Introduction
Relatively recent developments in our understanding of sarcomeric proteins have expanded their role from the home of molecular motors generating force and shortening to a cellular organelle fully integrated in the control of structural, electrical, mechanical, chemical, and metabolic homeostasis. Even so, in some cases these diverse functions of sarcomeric proteins appear to remain a curiosity, not fully appreciated in the analysis of major controllers of cardiac function. This attitude toward the function of sarcomeric proteins in cardiac myocytes is summarized in the following definition of “curiosity,” which seems particularly apropos: “meddlesome; thrusting oneself into and taking an active part in others’ affairs.” We focus in this Perspective on how sarcomeric proteins function in integration with membrane channels and transporters in control of cardiac dynamics, especially in adrenergic control of cardiac function. Understanding these mechanisms at the level of cardiac sarcomeres took on special significance with the identification of mutations in sarcomeric proteins as the most common cause of familial hypertrophic and dilated cardiomyopathies. These mutations commonly lead to structural, electrical, and metabolic remodeling and to sudden death. These disorders indicate a critical role of processes at the level of the sarcomeres in homeostatic control of cardiac energetics, dynamics, and structure. Yet, control of Ca2+ delivery to and removal from the myofilaments has dominated discussions of mechanisms regulating cardiac contractility. We first provide an alternative perspective in which rate processes at the level of the sarcomeres appear to be dominant during the rise and maintenance of systolic elastance and of isovolumic relaxation. A discussion of established adrenergic mechanisms and newly understood anti-adrenergic mechanisms controlling sarcomere response to Ca2+ follows and expands on this perspective.
Are kinetic processes intrinsic to the cardiac sarcomeres rate limiting in the contraction–relaxation cycle of the heart?
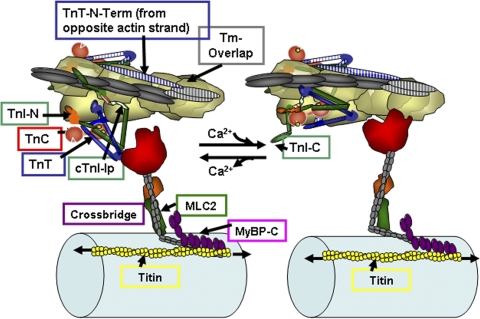
During the cardiac cycle, there is universal agreement that Ca2+ released from stores in the myocytes binds to troponin C (TnC) and sets into motion a set of protein–protein interactions, which promote an ATP-dependent reaction of cross-bridges with actin, inducing force (pressure) and shortening (ejection). Reversal of these protein–protein interactions occurs with Ca2+ removal from the sarcoplasm. Fig. 1 illustrates protein–protein interactions among sarcomeric proteins in thin filament functional regulatory units: seven actins in 1:1:1:1 complex with tropomyosin (Tm), TnC, troponin I (TnI), and troponin T (TnT). The thin filament unit on the left is relaxed, and the one on the right has been activated by Ca2+ binding to TnC. Fig. 1, which also shows a depiction of the thick filament with cross-bridges and a portion of titin, emphasizes the rich array of potential protein–protein interactions for control of force and shortening downstream of Ca2+ binding to TnC. The origins of the cartoon in Fig. 1 have been reviewed elsewhere (Kobayashi and Solaro, 2005) and include recent structural information (Galińska-Rakoczy et al., 2008). In diastolic levels of cellular Ca2+, the thin filament regulatory units remain relaxed via the actions of components of Tn, which immobilize Tm and depress the reactivity of actin with myosin. Fig. 1 illustrates a new concept of the protein interactions, which wedge and immobilize Tm with actin-binding peptides of TnI binding on one side of Tm and the N-terminal extension of TnT arising from a Tn on the adjacent actin strand binding to Tm on the other side. Cardiac TnI (cTnI) interacts with actin via two regions, a highly basic inhibitory peptide (Ip) and a second actin-binding region. These regions flank a switch peptide, which binds to cTnC when Ca2+ binds to cTnC. The C lobe of TnC intersects with near N-terminal regions of TnI with C-terminal regions of TnT. New evidence indicates that the C-terminal region or mobile domain of cTnI (cTnI-C in Fig. 1) binds to the thin filament strand of actins with the far C terminus lying across and directly interacting with Tm. This state of the thin filament either blocks cross-bridges from reacting with actin or holds weakly bound cross-bridges in a nonforce-generating state. Both blocked and weakly bound cross-bridges may also coexist. Binding of Ca2+ to a single regulatory site at the N lobe of TnC activates the thin filament regulatory unit. Key reactions in this activation are an altered interaction of TnT with Tm and the promotion of an interaction of the TnI switch peptide with TnC, thereby releasing TnI actin-binding peptides from their inhibitory location. The mobile domain of TnI most likely no longer interacts with actin or Tm. Actin–cross-bridge reactions are promoted as Tm becomes mobile and actin reactivity is enhanced. The cross-bridge lever arm rotates as indicated in Fig. 1, thereby promoting force and shortening.
