatner D.E., Sato N., Kiuchi K., Shannon R.P., Vatner S.F. 1994. Decrease in myocardial ryanodine receptors and altered excitation-contraction coupling early in the development of heart failure. Circulation. 90:1423–1430 [DOI] [PubMed] [Google Scholar]
- Wang S.Q., Stern M.D., Ríos E., Cheng H. 2004. The quantal nature of Ca2+ sparks and in situ operation of the ryanodine receptor array in cardiac cells. Proc. Natl. Acad. Sci. USA. 101:3979–3984 10.1073/pnas.0306157101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano M., Yamamoto T., Ikeda Y., Matsuzaki M. 2006. Mechanisms of disease: ryanodine receptor defects in heart failure and fatal arrhythmia. Nat. Clin. Pract. Cardiovasc. Med. 3:43–52 10.1038/ncpcardio0419 [DOI] [PubMed] [Google Scholar]
- Zahradník I., Györke S., Zahradníková A. 2005. Calcium activation of ryanodine receptor channels—reconciling RyR gating models with tetrameric channel structure. J. Gen. Physiol. 126:515–527 10.1085/jgp.200509328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahradníková A., Zahradník I. 1996. A minimal gating model for the cardiac calcium release channel. Biophys. J. 71:2996–3012 10.1016/S0006-3495(96)79492-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahradníková A., Zahradník I., Györke I., Györke S. 1999. Rapid activation of the cardiac ryanodine receptor by submillisecond calcium stimuli. J. Gen. Physiol. 114:787–798 10.1085/jgp.114.6.787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahradníková A., Dura M., Györke I., Escobar A.L., Zahradník I., Györke S. 2003. Regulation of dynamic behavior of cardiac ryanodine receptor by Mg2+ under simulated physiological conditions. Am. J. Physiol. Cell Physiol. 285:C1059–C1070 [DOI] [PubMed] [Google Scholar]
- Zahradníková A., Kubalová Z., Pavelková J., Györke S., Zahradník I. 2004. Activation of calcium release assessed by calcium release-induced inactivation of calcium current in rat cardiac myocytes. Am. J. Physiol. Cell Physiol. 286:C330–C341 10.1152/ajpcell.00272.2003 [DOI] [PubMed] [Google Scholar]
- Zahradníková A., Jr., Poláková E., Zahradník I., Zahradníková A. 2007. Kinetics of calcium spikes in rat cardiac myocytes. J. Physiol. 578:677–691 10.1113/jphysiol.2006.117796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y.Y., Song L.S., Lakatta E.G., Xiao R.P., Cheng H. 1999. Constitutive beta2-adrenergic signalling enhances sarcoplasmic reticulum Ca2+ cycling to augment contraction in mouse heart. J. Physiol. 521:351–361 10.1111/j.1469-7793.1999.00351.x [DOI] [PMC free article] [PubMed] [Google Scholar]