Figure 1.
A section of cardiac thin filaments illustrating the position of troponin components with Tm in the OFF state (left) and ON state (right). Thin filaments are shown containing TnI, TnT, and TnC. cTnC, a dumbbell-shaped protein with an N lobe containing a single regulatory Ca-binding site (yellow spheres) and a C lobe containing slowly exchanged Ca2+/Mg2+ sites. In the OFF state, Tm is immobile and held in place by TnI, which is shown tethered to actin on an actin strand by an Ip and a second actin-binding region flanking a switch peptide (Sw), which interacts with cTnC upon Ca activation. Note that the Ip of cTnI binds to one actin strand, whereas the N-terminal tail of TnT interacts with Tm on the adjacent actin strand. The distal C-terminal end of cTnI drapes across azimuthal actins and may interact with Tm. TnI has a unique stretch of N-terminal amino acids, which contain phosphorylation sites at S23 and S24. The N peptide interacts with the N lobe of cTnC, but it is released upon phosphorylation. With release of Ca2+, there is a release of cTnI from actin and binding of the switch peptide to cTnC. Activation is associated with release of Tm from an immobilized state by protein signaling to cTnT and possible release of Tm from an interaction with the C-terminal end of cTnI. See Are kinetic processes intrinsic to… for further description.
Fig. 1 also illustrates regulatory domains and proteins that significantly affect the response of the myofilaments to Ca2+. We emphasize here a unique N-terminal peptide of TnI, which contains two prominent phosphorylation sites at S23 and S24 that are substrates for PKA, PKG, PKD, and PKC-δ (Solaro, 2008). When S23 and S24 are not phosphorylated, the N-terminal peptide binds to the N lobe of TnC and Ca2+ affinity is relatively high. With phosphorylation of S23 and S24, the peptide is released from TnC and there is drop in Ca2+ affinity due to an enhanced off rate for Ca2+ exchange. Thus, the sensitivity of force generation to Ca2+ is lower when these TnI sites are phosphorylated. There is also evidence (Biesiadecki et al., 2007), albeit controversial (Sadayappan et al., 2005), that the phosphorylation of S23 and S24 also increases turnover kinetics of the actin–cross-bridge reaction. Fig. 1 also emphasizes the modulation of myofilament response to Ca2+ by thick filament–related mechanisms. These mechanisms involve myosin light chain 2 (MLC2), myosin-binding protein C (MyBP-C), and titin. All three of these proteins control the propensity of interaction of the cross-bridges with actin by altering the relative position of the cross-bridges with respect to the thick filament proper. MLC2 and MyBP-C restrict radial movements of cross-bridges and thus control their local concentration in the vicinity of the actins (Colson et al., 2010). Phosphorylation controls this action of MLC2 and MyBP-C. Fig. 1 shows only a small portion of the giant protein titin, which extends from the Z disk to the M line. Titin is largely responsible for the passive tension of cardiac myocytes and interacts with MyBP-C. Strong evidence indicates that stretch of titin in diastole can affect systolic function by an interaction with MyBP-C, which promotes the radial movement of cross-bridges away from the thick filament.
Sarcomeric mechanisms controlling dynamics of the heart beat
With the advent of convenient to use fluorescent probes, many laboratories gained the ability to measure the transient change in Ca2+ in isolated ventricular myocytes. Implicit in the interpretation of changes in the Ca2+-transient amplitude and dynamics was that they define contractility. Yet, in general, transient changes in Ca2+ are out of phase with the dynamics of the mechanical events. For example, during increases in isometric tension, Ca2+ rises faster than force and pressure (Kurihara and Allen, 1982; Backx et al., 1995; Baran et al., 1997; Stull et al., 2002). There is evidence that tension (pressure and systolic elastance) is maintained during the heart beat via processes at the level of the sarcomeric proteins. Relaxation also appears dominated by processes at the level of the sarcomeric proteins.
Studies of rate processes in the rise and maintenance of systole in the cardiac beat indicate an important rate limitation by the turnover kinetics of the force-generating reactions of the cross-bridges. During this rising phase of the heart beat, Ca2+ is released into the myofilament space. There are no direct measurements of the relative in situ binding, but investigations of rates of Ca2+ binding to cTnC in myofilament preparations with cycling cross-bridges indicate that the rate constant for binding is most likely diffusion limited and not rate limiting (Davis et al., 2007). The subsequent steps in the activation of thin filaments have been measured with the aid of fluorescent probes, and it is apparent that they may not be rate limiting as well (Stehle and Iorga, 2010). This indicates that the kinetic cycle of the cross-bridge reaction with actin is most likely the slow, rate-limiting process in the rise of ventricular pressure to the point of opening of the aortic valve. After the valve opens, ejection begins and systolic elastance is maintained. An important and unresolved issue is the state of the myofilaments and the relative amounts of bound Ca2+ during shortening. Again, we have no direct estimates of bound Ca2+, but indirect evidence indicates that the fall in Ca2+ bound to cTnC in an isometric twitch occurs well ahead of the drop in force. Peterson et al. (1991) estimated amounts of Ca2+ bound from the rise in tension after quick detachment of cross-bridges. Bound Ca2+ was estimated from the initial rate of force redevelopment. These studies demonstrated that when the isometric twitch force was near maximum, bound Ca2+ had fallen to ∼50% of its peak, whereas when force had fallen to 50% of its max, bound Ca2+ was <10% of maximum. Previous modeling (Burkhoff, 1994; Hinken and Solaro, 2007) had also predicted this temporal difference between activation of the myofilaments by Ca2+ and the activation of tension. This prediction raises the question as to the mechanism of maintenance of systolic elastance. In our judgment, an excellent candidate is an induction of cooperative activation of the myofilaments, which recruits cross-bridges into force-generating states in regulatory units that do not contain bound Ca2+. The dynamics of this cooperative activation have been estimated in rat hearts from measurements of the pressure change induced by rapid, specified volume changes during a beat. The data and the predictions indicated that the time course of activation by Ca2+ was relatively brief and of low relative magnitude compared with the time course of cooperative activation, which more closely coincided with tension, and was the dominant mechanism late in systole. An issue not fully resolved is how this cooperative activation reverses fast enough so as to not impair relaxation. Campbell et al. (2008) have addressed this issue in their model of the dynamics of left ventricular elastance, which incorporates a decay of the cooperative activation via what they term “elastic distortion dynamics.”
The role and mechanism of cooperative activation of the sarcomeres remains unclear
The molecular mechanism of cooperative activation also remains controversial. Measurements of the steady-state relation between force generation and Ca2+ provide strong evidence for cooperativity in activation of the sarcomeres. Hill coefficients for this relation are generally 3–5 despite the presence of a single regulatory site on cTnC (Pan and Solaro, 1987). Two general theories are based on either a thick filament–dominated process with positive feedback of cycling, force-generating cross-bridges on activation (Moss et al., 2004), or a thin filament–based longitudinal spread of activation (Tobacman and Butters, 2000; Sun et al., 2009), which allosterically recruits blocked cross-bridges to a force generation reaction or recruits weakly bound cross-bridges to a force-generating state. Data supporting a role for strongly bound cross-bridges in promotion of the active state of the thin filament are derived from studies using noncycling rigor cross-bridges or pseudo-strong cross-bridges in the form of NEM-S1 (Moss et al., 2004). Yet, when tested using cycling cross-bridges, a thick filament–based cooperative mechanism has not been supported. Moreover, two lines of experiments support the thin filament–based mechanism. In our studies (Davis et al., 2007), we measured Ca2+ binding to the regulatory site of cTnC in reconstituted thin filaments alone or reacting with myosin S-1 in the presence and absence of MgATP. There were similar cooperative Hill coefficients (nH = 1.65), Ca2+-binding affinities, and off rates of Ca2+ exchange with the cTnC regulatory site in both thin filaments alone and thin filaments plus S-1-MgATP. Ca2+ affinity increased with a significant decrease in cooperativity (nH = 0.85), and the off rate slowed in the presence of rigor cross-bridges. Studies using fluorescent probes as sensors also demonstrated no effect of cycling cross-bridges of the on state of cTnC (Solaro, 2009b; Sun et al., 2009). These data indicate that the influence of strongly bound cross-bridges on thin filament activation may be most relevant during pathological states in which long-lived cross-bridge interactions are present. The thin filament–based mechanism is appealing, but it requires more experimental proof. Ideally, it would be of great benefit to have an agent or a genetic approach specifically altering cooperative activation and applicable in the setting of the in situ beating heart.
Cross-bridge activity during systolic shortening and isovolumic relaxation
As outlined and discussed by Stehle and Iorga (2010), an unresolved issue is the question of what cross-bridges are doing during systolic shortening. Estimates of energy consumption indicate that the number of turnover cycles is about one per cross-bridge, yet the sarcomeres shorten by ∼10%. If sarcomere length is ∼2,000 nM, the thin filaments in a half-sarcomere move ∼100 nM. One cycle would be expected to move the thin filaments in a half-sarcomere by 8–10 nM. These considerations indicate that the cross-bridge may, in fact, act as thermal ratchet as proposed by Kitamura et al. (1999), repeatedly attaching and detaching from actin, but splitting only one ATP. In any case, the dwell time of the cross-bridge force-generating interaction with the thin filament would be expected to be an important determinant of the duration of systolic elastance. Evidence supporting this idea comes from the effects of an inotropic agent (CK-1827452) under clinical development and demonstrated to directly interact with myosin and to prolong the duration of the reaction of cross-bridges with actin and to prolong systole (Solaro, 2009a; Teerlink, 2009). Thick filament–associated proteins are also critical determinants of systolic elastance. Palmer et al. (2004) reported an abbreviation of the time course and peak amplitude of systolic elastance of hearts with myofilaments missing MyBP-C. Moreover, Scruggs et al. (2009) reported an abbreviation of the duration of systolic elastance in hearts expressing a non-phosphorylatable mutant, MLC2. These modifications appear to alter the radial movements of the myosin heads from the thick filament backbone, thereby altering the local concentrations of cross-bridges in the vicinity of the thin filaments (Colson et al., 2010).
With the end of systole, isovolumic relaxation begins, and there is strong evidence that this phase of the cardiac cycle is also dominated by processes at the level of the sarcomeres. Insights into what sarcomeres do during isovolumic relaxation have been substantially influenced by studies at the myofibrillar level of organization (Stehle et al., 2009). With a sudden drop in Ca2+, sarcomeres remain isometric for a time during which there is a relatively slow and relatively brief linear phase of relaxation of force, followed by a rapid fall in force associated with sequential propagated re-lengthening of sarcomeres. The initial slow phase of relaxation involves the reversal of thin filament activation initiated by Ca2+ release from cTnC, which is significantly slower than Ca2+ binding to cTnC (Davis et al., 2007). Whether the fall in force during this initial period of relaxation is rate limited by Ca2+ release from cTnC is not clear. Replacement of cTnI with slow skeletal TnI increases myofibrillar sensitivity to Ca2+ and slows the off rate of Ca2+ exchange with cTnC, but does not affect the relaxation kinetics of myofibrils held isometric. On the other hand, compared with wild-type hearts, ejecting hearts expressing slow skeletal TnI, a non-phosphorylatable isoform, demonstrate a significant impairment of relaxation kinetics, whereas isovolumic hearts do not (Layland et al., 2005). Similar findings have been reported with hearts expressing cTnI pseudo-phosphorylated at S23 and S24 or missing the N-terminal extension. Moreover, in studies with myofibrils, deletion of Lys184 in the mobile C-terminal domain of cTnI impaired the fast phase of relaxation (Iorga et al., 2008). This mutation alters the ability of cTn to switch off cross-bridge cycling but not turnover kinetics. These data indicate a role for thin filament–related mechanisms and emphasize the complexity of the processes at the level of the sarcomeres that determine relaxation kinetics.
With this perspective on the role of processes at the level of sarcomeres as major determinants of the dynamics of the cardiac cycle, it follows that we need to understand control mechanisms modifying these processes in physiological states as well in inherited and acquired disorders of the heart. We next discuss physiological control mechanisms with emphasis on a recently discovered anti-adrenergic pathway involving receptor signaling to protein phosphatase 2A (PP2A).
Yin and yang and adrenergic control of cellular Ca2+ fluxes and sarcomere response to Ca2+
The response of the heart to β-adrenergic stimulation provides an excellent example of how sarcomere response to Ca2+ and dynamics are coordinately controlled by the same signaling cascades that control Ca2+ fluxes. In exercise, heart rate and contractility increase, but at the same time, the overall cycle time of the heart beat must be abbreviated so as not to impair filling time. The activation of β-adrenergic signaling promotes phosphorylation of regulatory proteins (Ca2+ channel subunits, phospholamban, and ryanodine receptors) of channels and transporters and promotes an increased release of Ca2+ to the sarcomeres and increased uptake of Ca2+ by the sarcoplasmic reticulum (for review see Bers, 2000). However, in a yin and yang–like manner, there is depression of sarcomere responsiveness to Ca2+, owing largely to the phosphorylation of the unique N-terminal serines of TnI (Kobayashi and Solaro, 2005). The concept of yin and yang described as “how…seemingly contrary forces are interconnected and interdependent in the natural world, and how they give rise to each other in turn” is especially appropriate to describe alterations in thin filament mechanisms in β-adrenergic control of cardiac dynamics, There is strong evidence from many lines of investigation, nicely summarized in an editorial by Ramirez-Correa and Murphy (2007), that especially when the studies are performed at physiological temperatures, phosphorylation of TnI accounts is a major mechanism in the lusitrophic effect of β-adrenergic stimulation. With β-adrenergic stimulation, there is also evidence that cross-bridge turnover kinetics are enhanced. At the thick filament level there is phosphorylation of both MLC2 and MyBP-C, which stimulates cross-bridge turnover rate. Phosphorylation of cTnI has also been demonstrated to increase cross-bridge kinetics, but as mentioned, the effect is controversial (Tong et al., 2008). The movement of the cross-bridges away from the thick filament is controlled by phosphorylation of both MLC2 and MyBP-C (Colson et al., 2010) and is the most likely mechanism whereby these proteins control entry of cross-bridges into the force-generating state.
Novel control of sarcomeric and cardiac function by signaling to PP2A
One of the major unresolved questions in this yin and yang control process is phosphatase-regulated control of the processes at the level of the myofilaments. Studies in our laboratories support the hypothesis that a major control mechanism involves signaling to PP2A by p21-activated kinase (Pak) (for review see Sheehan et al., 2007). Pak is a multifunctional kinase activated by Cdc42 and Rac1 in a Gi-coupled receptor signaling pathway. We reported that Pak coimmunoprecipitated with PP2A in both ventricular and sino-atrial nodal cells. When expressed as a constitutively active enzyme in ventricular myocytes, Pak1 induced a faster time to peak shortening, but a significantly slower rate of [Ca2+]i decay and time of re-lengthening compared with controls expressing LacZ (Sheehan et al., 2009). The active Pak1 also induced an anti-adrenergic effect in blunting the response to isoproterenol. Moreover, spontaneous sarcoplasmic reticulum Ca2+ release sparks were significantly smaller in amplitude in active Pak1 than LacZ-expressing myocytes. These results fit with our findings of a Pak1-induced dephosphorylation of TnI and L-type Ca2+ channels. Surprisingly, there was no change in phospholamban phosphorylation at either Ser 16 or Ser 17 (Sheehan et al., 2009). These data indicated that the yin and yang of adrenergic stimulation involves an active control mechanism in which Ca2+ release unit activity is suppressed and sarcomeric Ca2+ sensitivity is promoted, but active uptake by the sarcoplasmic reticulum is not altered by PLN. There is evidence that the predominant control of PLN dephosphorylation is via protein phosphatase 1. An important aspect of the Pak1-related control mechanism is that it also opposes the effects of β-adrenergic stimulation on heart rate at the sino-atrial nodal cells (Sheehan et al., 2007). In summary, activated β-1 receptors signal through Gi to enhance heart rate and promote Ca2+ fluxes and cross-bridge turnover kinetics, but reduce thin filament Ca2+ responsiveness. Activation of receptor signaling through Gi in a cascade involving Pak1 does the opposite, except for PLN phosphorylation. Current studies are aimed at identifying the Gi-coupled receptors that signal though Pak1/PP2a.
Our laboratories have reported evidence that Gi-coupled signaling through bradykinin (Ke et al., 2010) and sphingolipid receptors (Egom et al., 2010a) is able to activate Pak1 and PP2A in cardiac myocytes. Both of these receptors had been demonstrated to activate Pak1 in other cell types. We think these pathways are significant not only in physiological control of cardiac output, but also as important mechanisms in cardio protection in ischemia/reperfusion injury (Egom et al., 2010b). Treatment of cardiac myocytes with bradykinin induced an increased auto-phosphorylation at Pak1 at Thr 423, and, as we had seen with the viral-driven expression of active Pak1, there was redistribution of Pak1 to the cytoplasm from sites localized at or near Z disk locations. In this receptor-stimulated pathway, there was a reduced phosphorylation of both PLN and cTnI, and, as expected, there was a reduced response to isoproterenol in myocytes treated with bradykinin. In our studies related to sphingolipid signaling and activation of Pak, we tested the effects of sphingosine-1-phosphate, which is well recognized as a critical lipid operating through a family of G protein–coupled receptors with downstream signaling to diverse cellular processes. We have also tested the effects of a sphingosine-1-phosphate analogue, FTY 720 (fingolimod, 2-amino-2-[-2-(4-octyphenyl)ethyl]propane-1,3-diolhydrochloride), derived from a fungus, Iscaria sinclarii, well known in Chinese herbal medicine. FTY 720 is undergoing current clinical trials for the treatment of primary progressive multiple sclerosis. Our studies demonstrated that FTY720 is able to prevent arrhythmias induced by simulated ischemia and reperfusion in association with the activation of Pak1. There was also activation of Akt. Based on our studies with active Pak1, our hypothesis is that the activation of the Pak1 signaling may be anti-arrhythmic by suppression of Ca2+ release from the sarcoplasmic reticulum and the myofilaments in ischemia reperfusion injury.
Future prospects
Current understanding of the relative role and control of the kinetics of sarcomeric processes in the dynamics of the heart beat demands full integration of this critical organelle into our perception of molecular processes regulating the heart beat. The implications are profound with regard to treatment and understanding of prevalent acquired and familial disorders of the heart, and to our understanding of how missense mutations of a sarcomeric protein link to a signaling cascade instigating malignant remodeling and growth of cardiac myocytes. We also need to more thoroughly understand the link between sudden death and altered myofilament Ca2+ signaling and buffering associated with mutations linked to hypertrophic and dilated cardiomyopathies. We think that further studies on the cascades signaling through PP2A by Pak1 also add a new dimension to the search for potential therapeutic agents for cardiac disorders.
Acknowledgments
Work described here is supported by grants from the National Institutes of Health (NHLBI RO1 HL 064035, RO1 HL 022231, and PO1 HL 062626 to R.J. Solaro) and by the Wellcome Trust (to M. Lei).
Footnotes
Abbreviations used in this paper:
- Ip
- inhibitory peptide
- MLC2
- myosin light chain 2
- MyBP-C
- myosin-binding protein C
- Pak
- p21-activated kinase
- PP2A
- protein phosphatase 2A
- Tm
- tropomyosin
- TnC
- troponin C
- TnI
- troponin I
- TnT
- troponin T
References
- Backx P.H., Gao W.D., Azan-Backx M.D., Marban E. 1995. The relationship between contractile force and intracellular [Ca2+] in intact rat cardiac trabeculae. J. Gen. Physiol. 105:1–19 10.1085/jgp.105.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baran D., Ogino K., Stennett R., Schnellbacher M., Zwas D., Morgan J.P., Burkhoff D. 1997. Interrelating of ventricular pressure and intracellular calcium in intact hearts. Am. J. Physiol. 273:H1509–H1522 [DOI] [PubMed] [Google Scholar]
- Bers D.M. 2000. Calcium fluxes involved in control of cardiac myocyte contraction. Circ. Res. 87:275–281 [DOI] [PubMed] [Google Scholar]
- Biesiadecki B.J., Kobayashi T., Walker J.S., John Solaro R., de Tombe P.P. 2007. The troponin C G159D mutation blunts myofilament desensitization induced by troponin I Ser23/24 phosphorylation. Circ. Res. 100:1486–1493 10.1161/01.RES.0000267744.92677.7f [DOI] [PubMed] [Google Scholar]
- Burkhoff D. 1994. Explaining load dependence of ventricular contractile properties with a model of excitation-contraction coupling. J. Mol. Cell. Cardiol. 26:959–978 10.1006/jmcc.1994.1117 [DOI] [PubMed] [Google Scholar]
- Campbell K.B., Simpson A.M., Campbell S.G., Granzier H.L., Slinker B.K. 2008. Dynamic left ventricular elastance: a model for integrating cardiac muscle contraction into ventricular pressure-volume relationships. J. Appl. Physiol. 104:958–975 10.1152/japplphysiol.00912.2007 [DOI] [PubMed] [Google Scholar]
- Colson B.A., Locher M.R., Bekyarova T., Patel J.R., Fitzsimons D.P., Irving T.C., Moss R.L. 2010. Differential roles of regulatory light chain and myosin binding protein-C phosphorylations in the modulation of cardiac force development. J. Physiol. 588:981–993 10.1113/jphysiol.2009.183897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J.P., Norman C., Kobayashi T., Solaro R.J., Swartz D.R., Tikunova S.B. 2007. Effects of thin and thick filament proteins on calcium binding and exchange with cardiac troponin C. Biophys. J. 92:3195–3206 10.1529/biophysj.106.095406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egom E.E., Ke Y., Musa H., Mohamed T.M., Wang T., Cartwright E., Solaro R.J., Lei M. 2010a. FTY720 prevents ischemia/reperfusion injury-associated arrhythmias in an ex vivo rat heart model via activation of Pak1/Akt signaling. J. Mol. Cell. Cardiol. 48:406–414 10.1016/j.yjmcc.2009.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egom E.E., Ke Y., Solaro R.J., Lei M. 2010b. Cardioprotection in ischemia/reperfusion injury: spotlight on sphingosine-1-phosphate and bradykinin signalling. Prog. Biophys. Mol. Biol. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galińska-Rakoczy A., Engel P., Xu C., Jung H., Craig R., Tobacman L.S., Lehman W. 2008. Structural basis for the regulation of muscle contraction by troponin and tropomyosin. J. Mol. Biol. 379:929–935 10.1016/j.jmb.2008.04.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinken A.C., Solaro R.J. 2007. A dominant role of cardiac molecular motors in the intrinsic regulation of ventricular ejection and relaxation. Physiology (Bethesda). 22:73–80 [DOI] [PubMed] [Google Scholar]
- Iorga B., Blaudeck N., Solzin J., Neulen A., Stehle I., Lopez Davila A.J., Pfitzer G., Stehle R. 2008. Lys184 deletion in troponin I impairs relaxation kinetics and induces hypercontractility in murine cardiac myofibrils. Cardiovasc. Res. 77:676–686 10.1093/cvr/cvm113 [DOI] [PubMed] [Google Scholar]
- Ke Y., Sheehan K.A., Egom E.E., Lei M., Solaro R.J. 2010. Novel bradykinin signaling in adult rat cardiac myocytes through activation of p21-activated kinase. Am. J. Physiol. Heart Circ. Physiol. 298:H1283–H1289 10.1152/ajpheart.01070.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura K., Tokunaga M., Iwane A.H., Yanagida T. 1999. A single myosin head moves along an actin filament with regular steps of 5.3 nanometres. Nature. 397:129–134 10.1038/16403 [DOI] [PubMed] [Google Scholar]
- Kobayashi T., Solaro R.J. 2005. Calcium, thin filaments, and the integrative biology of cardiac contractility. Annu. Rev. Physiol. 67:39–67 10.1146/annurev.physiol.67.040403.114025 [DOI] [PubMed] [Google Scholar]
- Kurihara S., Allen D.G. 1982. Intracellular Ca++ transients and relaxation in mammalian cardiac muscle. Jpn. Circ. J. 46:39–43 [DOI] [PubMed] [Google Scholar]
- Layland J., Solaro R.J., Shah A.M. 2005. Regulation of cardiac contractile function by troponin I phosphorylation. Cardiovasc. Res. 66:12–21 10.1016/j.cardiores.2004.12.022 [DOI] [PubMed] [Google Scholar]
- Moss R.L., Razumova M., Fitzsimons D.P. 2004. Myosin crossbridge activation of cardiac thin filaments: implications for myocardial function in health and disease. Circ. Res. 94:1290–1300 10.1161/01.RES.0000127125.61647.4F [DOI] [PubMed] [Google Scholar]
- Palmer B.M., Georgakopoulos D., Janssen P.M., Wang Y., Alpert N.R., Belardi D.F., Harris S.P., Moss R.L., Burgon P.G., Seidman C.E., et al. 2004. Role of cardiac myosin binding protein C in sustaining left ventricular systolic stiffening. Circ. Res. 94:1249–1255 10.1161/01.RES.0000126898.95550.31 [DOI] [PubMed] [Google Scholar]
- Pan B.S., Solaro R.J. 1987. Calcium-binding properties of troponin C in detergent-skinned heart muscle fibers. J. Biol. Chem. 262:7839–7849 [PubMed] [Google Scholar]
- Peterson J.N., Hunter W.C., Berman M.R. 1991. Estimated time course of Ca2+ bound to troponin C during relaxation in isolated cardiac muscle. Am. J. Physiol. 260:H1013–H1024 [DOI] [PubMed] [Google Scholar]
- Ramirez-Correa G.A., Murphy A.M. 2007. Is phospholamban or troponin I the “prima donna” in beta-adrenergic induced lusitropy? Circ. Res. 101:326–327 10.1161/CIRCRESAHA.107.158873 [DOI] [PubMed] [Google Scholar]
- Sadayappan S., Gulick J., Osinska H., Martin L.A., Hahn H.S., Dorn G.W., II, Klevitsky R., Seidman C.E., Seidman J.G., Robbins J. 2005. Cardiac myosin-binding protein-C phosphorylation and cardiac function. Circ. Res. 97:1156–1163 10.1161/01.RES.0000190605.79013.4d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scruggs S.B., Hinken A.C., Thawornkaiwong A., Robbins J., Walker L.A., de Tombe P.P., Geenen D.L., Buttrick P.M., Solaro R.J. 2009. Ablation of ventricular myosin regulatory light chain phosphorylation in mice causes cardiac dysfunction in situ and affects neighboring myofilament protein phosphorylation. J. Biol. Chem. 284:5097–5106 10.1074/jbc.M807414200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan K.A., Ke Y., Solaro R.J. 2007. p21-Activated kinase-1 and its role in integrated regulation of cardiac contractility. Am. J. Physiol. Regul. Integr. Comp. Physiol. 293:R963–R973 [DOI] [PubMed] [Google Scholar]
- Sheehan K.A., Ke Y., Wolska B.M., Solaro R.J. 2009. Expression of active p21-activated kinase-1 induces Ca2+ flux modification with altered regulatory protein phosphorylation in cardiac myocytes. Am. J. Physiol. Cell Physiol. 296:C47–C58 10.1152/ajpcell.00012.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solaro R.J. 2008. Multiplex kinase signaling modifies cardiac function at the level of sarcomeric proteins. J. Biol. Chem. 283:26829–26833 10.1074/jbc.R800037200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solaro R.J. 2009a. CK-1827452, a sarcomere-directed cardiac myosin activator for acute and chronic heart disease. IDrugs. 12:243–251 [PubMed] [Google Scholar]
- Solaro R.J. 2009b. Maintaining cooperation among cardiac myofilament proteins through thick and thin. J. Physiol. 587:3 10.1113/jphysiol.2008.166751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehle R., Iorga B. 2010. Kinetics of cardiac sarcomeric processes and rate-limiting steps in contraction and relaxation. J. Mol. Cell. Cardiol. 48:843–850 10.1016/j.yjmcc.2009.12.020 [DOI] [PubMed] [Google Scholar]
- Stehle R., Solzin J., Iorga B., Poggesi C. 2009. Insights into the kinetics of Ca2+-regulated contraction and relaxation from myofibril studies. Pflugers Arch. 458:337–357 10.1007/s00424-008-0630-2 [DOI] [PubMed] [Google Scholar]
- Stull L.B., Leppo M.K., Marbán E., Janssen P.M. 2002. Physiological determinants of contractile force generation and calcium handling in mouse myocardium. J. Mol. Cell. Cardiol. 34:1367–1376 10.1006/jmcc.2002.2065 [DOI] [PubMed] [Google Scholar]
- Sun Y.B., Lou F., Irving M. 2009. Calcium- and myosin-dependent changes in troponin structure during activation of heart muscle. J. Physiol. 587:155–163 10.1113/jphysiol.2008.164707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teerlink J.R. 2009. A novel approach to improve cardiac performance: cardiac myosin activators. Heart Fail. Rev. 14:289–298 10.1007/s10741-009-9135-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobacman L.S., Butters C.A. 2000. A new model of cooperative myosin-thin filament binding. J. Biol. Chem. 275:27587–27593 [DOI] [PubMed] [Google Scholar]
- Tong C.W., Stelzer J.E., Greaser M.L., Powers P.A., Moss R.L. 2008. Acceleration of crossbridge kinetics by protein kinase A phosphorylation of cardiac myosin binding protein C modulates cardiac function. Circ. Res. 103:974–982 10.1161/CIRCRESAHA.108.177683 [DOI] [PMC free article] [PubMed] [Google Scholar]